Abstract
Background
Although inflammation is presumed to contribute to colonic neoplasia in ulcerative colitis (UC), few studies have directly examined this relationship.
Aim
To determine whether severity of microscopic inflammation over time is an independent risk factor for neoplastic progression in UC.
Methods
A cohort of patients with UC undergoing regular endoscopic surveillance for dysplasia was studied. Degree of inflammation at each biopsy site had been graded as part of routine clinical care using a highly reproducible histological activity index. Progression to neoplasia was analyzed in proportional hazards models with inflammation summarized in three different ways, and each included as a time-changing covariate: 1) mean inflammatory score (IS-mean); 2) binary inflammatory score (IS-bin); and 3) maximum inflammatory score (IS-max). Potential confounders were analyzed in univariate testing, and, when significant, in a multivariable model.
Results
Of 418 patients who met inclusion criteria, 15 progressed to advanced neoplasia (high-grade dysplasia, HGD, or colorectal cancer, CRC) and 65 progressed to any neoplasia (low-grade dysplasia, HGD, or CRC). Univariate analysis demonstrated significant relationships between histological inflammation over time and progression to advanced neoplasia (HR=3.0; 95% CI 1.4-6.3 for IS-mean, HR=3.4; 95% CI 1.1-10.4 for IS-bin; and HR=2.2; 95%CI 1.2-4.2 for IS-max). This association was maintained in multivariable proportional hazards analysis.
Conclusion
The severity of microscopic inflammation over time is an independent risk factor for developing advanced colorectal neoplasia among patients with longstanding UC.
Introduction
Patients with ulcerative colitis (UC) face an increased risk for developing colorectal cancer (CRC).1-3 Established risk factors include prolonged disease duration (>7-8 years), extensive colonic involvement, a family history of colorectal cancer, coexistence of primary sclerosing cholangitis, and, in some studies, young age at disease onset.1 To lower this risk, patients are typically enrolled in colonoscopic surveillance programs. This serves to diminish the risk of CRC morbidity and mortality while also minimizing the use of prophylactic colectomy. Such programs call for periodic colonoscopic examinations during which multiple biopsies are taken and evaluated for neoplasia (dysplasia or CRC).1 Patients with biopsy-proven neoplasia are regarded as being at high risk for the coexistence of or further progression to CRC, and depending on the specific findings, are encouraged to undergo either colectomy or more frequent surveillance examinations.
Although acknowledged to be the most effective method of cancer prevention other than prophylactic colectomy, surveillance carries important limitations, including unproven efficacy, incomplete patient enrollment and adherence,4-6 invasiveness, high cost,7 endoscopic sampling variations,8, 9 and poor interobserver agreement in histopathological interpretation.10, 11 One way to improve the effectiveness of surveillance would be to identify additional risk factors for neoplastic progression, thereby permitting closer observation of high-risk patients. Curiously, although inflammation has been assumed to be a key factor contributing to higher risk of colonic neoplasia in UC,12 few studies have examined this issue. One well conducted retrospective case-control study recently reported that histologic inflammation was indeed associated with an increased risk of neoplastic progression.13
We reasoned that a cohort study design would better mimic the usual clinical situation of surveillance colonoscopy and would allow us to account for changes in inflammation over time, while also considering exposure to surveillance colonoscopies. The aim of the present study was to use such a study design to establish whether the degree of histologic inflammation was an independent risk factor for developing neoplasia in UC.
Materials and Methods
Following approval by The Mount Sinai School of Medicine Institutional Review Board and in accordance with Health Insurance Portability and Accountability Act (HIPAA) guidelines, our research group established a UC Surveillance Database. This Database contains all patients in the Mount Sinai Hospital Gastrointestinal Pathology and Surgical Pathology Registries who had undergone at least one surveillance colonoscopy between January 1996 and December 1997, a period chosen to allow for long-term follow-up. The Database includes demographic information, data on exposure to IBD-related medications, clinical history, and pathologic findings from colonoscopies and surgeries, including anatomic extent of disease (defined by greatest microscopic extent at any examination based on the findings of Mathy and colleagues),14 number of biopsies per examination, the presence and grade of any dysplasia, and the presence and severity of inflammation at each biopsied segment of colon.
