Abstract
The hypothalamic-pituitary-adrenal (HPA) axis habituates, or gradually decreases its activity, with repeated exposure to the same stressor. During habituation, the HPA axis likely requires input from cortical and limbic regions involved in processing of cognitive information that is important in coping to stress. Brain regions such as the medial prefrontal cortex (mPFC) are recognized as important in mediating these processes. The mPFC modulates stress-related behavior and some evidence suggests that the mPFC regulates acute and repeated stress-induced HPA responses. Interestingly, corticotropin releasing hormone(CRH)-1 receptors, which integrate neuroendocrine, behavioral and autonomic responses to stress, are localized in the mPFC but have not been specifically examined with respect to HPA regulation. We hypothesized that CRH receptor activity in the mPFC contributes to stress-induced regulation of HPA activity and anxiety-related behavior, and that CRH release in the mPFC may differentially regulate HPA responses in acutely- compared to repeatedly-stressed animals. In the present experiments, we found that blockade of CRH receptors in the mPFC with the non-selective receptor antagonist, D-Phe-CRH (50ng or 100ng) significantly inhibited HPA responses compared to vehicle regardless of whether animals were exposed to a single, acute 30min restraint or to the eighth 30min restraint. We also found that intra-mPFC injections of CRH (20ng) significantly increased anxiety-related behavior in the elevated plus maze in both acutely- and repeatedly-restrained groups compared to vehicle. Together, these results suggest an excitatory influence of CRH in the mPFC on stress-induced HPA activity and anxiety-related behavior regardless of prior stress experience.
Keywords: prefrontal cortex, corticotropin releasing hormone, restraint, anxiety, ACTH, corticosterone
1. Introduction
The medial prefrontal cortex (mPFC) has received considerable attention for its role in working memory, learning, attention and emotional behavior (Ragozzino, 2000; Birrell & Brown, 2000; Ridderinkhof et al, 2004) and is a site that is thought to perform the complex appraisals necessary to process the nature of aversive stimuli such as stressors (Quirk & Gehlert, 2003; Amat et al, 2005). There is substantial evidence that the mPFC influences activity in the main neuroendocrine system of the stress response, the hypothalamic-pituitary-adrenal (HPA) axis (Diorio et al, 1993; Sullivan & Gratton, 1999; Akana et al, 2001; Sullivan & Gratton, 2002; Figueiredo et al, 2003; Rangel et al, 2003; Spencer et al, 2005). Lesions of the mPFC potentiate HPA responses to processive (psychological/emotional) stressors such as restraint but often have no effect on responses to systemic stressors that represent an immediate threat to physiological homeostasis such as ether exposure (Diorio et al, 1993; Figueiredo et al, 2003). There are, however, exceptions to the latter case in which mPFC lesions have been reported to potentiate HPA responses to an immune challenge (Crane et al, 2003). Furthermore, the mPFC is sensitive to repeated stress (Bagley and Moghaddam, 1997; Finlay et al, 1997; Sullivan & Gratton, 1999; Akana et al, 2001). For example, acute tail shock elicits a two-fold greater increase in extracellular norepinephrine in the mPFC of repeatedly cold-stressed rats than in naive control rats (Finlay et al, 1997). Corticosterone implanted into the mPFC also inhibits ACTH responses to restraint in repeatedly, as well as acutely, cold-stressed rats (Akana et al, 2001). Importantly, mPFC lesions suppress restraint-induced corticosterone responses in repeatedly-, but not acutely-restrained rats (Sullivan and Gratton, 1999). The lack of effects in acutely-restrained rats in the latter study contradict the lesion studies described above that found that mPFC lesions potentiate HPA responses to acute restraint (Diorio et al, 1993; Figueiredo et al, 2003). This discrepancy may be due to differences in the size of mPFC lesions. Lesions in Sullivan & Gratton (1999) were relatively extensive and were observed in the entire mPFC including cingulate, prelimbic and infralimbic regions. Lesions in Diorio et al (1993) and Figueiredo et al (2003) were smaller and more confined