Abstract
We investigated the effects of morphine and other agonists on the human mu opioid receptor (MOP) expressed in M2 melanoma cells, lacking the actin cytoskeleton protein filamin A and in A7, a sub clone of the M2 melanoma cells, stably transfected with filamin A cDNA. The results of binding experiments showed, that after chronic morphine treatment (24 hr) of A7 cells, MOP binding sites were down-regulated to 63% of control, whereas, unexpectedly, in M2 cells, MOP binding was up-regulated to 188% of control naïve cells. Similar up-regulation was observed with the agonists methadone and levorphanol. The presence of antagonists (naloxone or CTAP) during chronic morphine treatment inhibited MOP down-regulation in A7 cells. In contrast, morphine-induced up-regulation of MOP in M2 cells was further increased by these antagonists. Chronic morphine desensitized MOP in A7 cells, i.e. it decreased DAMGO-induced stimulation of GTPγS binding. In M2 cells DAMGO stimulation of GTPγS binding was significantly greater than in A7 cells and was not desensitized by chronic morphine. Pertussis toxin treatment abolished morphine-induced receptor up-regulation in M2 cells, whereas it had no effect on morphine-induced down-regulation in A7 cells. These results indicate that, in the absence of filamin A, chronic treatment with morphine, methadone or levorphanol leads to up-regulation of MOP, to our knowledge, the first instance of opioid receptor up-regulation by agonists in cell culture.
Keywords: mu opioid receptor, up-regulation, filamin A, melanoma cells, morphine
1. Introduction
Opioid receptors (mu, delta and kappa) are members of the family of G protein coupled receptors (GPCRs). Similar to other GPCRs, agonist binding to the mu opioid receptor (MOP) induces phosphorylation of the receptor by protein kinases and promotes recruitment of β– arrestins to the plasma membrane. Association of β– arrestins with the receptor causes uncoupling of the receptor from G-proteins and leads to receptor desensitization. In addition, β– arrestins and protein kinases are involved in dynamin-dependent receptor endocytosis via clathrin-coated pits (Ferguson and Caron, 1998). Endosome-associated receptors can either be resensitized after dephosphorylation and recycled back to the plasma membrane or transported into lysosomes for degradation. Long-term exposure to agonist induces the loss of receptor binding sites due to receptor degradation, referred to as down-regulation.
The importance of the carboxyl tail in the down-regulation and trafficking of the opioid receptors is well established (Burd et al., 1998; Capeyrou et al., 1997; Chaipatikul et al., 2003; Finn and Whistler, 2001; Trapaidze et al., 1996). There are many examples of association of the GPCRs, including MOP with other proteins, such as beta-arrestins, (Lefkowitz, 1998), GRKs (Pitcher et al., 1998) and small G proteins (Mitchell et al., 1998). Different laboratories, including ours, have reported interaction of MOP C-tail with different proteins, such as periplakin (Feng et al., 2003), Phospholipase D2 (Koch et al., 2003), mPKCI (Guang et al., 2004), HSP40 {Ancevska-Taneva, 2006 #5292)and Filamin A (Onoprishvili et al., 2003).
Filamin A is a cytoskeleton protein that crosslinks actin filaments into an orthogonal network and maintains the integrity of the cell cytoskeleton. Filamin A is known to bind to various membrane and signaling molecules, see reviews (Stossel et al., 2001; van der Flier and Sonnenberg, 2001)}. More recently different laboratories, including our own, demonstrated that filamin A associates with various GPCRs, such as the D2 and D3 dopamine receptors (Li et al., 2000; Lin et al., 2001), the calcium-sensing receptor (CaR) (Awata et al., 2001; Hjalm et al., 2001), the metabotropic glutamate receptor type 7 (Enz, 2002), alpha1-adrenergic receptors (Zhang et al., 2004), calcitonin receptor (Seck et al., 2003) and the mu opioid receptor (MOP) (Onoprishvili et al., 2003). In some cases, such as the calcium sensing receptor and the MOP, the binding of filamin A is to the carboxyl tail, while for other receptors, such as the D2 and D3 dopamine receptors, the binding is to the third cytoplasmic loop.
Filamin A was found to bind directly to beta-arrestin2 and it has been suggested that D3R, filamin A, and beta-arrestin form a signaling complex (Kim KM, 2005). This suggests that filamin A has a role in signal transduction of GPCRs, in addition to its actions as an actin cytoskeleton protein.
We have reported (Onoprishvili et al., 2003) the finding that filamin A binds to the carboxyl tail of the MOP. This interaction drastically reduces the effects of chronic treatment with mu agonist, such as down-regulation, desensitization and trafficking of the MOP, as shown in the melanoma cell line M2, which does not express endogenous filamin A. These major changes in the absence of filamin A indicate that the interaction between this protein and the MOP plays a significant role in the regulation, trafficking and, possibly, function of the MOP. This laboratory has continued research on this interaction with the aim of learning more about the physiological significance and the mechanisms involved. The current studies were all carried out in the melanoma cell line M2 (and its filamin A-expressing control subclone A7), which is the only known system lacking filamin A and has been used in all of the functional studies on filamin A cited above. It is our intent to extend these studies to other systems, including primary neuronal cultures and whole animals.
Our previously reported studies on the filamin A-MOP interaction (Onoprishvili et al., 2003) were carried out exclusively with the synthetic mu-selective peptide agonist DAMGO. The current study was designed to test the effect of morphine, as well as other opioids, in the cell line lacking filamin A (M2) and compare them with effects in a subclone of the cell line (A7), expressing filamin A. We report here a very surprising and interesting finding. Morphine and several other agonists cause significant up-regulation of MOP in M2 cells, not observed in the filamin A-expressing A7 cells or other cell lines. While up-regulation by opioid antagonists is well established, this is, to the best of our knowledge, the first observation of opioid receptor up-regulation by morphine or other opioid agonists. We feel that this observation should be of considerable interest to researchers in the opioid field. This paper includes experiments designed to elucidate the mechanism of the up-regulation described, but the mechanism is not yet clear and is under further investigation.
2. Results
2.1. Regulation of MOP binding in melanoma cells
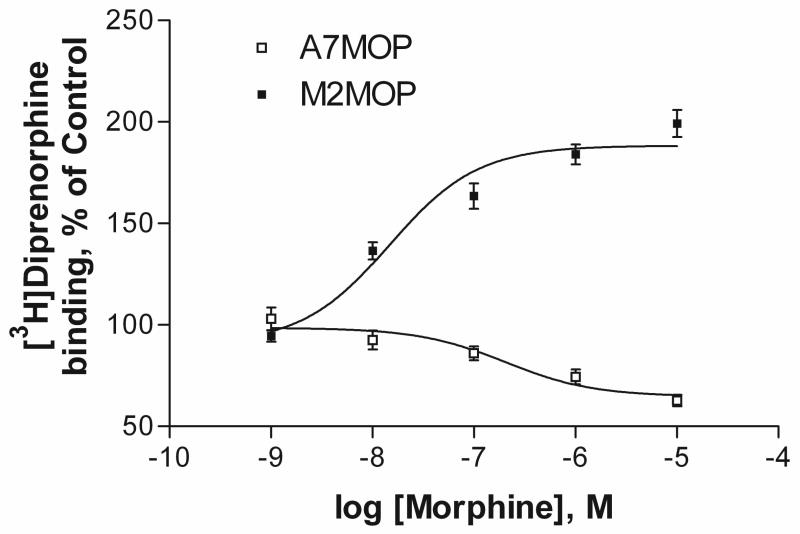
Melanoma cell line M2, which does not express filamin A endogenously and A7 cells, a subclone of the M2 cell line in which filamin A is expressed, were stably transfected with the cDNA encoding myc-tagged human mu opioid receptors (MOP). These cells were used for all studies. The affinity (KD) and BMAX of the nonselective opioid antagonist [3H]diprenorphine and of the mu agonist [3H]DAMGO were very similar in the A7 and M2 cell lines expressing MOP (Onoprishvili et al., 2003). Chronic treatment of melanoma cells with morphine caused a concentration and time-dependent change in MOP density as determined by radioligand binding assays using [3H]diprenorphine. As shown in Figure 1, treatment for 24 hours with different concentrations of morphine caused a concentration-dependent decrease in MOP density (down-regulation) in A7 cells. The EC50 for morphine-induced down-regulation was 205±0.3 nM and receptor binding decreased to 63±3.7% of control. These data are consistent with previously published results from different laboratories in various cell lines (Chakrabarti et al., 2005; Chaturvedi et al., 2001; Horner and Zadina, 2004). In contrast, in M2 cells lacking filamin A, chronic morphine treatment induced a marked concentration-dependent increase in MOP binding sites to 188±4.8 % of control (Fig. 1). The EC50 value for receptor up-regulation was 14±0.2 nM. Radioligand binding studies, using the mu opioid selective agonist [3H]DAMGO, revealed similar results (not shown). No difference in the affinity (Kd) of DAMGO or diprenorphine was noticed before or after morphine treatment in either A7 or M2 cell lines.
