Abstract
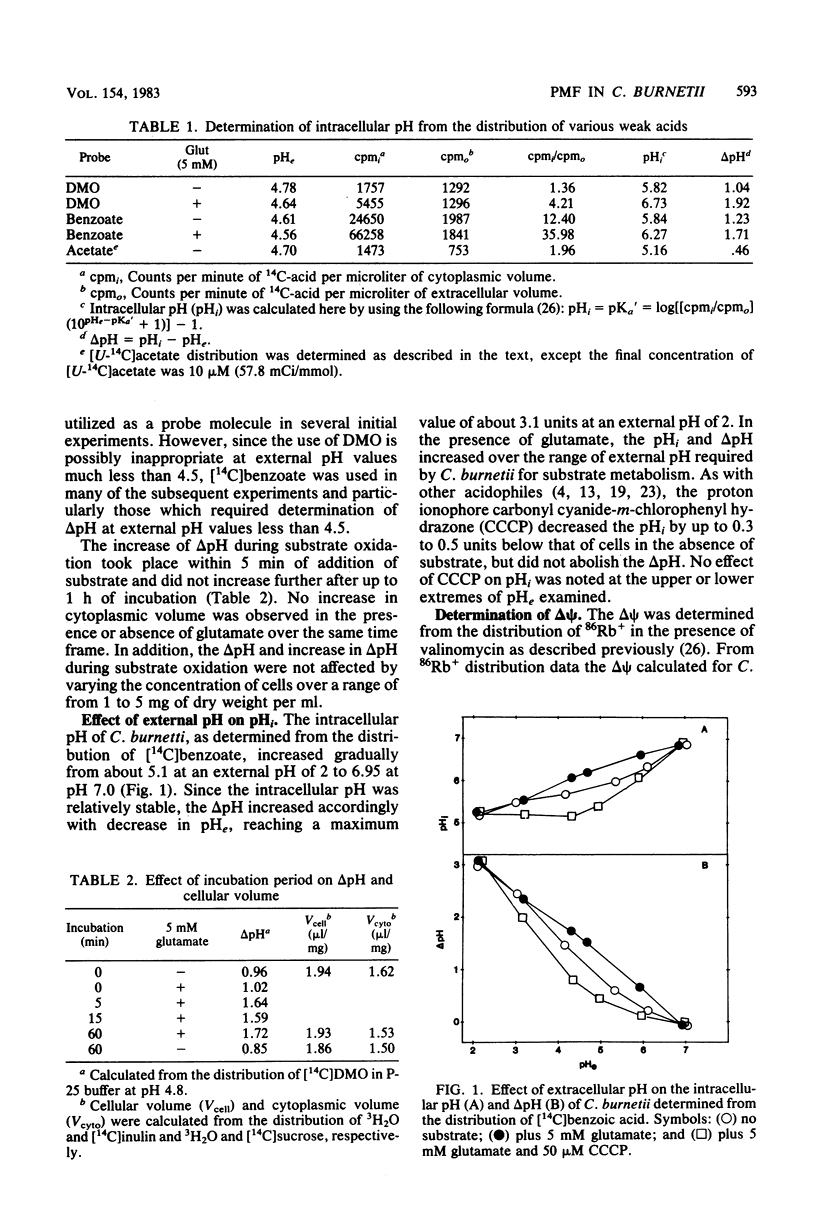
The magnitude of the proton motive force generated during in vitro substrate oxidation by Coxiella burnetii was examined. The intracellular pH of C. burnetii varied from about 5.1 to 6.95 in resting cells over an extracellular pH range of 2 to 7. Similarly, delta psi varied from about 15 mV to -58 mV over approximately the same range of extracellular pH. Both components of the proton motive force increased during substrate oxidation, resulting in an increase in proton motive force from about -92 mV in resting cells to -153 mV in cells metabolizing glutamate at pH 4.2. The respiration-dependent increase in proton motive force was blocked by respiratory inhibitors, but the delta pH was not abolished even by the addition of proton ionophores such as carbonyl cyanide-m-chlorophenyl hydrazone or 2,4-dinitrophenol. Because of this apparently passive component of delta pH maintenance, the largest proton motive force was obtained at an extracellular pH too low to permit respiration. C. burnetii appears, therefore, to behave in many respects like other acidophilic bacteria. Such responses are proposed to contribute to the extreme resistance of C. burnetii to environmental conditions and subsequent activation upon entry into the phagolysosome of eucaryotic cells in which this organism multiplies.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addanki A., Cahill F. D., Sotos J. F. Determination of intramitochondrial pH and intramitochondrial-extramitochondrial pH gradient of isolated heart mitochondria by the use of 5,5-dimethyl-2,4-oxazolidinedione. I. Changes during respiration and adenosine triphosphate-dependent transport of Ca++, Mg++, and Zn++. J Biol Chem. 1968 May 10;243(9):2337–2348. [PubMed] [Google Scholar]
- Burton P. R., Kordová N., Paretsky D. Electron microscopic studies of the rickettsia Coxiella burneti: entry, lysosomal response, and fate of rickettsial DNA in L-cells. Can J Microbiol. 1971 Feb;17(2):143–150. doi: 10.1139/m71-025. [DOI] [PubMed] [Google Scholar]
- Burton P. R., Stueckemann J., Welsh R. M., Paretsky D. Some ultrastructural effects of persistent infections by the rickettsia Coxiella burnetii in mouse L cells and green monkey kidney (Vero) cells. Infect Immun. 1978 Aug;21(2):556–566. doi: 10.1128/iai.21.2.556-566.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. C., Nicholls D. G., Ingledew W. J. Transmembrane electrical potential and transmembrane pH gradient in the acidophile Thiobacillus ferro-oxidans. Biochem J. 1979 Jan 15;178(1):195–200. doi: 10.1042/bj1780195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guffanti A. A., Davidson L. F., Mann T. M., Krulwich T. A. Nigericin-induced death of an acidophilic bacterium. J Gen Microbiol. 1979 Sep;114(1):201–206. doi: 10.1099/00221287-114-1-201. [DOI] [PubMed] [Google Scholar]