to prelimbic and infralimbic regions. Together, the evidence discussed above suggests that activity in the mPFC regulates both acute and repeated stress-induced HPA activity. Repeated exposure to the same, homotypic stressor can produce a gradual decrement, or habituation, of HPA activity (Bhatnagar & Meaney, 1995; Li & Sawchenko, 1998; Garcia et al, 2000; Viau and Sawchenko, 2002; Jaferi & Bhatnagar, 2006). Habituation to repeated stress may involve discrimination of the stressor as a familiar and previously encountered stressor as opposed to one that is novel. During this process, the hypothalamic paraventricular nucleus (PVN) likely requires input from cortical and limbic regions involved in cognitive processing such as the mPFC (Melia et al, 1994; Cullinan et al, 1996). In addition to regulating HPA responses to stress, the mPFC also modulates anxiety-related behaviors. In particular, mPFC lesions generally decrease anxiety-related behavior as shown by increased time spent in the open arms of the elevated plus maze (Gonzalez et al, 2000; Lacroix et al, 2000; Shah & Treit, 2003), increased social interaction (Gonzalez et al, 2000; Shah & Treit, 2003), increased open field exploration (Lacroix et al, 2000), and lower rates of burying in the shock probe burying test (Shah & Treit, 2003). These lesion studies suggest that, normally, stimulation of the mPFC increases anxiety-related behavior. However, little is known about the specific neurotransmitters that mediate mPFC regulation of anxiogenic behavior or regulation of HPA activity.
Interestingly, corticotropin-releasing-hormone (CRH)-1 receptors, which play an intricate role in integrating neuroendocrine, behavioral and autonomic responses to stress (Bale & Vale, 2004), are found in large densities in the mPFC as demonstrated by immunohistochemistry and in situ hybridization (Radulovic et al, 1998; Van Pett et al, 2000). The presence of CRH-2 receptors has not been reported in the rodent mPFC (Chalmers et al, 1995). CRH stimulates HPA responses to a variety of acute stressors (Deak et al, 1999; Habib et al, 2000; McElroy et al, 2002; Rivier et al, 2003). In addition, CRH also plays a prominent role in inducing anxiety-related behavior (Landgraf, 2001). Intracerebroventricular administration of CRH reduces open arm exploration on the elevated plus maze (EPM) (Baldwin et al, 1991; Adamec & McKay, 1993) and also has anxiogenic effects in other common tests of anxiety (Dunn & File, 1987; Takahashi et al, 1989). These anxiogenic-type effects of CRH appear to be mediated by the CRH-1 receptor subtype in particular (Heinrichs et al, 1997). However, whether CRH release in the mPFC regulates HPA activity or anxiety-related behavior has not been studied. In the present studies, we examined HPA responses to acute restraint or behavior in the elevated plus maze after CRH receptor blockade in the mPFC. We also assessed these measures in repeatedly-stressed animals because repeatedly, cold-stressed rats exhibit sensitized norepinphrine release in the mPFC after intraventricular CRH compared to acutely-stressed rats (Finlay et al., 1997), suggesting that the mPFC may be sensitized to CRH in repeatedly-stressed rats. In addition, activity in the mPFC is important for repeated stress-induced HPA activity, as described above. Therefore, in the first experiment, we hypothesized that CRH receptor activation in the mPFC stimulates HPA responses to acute and repeated stress, and that the actions of CRH at the mPFC on HPA activity will be of a greater magnitude in repeatedly-stressed animals. We tested this hypothesis in the first experiment by examining HPA responses to the 1st or 8th restraint exposure after a single intra-mPFC injection of the non-selective CRH receptor antagonist, [DPhe12, Nle21,38, CalphaMe Leu37] r/h CRH(12–41) (D-Phe-CRH). In the second experiment, we hypothesized that CRH receptor activity in the mPFC would increase anxiety-related behavior in both acutely- and repeatedly-stressed animals and that the effects of CRH in the mPFC will be of a greater magnitude in repeatedly-stressed animals. To test this hypothesis, we measured behavior in the EPM after intra-mPFC injections of vehicle or CRH in animals that were previously exposed to one or eight days of restraint.