Fig. 1.
Concentration curves for the effect of chronic morphine treatment on [3H]diprenorphine binding in melanoma cells expressing MOP. A7(+filamin A) and M2(−filamin A) cells expressing MOP (A7MOP and M2MOP) were pretreated with various concentrations of morphine for 24 hours at 37°C. Radioligand binding was performed with saturating concentration of [3H]diprenorphine (1.5nM). Results are shown as binding site levels in morphine-treated cells, expressed as % of binding site levels in untreated cells (A7- open squares and M2 filled squares). Data are the mean ± SEM values from at least five independent experiments.
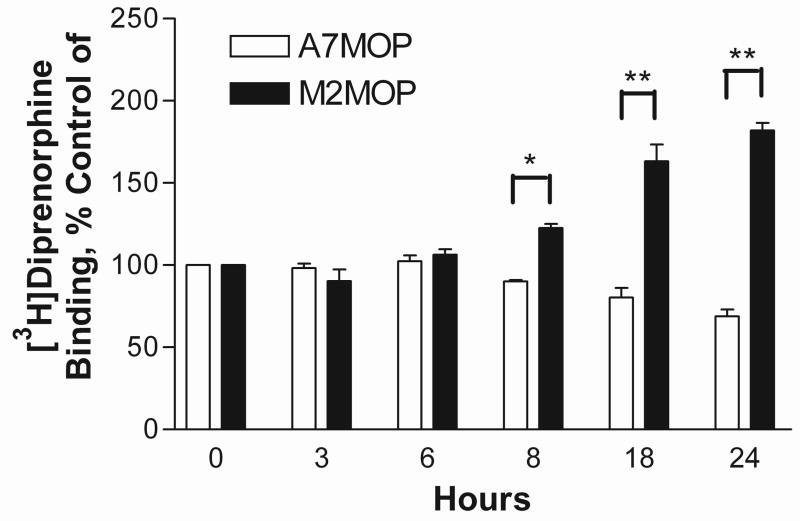
The time course of morphine-induced MOP up-regulation in M2 cultures and down-regulation in A7 cells was determined by exposure of the cells to 1 μM morphine for various periods of time. As depicted on fig. 2, the down-regulation in A7 and the up-regulation in M2 cells were first observed after 8 hours of morphine treatment and reached a maximum after 18 hrs.
Fig. 2.
Time course of the effect of morphine treatment on [3H]diprenorphine binding in melanoma cells expressing MOP. A7, +filamin A (open bars) and M2, −filamin A (filled bars) cells expressing MOP were pretreated with 1μM morphine for indicated time intervals. Radioligand binding was performed with a saturating concentration of [3H]diprenorphine (1.5nM). Results are plotted as binding site level of morphine-treated cells, expressed as % of binding site level in untreated cells. Data are the mean ± SEM values from three independent experiments. Asterisks indicate significant differences between morphine-treated and untreated samples, *, P < 0.05 and **, P < 0.001.
2.2 [35S]GTPγS binding and MOP desensitization
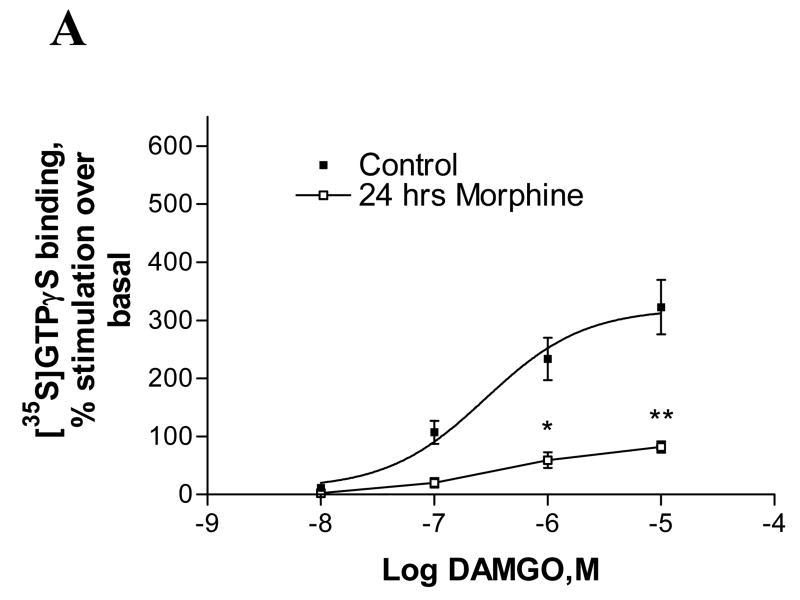
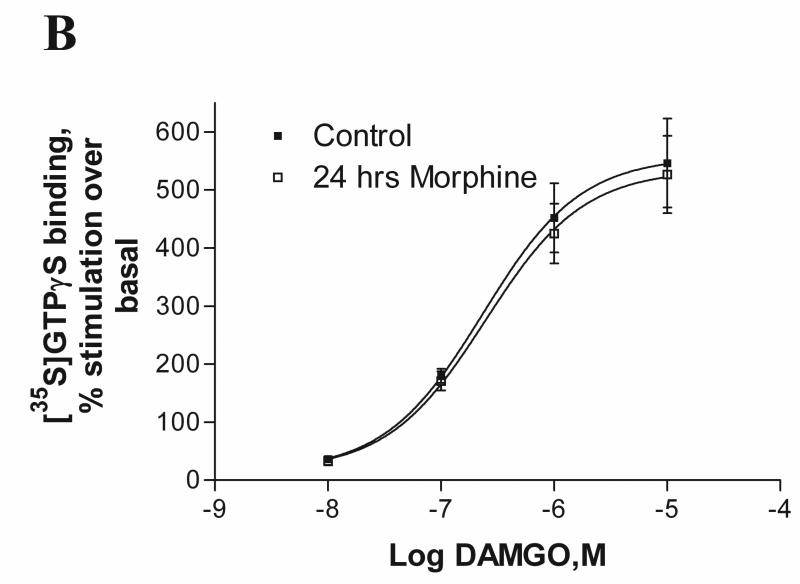
To determine morphine-induced functional desensitization of MOP, melanoma cells were pretreated with 1μM morphine for 24 hours and cell membranes were prepared from control and morphine-treated cells. Receptor desensitization was measured as the decreased ability of the agonist, DAMGO, to stimulate [35S]GTPγS binding to membranes of A7 and M2 cells after agonist treatment. As depicted in Fig. 3A, in A7 cell membranes expressing MOP, DAMGO exhibited reduced ability to stimulate [35S]GTPγS binding after chronic morphine exposure (EMAX decreased from 322±47 % to 82±9.5 % of control), indicating that the receptors were desensitized. In contrast, as shown in Fig. 3B, in M2, functional desensitization of MOP after chronic morphine exposure was abolished (546±76% of control before and 526±66% after morphine treatment). It is noteworthy that the maximal stimulation (Emax) of GTPγS binding in naive M2 cells (546%) is significantly higher than the Emax in naïve A7 cells(322%).
Fig. 3.
Effect of chronic morphine treatment on [35S]GTPγS binding to membranes of melanoma cells. The cell membranes of control and morphine-pretreated (1μM for 24 hours) A7MOP, +filamin A (A) and M2MOP, −filamin A (B) cells were used to measure DAMGO-stimulated [35S]GTPγS binding. Data are expressed as percent increase in [35S]GTPγS binding compared to unstimulated basal levels. Data are the mean ± SEM values from four independent experiments. The results were analyzed by nonlinear regression using the GraphPad Prizm program. Asterisks indicate significant differences between treated and untreated samples, *, p < 0.01 and **, p < 0.001.
