Abstract
Monoclonal antibodies with reactivity to vaccinia virus specific proteins are useful reagents to study the proteins as well as to help understand aspects of the poxvirus life cycle. Using a vaccinia virus proteomics microarray, we found a hybridoma (MAb 3015B2) from a vaccinia virus vaccinated mouse that reacted with the product of the E3L gene. The specificity to the E3 protein was confirmed by Western blotting and immunofluorescence of cells infected with either wild-type vaccinia virus or a mutant virus with the E3L gene deleted. Antibody reactivity with E3 was also seen in cells transfected with a plasmid expressing the E3 protein. A panel of mutated vaccinia viruses with truncations in the E3L gene revealed that while MAb 3015B2 reacted with E3 lacking the C-terminal 7 amino acids, it lost reactivity with a mutant E3 lacking the C-terminal 26 amino acids. This indicates that the antigenic site recognized by 3015B2 is on the C-terminus, somewhere between amino acids 164 through 183. The antibody also recognizes the E3 protein encoded by other orthopoxviruses. This antibody will be useful for further investigations of the E3 protein as well as a useful reagent to indicate vaccinia virus early protein expression.
Keywords: Vaccinia virus, E3L, monoclonal antibody, early protein expression
Vaccinia virus (VACV) is a large double-stranded DNA virus and a member of the Orthopoxviridae genus. This virus genus includes the variola virus, the causative agent of smallpox. VACV contains an estimated 190 genes and replicates in the cytoplasm of infected cells. The process of VACV gene expression is divided into early, intermediate, and late. Early gene transcription begins upon viral entry into cells; however, in order for transcription of the intermediate and then late genes, viral DNA replication must occur (Moss, 2001).
One early gene is the E3L gene (WR059), which encodes a 190-amino acid protein (Chang and Jacobs, 1993). There are two domains, and each of these domains appears to play a role in evading the cellular antiviral response. The C-terminal domain binds double-stranded RNA (dsRNA) (Chang and Jacobs, 1993), while the N-terminal domain has been shown to bind Z-DNA (Kwon and Rich, 2005; Langland et al., 2006). It is the C-terminal dsRNA-binding domain that has been shown to be responsible for the interferon (IFN) resistance in VACV-infected cells. In fact, when this domain is deleted, VACV is no longer IFN resistant (Shors et al., 1998). Because E3 binds dsRNA, protein kinase R and 2′– 5′ A oligoadenylate synthetase are not activated, and translation can occur within the infected cell, along with viral replication (Chang et al., 1992; Rivas et al., 1998). One function the N-terminal domain is thought to have is to regulate host gene expression by binding Z-DNA (Kwon and Rich, 2005; Langland et al., 2006). Unlike the C-terminal domain, the N-terminal domain is not needed for IFN resistance of VACV; however, it is needed for VACV virulence in mice (Brandt and Jacobs, 2001; Kim et al., 2003). There are conflicting data as to whether host gene expression is activated or repressed by binding to the N-terminal of E3 to Z-DNA. Using microarray analysis of cells infected with VACV virus that have the N-terminal deleted, it was shown that the expression of some genes involved in the inflammatory response were increased. This led researchers to conclude that when the N-terminal of E3 binds Z-DNA, it blocks the expression of these inflammatory response genes (Langland et al., 2006). In a different study, using the transfection of a plasmid expressing E3 in uninfected cells, it was shown that when the N-terminal of E3 binds Z-DNA, it activates certain host genes that are involved a wide array of cellular activities including apoptosis and the immune response (Kwon and Rich, 2005). Thus the exact effect of Z-DNA binding by the N-terminal domain of E3 during a VACV infection is not known.
Monoclonal antibodies with reactivity to vaccinia virus specific proteins are useful reagents to study the proteins as well as to help understand aspects of the poxvirus life cycle. To generate anti-VACV hybridomas, a BALB/c mouse was vaccinated with VACV (strain WR, ~4 × 106 pfu) intraperitoneally two times at one-month intervals. Two months after a second vaccination, the mouse was sacrificed, and the spleen was harvested for fusion. Initial hybridomas were screened using a combination of ELISA reactivity to lysates of VACV-infected cells, as well as a viral growth inhibition assay. We then selected a panel of hybridomas to study using a VACV proteomics microarray (Davies et al., 2005) to identify viral proteins the selected hybridoma supernatants were reacting with. This screen revealed that one of the hybridoma supernatants (designated 3015B2-B5; IgG subtype 3) appeared to react with the VACV protein encoded by the E3L gene (Figure 1A).
