Abstract
Different stressors likely elicit different physiological and behavioral responses. Previously reported differences in the effects of stressors on immune function may reflect qualitatively different physiological responses to stressors; alternatively, both large and subtle differences in testing protocols and methods among laboratories may make direct comparisons among studies difficult. Here we examine the effects of chronic stressors on plasma corticosterone concentrations, leukocyte redistribution, and skin delayed-type hypersensitivity (DTH) and the effects of acute stressors on plasma corticosterone and leukocyte redistribution. The effects of several commonly used laboratory stressors including restraint, forced swim, isolation, and low ambient temperatures (4°C) were examined. Exposure to each stressor elevated corticosterone concentrations, with restraint (a putative psychological stressor) evoking a significantly higher glucocorticoid response than other stressors. Chronic restraint and forced swim enhanced the DTH response compared to the handled, low temperature, or isolation conditions. Restraint, low temperature, and isolation significantly increased trafficking of lymphocytes and monocytes compared to forced swim or handling. Generally, acute restraint, low temperature, isolation, and handling increased trafficking of lymphocytes and monocytes. Considered together, our results suggest that the different stressors commonly used in psychoneuroimmunology research may not activate the physiological stress response to the same extent. The variation observed in the measured immune responses may reflect differential glucocorticoid activation, differential metabolic adjustments, or both processes in response to specific stressors.
Keywords: Restraint, Glucocorticoids, Low Temperature, Stressors, Stress, Isolation, Mice, Delayed-type Hypersensitivity
Introduction
Stress is an integral part of daily life (Selye, 1950). Psychological stressors such as confinement or predator odors, as well as physical stressors such as low temperature or food shortage, evoke physiological changes that perturb homeostasis. In response to such perturbations, several physiological mechanisms are engaged to restore homeostasis by a process termed “allostasis” (Dhabhar et al., 2000; McEwen, 1998; McEwen and Wingfield, 2003; Schulkin et al., 1994). Although biological function is returned to “normal,” or baseline, after perturbations, stress can have enduring effects on individuals.
Traditionally, specific stressors, regardless of source (e.g., excessive cold, exercise, and phobias), were thought to activate a common neurological and physiological network (Selye, 1950). Recent work, however, suggests that different types of stressors may not necessarily elicit parallel biochemical and physiological changes. Specific stressors induce differential physiological responses, including: brain activation patterns (Dayas et al., 2001; Reyes et al., 2003), Fos immunoreactivity (Pacak and Palkovits, 2001), , receptor expression (Ghi et al., 1995), and neurotransmitter release (Pacak, 2000). More specifically, the immune system has been found to be differentially influenced by stressors, particularly in macrophage activity (Palermo-Neto et al., 2003), antibody production (Karp et al., 2000), and sensitivity to the antigen DNFB (Blecha et al., 1982). Nevertheless, the physiological effects of a wide spectrum of stressors have rarely been directly compared within a single study, making it difficult to ascertain effects of different stressors. In the present experiment, we make direct comparisons of the effects of several stressors to provide a better understanding of the overall effects they have.
The duration of a stressor is thought to be important when considering its impact on individuals. Many differences exist in the ways that acute (short-term) and chronic (long-term) stressors affect physiology and behavior. For example, chronic restraint (6h/day for 3–5 weeks) significantly suppresses delayed-type hypersensitivity (DTH) responses and decreases leukocyte mobilization in the skin of rodents; when administered acutely (single 2 h session), restraint enhances the DTH response and increases leukocyte redeployment (Dhabhar and McEwen, 1997). In humans, chronic stressful life events (lasting more than one month), but not acute stressful life events (lasting less than one month), increase susceptibility to the common cold (Cohen et al., 1998).
The use of various stress protocols, combined with the wide spectrum of specific stressor-evoked changes that are observed, has complicated the understanding of the precise mechanisms involved in processing and coping with stressors, and makes comparisons among studies challenging. For example, several stress models are commonly used to evoke depression-like symptoms in animals, including restraint (reviewed in (Thiebot et al., 1992)) and chronic mild stress (CMS), a widely used regimen that consists of a schedule of confinement, food/water restriction, novel environment, wet bedding, and overnight cage tilt (Konkle et al., 2003). Furthermore, chronic unpredictable stress has been used to test nociception (Pinto-Ribeiro et al., 2004), gene expression (Qin et al., 2004), and cortical histamine receptor expression (Ghi et al., 1995) in rats; however, different stress paradigms were operationally defined as “chronic unpredictable stress” for each study. The extent to which each of these stressors affects the HPA axis or immune function remains unspecified.
The goal of this study was to determine whether intrinsically different stressors (e.g., psychological, physical) have similar or unique influences on several responses: corticosterone, delayed-type hypersensitivity, and leukocyte trafficking. Mice were subjected to restraint, a largely psychological stressor (Dayas et al., 2001; Madrigal et al., 2003; Schmitz et al., 2002), as well as other commonly used laboratory stressors, including: low temperature, a physical and metabolic (energetic) stressor (Baffi and Palkovits, 2000; Miyata et al., 1995; Zoeller et al., 1990); isolation, a social stressor (Bartolomucci et al., 2003; Palanza, 2001); and forced swim, a psychological, physical and metabolic stressor (Dayas et al., 2001; Leitner, 1989; Rauch and Lieberman, 1990). We also included a group of mice that were handled each day to control for any potential influences of daily manipulation (Balcombe et al., 2004; Neigh et al., 2004). Finally, stressor effects on plasma corticosterone and leukocyte redistribution in response to a single exposure were compared to the effects of multiple exposures to a stressor.
Delayed-type hypersensitivity (DTH) reactions are antigen-specific, T cell-mediated immune responses, and have generally been considered a measure of effector T cell function, but also involve the development of immunological memory for a specific antigen (Dhabhar and McEwen, 1997; Dhabhar et al., 1995). Inflammation occurs at the site of antigenic challenge as a result of monocytes and lymphocytes infiltrating into the epidermis and dermis (Janeway et al., 1999; Malorny et al., 1990), and a positive correlation exists between the intensity of the swelling and the immune response (Buunen et al., 2004; Galli and Hammel, 1984; Phanupak et al., 1974). In addition, blood leukocyte populations are known to change during stress. Acute stress decreases blood leukocyte subpopulations, specifically T cells, B cells, and monocytes (Dhabhar et al., 1995; 1996; Dhabhar et al., 1994). These cell population adjustments are not an indication of cell death; rather, they reflect a redistribution of cells to areas such as the skin, to prepare the organism for potential injury or infection (Dhabhar and McEwen, 1996a; 1997; Dhabhar et al., 1996).