2.3 Effects of Pertussis Toxin treatment
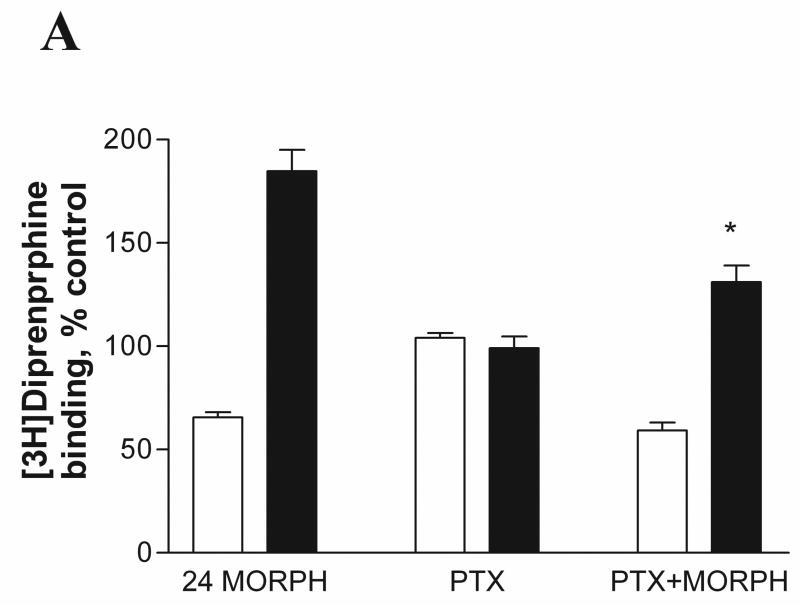
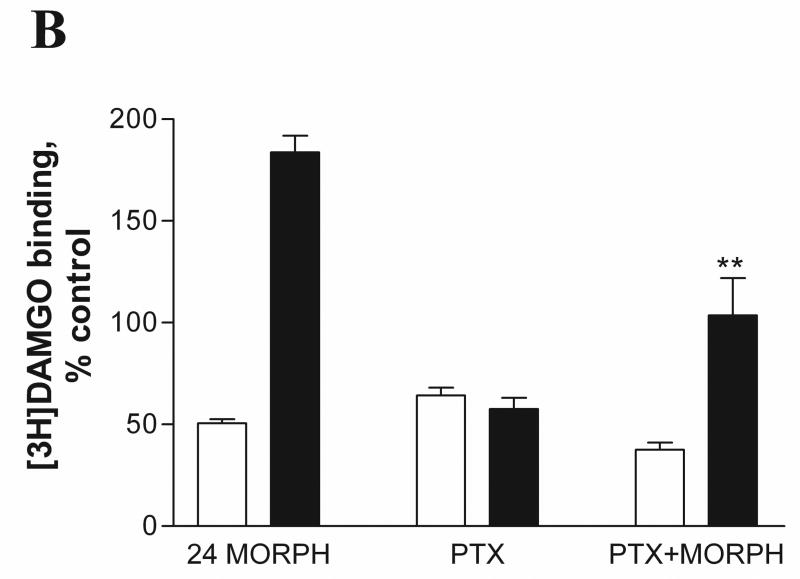
We investigated whether uncoupling of receptors from G proteins interferes with morphine-induced MOP up-regulation in M2 cells. It is well known that pertussis toxin (PTX) treatment ADP-ribosylates inhibitory G proteins and uncouples GPCRs from Gi/o proteins (Kurose et al., 1983). As described in Materials and Methods, A7 and M2 cells were pretreated with 100ng/ml PTX for 24 hrs in the absence or presence of 1μM morphine at 37°C. The cells were collected and radioligand binding was performed using the non-selective opioid antagonist [3H]diprenorphine and mu-selective agonist [3H]DAMGO. As expected, [3H]DAMGO binding (Fig. 4B) was inhibited after PTX treatment compared to PTX-untreated controls due to uncoupling of the receptors from their cognate Gi/o proteins in both A7 (64 ± 3.7 % of control) and M2 (57 ± 5.6 % of control) cell lines, whereas [3H]diprenorphine binding (Fig. 4A) was unaffected. PTX treatment in the presence of morphine had little or no effect on morphine-induced down-regulation of MOP (binding of either DAMGO or diprenorphine) in cells expressing filamin A (A7) (59 ± 3.9 % of control) compared to morphine treatment alone (65 ± 2.5 % of control). In contrast, PTX treatment of M2 cells partially reduced up-regulation of MOP binding sites by morphine to 131± 8.0 % of control compared to morphine treatment alone (184 ± 10.0 %), as measured by [3H]diprenorphine binding. When binding was performed with [3H]DAMGO in morphine and PTX-treated M2 cells, the up-regulation was virtually abolished (from 183±8.1% to 103±18.3%)(Fig. 4B).
Fig. 4.
Effect of pertussis toxin treatment on agonist and antagonist binding in melanoma cells expressing MOP. A7MOP, + filamin A ( open bars) and M2MOP, −filamin A (filled bars) cells were incubated with 100ng/ml pertussis toxin (PTX) for 24 hours in the absence or presence of 1μM morphine. After treatment, radioligand binding was performed with [3H]diprenorphine (1.5nM) (A) and [3H]DAMGO (3.5nM) (B). Data are the mean ± SEM values from four independent experiments. The statistical significance of differences between morphine-treated and PTX/morphine treated cells is indicated by asterisks (*, P < 0.05 and **, P < 0.001).
2.4. Treatment with Different ligands
Our next approach was to determine the effects of various ligands on MOP density in A7 and M2 cells. In particular, we wanted to determine whether any other agonists resemble morphine in exhibiting the ability to up-regulate MOP in M2 cells. Melanoma cells were pretreated for 24 hrs with 1 μM of each ligand (except etorphine, 0.1μM), as described in Materials and Methods. As shown in Table 1, in A7 cells all agonists caused down-regulation of MOP binding sites. DAMGO, etorphine and etonitazine were somewhat more effective than morphine, methadone and levorphanol. Treatment with the mu opioid selective peptide antagonist CTAP and the non-selective antagonist diprenorphine had no effect on MOP density. The nonselective antagonist naloxone induced an increase in MOP density to 130± 2.6 % of control, in accordance with published results (Zadina et al., 1994).
Table 1.
Ligand-induced changes in MOP binding site levels in melanoma cells
| Receptor Level (% of control)
|
||
|---|---|---|
| Ligand (1μM) | M2-MOP | A7-MOP |
| Agonists | ||
| DAMGO | 109±3.3 | 30±0.9* |
| Morphine | 188±4.8 * | 63±3.7* |
| Etorphine# | 108±3.8 | 27±3.2* |
| Methadone | 173±8.3* | 56±2.0* |
| Levorphanol | 179±6.9 * | 47±1.5* |
| Etonitazene | 110±11.1 | 33±2.5* |
| Antagonists | ||
| CTAP | 128±3.6* | 104±11.5 |
| Naloxone | 164±2.6* | 130±2.6* |
| Diprenorphine | 98±5.1 | 95±5.6 |
A7(+filamin A) and M2 (−filamin A) cells, expressing MOP, were pretreated with 1μM ligand (#except etorphine 0.1 μM), as listed in the table, for 24 hours. Changes in MOP binding was detected by [3H]diprenorphine (1.5nM) binding experiments. The results are the mean ± SEM of at least three separate experiments. Asterisks indicate significant differences between ligand-treated and untreated samples,
P < 0.001.
With M2 cells, the effects of various ligands were quite different, as shown in Table 1. Thus, exposure of M2 cells for 24 hrs to DAMGO, etorphine or etonitazene did not produce any change in MOP density, either up or down. In contrast, treatment with methadone or levorphanol, induced large increases in MOP binding sites in the M2 cells lacking filamin A (170–180% of control), similar to those observed after chronic morphine. Treatment with the antagonist, diprenorphine, had no effect on MOP density, whereas CTAP and naloxone increased MOP binding sites to 128± 3.6 % and 164± 2.6 % of control, respectively.