Figure 1.
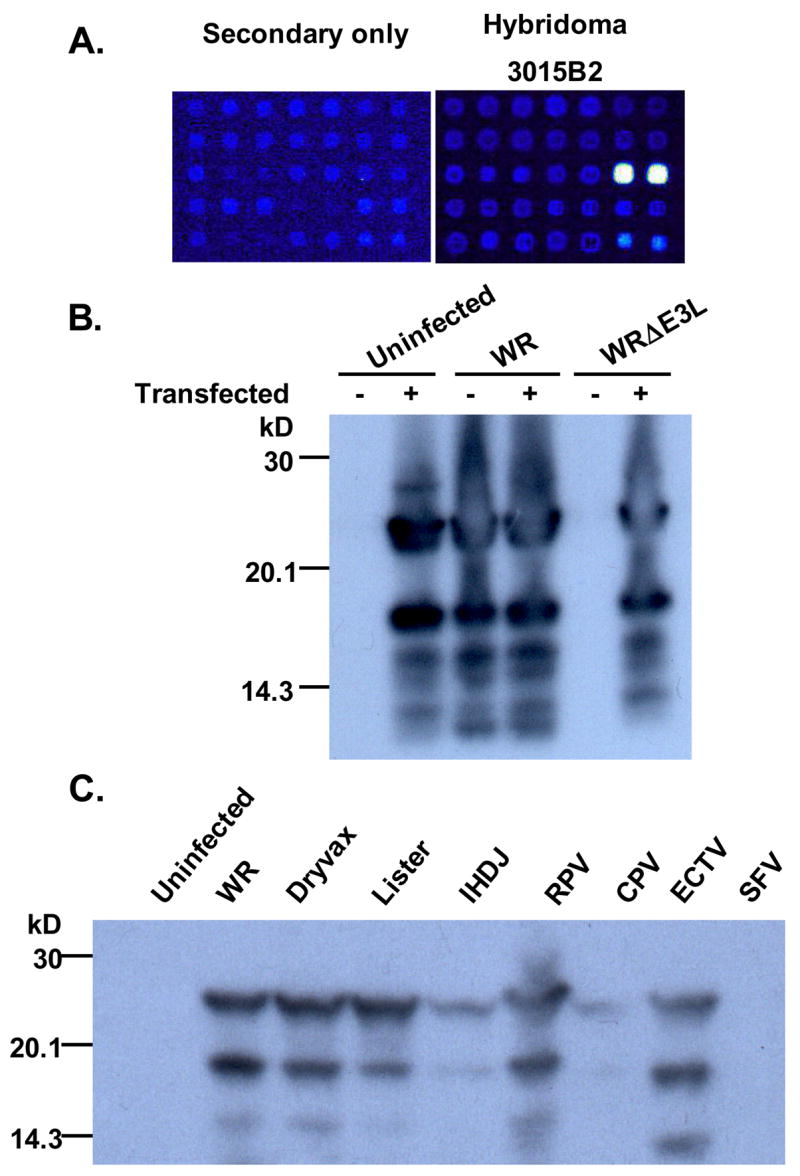
Identification of MAb 3015B2 binding to the vaccinia virus (VACV) E3 protein. (A) A region of the proteome microarray surrounding the E3 spots printed in duplicate. WR proteome arrays were fabricated as described (Davies et al., 2005) and probed with undiluted hybridoma culture supernatants (hybridoma 3015B2), or blocking buffer alone as a control (secondary only). This was followed by mouse anti-IgG (gamma chain) secondary antibody (Jackson Immunoresearch) and bound antibodies visualized with streptavivdin-PBXL3 (Martek Biosciences). After washing slides were air dried under brief centrifugation and examined in a ScanArray ExpressHT confocal Scanner (PerkinElmer). (B) A Western blot showing that MAb 3015B2 is reacting specifically to VACV E3 protein. The indicated lanes contain RK-13 cell lysates from a 6-well plate that were either untransfected or transfected for 24 hours with 2 μg pMT-E3L, a plasmid expressing the VACV E3 protein, using FuGene 6 (Roche) according to the manufacturer’s instructions. Cells were then either left uninfected or infected with wild-type virus (WR) or the E3L-deletion virus (WRΔE3L) for 24 hours after transfection. Cells were harvested, resuspended in RIPA buffer with protease inhibitors, and resolved on a pre-cast 16% Tris-glycine acrylamide gel (Invitrogen) under reducing and denaturing conditions. After transfer to a PVDF membrane (Amersham), blocking with 10% non-fat dry milk in phosphate-buffered saline (PBS) containing 0.2% Tween-20 (PBS-T), the blot was probed with hybridoma generated mouse ascites at a 1:500 dilution. The blot was then washed with PBS-T, probed with horse anti-mouse HRP (Vector Labs) at 1:20,000 dilution, and washed with PBS-T. Protein-antibody interactions were visualized by adding a chemiluminescent reagent (Pierce) after which the membranes were exposed to film (Kodak). (C) A Western blot showing that MAb 3015B2 is reacting to E3 protein from different orthopoxviruses. RK-13 cells were either uninfected or infected with vaccinia virus strains WR, Dryvax®, Lister, and IHDJ, or infected with cowpox virus (CPV) or ectromelia virus (ECTV) or Shope fibroma virus (SFV) in a 6-well plate for 48 hours. Lysates were run on a pre-cast 16% Tris-glycine acrylamide gel (Invitrogen), and after transferring, the membrane was probed with MAb 3015B2 (purified IgG at a concentration of 2 μg/ml). The blot was washed with PBS-T, probed with horse anti-mouse HRP (Vector Labs) at a 1:10,000 dilution, and washed again with PBS-T.