Inclusion and Exclusion Criteria
Patients were included if they had: 1) a colonoscopic examination in 1996 or 1997; 2) an established clinical diagnosis of UC for ≥ 7 years based on symptoms, radiology, or endoscopy, 3) no known dysplasia or CRC prior to, or at the time of, their first colonoscopy at our institution, and 4) at least one subsequent endoscopic or surgical follow-up examination with biopsies of the colon. Patients were excluded who had undergone prior segmental resection of colitic colon or carried a diagnosis of either Crohn’s disease or indeterminate colitis.
Grading of Histological Inflammation
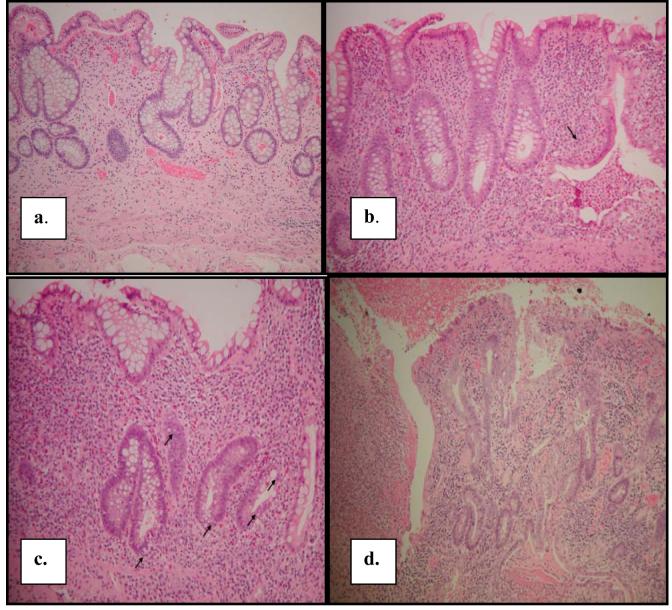
The severity of histological inflammation in each biopsy was extracted from the pathology reports for all available surgical and colonoscopic specimens (surveillance and non-surveillance) prior to, at, and subsequent to each subject’s inclusion colonoscopy in 1996 or 1997. The histological criteria for grading severity of inflammation was developed by one of the authors (N.H.) and placed into routine use at The Mount Sinai Hospital in 1988. These criteria have remained unchanged since their introduction. All reports had been issued by one of a small group of gastrointestinal pathologists, trained by N.H., using the narrative equivalent of a standardized histological activity index (HAI). The degree of inflammation for each biopsy site was scored as follows: (0) inactive/absent, (1) mild, (2) moderate or (3) severe. Histologic criteria are listed in Table 1, with examples provided in Figure 1. A previous study has verified that the grading of HAI achieves high levels of intra- and interobserver agreement with kappa values of approximately 0.9 regardless of level of training.15 By convention, when more than one biopsy was taken from a particular segment of the colon, the highest HAI from that segment was recorded. Results of a particular colonoscopy were summarized into a single value, the inflammatory score (IS), which was equal to the sum of HAI’s at all sites divided by the number of sites.
Table 1.
Histological Activity Index (HAI)
| Inflammatory Activity | Score | Histopathologic Defining Characteristics |
|---|---|---|
| Inactive/ Quiescent/ Normal | 0 | No epithelial infiltration by neutrophils. |
| Mildly Active | 1 | Neutrophil infiltration of <50% of sampled crypts or cross sections, no ulcers or erosions. |
| Moderately Active | 2 | Neutrophil infiltration of ≥ 50% of sampled crypts or cross sections, no ulcers or erosions. |
| Severely Active | 3 | Erosion or ulceration, irrespective of other features. |
a. Inactive colitis with no cryptitis or crypt abscesses; HAI=0.
b. Mildly active colitis with one crypt abscess (arrow); HAI=1
c. Moderately active colitis with cryptitis involving >50% of crypts (arrows); HAI=2.
d. Severely active colitis with ulceration; HAI=3.
Figure 1.
- Inactive colitis with no cryptitis or crypt abscesses; HAI=0.
- Mildly active colitis with one crypt abscess (arrow); HAI=1
- Moderately active colitis with cryptitis involving >50% of crypts (arrows); HAI=2.
- Severely active colitis with ulceration; HAI=3.