2. Results
Experiment 1a: Effect of intra-mPFC injections of 50ng of D-Phe-CRH on HPA responses to acute or repeated restraint
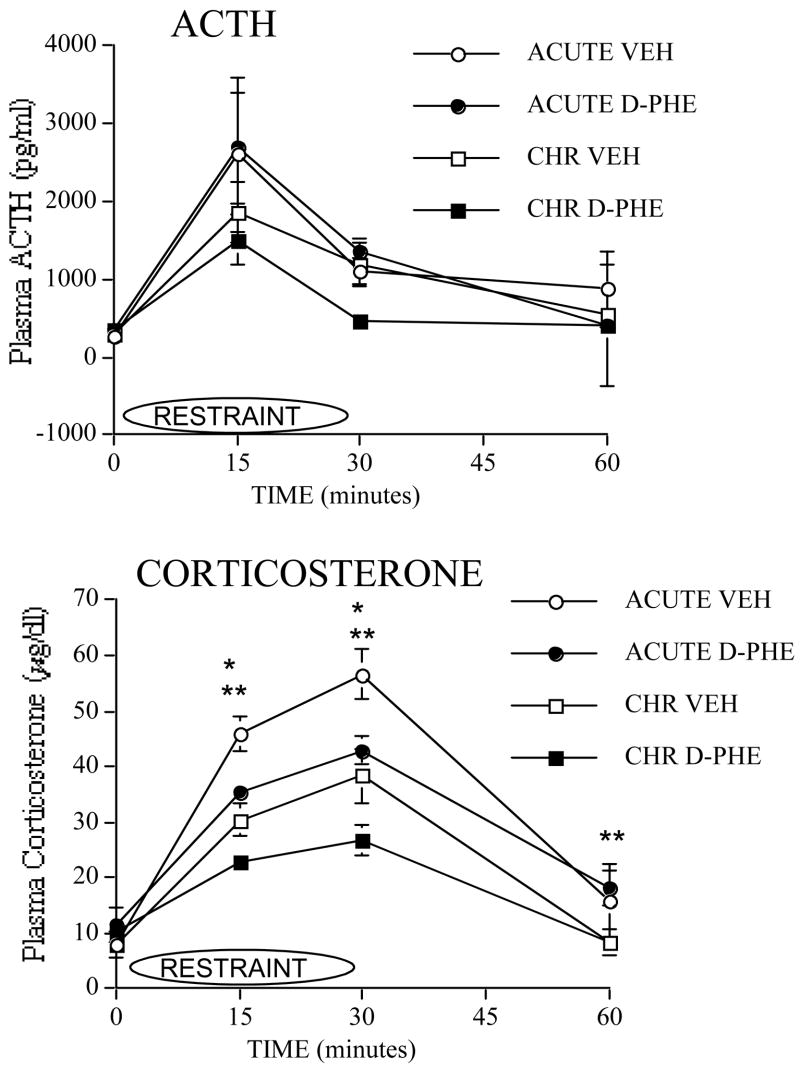
Cannula were stereotaxically localized to the mPFC and confirmed for correct placement as shown in Figure 1. Plasma ACTH responses to the 1st or 8th restraint after intra-mPFC injections of 50ng of D-Phe-CRH or vehicle are shown in Figure 2. At 0, 15 and 30 min, no significant effects on ACTH were observed. At 30 min, although there was a trend towards a Stress Group × Drug Treatment Interaction (F(1,41)=3.6, p=0.06), this effect was not significant. At 60 min, no significant effects on ACTH were observed.
Figure 1.
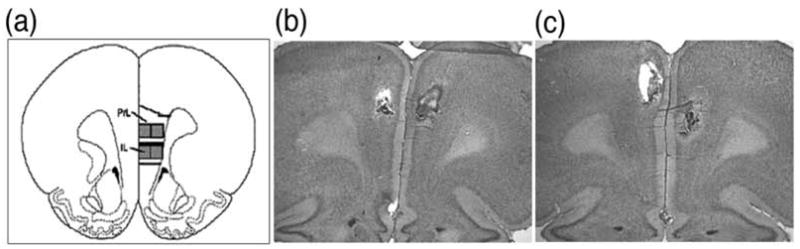
Images of cannula placements in mPFC. The dorsal (prelimbic- PL) and ventral (infralimbic- IL) subregions of the mPFC (+3.2mm from bregma) based on the atlas of Paxinos and Watson are shown in a. Shown in b and c are representative cresyl violet-stained sections. Shown in b is an animal with the tracks of both injector cannulae placed in the dorsal mPFC (anterior cingulate, prelimbic). Shown in c is an animal with one cannula in the dorsal mPFC and other in the ventral mPFC (infralimbic). The majority of cannula placements in each experiment were bilaterally in the dorsal mPFC (65% in Experiment 1a; 100% in Experiment 1b; 93% in Experiment 2).