Given the importance of the relationship between stress and immune function, in the present series of studies we examined the effects of chronic stressors on plasma corticosterone concentrations, leukocyte redistribution, and skin DTH and the effects of acute stressors on plasma corticosterone and leukocyte redistribution.
Methods
Animals
Adult male C57BL/6 mice (3–4 months of age), obtained from Charles River Laboratories (Raleigh, NC), were group-housed 2–3 per cage, in polycarbonate cages (28 × 17 × 12 cm), in a colony room illuminated for 14 h/day (light:dark 14:10, lights on at 0000 h Eastern Standard Time [EST]). The colony room was maintained at a temperature of 20 ± 4ºC and a relative humidity of 50 ± 5%. Food (LabDiet 5001, PMI Nutrition, Brentwood, MO) and filtered tap water were available ad libitum throughout the study, unless otherwise indicated. Prior to the start of the experiment, all animals were allowed 1 wk to acclimate to the colony environment.
Experiment 1: Chronic Stress and DTH
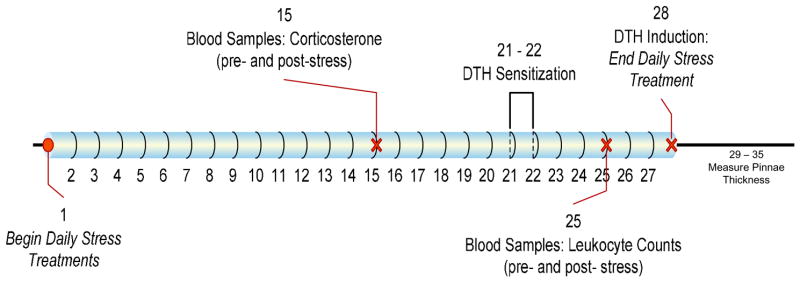
Refer to Figure 1 for a timeline of this experiment. The animals were assigned to one of five experimental groups: restraint, forced swim, low ambient temperature, isolation, and handled. All animals in a cage were assigned to the same treatment group in order to avoid stressor ‘contamination’ among cagemates. The daily stressors were randomly timed between 0800 and 1200 h EST, and persisted for 4 weeks, through the beginning of the challenge phase of DTH induction. Food, water, and bedding were not available during exposure to the stressors. The experimental treatment conditions were as follows:
Figure 1.
Timeline for Experiment 1.
Restraint: (n=9) Mice were placed in ventilated plexiglas restrainers [11.5 cm (length) × 3 cm (diameter) with 8–0.5 cm diameter air holes] for 2 h (Dayas et al., 2001; Madrigal et al., 2003; Schmitz et al., 2002).
Forced Swim: (n=8) Mice were required to swim for 2 min in room temperature (22º C) tap water (10 cm deep) in an uncovered, cylindrical Plexiglas container [30.5 cm (height) × 30.5 cm (diameter)] (Dayas et al., 2001; Leitner, 1989; Rauch and Lieberman, 1990). Water was changed between each animal.
Low Temperature: (n=9) Mice were separated into individual polycarbonate cages and placed into a ventilated cold chamber (4º C) for 2 h. The cages were cleaned each day and empty cages remained in the cold chamber overnight (Baffi and Palkovits, 2000; Miyata et al., 1995; Zoeller et al., 1990).
Isolation: (n=12) Animals were separated into individual polycarbonate cages for 2 h, and remained in the animal colony room (Bartolomucci et al., 2003; Palanza, 2001).
Handled: (n=11) Mice were briefly picked up and allowed to walk on the experimenters’ hand (~30s)(Balcombe et al., 2004; Neigh et al., 2004).
Experimental Procedures
Throughout the experiment, a total of five blood samples were obtained from each animal on days 0, 15, and 25 (day 1 indicates the start of the experiment), with two samples taken on days 15 and 25 (before and after daily experimental conditions). For each collection, animals were lightly anesthetized with isofluorane vapors (Abbot Laboratories, Chicago, IL) and rapidly bled (< 2 min) from the retro-orbital sinus into collection tubes. Following the ‘pre-stress’ collections on days 15 and 25, the animals were allowed at least 1 h to recover from the anesthetic prior to the start of their respective treatments. ‘Post-stress’ samples were taken immediately following daily experimental conditions. Sampling was arranged so that all blood collections, including ‘post-stress’ samples, would be completed before 1200 h to control for any circadian variation in corticosterone or leukocyte populations, and all animals were sampled at approximately the same time of day. On day 35, all animals were euthanized by CO2 inhalation overdose.
Blood samples for assessment of corticosterone concentrations
On days 0 and 15, approximately 0.20 ml of blood was drawn from the retro-orbital sinus. Animals received an intraperitoneal (i.p.) injection of sterile isotonic saline (0.5 ml) after each collection, and then were returned to their cages. Samples were allowed to clot, the clot was removed, and the samples centrifuged at 4º C for 30 min at 2500 rpm. Serum aliquots were aspirated and stored in sealable polypropylene microcentrifuge tubes at −70º C until assayed for serum corticosterone concentrations using a radioimmunoassay (RIA) (see below for details).
Blood samples for assessment of blood leukocyte populations
On day 25, approximately 0.10 ml of blood was drawn from the retro-orbital sinus. Animals again received an i.p. injection of sterile isotonic saline (0.5 ml), and were returned to their cages. All samples were immediately mixed with 0.005 ml of heparin to prevent clotting. Blood leukocyte populations were then separated and quantified using florescence activated cell sorter (FACS) analysis (see below).
DTH sensitization
On days 21 and 22 of the experiment, all groups were sensitized to the antigen 2–4-dinitro-1-flourobenzene (DNFB; Sigma, St. Louis, MO). On day 1 of sensitization, animals were lightly anesthetized with isoflurane vapor, and an area of approximately 2 × 3 cm was shaved on the dorsum. The shaved skin was lightly swabbed with 70% alcohol, and approximately 25 μl of DNFB [0.5% (wt/vol) in 4:1, acetone:olive oil vehicle] was applied to the shaved skin via pipette on day 1, and again on day 2, of sensitization. The thickness of both pinnae was measured prior to sensitization, using a constant-loading dial micrometer (Mitutoyo America, Aurora, IL), for determination of baseline pinna thickness.