Grading of Dysplasia
Dysplasia, the outcome measure in the present study, was either diagnosed initially or subsequently confirmed as part of routine clinical practice at our institution by one of the authors (N.H.) using the criteria of the IBD Dysplasia Morphology Study Group16 and was recorded as either negative for dysplasia (NoD), indefinite for dysplasia (IND), or positive for low-grade dysplasia (LGD), high-grade dysplasia (HGD) or CRC. Adenomatous polyps above the proximal-most extent of microscopic disease were excluded. Such polyps were defined as discrete polypoid dysplastic lesions located in segments of endoscopically and histologically normal colon (proximal to diseased colon). Polypoid dysplastic lesions within colitic mucosa were graded according to their histology (LGD, HGD, or CRC).
We defined the development of HGD or CRC at follow-up surveillance or colectomy as progression to advanced neoplasia and the development of LGD, HGD or CRC as progression to any neoplasia. For these outcome events, no distinction was made between flat and polypoid neoplasia. All subjects were followed from their first dysplasia-free colonoscopy (defined as time-zero) to one of the following endpoints: 1) neoplastic progression, 2) colectomy, or 3) last recorded colonoscopy without progression. Only two colectomies were performed for indications other than dysplasia. The interval between time zero and the endpoint was termed the follow-up interval.
Data Analysis
Medians and interquartile ranges were calculated for all continuous variables; proportions were calculated for all binary variables.
Hazard ratios with 95% confidence intervals were calculated using proportional hazards analysis (Cox regression).17 The primary variable of interest, IS (see below), was assessed for its relationships to progression to advanced or any neoplasia both in univariate models and jointly with other suspected risk factors in a multivariable analysis. Univariate analyses were performed initially to test for the influence of known and suspected confounders (duration and extent of disease, PSC, age of onset of disease, exposure to mesalamine-based compounds, and exposure to colonoscopy) on the development of any, or of advanced, neoplasia. Covariates were added, one at a time, to a multivariable model with IS-mean if they had a p-value <.20 in either the any or advanced neoplasia univariate analyses.
In the proportional hazards model, effects of covariates are assessed repeatedly for all patients still under observation at the time of each positive finding in any patient. Typically, values of the covariates are fixed at the time of entry into the study. In this study, two variables whose values are known to change over time, IS and exposure to colonoscopy, were coded as time-changing covariates. Use of time-changing covariates allows for values of variables to be updated so that they refer only to values up to and including the time of a particular comparison.18
The relationship between histological inflammation and progression to any neoplasia or to advanced neoplasia was assessed three ways, each based on the inflammation scores of all colonoscopies up to and including the time of a particular comparison. (As described previously, comparisons were made at the time of each positive outcome according to Cox proportional hazards modeling.)17
IS-mean is the average inflammation score of all of the colonoscopies registered in the model at the time of the comparison. It is a continuous variable, with high values if there are multiple high inflammation scores to date. An occasional high inflammation score will be diluted by a large number of colonoscopies with low scores and vice versa.
IS-bin is a binary variable that equals 1 if IS-mean at the time of comparison is ≥1, and 0 otherwise.
IS-max is a continuous variable whose value equals the maximum inflammation score among colonoscopies up to and including the time of a particular comparison.
Exposure to colonoscopy, given its irregular application in this non-protocolized surveillance cohort, was also evaluated as a time-changing covariate, defined at each time-point as the number of colonoscopies to that time divided by the number of years since the first. Exposure to medications was considered positive if there were at least 4 months of consecutive use. All analyses were performed with SAS software on a Windows-operated personal computer.
Results
Of 543 UC patients who underwent surveillance colonoscopy between 1996-1997, 125 patients were excluded for having dysplasia at their first colonoscopy at our institution, lack of histological follow-up, or prior colorectal surgery, leaving a cohort of 418 patients with no initial dysplasia. The demographic characteristics of the cohort, their surveillance patterns and medication use are provided in Table 2. Over 90% of patients had extensive colitis, and the median duration of UC was 16 years. Very few patients in this cohort had PSC. Patients were followed for a median of 6.7 years, received a median of 5 surveillance colonoscopies at a median interval of 0.8 years. The vast majority was taking 5-ASA compounds, more than half had used steroids, fewer than a third had taken 6-mercaptopurine or azathioprine, almost one third had taken folic acid, and very few received cyclosporine. Overall, 65 (15.6%) patients progressed to any neoplasia and 15 (3.6%) progressed to advanced neoplasia. Of these, five were adenocarcinomas, one was a raised HGD lesion, and nine were flat HGD.