2.5. Ligand combinations with morphine
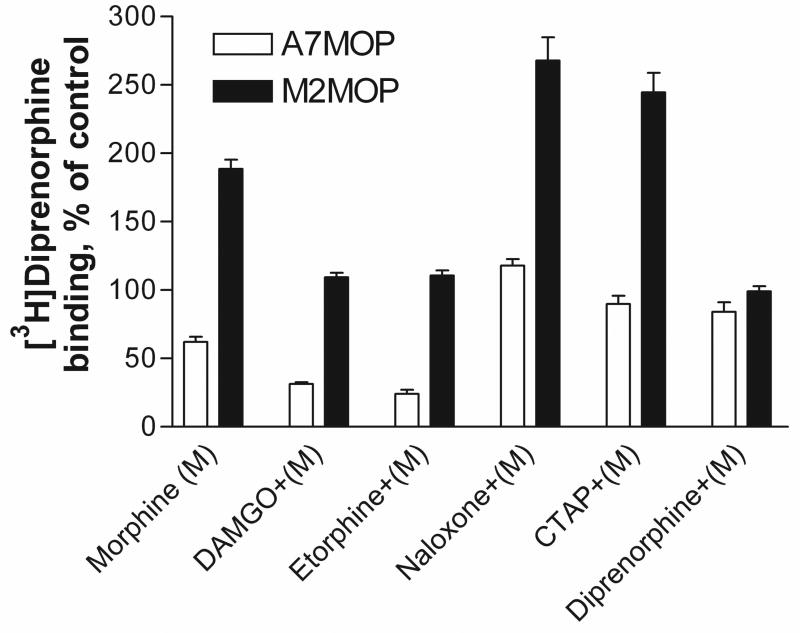
We tested the effect on the level of MOP binding sites of treatment of M2 and A7 cells with various opioids in combination with morphine. The cells were incubated for 24 hours with various ligands, in the absence (Table 1) and presence (Fig. 5) of morphine. MOP density was measured by [3H]diprenorphine binding, as described in Materials and Methods. As depicted in Fig. 5, in A7 cells, DAMGO and etorphine treatment in the presence of morphine further enhanced down-regulation to 31±1.4 % and 24±3.1 % of control, respectively. However, exposure of A7 cells to antagonists in the presence of morphine reversed the morphine-induced down-regulation from 62 ±3.6% to 117±4.6% (naloxone), 89 ±6.0% (CTAP) and 84 ±7.0% (diprenorphine). In M2 cells, morphine-induced up-regulation of MOP density was abolished when morphine was combined with either DAMGO or etorphine. Thus, the increase of MOP level by morphine to 188 ± 6.7 % of control was decreased to 109±3.3 % and 110±3.7 % of control after combined incubation with DAMGO or etorphine, respectively. The antagonist diprenorphine reduced morphine-induced up-regulation of MOP by morphine to control level (99±3.6%). In contrast, combination of naloxone or CTAP with morphine revealed further up-regulation of MOP binding sites to 263±20.0% for naloxone and 231±16.5% of control for CTAP, suggesting additive effects for these antagonists and morphine in M2 cells (Fig. 5).
Fig. 5.
Effect of treatment with various ligands on [3H]diprenorphine binding in melanoma cells in the presence of morphine. A7MOP, +filamin A ( open bars) and M2,MOP, −filamin A ( filled bars) cells were pretreated with various ligands (1μM), as indicated, in the presence of 1μM morphine (M) for 24 hours. Radioligand binding was performed with [3H]diprenorphine (1.5nM). Data are the mean ± SEM values from three to seven independent experiments. The statistical comparisons were performed by unpaired Student’s t-test and two-way ANOVA followed by Fisher’s Protected t-test. To control experiment-wise error a P<0.01 value was considered to be a statistically significant.
2.6. Western Blot Analysis of MOP levels
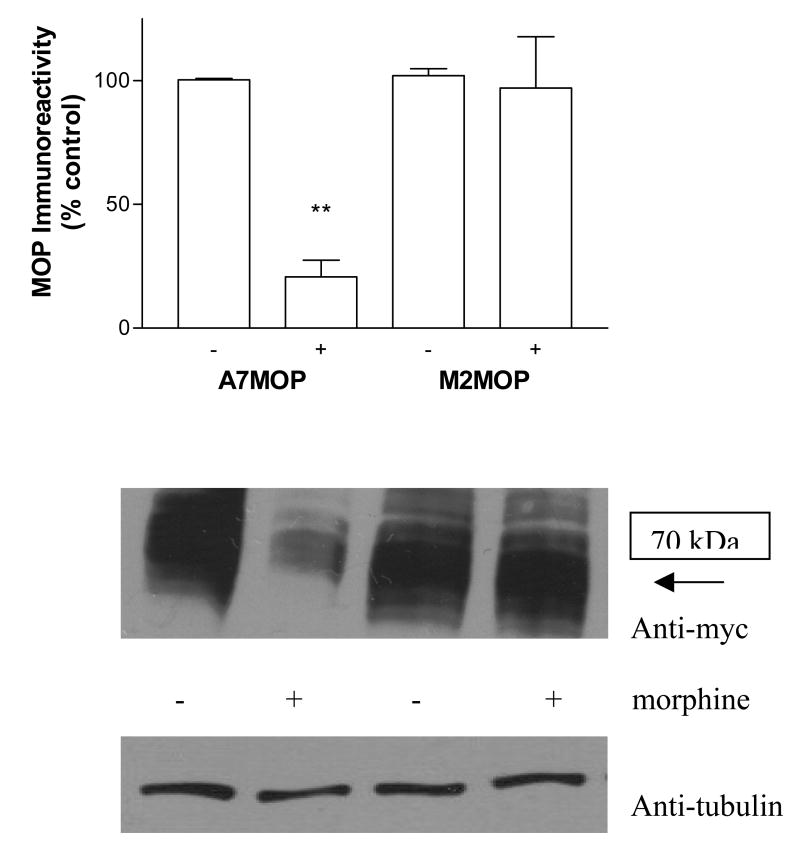
The change in myc-tagged MOP density in A7 and M2 cells was also examined by immunoblot analysis. Cell lysates extracted from A7 and M2 cells, before and after morphine treatment, were subjected to Western blot analysis and receptor protein levels in each sample were compared. Anti-myc antibodies detected bands migrating at a molecular mass corresponding to ca 70 kDa. As shown on Fig. 6, chronic morphine treatment of A7 cells significantly reduced the MOP protein level, as compared to untreated controls (to 21± 4 % of untreated, see histogram in Fig. 6). This is in good agreement with receptor down-regulation measured by binding. In contrast, in M2 cells the level of MOP protein remained steady after prolonged morphine treatment and was similar to that of the untreated control, although up-regulation of the binding sites (to 188% of controls) was observed.
Fig. 6.
Effect of chronic morphine treatment on MOP immunoreactivity in melanoma cells. Western blot analysis was performed on lysates from A7 (+filamin) and M2(−filamin) cells expressing myc-tagged MOP in the absence and presence of morphine (24 hrs), as described in “Materials and Methods”. The blots were probed with anti-myc antibodies to detect MOP. To ensure that approximately equal amounts of the proteins were loaded on the gel, nitrocellulose membranes were probed with anti-tubulin antibodies. The images of immunoblots from four independent experiments were quantified by densitometric scanning using NIH Image J 1.37v analysis software. Data are the mean ± SEM from four independent experiments shown on the histograms. **, p < 0.001 indicates significant differences from control samples. Representative immunoblots are shown below the histograms.
3. Discussion
In our previous studies, we identified the actin cytoskeleton protein filamin A as a direct binding partner for the human MOP. We found that the absence of filamin A has major effects on the regulation (desensitization and down-regulation) and trafficking of the MOP (Onoprishvili et al., 2003). Our previous studies were carried out exclusively with the mu-selective synthetic peptide agonist DAMGO. When we performed studies in melanoma cells using morphine, unexpected results were obtained with the M2 cells lacking filamin A. After chronic morphine treatment, the density of MOP binding sites was increased markedly, almost 2-fold, compared to untreated M2 cells. In contrast, in A7 cells expressing filamin A, chronic morphine treatment resulted in down-regulation of MOP binding sites, as seen in normal cultured cells.
To investigate further the mechanism of morphine–induced MOP up-regulation in M2 cells, we performed GTPγS binding assays. Our data showed, that in cells lacking filamin A, MOP exhibited enhanced coupling to G proteins and after chronic morphine treatment, the receptor’s ability to activate G protein was not desensitized, as seen by the absence of a decrease in agonist-stimulated GTPγS binding. In contrast, MOP expressed in A7 cells exhibited normal desensitization of GTPγ binding after chronic morphine treatment. One explanation for our results is that filamin A may be important for the proper receptor conformation or proper positioning of the MOP in the cell membrane, required for receptor uncoupling and desensitization after agonist treatment. Whether MOP is phosphorylated after chronic agonist treatment in M2 cells is not known and further studies are necessary to determine this.
We were interested in examining the relationship between morphine-induced up-regulation and MOP coupling to Gi/o proteins. After pertussis toxin (PTX) treatment, we observed a marked reduction in morphine-promoted MOP up-regulation in M2 cells, which indicates the importance of receptor-Gi/o protein interaction for agonist-induced MOP up-regulation. In contrast, PTX treatment did not have any effect on morphine-induced down-regulation of MOP in A7 cells. Yabaluri et al (Yabaluri and Medzihradsky, 1997) reported that PTX treatment did not block agonist-induced down-regulation of MOP in C6 glioma cells. However, it should be noted that PTX treatment of N2A cells expressing MOP blocked etorphine-induced MOP down-regulation (Chakrabarti, 1997).
Our studies demonstrated that, not only morphine, but also methadone and levorphanol induce considerable up-regulation of MOP binding sites in M2 cells. On the other hand, no significant increase in MOP density was observed after treatment of M2 cells for 24 hrs with DAMGO, etorphine or etonitazene.