Figure 2.
Effect of 50ng of D-Phe-CRH in mPFC on ACTH and corticosterone responses to acute or repeated stress. Plasma ACTH and plasma corticosterone responses to the first restraint (acute restraint) or to the eighth restraint (repeated restraint) are shown in rats that received intra-mPFC injections of 50ng of D-Phe-CRH or VEH 30min prior to the 1st or 8th restraint. (The following are sample sizes at each time point of the ACTH response. For acute vehicle, n=8 at 0 min, n=9 at 15 min, n=11 at 30 min, and n=10 at 60 min. For acute drug, n=7 at 0 min, n=8 at 15 min, n=10 at 30 min, n=9 at 60 min. For repeated vehicle, n=7 at 0 min and 15 min, n=12 at 30 min, and n=10 at 60 min. Lastly, for repeated drug, n=8 at 0 min, n=7 at 15 min, n=12 at 30 min, and n=10 at 60 min. The following are sample sizes at each time point of the corticosterone response. For acute vehicle, n=11 at 0 min, n=14 at 15 min, n=13 at 30 min, and n=7 at 60 min. For acute drug, n=11 at 0 min, n=14 at 15 min and 30 min, and n=10 at 60 min. For repeated vehicle, n=12 at 0 min, n=14 at 15 min, n=13 at 30 min, and n=14 at 60 min. Lastly, for repeated drug, n=8 at 0 min, n=12 at 15 min, n=9 at 30 min, and n=15 at 60 min.)
*D-Phe-CRH-injected groups are significantly lower than vehicle-injected groups regardless of whether animals were acutely or repeatedly stressed, reflecting a significant Main effect of Drug Treatment. p≤ 0.05.
** Repeatedly-stressed groups are significantly lower than acutely-stressed groups regardless of drug treatment, reflecting a significant Main effect of Stress Group, p≤ 0.05.
Plasma corticosterone responses to the 1st or 8th restraint after intra-mPFC injections of 50ng of D-Phe-CRH or vehicle are also shown in Figure 2. No significant effects were observed at 0 min. At 15 min, there was a significant Stress Group effect (F(1,50)=26.9, p≤0.001) and a significant Drug Treatment effect (F(1,50)=12.4, p≤0.001). Similarly, at 30 min, there was a significant Stress Group effect (F(1,45)=19.2, p≤0.001) and a significant Drug Treatment effect (F(1,45)=10.2, p≤0.01). At 60 min, we observed a significant Stress Group effect (F(1,41)=5.9, p≤0.01). For significant Stress Groups effects at 15, 30 and 60 min, repeatedly-restrained groups had lower corticosterone than acutely-restrained groups regardless of drug treatment. For significant Drug Treatment effects at 15 and 30 min, groups that received D-Phe-CRH treatment had lower corticosterone than vehicle groups regardless of whether or not they were repeatedly-stressed.
To summarize, repeatedly-restrained groups overall displayed significantly lower corticosterone responses to restraint than acutely-restrained groups at 15, 30 and 60 min (regardless of Drug treatment), providing evidence of habituation. D-Phe-CRH treated groups overall displayed significantly lower corticosterone than vehicle-treated groups at 15 and 30 min (regardless of stress group assignment).