To confirm the reactivity of this MAb to E3, we performed Western blots using both wild-type VACV (strain WR) infected cells and cells infected with an E3L-deletion virus (WRΔE3L) (Chang et al., 1995), as well as cells transfected with a plasmid expressing E3L (pMT-E3L) under an adenovirus major late promoter (Chang et al., 1995). Because WRΔE3L replicates and produces progeny in RK-13 cells, but not BSC-1 cells (Chang et al., 1995), we performed all infections and transfections in RK-13 cells to ensure expression of all viral proteins in WRΔE3L infected cells. As seen in Figure 1B, uninfected/untransfected cells do not react with the antibody, while cells transfected with pMT-E3L results in two major bands of 25- and 19-kD. The same bands are seen in cells infected with the wild-type virus (WR). Importantly, no reactivity is seen in cells infected with the E3L-deletion virus (WRΔE3L). However, reactivity is once again seen in cells initially transfected with the pMT-E3L and then infected with the WRΔE3L virus. These two bands of 25- and 19-kD for the E3L gene product have been previously reported and are thought to be due to two potential initiation sites (Yuwen et al., 1993). We also tested another previously isolated anti-E3 MAb (TW2.3) (Yuwen et al., 1993) by Western blotting but did not find reactivity with this antibody to VACV-infected cell lysates or with cells transfected with pMT-E3L (data not shown).
Because a future use of MAb 3015B2 may be as a marker of VACV early protein transcription, we performed infections in the presence of cytosine arabinoside (Ara-C). Ara-C inhibits VACV DNA synthesis, and thus only early viral protein expression occurs (Cochran et al., 1985). RK-13 cells were infected with WR virus or with the WRΔE3L virus and incubated for 18 hours in the presence or absence of Ara-C. Cells were lysed and Western blots were performed. Similar to Figure 1B, Western blots revealed no E3-specific bands in WRΔE3L but bands in WR-infected cells in the presence or absence of Ara-C (data not shown). This confirms the ability of the antibody to recognize early protein expression in VACV-infected cells by Western blotting. We also wanted to confirm that the antibody could recognize the E3 protein ortholog in other orthopoxviruses. We therefore infected RK-13 cells with the viruses indicated in Figure 1C and found some reactivity in all the orthopoxviruses indicating that the MAb recognized E3 from multiple viruses. This is not surprising given the high amino acid homology of E3’s (>90% similarity) among the orthopoxvirus genus. We did not find an E3-specific band in RK-13 cells infected with a Leporipoxvirus (rabbit fibroma virus), which has only ~70% similarity over the C-terminal dsRNA-binding domain.