Induction of DTH
On day 28 of the experiment (7 days post-sensitization), pinnae thickness was again measured to establish a baseline measurement for each animal. Animals were again lightly anesthetized with isoflurane vapors for the procedure. Mice received a challenge of approximately 20 μl of DNFB [0.2% (wt/vol) in 4:1, acetone:olive oil vehicle] to the skin of the dorsal surface of the right pinna. The left pinna was treated with 20 μl of the acetone:olive oil vehicle only. Pinnae thickness was measured every 24 h for the subsequent seven days at 1000–1100 h. All measurements were made on the same relative region of the pinnae.
Experiment 2: Acute Stress
Additional group-housed animals were assigned to the same experimental groups as described above: 2 h restraint (n=6), 2 min forced swim (n=5), 2 h (4°C) low temperature (n=5), 2 h isolation (n=4), and brief handling (n=5). In this experiment, however, the animals were only tested for one day. Blood samples from each animal were obtained 1 h prior to and immediately following their assigned stress condition. The samples were analyzed for both pre- and post-stress serum corticosterone concentrations, and pre- and post-stress blood leukocyte populations. Blood collection and analysis were performed as explained above for the chronic stress procedures.
Flow Cytometry
Total leukocyte numbers and lymphocyte–neutrophil differentials were obtained on a hematology analyzer (F800, Sysmex, McGraw Park, IL) as described in detail previously (Dhabhar et al., 2000). Specific leukocyte subtypes were measured by immunofluorescent Ab staining and analyzed using single color flow cytometry (FACSCalibur, Becton Dickinson, San Jose, CA). Lymphocyte, neutrophil, and monocyte subpopulations were identified and gated using forward versus side scatter characteristics. T cells were identified using CyChr-labeled anti-CD3 (clone 145-2C11), and B cells using phycoerythrin-labeled anti-B220 (RA3-6B2). Neutrophils and monocytes were identified using forward versus side scatter patterns. All monoclonals were directly conjugated, rat anti-mouse Abs and were obtained from BD-PharMingen (San Diego, CA). Briefly, blood samples were incubated with antibody for 20 min at room temperature, washed with PBS, and read on the FACSCalibur; 3000 to 5000 events were acquired from each preparation. Control samples matched for each fluorochrome and each antibody isotype were used to set negative staining criteria. Data were analyzed using CELLQuest software (Becton Dickinson, San Jose, CA).
RIA
Total serum corticosterone concentrations for mice in both experiments were determined in duplicate in two assays using an ICN Diagnostics 125I double antibody kit (Costa Mesa, CA). The high and low limits of detectability of the assay were 1000 and 5 ng/ml, respectively. Intra-assay coefficients of variability were 2.8 and 1.3%, and the inter-assay coefficient of variability was 11%. All procedures were followed as described by the manufacturer except that the standard curve was assayed in triplicate.
Data Analysis
DTH responses were determined as percent increases of pinnae thickness over baseline for each animal, and compared among groups using a two-factor, repeated measures ANOVA, with stressor (between-subjects variable) x day (within-subjects variable). Following a significant F score, post-hoc tests (Tukey’s Honestly Significant Difference) were used to further distinguish between groups. Similarly, corticosterone concentrations were analyzed using a two-factor ANOVA, except that day was replaced by time as the within-subjects variable. Leukocytes were analyzed by cell type, and as percent change from pre-stress levels, using one-way ANOVAs and post-hoc tests to further distinguish between groups. Additionally, t tests were performed to determine whether the pre- and post-stress samples were different within each treatment and cell type. All mean differences were considered statistically significant if p<0.05. All statistics were performed using SigmaStat Statistical Software (Chicago, IL).
Results
Experiment 1: Chronic Stress and DTH
DTH
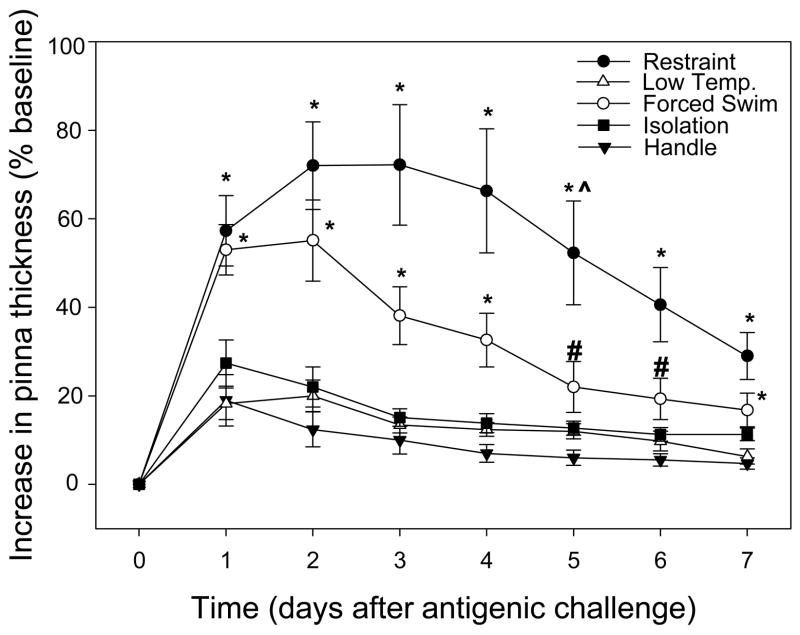
Our results show a significant interaction of group x day (F(6,294)=6.336, p<0.001). Restraint elicited a significantly higher DTH response than low temperature, isolation, and handling on all days (1–7) post-DNFB challenge (p<0.05 in all comparisons); in addition, the restraint response was significantly higher than forced swim on day 5 post-DNFB challenge (p<0.05; Fig. 2). Similarly, forced swim elicited a significantly higher DTH response than low temperature, isolation, and handled stressors on days 1–4 and 7 post-DNFB challenge (p<0.05; Fig. 2). On days 5 and 6, forced swim evoked significantly elevated DTH responses as compared to the isolation and handled groups only (p<0.05; Fig. 2). The ear treated with only the vehicle did not result in any increase in tissue thickness in any group (data not shown). Furthermore, there were no significant differences in DTH observed among low temperature, isolation, and handled stressors (p >0.05 in each case). It should be noted that the dosage of DNFB used was a low threshold dose, designed to elicit a minimal response (Dhabhar and McEwen, 1996a), thus allowing one to detect possible immuno-enhancement more easily.