Table 2.
Demographics
| Total | |
|---|---|
| Number of Patients | 418 |
| Male, n | 224 (54.0%) |
| Age at UC Diagnosis; years [IQ-range] | 26.8 [19.4, 36.1] |
| Extensive UC, n | 324 (92.0%) |
| Duration UC; years [IQ-range] | 16 [11.1, 22.9] |
| PSC, n | 6 (1.8%) |
| Surveillance Patterns | |
| Total Number of Colonoscopies | 2255 |
| Exams Per Patient; median [IQ-range] | 5 [3, 7] |
| Length of Follow-up; median [IQ-range] | 6.7 [4.2, 8.8] |
| Frequency of Exams per patient per year; median [IQ-range] | 0.8 [0.6, 1.1] |
| Medication Use | |
| 5ASA, n | 310 (90.9%*) |
| Steroids, n | 200 (58.7%*) |
| 6-MP/AZA, n | 117 (28.0%*) |
| Cyclosporine, n | 21 (6.1%*) |
| Folate, n | 105 (30.8%*) |
note: excludes patients for whom this information was missing
Median and interquartile range (IQ-range) given for continuous variables
Of the 2255 surveillance examinations, most exhibited little histologic inflammation in the colon. As seen in Table 3, only 83 of these examinations resulted in an IS-mean of ≥ 2, with 1832 showing values of < 1. Overall, the median IS was 0.33 with an interquartile range from 0 to 0.75.
Table 3.
Inflammatory Score for the 2255 colonoscopies performed
| IS | Number |
|---|---|
| 0 | 591 |
| > 0 - <1 | 1241 |
| 1 - < 2 | 340 |
| 2 - < 3 | 59 |
| >3 | 24 |
On univariate analysis, a significant relationship was found between inflammation and progression to advanced neoplasia (Table 4). Measuring inflammation as the mean over the length of surveillance (IS-mean), a three-fold increased risk for advanced neoplasia was observed (HR 3.0, 95% CI 1.4-6.3). Thus, for every unit increase in cumulative mean histological inflammation score, there was a three-fold increase in risk of advanced neoplasia. Likewise, when scored as the maximal inflammation score ever during surveillance (IS-max), a two-fold increase in risk was observed (HR 2.2; 95% CI 1.2-4.2). When the cumulative mean inflammation score ≥1 (IS-bin), we found the highest point estimate (HR 3.4, 95% CI 1.1-10.4)) for inflammation predicting advanced neoplasia.. When the endpoint of any neoplasia was used, there was a trend toward increased risk of neoplasia, with HR values ranging between 1.0 and 1.7 for the three IS measures, but these did not achieve statistical significance. To ensure that the effects of inflammation prior to surveillance were not somehow biasing our results, we removed the 34 patients in whom such examinations were available for analysis, and found that there was no change in risk to progression to advanced neoplasia, with IS-mean yielding a HR of 2.8 [95% CI 1.2-6.2].
Table 4.
Univariate Association between Predictors and Endpoint
| ENDPOINT, HR (95% CI) | ||
|---|---|---|
| Any Neoplasia (N=65) | Advanced Neoplasia (N=15) | |
| INFLAMMATION SCORE | ||
| IS-mean* | 1.4 (0.9, 2.3) | 3.0 (1.4, 6.3) |
| IS-bin* | 1.7 (0.9, 3.1) | 3.4 (1.1, 10.4) |
| IS-max* | 1.0 (0.7, 1.5) | 2.2 (1.2, 4.2) |
| OTHER FACTORS | ||
| 1 or More Colonoscopies/year | 1.7 (0.9, 3.0) | 3.9 (1.3, 11.4) |
| Extent of Disease | 1.1 (0.4, 3.5) | ** |
| Duration of Disease >15 | 1.6 (0.9, 2.8) | 2.0 (0.6, 6.3) |
| Age at diagnosis ≤ 25 | 0.7 (0.4, 1.2) | 1.6 (0.6, 4.5) |
| PSC | 1.1 (0.2,8.0) | ** |
| 5ASA | 0.6 (0.3, 1.2) | 0.5 (0.1, 2.4) |
| 6MP/AZA | 1.0 (0.6, 1.6) | 0.8 (0.3, 2.7) |
| Steroids | 0.6 (0.4, 1.1) | 0.6 (0.2, 1.7) |
| Cyclosporine | 0.8 (0.3, 2.6) | ** |
| Folate | 0.9 (0.5, 1.6) | 1.3 (0.4, 3.7) |
| Male | 1.5 (0.9, 2.4) | 2.5 (0.8, 7.8) |
defined in text
PSC-primary sclerosing cholangitis; 5ASA—mesalamine-based agents; 6MP/AZA—6-mercaptopurine or azathioprine
------- Hazard ratios not calculated as there was no advanced neoplasia in the non-extensive disease group, among patients getting PSC and cyclosporine.