In this context, Koch and colleagues (Koch et al., 2005) investigated the effect of different ligands on MOP endocytosis. Maximal (50%) MOP internalization after 60 min exposure to agonist was found with DAMGO, etonitazene and sufentanil. Methadone, beta-endorphin and fentanyl caused partial internalization (20–30%), while morphine, buprenorphine and oxycodone failed to induce detectable MOP endocytosis. Similar results were reported by Keith et al (Keith et al., 1998). Morphine, buprenorphine and codeine induced almost no internalization, fentanyl and methadone caused partial internalization, whereas etorphine and etonitazene triggered rapid endocytosis.
Interestingly, in our results, chronic treatment with DAMGO, etorphine and etonitazene, known as ligands with high potencies for inducing internalization, did not cause any up-regulation of MOP binding sites in M2 cells. After chronic treatment of M2 cells, morphine which promotes almost no internalization (Keith et al., 1998) and methadone which induces partial internalization (Koch et al., 2005), promoted up-regulation of MOP binding sites. In addition, levorphanol also caused MOP up-regulation in M2 cells. While we did not find any papers regarding MOP internalization by levorphanol, Bot et al (Bot et al., 1997) demonstrated, that levorphanol, a close analogue of morphine, did not induce internalization of delta opioid receptors in HEK293 cells. As expected, chronic treatment of A7 cells with all of the agonists used induced MOP down-regulation.
MOP up-regulation could work via several possible mechanisms. Increased levels of MOP binding sites could be due to altered receptor trafficking, inhibition of receptor degradation, increased receptor synthesis and/or change in receptor conformation after agonist treatment. In Western blot analyses, we saw no change in protein level, suggesting, in a preliminary way, that protein synthesis is not involved.
The carboxyl terminal tail of MOP has an important role in receptor down-regulation and trafficking. Agonist-induced receptor phosphorylation of the carboxyl tail is frequently involved in receptor desensitization. Several laboratories reported (see review by Law et al (Law et al., 1999) that mutations and/or deletions of residues or motifs in the carboxyl terminal tail of MOP resulted in changes of function, regulation and trafficking of the receptor. For example, mutation of Ser356 and Ser363 to alanine attenuated agonist-induced MOP down-regulation without having any effect on receptor phosphorylation (Burd et al., 1998). Thus, these residues are not phosphorylated and may be involved in interaction with other proteins, necessary for down-regulation.
In addition, it was shown (Capeyrou et al., 1997) that when all serine and threonine residues within the third intracellular loop and carboxyl terminal tail of MOP were mutated to alanine, an increase in MOP binding sites was seen after chronic agonist treatment. As suggested by the authors, a receptor conformation involved in MOP internalization, degradation and recycling pathways could be disrupted in the mutant receptor, resulting in a lower rate of receptor degradation than receptor synthesis, leading to receptor up-regulation. It is unclear whether the mutant receptor has impaired ability to internalize into endosomes or has an increased rate of recycling, due to the unphosphorylated state of the receptor (Capeyrou et al., 1997).
In the light of our results, we suggest that mutation of Ser/Thr residues in the C-tail of MOP may induce disruption of the MOP-filamin A association, resulting in up-regulation of MOP. This hypothesis is under investigation.
As observed with other GPCRs, agonist treatment promotes opioid receptor endocytosis via clathrin-coated pits, followed by trafficking to the lysosomes for receptor down-regulation upon prolonged treatment with an agonist. Receptor down-regulation is defined as a reduction in radioligand binding sites as well as in receptor protein.
Ubiquitination mediated by proteasomes has also been implicated in down-regulation of GPCRs, including the MOP (Chaturvedi et al., 2001; Tsao and von Zastrow, 2000). Extensive studies in many different cell lines demonstrated MOP down-regulation by different agonists (Baumhaker et al., 1993; Chakrabarti et al., 1995; Kato et al., 1998; Yabaluri and Medzihradsky, 1997). Chronic morphine-induced MOP down-regulation has been well investigated and characterized in a various cell culture systems, such as SH-SY5Y (Zadina et al., 1993), HEK (Onoprishvili et al., 1999), CHO (Kato et al., 1998), C6 (Yabaluri and Medzihradsky, 1997), Neuro2a (Chakrabarti et al., 1995), 7315c (only 20%) (Puttfarcken and Cox, 1989) and SK-N-SH (Baumhaker et al., 1993).
Opioid receptor up-regulation by antagonist treatment is a well-established phenomenon in animals and cell cultures. Chronic treatment with opioid antagonists, such as naloxone or naltrexone, induces up-regulation of opioid receptor density (Bmax) in cell cultures as well as in animal models. Receptor up-regulation was determined by radioligand binding, autoradiography and quantitative immuno-localization (Lesscher et al., 2003; Unterwald et al., 1998; Yoburn et al., 1989; Zadina et al., 1993; Zukin and Tempel, 1986). Antagonist-induced MOP up-regulation results in an increase in mu agonist-stimulated G-protein activation (Narita et al., 2001). Studies have demonstrated that up-regulation of radioligand binding sites of MOP in vivo due to antagonist treatment is not accompanied by an increase in MOP mRNA (Unterwald et al., 1995) and a protein synthesis inhibitor, cycloheximide, did not block antagonist-induced MOP up-regulation in cultures of mouse spinal cord-ganglion explants (Tempel et al., 1986). It has been found that, after antagonist treatment, the number of binding sites is increased without change in the total receptor number in some brain regions, whereas in other regions, receptors binding sites are increased with concomitant changes in total receptor number. This was assessed in adjacent tissue sections of the brain regions by MOP immunohistochemistry and autoradiography after antagonist administration (Unterwald et al., 1998). The mechanism of chronic opioid antagonist induced up-regulation is not fully understood.
As stated, up-regulation of opioid receptors by agonists in cell culture has, to our knowledge, not been reported previously. However, such studies in cell cultures have been published for several other GPCRs. Different laboratories have reported an increase in the density of D2 (but not D1) dopamine receptors after agonist treatment (Filtz et al., 1994; Ng et al., 1997; Starr et al., 1995; Zhang et al., 1994). Construction of chimeric mutants of D1/D2 receptors suggested that trans-membrane region V and the third cytoplasmic loop are involved in agonist-induced D2 dopamine receptor up-regulation (Starr et al., 1995). Filtz at el (Filtz et al., 1994) also reported up-regulation of D2 dopamine receptors expressed in HEK-293 cells. Agonist-induced receptor up-regulation was found for 5-HT1A receptors, stable expressed in CHO cells (Cowen et al., 1997). Both Cowen et al and Filtz et al suggested, based on their evidence, that up-regulation of GPCRs by agonists involves a different mechanism from up-regulation by antagonists..
The effects of chronic morphine administration on MOP binding site density have also been investigated in animal brain and brain regions. These studies produced all possible changes in MOP density, namely, up-regulation (Besse et al., 1992; Brady et al., 1989; Fabian et al., 2002; Fabian et al., 2003; Holaday et al., 1982; Ray et al., 2004; Rothman et al., 1991; Schmidt et al., 2003; Vigano et al., 2003), down-regulation (Bhargava and Gulati, 1990; Meuser et al., 2003; Werling et al., 1989) or no change (Polastron et al., 1994; Stafford et al., 2001; Turchan et al., 1999). Some of these results appear to be regional differences, but others, performed on whole brain or the same regions of the same animal, appear to be discrepancies between different laboratories.
In conclusion, the present study reveals that chronic treatment with morphine and several other opioid agonists induces a marked increase in receptor binding sites in cells lacking filamin A. To the best of our knowledge, this is the first report of up-regulation of MOP by morphine and related agonists in cell culture.
4. Experimental Procedure
4.1. Materials
[3H]Diprenorphine (50–55 Ci/mmol) was purchased from PerkinElmer Life and Analytical Sciences, [35S]GTPγS (1100–1200 Ci/mmol) was obtained from GE Healthcare. [3H]DAMGO (44–48 Ci/mmol), Morphine sulfate, DAMGO, naloxone and other drugs were supplied by the National Institute on Drug Abuse. Minimum Essential Medium, Lipofectamine and 0.25% Trypsin-EDTA were obtained from Invitrogen. Pertussis toxin (isolated from B. pertussis) was purchased from List Biologicals (Cat. # 181)
4.2. Cell culture and treatment
Human melanoma cell lines M2 and A7 expressing myc-tagged human mu opioid receptor (MOP) (Onoprishvili et al., 2003) were maintained in Minimum Essential Medium (MEM) supplemented with 8% newborn calf serum and 2% fetal bovine serum. M2 cells do not express filamin A endogenously, whereas the M2 subclone, A7, is stably transfected with human filamin A cDNA and expresses filamin A (Cunningham et al., 1992). Cells were incubated in serum-free MEM containing the ligand to be studied at 1μM concentration for 24 hours at 37°C, unless indicated otherwise. To ensure complete removal of the drug after treatment, additional control samples of the cells were used, where drugs were added to the cells and removed immediately (0 min treatment). The difference of receptor density between untreated control and 0 min treatment controls was less then 5%. For toxin treatment, cells were incubated with 100ng/ml pertussis toxin (PTX) for 24 hours in a serum-free MEM in the absence or presence of 1μM morphine. After the treatment, the cells were washed 3 times with PBS, detached from flasks and washed again 3 times with PBS by centrifugation to remove any residual ligand.