Experiment 1b: Effect of intra-mPFC injections of 100ng of D-Phe-CRH on HPA responses to acute or repeated restraint
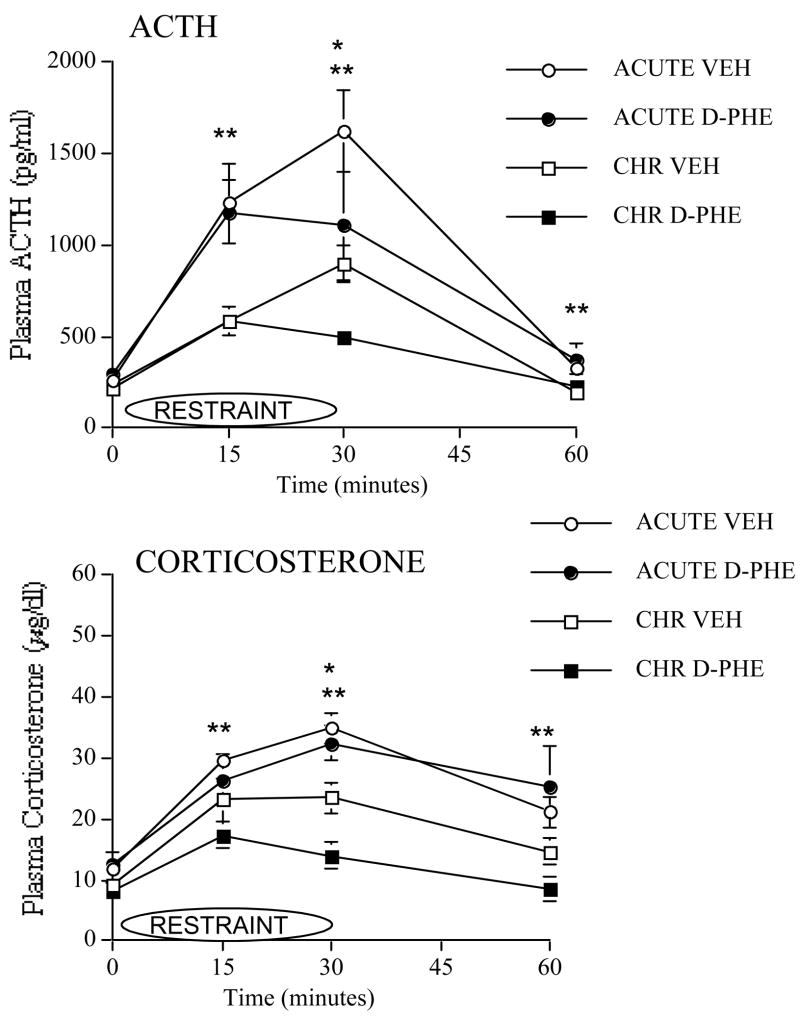
Plasma ACTH responses to the 1st or 8th restraint after intra-mPFC injections of 100ng of D-Phe-CRH or vehicle are shown in Figure 3. No significant effects on ACTH were observed at 0 min. At 15 min, there was a significant Stress Group effect (F(1,36)=15.8, p≤0.001). At 30 min, there was a significant Stress Group effect (F(1,29)=10.4, p≤0.01) and a significant Drug Treatment effect (F(1,29)=4.9, p≤0.05) in which groups that received D-Phe-CRH injection had lower ACTH than vehicle-injected groups. At 60 min, we observed a significant Stress Group effect (F(1,34)=9.7, p≤0.01). For significant Stress Group effects at 15, 30 and 60 min, repeatedly-restrained groups had lower ACTH than acutely-restrained groups regardless of drug treatment.
Figure 3.
Effect of 100ng of D-Phe-CRH in mPFC on ACTH and corticosterone responses to acute or repeated stress. Plasma ACTH and plasma corticosterone responses to the first restraint (acute restraint) or to the eighth restraint (repeated restraint) are shown in rats that received intra-mPFC injections of 100ng of D-Phe-CRH or VEH prior to day 1 or 8 of restraint. (The following are sample sizes at each time point of the ACTH response. For acute vehicle, n=11 at 0, 15 and 30 min, and n=10 at 60 min. For acute drug, n=10 at 0 min and 15 min, n=7 at 30 min, and n=9 at 60 min. For repeated vehicle, n=10 at 0 min and 15 min, n=7 at 30 min, n=9 at 60 min. Lastly, for repeated drug, n=10 at 0 min, n=9 at 15 min, n=8 at 30 min, and n=10 at 60 min. The following are sample sizes at each time point of the corticosterone response. For acute vehicle, n=9 at 0 min, n=11 at 15 min, n=8 at 30 min, and n=10 at 60 min. For acute drug, n=7 at all time points. For repeated vehicle, n=11 at 0 min and 15 min, and n=9 at 30 min and 60 min. Lastly, for repeated drug, n=7 at 0 min, and n=10 at 15 min and 30 min, and n=11 at 60 min.)