As shown in Table 4, the relationship between most suspected clinical variables and neoplastic progression was not significant in univariate testing, including disease extent, duration, age at diagnosis, presence of PSC, or the use of aminosalicylates, purine analogue immunomodulators, corticosteroids or folic acid. Because it met the pre-defined threshold of p<0.20 for progression to any neoplasia in univariate testing, 5-ASA was included in the multivariable model, but it was neither independently significant (p=0.12 for any neoplasia and p=0.60 for advanced neoplasia), nor did it alter the relationship between inflammation and either any neoplasia or advanced neoplasia. There was a significant relationship between exposure to surveillance colonoscopy and the subsequent detection of advanced neoplasia. IS-mean and Frequency of Colonoscopy were therefore considered together in multivariable analyses (Table 5). The modest changes in the IS-mean odds ratios and their confidence intervals as compared to their univariate counterparts indicate that the effect of inflammation remains significant or near-significant when controlling for the frequency of colonoscopy. The results of the multivariable analysis were similar when inflammation score was characterized as IS-bin or IS-max.
Table 5.
Association between Mean Inflammation Score and Endpoint after adjusting for frequency of scopes per year ≥ 1
| ENDPOINT, HR (95% CI) | ||
|---|---|---|
| Any Neoplasia (65) | Advanced Neoplasia (15) | |
| IS-mean | 1.4 (0.9, 2.3) | 3.8 (1.7, 8.6) |
| 1 or More Colonoscopies per Year | 1.7 (0.9, 3.1) | 5.4 (1.7, 17.0) |
Note: IS-Mean and Frequency of Colonoscopy were both modeled as time-changing covariates
Discussion
Few studies have addressed the severity of colonic inflammation over time as an independent risk factor for progression to neoplasia. Two attempts to determine whether the risk of CRC was associated with frequency of clinical exacerbations found no such relationship.19, 20 Rutter and colleagues, however, successfully demonstrated a correlation between histologic inflammation and neoplastic progression in a case-control study involving 68 cases and 136 matched controls.13 They also showed that endoscopic disease activity correlated with neoplastic progression, although this relationship did not hold up in multivariable testing.13 While that study strongly supports the hypothesis that inflammation contributes to neoplasia, there were potential limitations based on its design: their use of a single value of inflammation for each subject’s history does not account for changes in inflammation status over the course of disease, their inflammation scoring system has not been measured for inter-observer agreement, and case-control methodology is known to be susceptible to unidentified biases.21
The present study attempted to overcome some of these potential biases. We used a cohort study design based on our UC Surveillance Database, which permitted us to determine the effect of changes in inflammation over time. Thus, inflammation scores (measured three ways) were continuously updated as they changed with the passage of time. In addition, we used a highly reproducible histologic scoring system to determine the severity of inflammation.
In our cohort of 418 patients, all of whom entered surveillance without known dysplasia, we found a significant relationship between the severity of histological inflammation over time and subsequent neoplastic progression to HGD or CRC. Although it did not reach statistical significance, a similar trend toward increased risk for any neoplasia was observed.