Figure 2.
Delayed-type hypersensitivity (DTH) responses after 4 wks of stressor treatments. All increases are significantly different from zero. Restraint and forced swim elicited significantly higher DTH responses post-challenge than the low temperature, isolation, or handled stressors.
*as compared to low temperature, isolation, and handle; # as compared to isolation and handle; ^ as compared to swim (p<0.05 for all)
Corticosterone concentrations
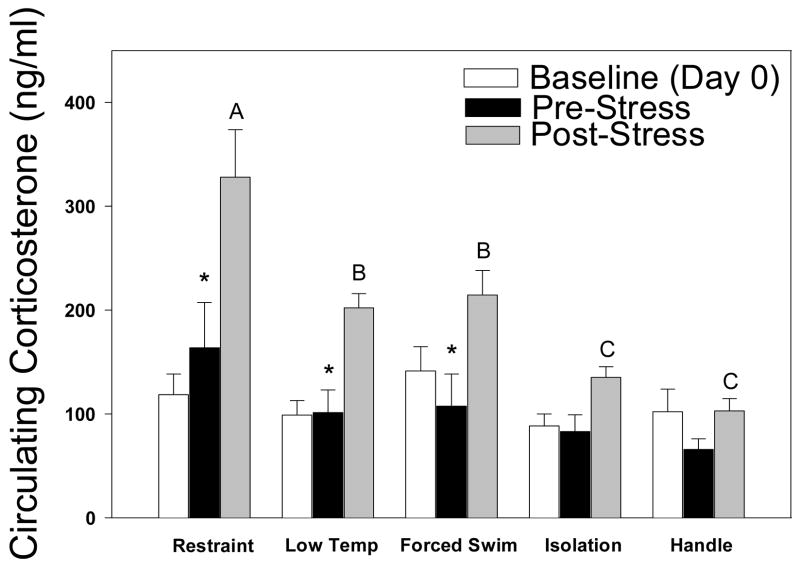
After two weeks of the experiment, none of the stressor treatments significantly elevated basal (pre-stress) corticosterone concentrations compared to day 0 baseline concentrations (Fig. 3). There was a significant interaction of group x time (F(8,49)=3.111, p=0.024) for pre- versus post-stress concentrations on day 15. Restraint significantly elevated corticosterone concentrations on day 15, as compared to all other groups (“A” in Fig. 3). Additionally, forced swim and low temperature elicited significantly higher corticosterone concentrations than isolation or handled groups on day 15 post-stressor (p< 0.05 in all comparisons; “B” and “C” in Fig. 3). Post-stressor corticosterone concentrations on day 15 were significantly elevated compared to pre-stress concentrations among mice in the restraint, forced swim, and low temperature conditions (p<0.05; indicated by asterisks in Fig. 3).
Figure 3.
Baseline (day 0), pre-, and post-stress (day 15) corticosterone concentrations. Restraint elicited higher corticosterone values than all other stressor treatments. Only restraint, forced swim, and low temperature induced significantly elevated corticosterone concentrations following stressor treatment compared to their respective day 15 baseline.
*Significant difference between pre- and post-stress; “A” is higher than all other groups, “B” higher than “C.”
Blood Leukocyte Populations
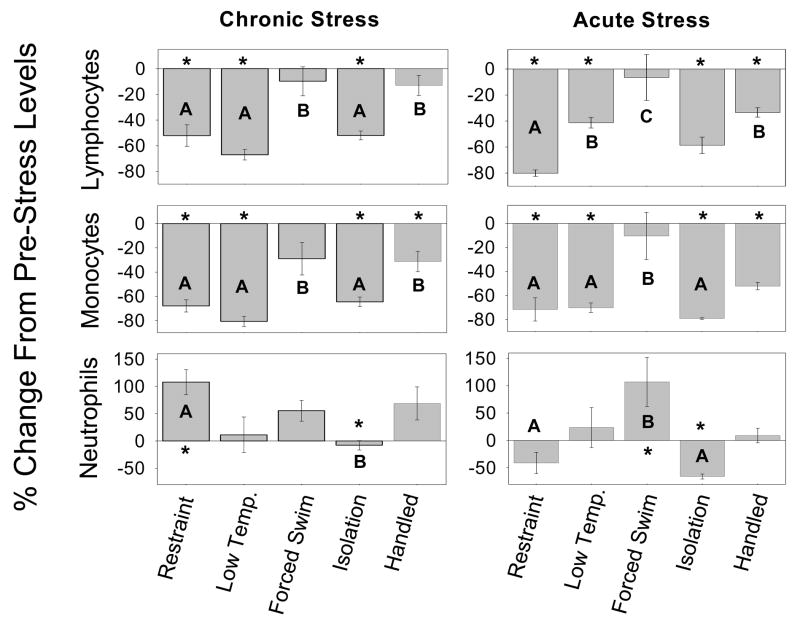
An overall effect dependent on ‘group’ was found for all cell types analyzed: total lymphocytes (F(4,48)=12.545, p<0.001), T cells (F(4,48)=11.205, p<0.001), B cells (F(4,48)=13.770, p<0.001), monocytes (F(4,48)=9.731, p<0.001), and neutrophils (F(4,48)=3.865, p=0.009). Specifically, restraint, low temperature, and isolation significantly decreased circulating total lymphocytes, as compared to forced swim and handling (p<0.05). Both T cells and B cells were significantly decreased in the restraint, low temperature, and isolation groups (T cells: −33.184±11.090, −49.683 ±17.902, and −36.566±4.182, respectively; B cells: −59.458±7.465, −74.265±3.266, and −57.565 ±3.302, respectively). Circulating monocyte levels were also significantly decreased in the restraint, low temperature, and isolation groups (p<0.05; Fig. 4). Restraint significantly increased circulating neutrophil levels compared to low temperature, forced swim, and isolation (p<0.05; Fig. 4). Significant differences from pre-stress levels (within the same treatment) are denoted by asterisks in Figure 4.