4.3. Receptor Radioligand Binding
For radioligand binding assays, the cells were washed with PBS and the final pellets were homogenized in 50mM Tris/1mM EDTA buffer (cell homogenate). For saturation experiments, increasing concentrations of either [3H]DAMGO (0.25–5nM) or [3H]diprenorphine (0.05–2nM) were used. Down-regulation experiments were performed, using 1.5nM or increasing concentrations (0.2–2nM) of [3H]diprenorphine or [3H]DAMGO (3.5nM or increasing concentrations 0.25–5nM). Nonspecific binding was determined in the presence of 1 μM naloxone. For all binding experiments cell homogenates or cell membranes (Onoprishvili et al., 1999) were incubated at room temperature for 1 hour. The samples were filtered through glass fiber filters and filter-retained radioactivity was measured in a Beckman scintillation counter using EcoScint A.
4.4. [35S]GTPγS binding assays
Cell membranes from control and morphine-treated (1μM for 24 hrs) M2 and A7 cells expressing myc-tagged MOP were prepared as previously described (Onoprishvili et al., 1999). An aliquot of frozen membranes were diluted in a 50 mM Tris–HCl buffer, pH 7.4, containing 3 mM MgCl2, 0.2 mM EGTA, 100 mM NaCl, 10 μM GDP and 10μg/ml Saponin. Samples (25–30 μg) were pre-incubated for 15 min with DAMGO (10−8–10−5M) in a 1 ml volume. [35S]GTPγS (GE Healthcare; 1100–1200 Ci/mmol) was added at a concentration of 0.05 nM to each sample and incubated for an additional 45 min at 30°C. Nonspecific binding was determined in the presence of 10 μM GTPγS and the basal binding was assessed in the absence of the mu opioid agonist DAMGO. Each experiment was carried out on triplicate samples. The reaction was terminated by rapid filtration. Bound radioactivity was determined in a Beckman liquid scintillation counter using Ecoscint A (National Diagnostics).
4.5. Western blot analysis
Melanoma cells were incubated with or without 1μM morphine in MEM serum free medium for 24 hours at 37°C. After treatment, the cells were washed in PBS and then lysed in a buffer containing 50 mM Tris, pH 7.4, 1% Triton X-100, 10% glycerol, 300 mM NaCl, 1.5mM MgCl2, 1mM CaCl2 and protease inhibitors. Cell lysates were kept on ice for 30 min and then centrifuged at 15,000 rpm for 15 min. The supernatants were saved and protein concentration was determined using Bio-Rad DC Protein Assay Kit (Bio-Rad Laboratories). Equal amounts of protein (10 μg) was loaded on polyacrylamide gels (ISC) and separated by electrophoresis. After transferring the samples onto nitrocellulose membranes, they were blocked in a buffer containing 5% milk, 25mM Tris, 100mM NaCl and 0.05% Tween-20. Western blot analysis was performed using 1: 1,500 dilution of polyclonal anti-myc (Santa Cruz). To ensure that approximately equal amounts of the proteins were loaded on the gel, nitrocellulose membranes were probed with 1: 10,000 dilution of monoclonal anti-tubulin antibodies (Sigma). Immunoblots were visualized by the enhanced chemiluminescence method (Pierce).
4.6. Data analysis
The radioligand binding and the concentration-response data were analyzed by nonlinear regression and sigmoidal dose-response curves, respectively, using GraphPad Prism software. The data from multiple experiments (from three to seven) were averaged and expressed as mean ± SEM values. The statistical comparisons were performed by unpaired Student’s t-test and/or Two-way ANOVA followed Bonferroni test or Fisher’s Protected t-test.
Acknowledgments
We thank Drs. Thomas P. Stossel and Y. Ohta for providing us with the M2 and A7 melanoma cells. This work was supported by NIDA grant DA00017 to EJS and a Fellowship from NIDA Training Grant DA-07254 to IO. We thank our colleague, Dr. Maarten Reith, for critical reading of the manuscript.
Abbreviations
- GPCR
G protein-coupled receptor
- PTX
Pertussis toxin
- MOP
mu opioid receptor
- DAMGO
[D-Ala2,N-MePhe4-Gly-ol5]enkephalin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ancevska-Taneva N, Onoprishvili I, Andria ML, Hiller JM, Simon EJ. A member of the heat-shock protein 40 family, hlj 1, binds to the carboxyl tail of the human mu opioid receptor. Brain Res. 2006;1081:228–233. doi: 10.1016/j.brainres.2006.01.125. [DOI] [PubMed] [Google Scholar]
- Awata H, Huang C, Handlogten ME, Miller RT. Interaction of the calcium-sensing receptor and filamin, a potential scaffolding protein. J Biol Chem. 2001;276:34871–34879. doi: 10.1074/jbc.M100775200. [DOI] [PubMed] [Google Scholar]
- Baumhaker Y, Gafni M, Keren O, Sarne Y. Selective and interactive down-regulation of mu- and delta-opioid receptors in human neuroblastoma SK-N-SH cells. Mol Pharmacol. 1993;44:461–467. [PubMed] [Google Scholar]
- Besse D, Lombard MC, Besson JM. Time-related decreases in mu and delta opioid receptors in the superficial dorsal horn of the rat spinal cord following a large unilateral dorsal rhizotomy. Brain Research. 1992;578:115–121. doi: 10.1016/0006-8993(92)90237-4. [DOI] [PubMed] [Google Scholar]
- Bhargava HN, Gulati A. Down-regulation of brain and spinal cord mu-opiate receptors in morphine tolerant-dependent rats. Eur J Pharmacol. 1990;190:305–311. doi: 10.1016/0014-2999(90)94194-3. [DOI] [PubMed] [Google Scholar]
- Bot G, Blake AD, Li S, Reisine T. Opioid regulation of the mouse delta-opioid receptor expressed in human embryonic kidney 293 cells. Mol Pharmacol. 1997;52:272–281. doi: 10.1124/mol.52.2.272. [DOI] [PubMed] [Google Scholar]
- Brady LS, Herkenham M, Long JB, Rothman RB. Chronic morphine increases mu-opiate receptor binding in rat brain: a quantitative autoradiographic study. Brain Res. 1989;477:382–386. doi: 10.1016/0006-8993(89)91432-7. [DOI] [PubMed] [Google Scholar]
- Burd AL, El-Kouhen R, Erickson LJ, Loh HH, Law P-Y. Identification of serine 356 and serine 363 as the amino acids involved in etorphine-induced down-regulation of the mu-opioid receptor. Journal of Biological Chemistry. 1998;273:34488–34495. doi: 10.1074/jbc.273.51.34488. [DOI] [PubMed] [Google Scholar]
- Capeyrou R, Riond J, Corbani M, Lepage JF, Bertin B, Emorine LJ. Agonist-induced signaling and trafficking of the μ-opioid receptor: role of serine and threonine residues in the third cytoplasmic loop and C-terminal domain. FEBS Letters. 1997;415:200–205. doi: 10.1016/s0014-5793(97)01124-1. [DOI] [PubMed] [Google Scholar]
- Chaipatikul V, Erickson-Herbrandson LJ, Loh HH, Law PY. Rescuing the traffic-deficient mutants of rat mu-opioid receptors with hydrophobic ligands. Mol Pharmacol. 2003;64:32–41. doi: 10.1124/mol.64.1.32. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Law PY, Loh HH. Neuroblastoma Neuro2A cells stably expressing a cloned mu-opioid receptor: a specific cellular model to study acute and chronic effects of morphine. Brain Res Mol Brain Res. 1995;30:269–278. doi: 10.1016/0169-328x(95)00014-j. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Regec A, Gintzler AR. Biochemical demonstration of mu-opioid receptor association with Gsalpha: enhancement following morphine exposure. Brain Res Mol Brain Res. 2005;135:217–224. doi: 10.1016/j.molbrainres.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Chaturvedi K, Bandari P, Chinen N, Howells RD. Proteasome Involvement in Agonist-induced Down-regulation of {micro} and delta Opioid Receptors. J Biol Chem. 2001;276:12345–12355. doi: 10.1074/jbc.M008054200. [DOI] [PubMed] [Google Scholar]