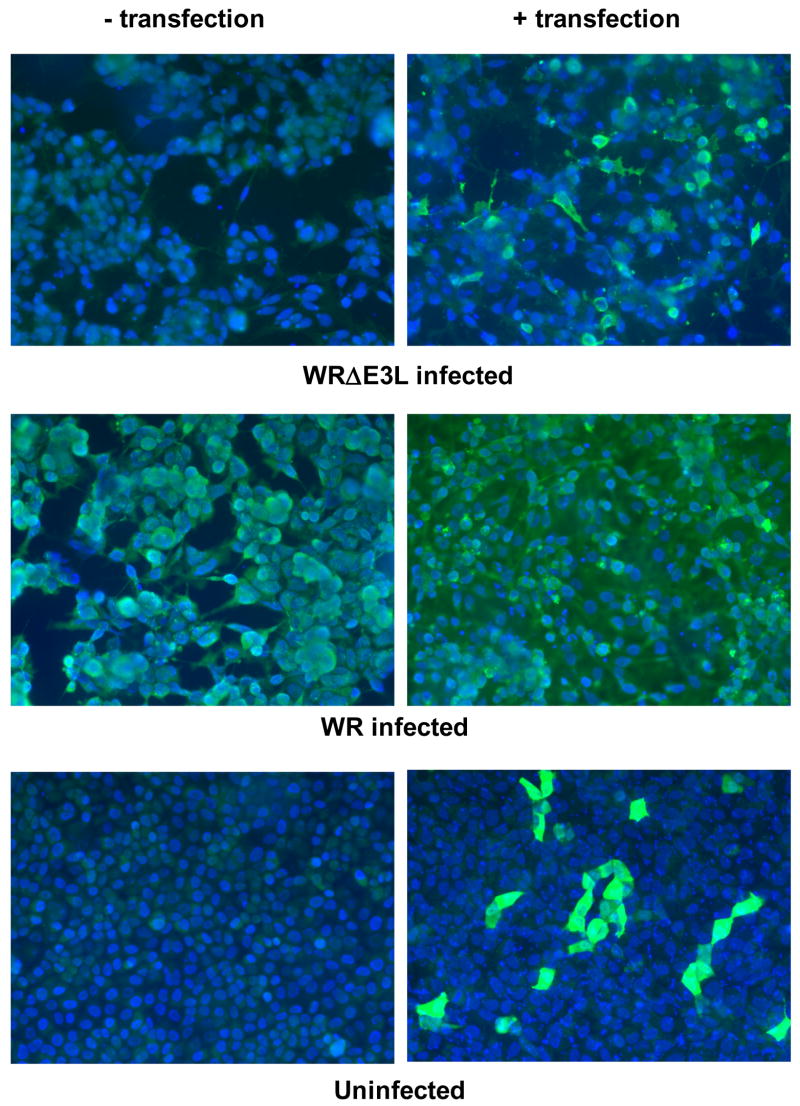
We next examined reactivity of 3015B2 with permeabilized cells by immunofluorescence (IF). As shown in Figure 2, uninfected/untransfected cells do not react with the antibody, while cells transfected with pMT-E3L result in a signal from transfected cells. This signal is also seen in cells infected with wild-type virus (WR). Importantly, no signal is seen in cells infected with the E3L-deletion virus (WRΔE3L). However, a signal above background is again seen in cells initially transfected with pMT-E3L and then infected with WRΔE3L. As opposed to the inability of TW2.3 to work on Western blots, TW2.3 showed a similar fluorescence signal by IF (data not shown). This was not surprising since the reactivity of TW2.3 by IF had been previously reported (Yuwen et al., 1993).
Figure 2.
Immunofluorescence (IF) showing that MAb 3015B2 is reacting specifically to VACV E3 protein. RK-13 cells seeded on an 8-chamber glass microscope slide (Nunc) were either untransfected or transfected for 24 hours with 2 μg of pMT-E3L using FuGene 6 reagent (Roche). Cells were then either left uninfected or infected with WR or WRΔE3L viruses for 24 hours. Cells were then fixed with 4% formalin, permeabilized with 0.1% Triton X-100 in PBS, and then probed with hybridoma generated mouse ascites at a 1:200 dilution. Cells were then washed with 3% bovine serum albumin (3% BSA-PBS), probed with goat anti-mouse immunoglobulin G conjugated to fluorescein isothiocyanate (FITC) (Zymed) at a 1:200 dilution, and washed with PBS. The nuclei of the cells were stained with DAPI (Vector Labs). Cells were visualized using a Nikon Eclipse E1000 fluorescence microscope with the appropriate filters and analyzed using Phase 3 Imaging software.