Figure 4.
Blood leukocyte populations on day 25(chronic) or after one stressor treatment (acute). Chronic -Restraint, low temperature, and isolation significantly increased trafficking of lymphocytes and monocytes to a greater extent than forced swim or handling. Restraint induced a larger increase in circulating neutrophils than isolation. Acute - Restraint resulted in higher stress-induced changes in lymphocytes than low temperature, forced swim, and handling; in addition, isolation induced larger changes than forced swim. Monocytes trafficked significantly more out of the blood in the restraint, low temperature, and isolation groups than in the forced swim group. Restraint and isolation also resulted in significantly different changes in neutrophil populations than forced swim.
Significance is specific to each panel (cell type and stressor duration): * significant difference between pre- and post-stress levels within treatment; “A” is significantly different from “B” which is different from “C”.
Experiment 2: Acute Stress
Corticosterone concentrations
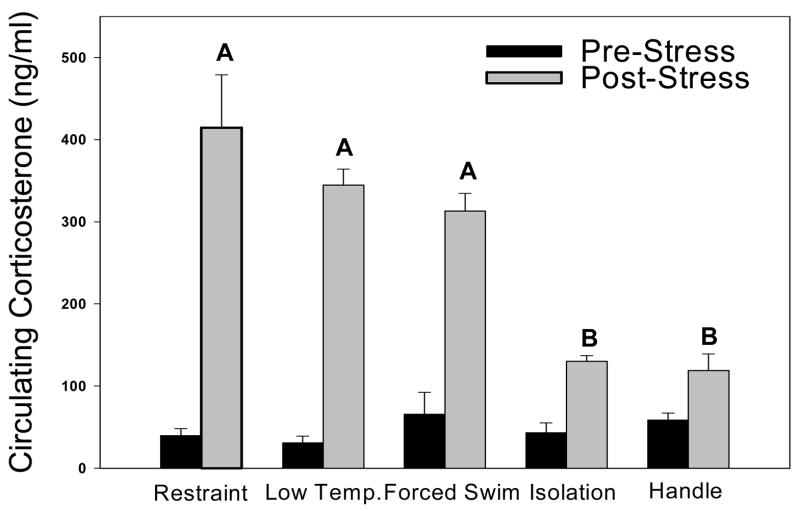
Mice in all experimental treatment groups displayed significantly elevated corticosterone concentrations after one session of stress as compared to baseline values (F(8,49)=9.542, p<0.001, Fig. 5). There was a significant interaction of group x time (F(8,49)=11.306, p<0.001) for pre- versus post-stress concentrations. In addition, restraint, forced swim, and low temperature stressors elicited significantly higher corticosterone concentrations than isolation or handled stressors (p< 0.05 in all comparisons).
Figure 5.
Corticosterone concentrations after one (acute) session of treatment. All stressor treatments significantly elevated corticosterone concentrations compared to baseline. “A” is significantly different from “B”.
Blood Leukocyte Populations
There were no differences in leukocyte numbers among the experimental groups prior to the onset of stress (F(4,24)=1.72, p=0.185). Overall effect of group was found for each cell type analyzed: total lymphocytes (F(4,24)=10.875, p<0.001), T cells (F(4,24)=9.102, p<0.001), B cells (F(4,24)=12.915, p<0.001), monocytes (F(4,24)=6.811, p<0.001), and neutrophils (F(4,24)=5.247, p=0.005). Specifically, low temperature and handling induced greater population changes in blood leukocytes than did forced swim (p<0.05). Total lymphocytes displayed more stress-induced changes in the restraint group than in any other group (Fig. 4). Restraint, low temperature, isolation, and handling all resulted in greater circulating monocyte changes than did forced swim (p<0.05; Fig. 4). Additionally, circulating neutrophil levels increased significantly more following forced swim than following restraint, isolation, or handling (p<0.05; Fig. 4). Significant differences from pre-stress levels (within the same treatment) are denoted by asterisks in Figure 4.
Discussion
This set of experiments was designed to test differences in some of the physiological alterations that commonly-used laboratory stressors have on animals. In Experiment 1, post-stress blood corticosterone concentrations were elevated after two weeks of repeated treatment for every type of stressor except isolation. Chronic restraint evoked the most robust increase in corticosterone concentrations compared to basal values, and produced the largest DTH response among all stressors; forced swim also showed similar changes, but to a lesser extent than restraint. Exposure to low temperatures significantly increased corticosterone concentrations, but did not robustly elevate the DTH response, likely due to low temperature-induced vasoconstriction (discussed below). Restraint, low temperature, and isolation significantly increased trafficking of lymphocytes and monocytes to a greater extent than forced swim or handling.
The DTH results and corticosterone concentrations observed in Experiment 1 generally follow similar patterns; restraint induced the greatest DTH response and elevation in serum corticosterone, whereas handling and isolation yielded the smallest effects. Forced swim generated ambiguous results, which is consistent with other studies (Connor et al., 2005; Dayas et al., 2001; Neumann et al., 1998; Shanks et al., 2000). Mice in this group displayed elevated corticosterone concentrations and an enhanced DTH response, but displayed highly variable changes in leukocyte populations. Similarly, the low temperature group displayed stressor-induced increases in corticosterone concentrations and decreases in blood leukocyte populations, but did not produce an enhanced DTH response (likely due to low temperature-induced vasoconstriction). Based on these inconsistencies, our results seem to provide further evidence that the stress-response is not entirely glucocorticoid-mediated (Blecha et al., 1982b). It is also possible that an analysis of free corticosterone, rather than total (which includes hormone bound by corticosterone binding globulin), would reveal a pattern of activation more similar to the immune data, and this remains to be determined in future studies.
The dissimilarity seen between DTH and corticosterone concentrations observed in the low temperature group could be due to vasoconstriction associated with low ambient temperatures. Decreased blood flow to skin might inhibit cell trafficking, and possibly decrease leukocyte infiltration into the skin. It is difficult to interpret the corticosterone results and leukocyte data together in Experiment 1, as the samples for each were taken on different days (day 15 and 25, respectively). Animals display HPA axis habituation (Dhabhar et al., 1997; Viau and Sawchenko, 2002), and the extent to which the animals habituated to the particular stressors between days 15 and 25 is indecipherable in the present study.