An additional strength is our use of a relatively simple histological activity index to grade inflammation. In contrast to other histological grading systems, the HAI is assessed primarily on the basis of neutrophil infiltration of the colonic epithelium. It disregards a variety of secondary cumulative changes in IBD that are indicative of disease chronicity but not necessarily activity. HAI therefore limits the number of histological variables considered, and affords both ease of application and high levels of interobserver agreement.15 Furthermore, to account for variations in inflammation and endoscopic sampling, we utilized the mean of the HAI values to define an inflammation score to describe the overall inflammation at each colonoscopy, and three different summaries of accumulated inflammation scores to assess the cumulative history of disease activity: IS-mean, IS-bin, and IS-max. Although it remains to be seen which of these measures best correlates with an individual’s cancer risk, our results suggest that histologic severity of inflammation should be considered an independent risk factor for neoplastic progression in UC. Such a relationship is indeed supported by current concepts relating inflammation to molecular mechanisms of neoplasia.12
There are several potential limitations to this study. First, despite being a cohort study, it is retrospective. A corresponding prospective investigation would be difficult if not unfeasible due to the long time intervals required for patients with no initial dysplasia to progress to cancer. Second, inflammation in IBD may be regional or patchy and can change with time. Although the time-changing covariate model, the use of three measures of histological activity, and the multiplicity of surveillance examinations and biopsies were intended to compensate as much as possible for these effects, our methodology is nonetheless based on the presumption that the biopsy results captured at each location and time point accurately represent the intensity of inflammation in the colon throughout each interval. Additionally, we were unable to accurately and reliably capture the histological inflammatory state of our subjects’ colons in the period preceding entrance into a surveillance system, owing in part to the referral nature of many of the practices and the variability with which clinicians perform endoscopic evaluations in the period preceding surveillance.
A third point for discussion is the selection of multiple end-points to study neoplastic progression. Although LGD, HGD and CRC have different clinical and prognostic implications, each, by definition, corresponds to unequivocal neoplasia16. Since so little is known regarding the role of inflammation in the pathogenesis of neoplasia, a comprehensive choice of end points seems justified. Additionally, whereas the diagnosis of dysplasia is less climactic than that of CRC, the current consensus is that it represents an indication for colectomy, certainly in the case of HGD22, and as recommended by some authorities for flat or unresectable LGD.6, 23-26 We purposely excluded polypoid dysplastic lesions proximal to areas of colitis since those are considered sporadic adenomas and therefore, do not represent an indication for colectomy.1 We did not attempt to subclassify polypoid dysplastic lesions within the diseased colon into DALMs and adenomas, since the endoscopists generally did not make this distinction themselves or provide sufficient documentation to permit retrospective determinations. It should also be noted that many of the procedures in this study, including that of the patient with polypoid HGD, were carried out prior to publications in 1999 demonstrating the safety of endoscopic resection of polypoid dysplasia in diseased mucosa of UC.27, 28 However, only one of the 15 patients who progressed to advanced neoplasia potentially could have had an adenoma rather than a DALM, thus it is unlikely that misclassification of adenomas as advanced neoplasia has substantially biased the results. Interestingly, while active inflammation has been claimed to interfere with the interpretation of surveillance biopsy specimens and cited as a reason for lower levels of observer agreement when specimens with indefinite for dysplasia and LGD histology have been studied,11 we found a lesser association with LGD (a constituent of any neoplasia) than with more advanced pathology.
Additionally, we noted a strong association between exposure to colonoscopy and the subsequent development of dysplasia and cancer. It is unclear exactly why such patients were more likely to develop neoplasia at a later date (as this variable was measured as a time-changing covariate). The most likely explanation is that a form of detection bias occurred, with a more intensive examination schedule yielding a higher frequency of positive findings. To what extent confounding (unreported endoscopic findings such as incomplete reporting of psueodopolyps, recently noted to be an independent risk factor for neoplasia,27 for example) may have additionally contributed to the association between colonoscopic frequency and neoplasia is unknown. Nonetheless, more frequent colonoscopy did not alter the association between inflammation and neoplasia in the multivariable analysis.
Finally, the system employed to grade histological activity in this study, although used routinely by gastrointestinal pathologists at The Mount Sinai Hospital for many years, is not standard among pathologists at large. Indeed, no such standard system exists largely because of issues related to complexity and unproven generalizability.28-30 The relative simplicity and high rates of intra- and interobserver agreement of the HAI system used in this study15 are therefore advantageous.