Once we showed that the MAb 3015B2 specifically binds the E3 protein, we mapped where protein binding occurs using available mutant VACV containing N- or C-terminal deletions (Kibler et al., 1997; Shors et al., 1997). We obtained two C-terminal domain deletion viruses, WRΔ7C and WRΔ26C, and one N-terminal domain deletion virus, WRΔ37N (Kibler et al., 1997; Shors et al., 1997). The WRΔ7C virus has 7 amino acids (184 through 190) deleted on the C-terminal domain, while the WRΔ26C virus has 26 amino acids (165 through 190) deleted from the C-terminus of the protein. In vivo, the WRΔ26C deletion virus has a phenotype similar to the WRΔE3L virus (Langland et al., 2006). This is most likely due to the fact that binding of dsRNA occurs at the C-terminal and is no longer functional in the WRΔ26C virus (Chang and Jacobs, 1993; Shors et al., 1997). The WRΔ37N virus has amino acids 1 through 37 deleted on the N-terminal domain. This deletion has not been shown to have an effect on WR virus replication in cultured cells; however, the N-terminal domain of E3 is required for full pathogenesis of WR virus in mice (Brandt and Jacobs, 2001; Kim et al., 2003). We performed Western blot analysis and immunofluorescence using this panel of viruses. As seen in Figure 3A, Western blotting with MAb 3015B2 of gels run under reduced and denatured conditions (Figure 3A, top), as well as gels run under “native” (non-reduced, non-denatured conditions; Figure 3A, bottom) results in similar bands from cells infected with wild-type virus (WR) and the E3L mutant virus lacking the C-terminal 7 amino acids (WRΔ7C). However, no reactivity is seen in cells infected with the E3L-mutant virus lacking the C-terminal 26 amino acids (WRΔ26C). We confirmed proper loading of sample by probing the same blot with an anti-β-actin antibody. This indicates that the MAb requires the amino acids between 164 and 183 for binding. It is interesting to note that this region of the protein in cowpox virus (Brighton) contains a single amino acid difference when compared to vaccinia virus strain WR at residue 181 (lysine to threonine). Since we saw a dim band on western blots in cells infected with cowpox virus (Figure 1C), this may mean that this amino acid is important for antibody binding. It is also interesting to note that a similar weak reactivity is seen in cells infected with vaccinia virus strain IHDJ. While this strain of vaccinia virus has not been sequenced, based on the reactivity we see on western blot (Figure 1C), we might predict that there is amino acid change(s) between 164 and 183. MAb 3015B2 reacted with the mutant E3L virus with a 37 amino acid N-terminal truncation. Because this protein is missing 37 amino acids, the band is smaller than wild-type and is missing the second initiation start site. Again, the previously isolated anti-E3 MAb TW2.3 (Yuwen et al., 1993) did not react on western blots, even on gels run under “native” (non-reducing, non-denaturing) conditions (data not shown).
Figure 3.
MAb 3015B2 requires the C-terminal 26 amino acids for optimal reactivity. (A) A Western blot showing that MAb 3015B2 does not react with WRΔ26C-infected cell lysate. RK-13 cells were infected with the indicated virus in a 6-well plate for 24 hours, and lysates were run on a pre-cast 16% Tris-glycine acrylamide gel (Invitrogen) and after transferring, the membrane was probed with MAb 3015B2 (purified IgG at a concentration of 2 μg/ml). The blot of a gel run under reducing and denaturing conditions (top) or a gel run under “native” (non-reducing, non-denaturing) conditions (bottom) were washed with PBS-T, probed with horse anti-mouse HRP (Vector Labs) at 1:10,000 dilution, and washed again with PBS-T. As a loading control, the blot was probed with an anti-β-actin antibody (Santa Cruz Biotechnology Inc.) at 1:2000 dilution. (B) Immunofluorescence showing that MAb 3015B2 gives a much lower signal to WRΔ26C-infected cells than to cells infected with either WR, WRΔ7C, or WRΔ37N. RK-13 cells were infected with the indicated virus for 24 hours. Permeabilized cells were probed with mouse ascites at a 1:200 dilution, washed with 3% BSA-PBS, probed with goat anti-mouse immunoglobulin G conjugated-FITC at a 1:200 dilution, and washed with 1X PBS. The nuclei of the cells were stained with DAPI (Vector Labs). Cells were visualized using a Nikon Eclipse E1000 fluorescence microscope with the appropriate filters and analyzed using Phase 3 Imaging software.