Following acute stressor exposure in Experiment 2, all treatment groups displayed elevated circulating corticosterone concentrations. Restraint, low temperature, and forced swim increased corticosterone concentrations more than isolation or handling. Generally, restraint, low temperature, isolation, and handling resulted in more stress-induced changes in lymphocytes and monocytes than forced swim. It is hypothesized that during stress leukocytes traffic out of the blood and into the skin, lymph nodes, and bone marrow in order to prepare the organism for any potential assault (Dhabhar and McEwen, 1997). The increase in immune cell trafficking and in serum corticosterone after one session of stressor exposure correspond with previous reports (Dhabhar and McEwen, 1996a, 1997; Dhabhar et al., 1996).
To maintain a consistent protocol, all post-stress blood samples were collected immediately after cessation of the stressor treatment. A possible explanation for the largely variable leukocyte population results obtained for the forced swim and handled groups is the timing of the post-stress blood sample in relation to the beginning of stressor treatment, 2 minutes and 30 seconds, respectively. Because changes in leukocyte populations may require 30–60 min to detect (Dhabhar et al., 1995), it is possible that the leukocyte data obtained from these groups do not accurately reflect stress-induced blood leukocyte populations, as the post-stress blood samples were obtained within several minutes of the conclusion of each stressor. This timeline allows for changes in blood corticosterone concentrations, but possibly not for leukocyte changes, because forced swim was two minutes in duration, handling lasted thirty seconds, and the other stressors lasted two hours. This variation makes interpretation difficult, and these types of temporal inconsistencies illustrate an inherent difficulty in evaluating results of stress studies.
A difficulty encountered in the present study was having a truly naïve, controlled group. It is necessary to repeatedly handle the animals in order to measure the DTH response. Therefore, the problem with having ‘undisturbed’ animals is that their DTH response could be exaggerated merely from being handled to induce and measure the swelling in response to antigen stimulation. Thus, we attempted to control for the effects of daily manipulation on stressor-exposed groups. Several studies have included handled control groups in experiments (e.g., Balcombe et al., 2004; Neigh et al., 2004).
By testing the effects of novel, short-term stressors in Experiment 2, we examined two different temporal patterns (acute and chronic) of inducing changes in leukocyte distribution and plasma corticosterone concentrations. All types of stressors significantly increased corticosterone concentrations after a single session, but only the restraint, forced swim, and low temperature treatments evoked enduring increases in corticosterone concentrations after 15 days. Similarly, neutrophil populations seem to be differentially influenced by acute and chronic stressors, which could merely reflect the unique responses elicited by particular stressors. It is also possible that animals habituate to each stressor at different rates and to different degrees, likely due to variations in severity of the stressor. We cannot make a strong conclusion about the degree to which the immune system habituated to each stressor, as we did not obtain baseline leukocyte population data in the first experiment, and did not conduct DTH measurements in the second.
The increase, rather than decrease, in DTH following chronic restraint was somewhat surprising, given previous reports that chronic stress is detrimental to immune function (Basso et al., 1993; Bonneaud et al., 2004; Dhabhar and McEwen, 1997); however, a few studies have reported that some aspects of immune function are enhanced and some are suppressed by chronic stress (Cunnick et al., 1988; Mizoguchi et al., 2001; Nakano, 2004; Van Raaij et al., 1996). There were also notable differences between the current study and previous experiments documenting the suppressive influence of chronic stress on DTH responses (Dhabhar and McEwen, 1997). Previous studies utilized 6 h of restraint/day (Dhabhar & McEwen, 1997), or an extended regimen of stress-induced levels of corticosterone, whereas this study involved 2h (restraint, low temperature, or isolation), or 2 min (forced swim) of stress per day. Moreover, in the previous study (Dhabhar and McEwen, 1997), the chronic stressor was accompanied by a significant decrease in leukocyte redistribution that was not observed in this study. Importantly, the previous study (Dhabhar and McEwen, 1997) showed that the chronic stressor resulted in a disruption of the circadian corticosterone rhythm (increased baseline corticosterone levels at the beginning of the inactive period) that has been suggested to be an important marker for the deleterious effects of chronic stress (Dhabhar et al., 2002). However, such a disruption was not observed in the present study. We report here that daily 2 h restraint or 2 min forced swim stress exposure for 4 weeks resulted in augmented DTH responses compared to handled controls. We believe this finding is quite informative and important, as it suggests that animals maintain the ability to respond adaptively to acute stress superimposed upon a repeated series of stressors, as long as they maintain a regular circadian corticosterone rhythm and the ability to redistribute leukocytes during stress.
One issue that was not fully addressed by the current study, but remains a critical issue in stress biology, is the nature and timing of the switch that causes a repeated stressor to evoke the ‘chronic stress’ phenotype. In other words, how much time/intensity is required for an acute stressor to be perceived as chronic? Is the switch from acute to chronic stress responses graded or discrete? Do different stressors engage the same switch at the same time? These issues must be resolved in order to have a complete understanding of allostasis/stress biology.
Many attempts have been made to categorize stressors (Dayas et al., 2001). In the design of the present experiment, we chose a variety of stressors from several categories (i.e., psychological or physical), defined by other experimenters. In general, however, stressor categorizations seem to be context-dependent, and often based on the specific behavioral or physiological endpoints examined.
We have provided evidence for specific effects of distinctive stressors. These effects differ based on the type and duration of the stressor, and presumably, previous experience with exposures to stressors that cannot be controlled (e.g., social status, birth order, etc.). Only a few of the many possible influences of stressor exposure were measured in the present study, and it is likely that many other factors play into the way an individual responds to stress. Further research is needed to examine the effects of stressors on various systems, not just particular receptors or cells, to elucidate the overall effects stress has on individuals.