* D-Phe-CRH-injected groups are significantly lower than vehicle-injected groups regardless of whether animals were acutely or repeatedly stressed, reflecting a significant Main effect of Drug Treatment, p ≤ 0.05.
** Repeatedly stressed groups are significantly lower than acutely stressed groups regardless of drug treatment, reflecting a significant Main effect of Stress Group, p ≤ 0.05.
Plasma corticosterone responses to the 1st or 8th restraint after intra-mPFC injections of 100ng of D-Phe-CRH or vehicle are also shown in Figure 3. No significant effects on corticosterone were observed at 0 min. At 15 min, there was a significant Stress Group effect (F(1,35)=6.9, p≤0.01). At 30 min, we observed a significant Stress effect (F(1,30)=35.1, p≤0.001) and a significant Drug Treatment effect (F(1,30)=5.6, p≤0.05) in which groups that received D-Phe-CRH treatment had lower corticosterone than vehicle-injected groups. At 60 min, there was a significant Stress Group effect (F(1,33)=12.1, p≤0.001). For significant Stress Group effects at 15, 30 and 60 min, repeatedly-restrained groups had lower corticosterone than acutely-restrained groups.
To summarize, repeatedly-restrained groups overall displayed significantly lower ACTH and corticosterone responses to restraint than acutely-restrained groups at 15, 30 and 60 min (regardless of drug treatment), providing evidence of habituation. D-Phe-CRH treated groups overall displayed significantly lower ACTH and corticosterone than vehicle-treated groups at 30 min regardless of whether or not they were repeatedly-stressed.
Experiment 2: Effect of intra-mPFC injection of CRH on anxiety-related behavior in the elevated plus maze after acute or repeated restraint
Anxiety-related behaviors in the elevated plus maze after intra-mPFC injection of vehicle or 20ng CRH in acutely- and repeatedly-restrained rats are shown in Figure 4. No significant Stress Group effects were observed on any of the behaviors measured. There were significant Drug Treatment effects on the number of open arm entries (F(1,23)=4.5, p≤0.05) and the percentage of total time spent in the open arms (F(1,23)=5.0, p≤0.05) in which CRH-treated groups had fewer open arm entries and spent less time in the open arms compared to vehicle-treated groups regardless of stress condition. We also observed a significant Drug Treatment effect on the percentage of total time spent in the closed arms (F(1,23)=5.5, p≤0.05) in which CRH-treated groups spent more time in the closed arms than vehicle-treated groups.
Figure 4.
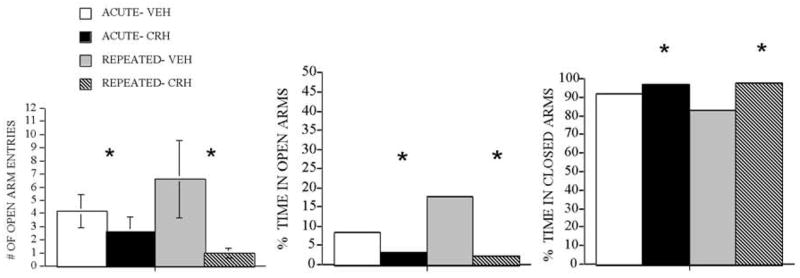
Effect of administration of 20ng CRH in mPFC on behavior in elevated plus maze in acutely and repeatedly stressed animals. (a) number of open arm entries, (b) percentage of total time spent in open arms and (c) percentage of total time spent in closed arms in the elevated plus maze following intra-mPFC injection of 20ng of CRH or vehicle (VEH) are shown in rats previously exposed to 1 restraint (acute) or 8 restraints (repeated), n= 6–8.