Immunofluorescence of infected cells led to similar conclusions (Figure 3B). MAb 3015B2 gives an obvious signal for permeabilized cells infected with WR, WRΔ7C virus, and WRΔ37N virus. The WRΔ26C virus infected cells gave a much lower and more variable intensity of signal when compared to cells infected with the other viruses. It is not clear why on Western blots this antibody results in no signal with cells infected with WRΔ26C, while there is a variable signal by IF. Perhaps IF reveals that a small portion of the epitope recognized by MAb 3015B2 is still present and full IF reactivity is partially dependent on protein conformation. It is interesting to note that MAb TW2.3 (Yuwen et al., 1993) showed similar results to MAb 3015B2 when tested by IF with cells infected with WR, WRΔ7C, and WRΔ37N. However, unlike MAb 3015B2, MAb TW2.3 gives no signal in WRΔ26C virus infected cells (data not shown). Thus MAb TW2.3 recognizes an epitope on E3 that relies on amino acids between 164 and 183. This is consistent with prior immunoprecipitation data using in vitro translation products of N-terminal truncations of E3 that showed that MAb TW2.3 was binding to the C-terminal of E3 (Yuwen et al., 1993). However, because MAb TW2.3 does not work on denaturing Western blots, TW2.3 likely recognizes a conformational epitope.
Here we report the identification of an anti-E3 monoclonal antibody, MAb 3015B2. MAb 3015B2 reacts with E3 on Western blots of reduced and denatured gels and in IFs of infected or transfected cells, indicating that it likely recognizes a non-conformational epitope on E3. TW2.3 does not react to E3 by Western blot, but by IF, indicating that it likely is recognizing a conformational epitope. The independently isolated MAbs also appear to react to the same portion of the E3 protein, the C-terminus. This indicates that this area is quite immunogenic in BALB/c mice. It is worth noting that proteomic screening of vaccinia immune globulin, as well as sera from individuals vaccinated with Dryvax® or individuals who recovered from smallpox infection also revealed an antibody response to the E3 protein (Davies et al., 2005; Davies et al., 2007). Thus, it appears that antibody responses to E3 are part of the host response to orthopoxvirus infections. Given that E3 is an intracellular protein, it is doubtful that the anti-E3 antibody response helps control the infection. E3 was also recently found to be part of the mature virion (Resch et al., 2007), although exactly where the protein is in the virion is not known. Finding an antibody to the VACV E3 protein that reacts both on Western blots and IFs can be of great use to the poxvirus community. Given the high homology of the E3 protein among the orthopoxvirus genus it is not surprising that MAb 3015B2 reacts with a number of orthopoxviruses like various stains of vaccinia virus, ectromelia virus, and weakly with cowpox virus. Thus this antibody may be useful to those studying the E3 protein, as well as a reagent to detect early protein expression in orthopoxvirus infected cells.