Acknowledgments
We thank Jean Tillie and Rob Rengel for technical assistance, Tricia Uhor for expert animal care, and Zachary Weil for manuscript comments. This research was supported by NIH grant MH66144 and NSF grant 04-16897.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baffi JS, Palkovits M. Fine topography of brain areas activated by cold stress. A fos immunohistochemical study in rats. Neuroendocrinology. 2000;72:102–113. doi: 10.1159/000054577. [DOI] [PubMed] [Google Scholar]
- Balcombe JP, Barnard ND, Sandusky C. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci. 2004;43:42–51. [PubMed] [Google Scholar]
- Bartolomucci A, Palanza P, Sacerdote P, Ceresini G, Chirieleison A, Panerai AE, Parmigiani S. Individual housing induces altered immuno-endocrine responses to psychological stress in male mice. Psychoneuroendocrinology. 2003;28:540–558. doi: 10.1016/s0306-4530(02)00039-2. [DOI] [PubMed] [Google Scholar]
- Basso AM, Depiante-Depaoli M, Cancela L, Molina V. Seven-day variable-stress regime alters cortical beta-adrenoceptor binding and immunologic responses: reversal by imipramine. Pharmacol Biochem Behav. 1993;45:665–672. doi: 10.1016/0091-3057(93)90522-u. [DOI] [PubMed] [Google Scholar]
- Blecha F, Barry RA, Kelley KW. Stress-induced alterations in delayed-type hypersensitivity to SRBC and contact sensitivity to DNFB in mice. Proc Soc Exp Biol Med. 1982;169:239–246. doi: 10.3181/00379727-169-41338. [DOI] [PubMed] [Google Scholar]
- Blecha F, Kelley KW, Satterlee DG. Adrenal involvement in the expression of delayed-type hypersensitivity to SRBC and contact sensitivity to DNFB in stressed mice. Proc Soc Exp Biol Med. 1982b;169:247–252. doi: 10.3181/00379727-169-41339. [DOI] [PubMed] [Google Scholar]
- Bonneaud C, Mazuc J, Chastel O, Westerdahl H. Terminal investment induced by immune challenge and fitness traits associated with major histocompatibility complex in the house sparrow. Evolution. 2004;58:2823–2830. doi: 10.1111/j.0014-3820.2004.tb01633.x. [DOI] [PubMed] [Google Scholar]
- Buunen M, Gholghesaei M, Veldkamp R, Meijer DW, Bonjer HJ, Bouvy ND. Stress response to laparoscopic surgery: a review. Surg Endosc. 2004;18:1022–1028. doi: 10.1007/s00464-003-9169-7. [DOI] [PubMed] [Google Scholar]
- Cohen S, Frank E, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM., Jr Types of stressors that increase susceptibility to the common cold in healthy adults. Health Psychol. 1998;17:214–223. doi: 10.1037//0278-6133.17.3.214. [DOI] [PubMed] [Google Scholar]
- Connor TJ, Brewer C, Kelly JP, Harkin A. Acute stress suppresses pro-inflammatory cytokines TNF-alpha and IL-1 beta independent of a catecholamine-driven increase in IL-10 production. J Neuroimmunol. 2005;159:119–128. doi: 10.1016/j.jneuroim.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Cunnick JE, Lysle DT, Armfield A, Rabin BS. Shock-induced modulation of lymphocyte responsiveness and natural killer activity: differential mechanisms of induction. Brain Behav Immun. 1988;2:102–113. doi: 10.1016/0889-1591(88)90011-6. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci. 2001;14:1143–1152. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, Miller AH, Stein M, McEwen BS, Spencer RL. Diurnal and acute stress-induced changes in distribution of peripheral blood leukocyte subpopulations. Brain Behav Immun. 1994;8:66–79. doi: 10.1006/brbi.1994.1006. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Effects of stress on immune cell distribution. Dynamics and hormonal mechanisms. J Immunol. 1995;154:5511–5527. [PubMed] [Google Scholar]
- Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Stress-induced changes in blood leukocyte distribution. Role of adrenal steroid hormones. J Immunol. 1996;157:1638–1644. [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Stress-induced enhancement of antigen-specific cell-mediated immunity. J Immunol. 1996a;156:2608–2615. [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS, Spencer RL. Adaptation to prolonged or repeated stress--comparison between rat strains showing intrinsic differences in reactivity to acute stress. Neuroendocrinology. 1997a;65:360–368. doi: 10.1159/000127196. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, Satoskar AR, Bluethmann H, David JR, McEwen BS. Stress-induced enhancement of skin immune function: A role for gamma interferon. Proc Natl Acad Sci U S A. 2000;97:2846–2851. doi: 10.1073/pnas.050569397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS. Stress-induced augmentation of immune function--the role of stress hormones, leukocyte trafficking, and cytokines. Brain Behav Immun. 2002;16:785–798. doi: 10.1016/s0889-1591(02)00036-3. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Hammel I. Unequivocal delayed hypersensitivity in mast cell-deficient and beige mice. Science. 1984;226:710–713. doi: 10.1126/science.6494907. [DOI] [PubMed] [Google Scholar]
- Ghi P, Ferretti C, Blengio M. Effects of different types of stress on histamine-H3 receptors in the rat cortex. Brain Res. 1995;690:104–107. doi: 10.1016/0006-8993(95)00542-x. [DOI] [PubMed] [Google Scholar]
- Janeway C, Travers P, Walport M, Capra J. Immunobiology: the Immune System in Health and Disease. Elsevier; Garland, NY: 1999. [Google Scholar]
- Karp JD, Smith J, Hawk K. Restraint stress augments antibody production in cyclophosphamide-treated mice. Physiol Behav. 2000;70:271–278. doi: 10.1016/s0031-9384(00)00267-5. [DOI] [PubMed] [Google Scholar]
- Konkle AT, Baker SL, Kentner AC, Barbagallo LS, Merali Z, Bielajew C. Evaluation of the effects of chronic mild stressors on hedonic and physiological responses: sex and strain compared. Brain Res. 2003;992:227–238. doi: 10.1016/j.brainres.2003.08.047. [DOI] [PubMed] [Google Scholar]
- Leitner DS. Multisensory deficits in rats produced by acute exposure to cold swim stress. Behav Neurosci. 1989;103:151–157. doi: 10.1037//0735-7044.103.1.151. [DOI] [PubMed] [Google Scholar]