- Hackstadt T., Williams J. C. Biochemical stratagem for obligate parasitism of eukaryotic cells by Coxiella burnetii. Proc Natl Acad Sci U S A. 1981 May;78(5):3240–3244. doi: 10.1073/pnas.78.5.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T., Williams J. C. Stability of the adenosine 5'-triphosphate pool in Coxiella burnetii: influence of pH and substrate. J Bacteriol. 1981 Nov;148(2):419–425. doi: 10.1128/jb.148.2.419-425.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T., Williams J. C. pH dependence of the Coxiella burnetii glutamate transport system. J Bacteriol. 1983 May;154(2):598–603. doi: 10.1128/jb.154.2.598-603.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsung J. C., Haug A. Intracellular pH of Thermoplasma acidophila. Biochim Biophys Acta. 1975 May 21;389(3):477–482. doi: 10.1016/0005-2736(75)90158-3. [DOI] [PubMed] [Google Scholar]
- Hsung J. C., Haug A. Membrane potential of Thermoplasma acidophila. FEBS Lett. 1977 Jan 15;73(1):47–50. doi: 10.1016/0014-5793(77)80011-2. [DOI] [PubMed] [Google Scholar]
- Krulwich T. A., Davidson L. F., Filip S. J., Jr, Zuckerman R. S., Guffanti A. A. The protonmotive force and beta-galactoside transport in Bacillus acidocaldarius. J Biol Chem. 1978 Jul 10;253(13):4599–4603. [PubMed] [Google Scholar]
- Maloney P. C., Kashket E. R., Wilson T. H. A protonmotive force drives ATP synthesis in bacteria. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3896–3900. doi: 10.1073/pnas.71.10.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A., Wilson B., Zychlinsky E., Matin M. Proton motive force and the physiological basis of delta pH maintenance in thiobacillus acidophilus. J Bacteriol. 1982 May;150(2):582–591. doi: 10.1128/jb.150.2.582-591.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaul T. F., Williams J. C. Developmental cycle of Coxiella burnetii: structure and morphogenesis of vegetative and sporogenic differentiations. J Bacteriol. 1981 Sep;147(3):1063–1076. doi: 10.1128/jb.147.3.1063-1076.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Oshima T., Arakawa H., Baba M. Biochemical studies on an acidophilic, thermophilic bacterium, Bacillus acidocaldarius: isolation of bacteria, intracellular pH, and stabilities of biopolymers. J Biochem. 1977 Apr;81(4):1107–1113. doi: 10.1093/oxfordjournals.jbchem.a131535. [DOI] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Schuldiner S. pH homeostasis in bacteria. Biochim Biophys Acta. 1981 Dec;650(2-3):151–166. doi: 10.1016/0304-4157(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Raven J. A., Smith F. A. The evolution of chemiosmotic energy coupling. J Theor Biol. 1976 Apr;57(2):301–312. doi: 10.1016/0022-5193(76)90003-5. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Rauch B., Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1977 Nov 10;252(21):7850–7861. [PubMed] [Google Scholar]
- Wiebe M. E., Burton P. R., Shankel D. M. Isolation and characterization of two cell types of Coxiella burneti phase I. J Bacteriol. 1972 Apr;110(1):368–377. doi: 10.1128/jb.110.1.368-377.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Peacock M. G., McCaul T. F. Immunological and biological characterization of Coxiella burnetii, phases I and II, separated from host components. Infect Immun. 1981 May;32(2):840–851. doi: 10.1128/iai.32.2.840-851.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