- Cowen DS, Molinoff PB, Manning DR. 5-hydroxytryptamine1A receptor-mediated increases in receptor expression and activation of nuclear factor-kappaB in transfected Chinese hamster ovary cells. Mol Pharmacol. 1997;52:221–226. doi: 10.1124/mol.52.2.221. [DOI] [PubMed] [Google Scholar]
- Cunningham CC, Gorlin JB, Kwiatkowski DJ, Hartwig JH, Janmey PA, Byers HR, Stossel TP. Actin-binding protein requirement for cortical stability and efficient locomotion. Science. 1992;255:325–327. doi: 10.1126/science.1549777. [DOI] [PubMed] [Google Scholar]
- Enz R. The actin-binding protein filamin-A interatcs with the metabotropic glutamate receptor type 7. FEBS Lett. 2002;514:184–188. doi: 10.1016/s0014-5793(02)02361-x. [DOI] [PubMed] [Google Scholar]
- Fabian G, Bozo B, Szikszay M, Horvath G, Coscia CJ, Szucs M. Chronic morphine-induced changes in mu-opioid receptors and G proteins of different subcellular loci in rat brain. J Pharmacol Exp Ther. 2002;302:774–780. doi: 10.1124/jpet.102.036152. [DOI] [PubMed] [Google Scholar]
- Fabian G, Tombor B, Nemeth I, Kicsi EG, Szikszay M, Horvath G, Szucs M. Upregulation of mu opioid receptors by voluntary morphine administration in drinking water. Acta Biol Hung. 2003;54:157–166. doi: 10.1556/ABiol.54.2003.2.4. [DOI] [PubMed] [Google Scholar]
- Feng GJ, Kellett E, Scorer CA, Wilde J, White JH, Milligan G. Selective interactions between helix VIII of the human mu-opioid receptors and the C terminus of periplakin disrupt G protein activation. J Biol Chem. 2003;278:33400–33407. doi: 10.1074/jbc.M305866200. [DOI] [PubMed] [Google Scholar]
- Ferguson SS, Caron MG. G protein-coupled receptor adaptation mechanisms. Semin Cell Dev Biol. 1998;9:119–127. doi: 10.1006/scdb.1997.0216. [DOI] [PubMed] [Google Scholar]
- Filtz TM, Guan W, Artymyshyn RP, Facheco M, Ford C, Molinoff PB. Mechanisms of up-regulation of D2L dopamine receptors by agonists and antagonists in transfected HEK-293 cells. J Pharmacol Exp Ther. 1994;271:1574–1582. [PubMed] [Google Scholar]
- Finn AK, Whistler JL. Endocytosis of the mu opioid receptor reduces tolerance and a cellular hallmark of opiate withdrawal. Neuron. 2001;32:829–839. doi: 10.1016/s0896-6273(01)00517-7. [DOI] [PubMed] [Google Scholar]
- Guang W, Wang H, Su T, Weinstein IB, Wang JB. Role of mPKCI, a novel mu-opioid receptor interactive protein, in receptor desensitization, phosphorylation, and morphine-induced analgesia. Mol Pharmacol. 2004;66:1285–1292. doi: 10.1124/mol.66.5.. [DOI] [PubMed] [Google Scholar]
- Hjalm G, macLeod RJ, Kifor O, Chattopadhyay N, Brown EM. Filamin-A binds to the carboxyl-terminal tail of the calcium-sensing receptor, an interaction that participates in CaR-mediated activation of mitogen-activated protein kinase. Journal of Biological Chemistry. 2001;276:34880–34887. doi: 10.1074/jbc.M100784200. [DOI] [PubMed] [Google Scholar]
- Holaday JW, Hitzemann RJ, Curell J, Tortella FC, Belenky GL. Repeated electroconvulsive shock or chronic morphine treatment increases the number of 3H-D-Ala2,D-Leu5-enkephalin binding sites in rat brain membranes. Life Sci. 1982;31:2359–2362. doi: 10.1016/0024-3205(82)90156-4. [DOI] [PubMed] [Google Scholar]
- Horner KA, Zadina JE. Internalization and down-regulation of mu opioid receptors by endomorphins and morphine in SH-SY5Y human neuroblastoma cells. Brain Res. 2004;1028:121–132. doi: 10.1016/j.brainres.2004.07.055. [DOI] [PubMed] [Google Scholar]
- Kato S, Fukuda K, Morikawa H, Shoda T, Mima H, Mori K. Adaptations to chronic agonist exposure of mu-opioid receptor-expressing Chinese hamster ovary cells. Eur J Pharmacol. 1998;345:221–228. doi: 10.1016/s0014-2999(98)00023-5. [DOI] [PubMed] [Google Scholar]
- Keith DE, Anton B, Murray SR, Zaki PA, Chu PC, Lissin DV, Monteillet-Agius G, Stewart PL, Evans CJ, von Zastrow M. Mu-opioid receptor internalization: opiate drugs have differential effects on a conserved endocytic mechanism in vitro and in the mammalian brain. Molecular Pharmacology. 1998;53:377–384. [PubMed] [Google Scholar]
- Kim KM, Gainetdinov RR, Laporte SA, Caron MG, Barak LS. G protein coupled receptor kinase regulates dopamine D3 receptor signaling by modulating the stability of a receptor-filamin-beta-arrestin complex. A case of autoreceptor regulation. J Biol Chem. 2005;280:12774–12780. doi: 10.1074/jbc.M408901200. [DOI] [PubMed] [Google Scholar]
- Koch T, Brandenburg LO, Schulz S, Liang Y, Klein J, Hollt V. ADP-ribosylation factor-dependent phospholipase D2 activation is required for agonist-induced mu-opioid receptor endocytosis. J Biol Chem. 2003;278:9979–9985. doi: 10.1074/jbc.M206709200. [DOI] [PubMed] [Google Scholar]
- Koch T, Widera A, Bartzsch K, Schulz S, Brandenburg LO, Wundrack N, Beyer A, Grecksch G, Hollt V. Receptor endocytosis counteracts the development of opioid tolerance. Mol Pharmacol. 2005;67:280–287. doi: 10.1124/mol.104.004994. [DOI] [PubMed] [Google Scholar]
- Kurose H, Katada T, Amano T, Ui M. Specific uncoupling by islet-activating protein, pertussis toxin, of negative signal transduction via alpha-adrenergic, cholinergic, and opiate receptors in neuroblastoma x glioma hybrid cells. Journal of Biological Chemistry. 1983;258:4870–4875. [PubMed] [Google Scholar]
- Law PY, Wong YH, Loh HH. Mutational analysis of the structure and function of opioid receptors. Biopolymers. 1999;51:440–455. doi: 10.1002/(SICI)1097-0282(1999)51:6<440::AID-BIP6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ. G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. Journal of Biological Chemistry. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- Lesscher HM, Bailey A, Burbach JP, Van Ree JM, Kitchen I, Gerrits MA. Receptor-selective changes in mu-, delta- and kappa-opioid receptors after chronic naltrexone treatment in mice. Eur J Neurosci. 2003;17:1006–1012. doi: 10.1046/j.1460-9568.2003.02502.x. [DOI] [PubMed] [Google Scholar]
- Li M, Bermak JC, Wang ZW, Zhou QY. Modulation of dopamine D2 receptor signaling by actin-binding protein (ABP-280. Molecular Pharmacology. 2000;57:446–452. doi: 10.1124/mol.57.3.446. [DOI] [PubMed] [Google Scholar]
- Lin R, Karpa K, Kabbani N, Goldman-Rakic P, Levenson R. Dopamine D2 and D3 receptors are linked to the actin cytoskeleton via interaction with filamin A. Proceedings of the National Academy of Sciences USA. 2001;98:5258–5263. doi: 10.1073/pnas.011538198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuser T, Giesecke T, Gabriel A, Horsch M, Sabatowski R, Hescheler J, Grond S, Palmer PP. Mu-opioid receptor mRNA regulation during morphine tolerance in the rat peripheral nervous system. Anesth Analg. 2003;97:1458–1463. doi: 10.1213/01.ANE.0000081721.75663.87. [DOI] [PubMed] [Google Scholar]
- Mitchell R, McCulloch D, Lutz E, Johnson M, MacKenzie C, Fennell M, Fink G, Zhou W, Sealfon SC. Rhodopsin-family receptors associate with small G proteins to activate phospholipase D. Nature. 1998;392:411–414. doi: 10.1038/32937. [DOI] [PubMed] [Google Scholar]