* Animals injected with CRH in mPFC are overall significantly different from animals injected with VEH regardless of whether they were acutely or repeatedly stressed, reflecting a significant Main effect of Drug Treatment. p ≤ 0.05.
To summarize, overall, CRH administration in the mPFC significantly decreased the number of open arm entries as well as the percentage of total time spent in the open arms and significantly increased the percentage of total time spent in the closed arms compared to vehicle treatment.
3. Discussion
In the present studies, repeatedly-restrained rats in each experiment exhibited lower ACTH and/or corticosterone responses at 15 and/or 30min to the 8th restraint compared to acutely-restrained rats, providing clear evidence of habituation of HPA activity. However, the effects of CRH receptor blockade with either the 50 or 100ng dose of D-Phe-CRH were not specific to the repeated stress state. We found that CRH receptor blockade in the mPFC by the lower dose (50ng) of D-Phe-CRH significantly inhibited corticosterone responses to restraint overall in both acutely- and repeatedly-stressed groups at 15 and 30 minutes, but did not significantly affect ACTH. A higher dose (100ng) of D-Phe-CRH significantly inhibited both ACTH and corticosterone responses overall in all animals at 30 minutes. Assessment of behaviors in the elevated plus maze showed that intra-mPFC injections of CRH in both acutely- and repeatedly-restrained animals significantly decreased the percentage of time spent in the open arms as well as the number of open arm entries and significantly increased the percentage of time spent in the closed arms. These behaviors are considered to be indices of increased anxiety (Pellow et al, 1985; Pellow & File, 1986). Therefore, the present studies demonstrate a role for CRH in the mPFC in increasing HPA responses to restraint and anxiety-related behavior regardless of prior stress experience.
In Experiment 1, blockade of CRH receptors in the mPFC with either 50ng or 100ng of D-Phe-CRH produced an inhibition of ACTH and/or corticosterone responses, depending on the experiment, to acute and repeated restraint. These results suggest that, normally, CRH acting in the mPFC exerts an excitatory influence on HPA responses to acute and repeated stress. Sources of stress-related afferent input to the mPFC are numerous and include the hippocampal formation, basolateral amygdala and paraventricular thalamus (Sarter & Markowitsch, 1983; Conde et al., 1995; Pinto et al., 2003; Jaferi and Bhatnagar, 2006). These inputs provide the mPFC with cognitive and emotionally salient information related to the stressor (Herman et al., 1996) in addition to memories related to past stress experiences (Roozendaal et al, 2004). Although the mPFC does not directly innervate the PVN, it can modulate HPA activity through its connections to the bed nucleus of the stria terminalis (BNST) or peri-PVN, important afferent sites of the PVN (Swanson & Sawchenko, 1983; Roland & Sawchenko, 1993). The mPFC could also regulate HPA activity through regions such as the basolateral and central amygdala that project indirectly to the PVN through the BNST (Dong et al, 2001). Regardless of the path by which CRH release in the mPFC regulates HPA activity, the present data demonstrate that acute restraint-induced CRH release in the mPFC normally excites HPA activity.
In the present studies, the majority of our bilateral cannula placements were in the dorsal region of the mPFC although we also accepted one cannula in the dorsal mPFC and one cannula in the ventral mPFC (infralimbic). This is because it is highly likely that drug administered into the dorsal region also diffused into the ventral region. However, it is worth noting that evidence exists for neuroanatomical and functional differences between dorsal (prelimbic) versus ventral (infralimbic) subregions of the rodent mPFC (Heidbreder & Groenewegen, 2003). For example, the prelimbic subregion primarily projects to limbic sites that reportedly affect cognition (Vertes et al, 2004) while the infralimbic subregion projects extensively and directly to autonomic/visceral-related cell groups in the hypothalamus and brainstem (Terreberry & Neafsey, 1987; Hurley et al, 1991; Vertes et al, 2004). In addition to differences in neuroanatomical connectivity, differential functional contributions have been reported with respect to drug-induced behavioral sensitization (Tzschentke & Schmidt, 2000) and acquisition and extinction of conditioned fear (Morgan & LeDoux, 1995). Therefore, further investigation with more selective injections into the infralimbic mPFC, although difficult, may be useful in clearly determining whether CRH plays differential roles in the prelimbic versus infralimbic mPFC when regulating stress-induced HPA activity.