Acknowledgments
We would like to thank Drs. Jon Yewdell, Jack Bennink, and Bernie Moss (NIH) for TW2.3 hybridoma generated mouse ascites and Constance Chamberlain and Dr. Bert Jacobs (Arizona State University) for the E3L-deletion virus, the viruses with C- and N-terminal truncations in E3L, and plasmid pMT-E3L. This work was funded by the NIH NIAID Middle Atlantic Regional (MARCE) in Biodefense and Emerging Infectious Diseases grant U54 AI057168.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brandt TA, Jacobs BL. Both carboxy- and amino-terminal domains of the vaccinia virus interferon resistance gene, E3L, are required for pathogenesis in a mouse model. J Virol. 2001;75(2):850–6. doi: 10.1128/JVI.75.2.850-856.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HW, Jacobs BL. Identification of a conserved motif that is necessary for binding of the vaccinia virus E3L gene products to double-stranded RNA. Virology. 1993;194(2):537–47. doi: 10.1006/viro.1993.1292. [DOI] [PubMed] [Google Scholar]
- Chang HW, Uribe LH, Jacobs BL. Rescue of vaccinia virus lacking the E3L gene by mutants of E3L. J Virol. 1995;69(10):6605–8. doi: 10.1128/jvi.69.10.6605-6608.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HW, Watson JC, Jacobs BL. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc Natl Acad Sci U S A. 1992;89(11):4825–4829. doi: 10.1073/pnas.89.11.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran MA, Puckett C, Moss B. In vitro mutagenesis of the promoter region for a vaccinia virus gene: evidence for tandem early and late regulatory signals. J Virol. 1985;54:30–37. doi: 10.1128/jvi.54.1.30-37.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DH, Liang X, Hernandez JE, Randall A, Hirst S, Mu Y, Romero KM, Nguyen TT, Kalantari-Dehaghi M, Crotty S, Baldi P, Villarreal LP, Felgner PL. Profiling the humoral immune response to infection by using proteome microarrays: high-throughput vaccine and diagnostic antigen discovery. Proc Natl Acad Sci U S A. 2005;102(3):547–52. doi: 10.1073/pnas.0408782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DH, Molina DM, Wrammert J, Miller J, Hirst S, Mu Y, Pablo J, Unal B, Nakajima-Sasaki R, Liang X, Crotty S, Karem KL, Damon IK, Ahmed R, Villarreal L, Felgner PL. Proteome-wide analysis of the serological response to vaccinia and smallpox. Proteomics. 2007;19:19. doi: 10.1002/pmic.200600926. [DOI] [PubMed] [Google Scholar]
- Kibler KV, Shors T, Perkins KB, Zeman CC, Banaszak MP, Biesterfeldt J, Langland JO, Jacobs BL. Double-stranded RNA is a trigger for apoptosis in vaccinia virus-infected cells. J Virol. 1997;71(3):1992–2003. doi: 10.1128/jvi.71.3.1992-2003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YG, Muralinath M, Brandt T, Pearcy M, Hauns K, Lowenhaupt K, Jacobs BL, Rich A. A role for Z-DNA binding in vaccinia virus pathogenesis. Proc Natl Acad Sci U S A. 2003;100(12):6974–9. doi: 10.1073/pnas.0431131100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JA, Rich A. Biological function of the vaccinia virus Z-DNA-binding protein E3L: gene transactivation and antiapoptotic activity in HeLa cells. Proc Natl Acad Sci U S A. 2005;102(36):12759–64. doi: 10.1073/pnas.0506011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langland JO, Kash JC, Carter V, Thomas MJ, Katze MG, Jacobs BL. Suppression of proinflammatory signal transduction and gene expression by the dual nucleic acid binding domains of the vaccinia virus E3L proteins. J Virol. 2006;80(20):10083–95. doi: 10.1128/JVI.00607-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B. Poxviridae: The Viruses and Their Replication. In: Knipe DM, Howley PM, editors. Fields Virology. 4. Vol. 2.2. Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 2849–2883. [Google Scholar]
- Resch W, Hixson KK, Moore RJ, Lipton MS, Moss B. Protein composition of the vaccinia virus mature virion. Virology. 2007;358(1):233–47. doi: 10.1016/j.virol.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Rivas C, Gil J, Melkova Z, Esteban M, Diaz-Guerra M. Vaccinia virus E3L protein is an inhibitor of the interferon (i.f.n.)-induced 2–5A synthetase enzyme. Virology. 1998;243(2):406–14. doi: 10.1006/viro.1998.9072. [DOI] [PubMed] [Google Scholar]
- Shors ST, Beattie E, Paoletti E, Tartaglia J, Jacobs BL. Role of the vaccinia virus E3L and K3L gene products in rescue of VSV and EMCV from the effects of IFN-alpha. J Interferon Cytokine Res. 1998;18(9):721–9. doi: 10.1089/jir.1998.18.721. [DOI] [PubMed] [Google Scholar]
- Shors T, Kibler KV, Perkins KB, Seidler-Wulff R, Banaszak MP, Jacobs BL. Complementation of vaccinia virus deleted of the E3L gene by mutants of E3L. Virology. 1997;239(2):269–76. doi: 10.1006/viro.1997.8881. [DOI] [PubMed] [Google Scholar]
- Yuwen H, Cox JH, Yewdell JW, Bennink JR, Moss B. Nuclear localization of a double-stranded RNA-binding protein encoded by the vaccinia virus E3l gene. Virology. 1993;195:732–744. doi: 10.1006/viro.1993.1424. [DOI] [PubMed] [Google Scholar]