- Madrigal JL, Caso JR, de Cristobal J, Cardenas A, Leza JC, Lizasoain I, Lorenzo P, Moro MA. Effect of subacute and chronic immobilisation stress on the outcome of permanent focal cerebral ischaemia in rats. Brain Res. 2003;979:137–145. doi: 10.1016/s0006-8993(03)02892-0. [DOI] [PubMed] [Google Scholar]
- Malorny U, Goebeler M, Gutwald J, Roth J, Sorg C. Differences in migration inhibitory factor production by C57Bl/6 and BALB/c mice in allergic and irritant contact dermatitis. Int Arch Allergy Appl Immunol. 1990;92:356–360. doi: 10.1159/000235164. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Miyata S, Ishiyama M, Shido O, Nakashima T, Shibata M, Kiyohara T. Central mechanism of neural activation with cold acclimation of rats using Fos immunohistochemistry. Neurosci Res. 1995;22:209–218. doi: 10.1016/0168-0102(95)00900-x. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui DH, Tabira T. Chronic stress differentially regulates glucocorticoid negative feedback response in rats. Psychoneuroendocrinology. 2001;26:443–459. doi: 10.1016/s0306-4530(01)00004-x. [DOI] [PubMed] [Google Scholar]
- Nakano Y. Stress-induced modulation of skin immune function: two types of antigen-presenting cells in the epidermis are differentially regulated by chronic stress. Br J Dermatol. 2004;151:50–64. doi: 10.1111/j.1365-2133.2004.05980.x. [DOI] [PubMed] [Google Scholar]
- Neigh GN, Bowers SL, Pyter LM, Gatien ML, Nelson RJ. Pyruvate prevents restraint-induced immunosuppression via alterations in glucocorticoid responses. Endocrinology. 2004;145:4309–4319. doi: 10.1210/en.2003-1748. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Johnstone HA, Hatzinger M, Liebsch G, Shipston M, Russell JA, Landgraf R, Douglas AJ. Attenuated neuroendocrine responses to emotional and physical stressors in pregnant rats involve adenohypophysial changes. J Physiol. 1998;508 ( Pt 1):289–300. doi: 10.1111/j.1469-7793.1998.289br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacak K. Stressor-specific activation of the hypothalamic-pituitary-adrenocortical axis. Physiol Res. 2000;49(Suppl 1):S11–17. [PubMed] [Google Scholar]
- Pacak K, Palkovits M. Stressor specificity of the central neuroendocrine responses: implications for stress-related disorders. Endocr Rev. 2001;22 (4):502–48. doi: 10.1210/edrv.22.4.0436. [DOI] [PubMed] [Google Scholar]
- Palanza P. Animal models of anxiety and depression: how are females different? Neuroscience & Biobehavioral Reviews. 2001;25:219–233. doi: 10.1016/s0149-7634(01)00010-0. [DOI] [PubMed] [Google Scholar]
- Palermo-Neto J, de Oliveira Massoco C, Robespierre de Souza W. Effects of physical and psychological stressors on behavior, macrophage activity, and Ehrlich tumor growth. Brain Behav Immun. 2003;17:43–54. doi: 10.1016/s0889-1591(02)00057-0. [DOI] [PubMed] [Google Scholar]
- Phanupak P, Moorhead JW, Claman HN. Tolerance and contact sensitivity to DNFB in mice. 3. Transfer of tolerance with “suppressor T cells”. J Immunol. 1974;113:1230–1236. [PubMed] [Google Scholar]
- Pinto-Ribeiro F, Almeida A, Pego JM, Cerqueira J, Sousa N. Chronic unpredictable stress inhibits nociception in male rats. Neurosci Lett. 2004;359:73–76. doi: 10.1016/j.neulet.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Qin Y, Karst H, Joels M. Chronic unpredictable stress alters gene expression in rat single dentate granule cells. J Neurochem. 2004;89:364–374. doi: 10.1111/j.1471-4159.2003.02332.x. [DOI] [PubMed] [Google Scholar]
- Rauch TM, Lieberman HR. Tyrosine pretreatment reverses hypothermia-induced behavioral depression. Brain Res Bull. 1990;24:147–150. doi: 10.1016/0361-9230(90)90299-f. [DOI] [PubMed] [Google Scholar]
- Reyes TM, Walker JR, DeCino C, Hogenesch JB, Sawchenko PE. Categorically distinct acute stressors elicit dissimilar transcriptional profiles in the paraventricular nucleus of the hypothalamus. J Neurosci. 2003;23:5607–5616. doi: 10.1523/JNEUROSCI.23-13-05607.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz C, Rhodes ME, Bludau M, Kaplan S, Ong P, Ueffing I, Vehoff J, Korr H, Frye CA. Depression: reduced number of granule cells in the hippocampus of female, but not male, rats due to prenatal restraint stress. Mol Psychiatry. 2002;7:810–813. doi: 10.1038/sj.mp.4001118. [DOI] [PubMed] [Google Scholar]
- Schulkin J, McEwen BS, Gold PW. Allostasis, amygdala, and anticipatory angst. Neurosci Biobehav Rev. 1994;18:385–396. doi: 10.1016/0149-7634(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Selye H. Stress and the general adaptation syndrome. British Medical Journal. 1950;1:1383–1392. doi: 10.1136/bmj.1.4667.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks N, Windle RJ, Perks PA, Harbuz MS, Jessop DS, Ingram CD, Lightman SL. Early-life exposure to endotoxin alters hypothalamic-pituitary-adrenal function and predisposition to inflammation. Proc Natl Acad Sci U S A. 2000;97:5645–5650. doi: 10.1073/pnas.090571897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebot MH, Martin P, Puech AJ. Animal behavioural studies in the evaluation of antidepressant drugs. Br J Psychiatry Suppl. 1992:44–50. [PubMed] [Google Scholar]
- Van Raaij MT, Oortgiesen M, Timmerman HH, Dobbe CJ, Van Loveren H. Time-dependent differential changes of immune function in rats exposed to chronic intermittent noise. Physiol Behav. 1996;60:1527–1533. doi: 10.1016/s0031-9384(96)00327-7. [DOI] [PubMed] [Google Scholar]
- Viau V, Sawchenko PE. Hypophysiotropic neurons of the paraventricular nucleus respond in spatially, temporally, and phenotypically differentiated manners to acute vs. repeated restraint stress: rapid publication. J Comp Neurol. 2002;445:293–307. doi: 10.1002/cne.10178. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Kabeer N, Albers HE. Cold exposure elevates cellular levels of messenger ribonucleic acid encoding thyrotropin-releasing hormone in paraventricular nucleus despite elevated levels of thyroid hormones. Endocrinology. 1990;127:2955–2962. doi: 10.1210/endo-127-6-2955. [DOI] [PubMed] [Google Scholar]