Whether our results can be extrapolated to other patient populations, surveillance schedules, and pathologists awaits further study. In the meantime, our study supports the concept that severity of histological inflammation in UC patients is directly related to the likelihood of developing neoplasia, and that patient stratification based on this risk may ultimately contribute to modifications in their management and surveillance. Nonetheless, it should be kept in mind that the risk of neoplastic progression in IBD is probably multi-factorial. A better understanding of the molecular interplay between inflammation and carcinogenesis and of other potential risk factors offers the hope of decreasing the likelihood of developing colitis-associated CRC.
Acknowledgments
Supported by grants from the Doris Duke Foundation (RBG and SM), the American College of Gastroenterology (TU), and National Institutes of Health (K-08-DK069393) (TU)
There are no conflicts of interest to disclose in relation to this manuscript.
The authors would like to acknowledge and thank the many attending gastroenterologists at Mount Sinai who supported this effort.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Itzkowitz SH, Harpaz N. Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. Gastroenterology. 2004;126:1634–48. doi: 10.1053/j.gastro.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 2.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–35. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228–33. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 4.Connell WR, Lennard-Jones JE, Williams CB, Talbot IC, Price AB, Wilkinson KH. Factors affecting the outcome of endoscopic surveillance for cancer in ulcerative colitis [see comments] Gastroenterology. 1994;107:934–44. doi: 10.1016/0016-5085(94)90216-x. [DOI] [PubMed] [Google Scholar]
- 5.Lynch DA, Lobo AJ, Sobala GM, Dixon MF, Axon AT. Failure of colonoscopic surveillance in ulcerative colitis [see comments] Gut. 1993;34:1075–80. doi: 10.1136/gut.34.8.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woolrich AJ, DaSilva MD, Korelitz BI. Surveillance in the routine management of ulcerative colitis: the predictive value of low-grade dysplasia [see comments] Gastroenterology. 1992;103:431–8. doi: 10.1016/0016-5085(92)90831-i. [DOI] [PubMed] [Google Scholar]
- 7.Provenzale D, Wong JB, Onken JE, Lipscomb J. Performing a cost-effectiveness analysis: surveillance of patients with ulcerative colitis. Am J Gastroenterol. 1998;93:872–80. doi: 10.1111/j.1572-0241.1998.00314.x. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein CN, Weinstein WM, Levine DS, Shanahan F. Physicians’ perceptions of dysplasia and approaches to surveillance colonoscopy in ulcerative colitis [see comments] Am J Gastroenterol. 1995;90:2106–14. [PubMed] [Google Scholar]
- 9.Eaden JA, Ward BA, Mayberry JF. How gastroenterologists screen for colonic cancer in ulcerative colitis: an analysis of performance. Gastrointest Endosc. 2000;51:123–8. doi: 10.1016/s0016-5107(00)70405-6. [DOI] [PubMed] [Google Scholar]
- 10.Eaden J, Abrams K, McKay H, Denley H, Mayberry J. Inter-observer variation between general and specialist gastrointestinal pathologists when grading dysplasia in ulcerative colitis. J Pathol. 2001;194:152–7. doi: 10.1002/path.876. [DOI] [PubMed] [Google Scholar]
- 11.Melville DM, Jass JR, Morson BC, Pollock DJ, Richman PI, Shepherd NA, Ritchie JK, Love SB, Lennard-Jones JE. Observer study of the grading of dysplasia in ulcerative colitis: comparison with clinical outcome. Hum Pathol. 1989;20:1008–14. doi: 10.1016/0046-8177(89)90273-6. [DOI] [PubMed] [Google Scholar]
- 12.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 13.Rutter M, Saunders B, Wilkinson K, Rumbles S, Schofield G, Kamm M, Williams C, Price A, Talbot I, Forbes A.Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis Gastroenterology 2004. Accepted for publication [DOI] [PubMed] [Google Scholar]