- Narita M, Mizoguchi H, Nagase H, Suzuki T, Tseng LF. Up-regulation of spinal mu-opioid receptor function to activate G-protein by chronic naloxone treatment. Brain Res. 2001;913:170–173. doi: 10.1016/s0006-8993(01)02785-8. [DOI] [PubMed] [Google Scholar]
- Ng GY, Varghese G, Chung HT, Trogadis J, Seeman P, O’Dowd BF, George SR. Resistance of the dopamine D2L receptor to desensitization accompanies the up-regulation of receptors on to the surface of Sf9 cells. Endocrinology. 1997;138:4199–4206. doi: 10.1210/endo.138.10.5433. [DOI] [PubMed] [Google Scholar]
- Onoprishvili I, Andria M, Vilim F, Hiller J, Simon E. The bovine mu-opioid receptor: cloning of cDNA and pharmacological characterization of the receptor expressed in mammalian cells. Molecular Brain Research. 1999;73:129–137. doi: 10.1016/s0169-328x(99)00249-1. [DOI] [PubMed] [Google Scholar]
- Onoprishvili I, Andria ML, Kramer HK, Ancevska-Taneva N, Hiller JM, Simon EJ. Interaction between the mu opioid receptor and filamin A is involved in receptor regulation and trafficking. Mol Pharmacol. 2003;64:1092–1100. doi: 10.1124/mol.64.5.1092. [DOI] [PubMed] [Google Scholar]
- Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- Polastron J, Meunier JC, Jauzac P. Chronic morphine induces tolerance and desensitization of mu-opioid receptor but not down-regulation in rabbit. Eur J Pharmacol. 1994;266:139–146. doi: 10.1016/0922-4106(94)90103-1. [DOI] [PubMed] [Google Scholar]
- Puttfarcken PS, Cox BM. Morphine-induced desensitization and down-regulation at mu-receptors in 7315C pituitary tumor cells. Life Sci. 1989;45:1937–1942. doi: 10.1016/0024-3205(89)90548-1. [DOI] [PubMed] [Google Scholar]
- Ray SB, Gupta H, Gupta YK. Up-regulation of m-opioid receptors in the spinal cord of morphine-tolerant rats. J Biosci. 2004;29:51–56. doi: 10.1007/BF02702561. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Long JB, Bykov V, Xu H, Jacobson AE, Rice KC, Holaday JW. Upregulation of the opioid receptor complex by the chronic administration of morphine: a biochemical marker related to the development of tolerance and dependence. Peptides. 1991;12:151–160. doi: 10.1016/0196-9781(91)90182-o. [DOI] [PubMed] [Google Scholar]
- Schmidt P, Schmolke C, Musshoff F, Menzen M, Prohaska C, Madea B. Area-specific increased density of mu-opioid receptor immunoreactive neurons in the cerebral cortex of drug-related fatalities. Forensic Sci Int. 2003;133:204–211. doi: 10.1016/s0379-0738(03)00067-7. [DOI] [PubMed] [Google Scholar]
- Seck T, Baron R, Horne WC. Binding of filamin to the C-terminal tail of the calcitonin receptor controls recycling. J Biol Chem. 2003;278:10408–10416. doi: 10.1074/jbc.M209655200. [DOI] [PubMed] [Google Scholar]
- Stafford K, Gomes AB, Shen J, Yoburn BC. mu-Opioid receptor downregulation contributes to opioid tolerance in vivo. Pharmacol Biochem Behav. 2001;69:233–237. doi: 10.1016/s0091-3057(01)00525-1. [DOI] [PubMed] [Google Scholar]
- Starr S, Kozell LB, Neve KA. Drug-induced up-regulation of dopamine D2 receptors on cultured cells. J Neurochem. 1995;65:569–577. doi: 10.1046/j.1471-4159.1995.65020569.x. [DOI] [PubMed] [Google Scholar]
- Stossel TP, Condeelis J, Cooley L, Hartwig JH, Noegel A, Schleicher M, Shapiro SS. Filamins as integrators of cell mechanics and signalling. Nat Rev Mol Cell Biol. 2001;2:138–145. doi: 10.1038/35052082. [DOI] [PubMed] [Google Scholar]
- Tempel A, Crain SM, Peterson ER, Simon EJ, Zukin RS. Antagonist-induced opiate receptor upregulation in cultures of fetal mouse spinal cord-ganglion explants. Brain Res. 1986;390:287–291. doi: 10.1016/s0006-8993(86)80237-2. [DOI] [PubMed] [Google Scholar]
- Trapaidze N, Keith DE, Cvejic S, Evans CJ, Devi LA. Sequestration of the delta opioid receptor. Role of the C terminus in agonist-mediated internalization. Journal of Biological Chemistry. 1996;271:29279–29285. doi: 10.1074/jbc.271.46.29279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao P, von Zastrow M. Downregulation of G protein-coupled receptors. Current opinion in Neurobiology. 2000;10:365–369. doi: 10.1016/s0959-4388(00)00096-9. [DOI] [PubMed] [Google Scholar]
- Turchan J, Przewlocka B, Toth G, Lason W, Borsodi A, Przewlocki R. The effect of repeated administration of morphine, cocaine and ethanol on mu and delta opioid receptor density in the nucleus accumbens and striatum of the rat. Neuroscience. 1999;91:971–977. doi: 10.1016/s0306-4522(98)00637-x. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Anton B, Lam H, Evans CJ. Quantitative immunolocalization of mu opioid receptors: regulation by naltrexone. Neuroscience. 1998;85:897–905. doi: 10.1016/s0306-4522(97)00659-3. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Rubenfeld JM, Imai Y, Wang JB, Uhl GR, Kreek MJ. Chronic opioid antagonist administration upregulates mu opioid receptor binding without altering mu opioid receptor mRNA levels. Brain Res Mol Brain Res. 1995;33:351–355. doi: 10.1016/0169-328x(95)00143-g. [DOI] [PubMed] [Google Scholar]
- van der Flier A, Sonnenberg A. Structural and functional aspects of filamins. Biochim Biophys Acta. 2001;1538:99–117. doi: 10.1016/s0167-4889(01)00072-6. [DOI] [PubMed] [Google Scholar]
- Vigano D, Rubino T, Di Chiara G, Ascari I, Massi P, Parolaro D. Mu opioid receptor signaling in morphine sensitization. Neuroscience. 2003;117:921–929. doi: 10.1016/s0306-4522(02)00825-4. [DOI] [PubMed] [Google Scholar]
- Werling LL, McMahon PN, Cox BM. Selective changes in mu opioid receptor properties induced by chronic morphine exposure. Proceedings of the National Academy of Sciences USA. 1989;86:6393–6397. doi: 10.1073/pnas.86.16.6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabaluri N, Medzihradsky F. Down-regulation of mu-opioid receptor by full but not partial agonists is independent of G protein coupling. Mol Pharmacol. 1997;52:896–902. doi: 10.1124/mol.52.5.896. [DOI] [PubMed] [Google Scholar]
- Yoburn BC, Sierra V, Lutfy K. Chronic opioid antagonist treatment: assessment of receptor upregulation. Eur J Pharmacol. 1989;170:193–200. doi: 10.1016/0014-2999(89)90539-6. [DOI] [PubMed] [Google Scholar]
- Zadina JE, Chang SL, Ge LJ, Kastin AJ. Mu opiate receptor down-regulation by morphine and up-regulation by naloxone in SH-SY5Y human neuroblastoma cells. J Pharmacol Exp Ther. 1993;265:254–262. [PubMed] [Google Scholar]
- Zadina JE, Harrison LM, Ge LJ, Kastin AJ, Chang SL. Differential regulation of mu and delta opiate receptors by morphine, selective agonists and antagonists and differentiating agents in SH-SY5Y human neuroblastoma cells. J Pharmacol Exp Ther. 1994;270:1086–1096. [PubMed] [Google Scholar]
- Zhang LJ, Lachowicz JE, Sibley DR. The D2S and D2L dopamine receptor isoforms are differentially regulated in Chinese hamster ovary cells. Mol Pharmacol. 1994;45:878–889. [PubMed] [Google Scholar]
- Zhang T, Xu Q, Chen FR, Han QD, Zhang YY. Yeast two-hybrid screening for proteins that interact with alpha1-adrenergic receptors. Acta Pharmacol Sin. 2004;25:1471–1478. [PubMed] [Google Scholar]
- Zukin RS, Tempel A. Neurochemical correlates of opiate receptor regulation. Biochem Pharmacol. 1986;35:1623–1627. doi: 10.1016/0006-2952(86)90314-x. [DOI] [PubMed] [Google Scholar]