- 14.Mathy C, Schneider K, Chen YY, Varma M, Terdiman JP, Mahadevan U. Gross versus microscopic pancolitis and the occurrence of neoplasia in ulcerative colitis. Inflamm Bowel Dis. 2003;9:351–5. doi: 10.1097/00054725-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Fiel M, Qin L, Suriawinita A, Qui L, Bitar M, Lee L, Harpaz N. Histologic grading of disease activity in chronic IBD: inter- and intra-observer variation among pathologists with different levels of experience. Modern Pathology. 2003;16:118A. [Google Scholar]
- 16.Riddell RH, Goldman H, Ransohoff DF, Appelman HD, Fenoglio CM, Haggitt RC, Ahren C, Correa P, Hamilton SR, Morson BC, et al. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983;14:931–68. doi: 10.1016/s0046-8177(83)80175-0. [DOI] [PubMed] [Google Scholar]
- 17.Cox DR. Regression models and life tables. J Roy Statist Soc Ser B Metho. 1972;34:187–220. [Google Scholar]
- 18.Collett D. Modelling Survival Data in Medical Research. Chapman and Hall; 1994. [Google Scholar]
- 19.Eaden J, Abrams K, Ekbom A, Jackson E, Mayberry J. Colorectal cancer prevention in ulcerative colitis: a case-control study. Aliment Pharmacol Ther. 2000;14:145–53. doi: 10.1046/j.1365-2036.2000.00698.x. [DOI] [PubMed] [Google Scholar]
- 20.Pinczowski D, Ekbom A, Baron J, Yuen J, Adami HO. Risk factors for colorectal cancer in patients with ulcerative colitis: a case-control study. Gastroenterology. 1994;107:117–20. doi: 10.1016/0016-5085(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 21.Feinstein AR. Clinical Epidemiology. W.B. Saunders Company; 1985. [Google Scholar]
- 22.Itzkowitz SH, Present DH. Consensus conference: Colorectal cancer screening and surveillance in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:314–21. doi: 10.1097/01.mib.0000160811.76729.d5. [DOI] [PubMed] [Google Scholar]
- 23.Ullman TA, Loftus EV, Jr., Kakar S, Burgart LJ, Sandborn WJ, Tremaine WJ. The fate of low grade dysplasia in ulcerative colitis. Am J Gastroenterol. 2002;97:922–7. doi: 10.1111/j.1572-0241.2002.05610.x. [DOI] [PubMed] [Google Scholar]
- 24.Ullman TA.Patients with low-grade dysplasia should be advised to undergo colectomy Inflamm Bowel Dis 20039267–9. discussion 273-5 [DOI] [PubMed] [Google Scholar]
- 25.Ullman T, Croog V, Harpaz N, Sachar D, Itzkowitz S. Progression of flat low-grade dysplasia to advanced neoplasia in patients with ulcerative colitis. Gastroenterology. 2003;125:1311–9. doi: 10.1016/j.gastro.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 26.Bernstein CN, Shanahan F, Weinstein WM. Are we telling patients the truth about surveillance colonoscopy in ulcerative colitis? [see comments] Lancet. 1994;343:71–4. doi: 10.1016/s0140-6736(94)90813-3. [DOI] [PubMed] [Google Scholar]
- 27.Engelsgjerd M, Farraye FA, Odze RD.Polypectomy may be adequate treatment for adenoma-like dysplastic lesions in chronic ulcerative colitis Gastroenterology 19991171288–94. discussion 1488-91 [DOI] [PubMed] [Google Scholar]
- 28.Rubin PH, et al. Colonoscopic polypectomy in chronic colitis: conservative management after endoscopic resection of dysplastic polyps. Gastroenterology. 1999;117:1295–300. doi: 10.1016/s0016-5085(99)70279-9. [DOI] [PubMed] [Google Scholar]
- 29.Velayos FS, Loftus EV, Jr., Jess T, Harmsen WS, Bida J, Zinsmeister AR, Tremaine WJ, Sandborn WJ. Predictive and protective factors associated with colorectal cancer in ulcerative colitis: A case-control study. Gastroenterology. 2006;130:1941–9. doi: 10.1053/j.gastro.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 30.Xiao LF, Sutherland LR. Assessing disease activity and disease activity indices for inflammatory bowel disease. Curr Gastroenterol Rep. 2002;4:490–6. doi: 10.1007/s11894-002-0025-z. [DOI] [PubMed] [Google Scholar]
- 31.Geboes K. Is histology useful for the assessment of the efficacy of immunosuppressive agents in IBD and if so, how should it be applied? Acta Gastroenterol Belg. 2004;67:285–9. [PubMed] [Google Scholar]
- 32.Geboes K, Riddell R, Ost A, Jensfelt B, Persson T, Lofberg R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut. 2000;47:404–9. doi: 10.1136/gut.47.3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]