As mentioned above, the infralimbic mPFC projects directly to autonomic/visceral-related cell groups in the hypothalamus and brainstem that may play a role in modulating visceral responses to emotional stimuli (Terreberry & Neafsey, 1987; Hurley et al, 1991; Vertes et al, 2004). In turn, autonomic innervation of the adrenal gland can act as an extra-ACTH mechanism in regulation of adrenal corticosteroid secretion (Edwards & Jones, 1993; Engeland, 1998). This may explain the dissociation of ACTH and corticosterone secretion in Experiment 1a in which D-Phe-CRH administration in the mPFC significantly altered stress-induced corticosterone without significantly altering ACTH levels. Manipulations of the infralimbic mPFC may indirectly alter adrenal functioning via its direct influence on autonomic-related neurons. The results of Experiment 1b, in which a higher dose of D-Phe-CRH (100ng) was used, differed from the results of Experiment 1a in that the drug did significantly alter both ACTH and corticosterone. There was, however, a more noticeable effect on ACTH responses to acute restraint than on corticosterone responses. Perhaps additional systems that influence the ACTH response to an acute stressor were affected by the higher dose of CRH receptor antagonism in the mPFC, but not by the lower dose, which resulted in a different HPA response in Experiment 1a versus 1b.
In Experiment 2, we found that intra-mPFC administration of CRH increased behaviors that are indicative of anxiety on the EPM. Furthermore, CRH in the mPFC produced increased anxiety-related behavior compared to vehicle treatment to a similar extent in animals that were exposed to 1 or 8 days of restraint prior to testing. These findings are in line with mPFC lesion studies that suggest that the mPFC normally acts to increase anxiety in the elevated plus maze (Gonzalez et al, 2000; Lacroix et al, 2000; Shah & Treit, 2003), the social interaction test (Gonzalez et al, 2000; Shah & Treit, 2003), the open field (Lacroix et al, 2000), and the shock probe burying test (Shah & Treit, 2003). However, to our knowledge, the present findings are the first to show that CRH receptor activation in the mPFC is important for increases in anxiety-related behavior in stressed animals. In contrast to the results of the lesion studies above, another mPFC-lesion study by Morgan & Ledoux (1995) suggested that the mPFC normally decreases anxiety-related behaviors in a fear conditioning paradigm (Morgan & Ledoux, 1995). An inhibitory influence on anxiety-related behavior might be mediated by neurotransmitters other than CRH such as GABA, opioids or dopamine, all of which decrease anxiety-related behavior as shown by selective manipulation of their receptors in the mPFC (Espejo, 1997; Wall & Messier, 2000; Shah et al, 2004.) With respect to characterizing the role of CRH in the mPFC on anxiety-related behavior, one caveat of the present study is that we used a single behavioral test for anxiety. Multiple behavioral tests will need to be employed in future studies in order to more thoroughly assess whether CRH in the mPFC has a uniformly excitatory effect on anxiety-related behaviors.
Interestingly, in Experiment 2, acute and repeated stress did not differentially impact anxiety-related behavior in the EPM in vehicle-treated groups. Previous reports on the effects of repeated restraint on anxiety-related behavior have yielded mixed results. For example, repeated restraint ranging from 10–21 days had no effect on anxiety-related behavior 2 hours later in the EPM (Thorsell et al, 1999; Chadda & Devaud, 2005), or 24 hours later in the open field test (Perrot-Sinal et al, 2004; Gregus et al, 2005) or the social interaction test (Gregus et al, 2005) compared to unstressed controls. However, another study that exposed rats to 14 days of restraint (2 or 8 hours/day) reported a significant decrease in the percentage of time spent in the open arms and number of open arm entries in the EPM compared to unstressed groups 48 hours following the last stress exposure (Kim & Han, 2006). The differences in the literature on the effects of repeated restraint on behavior in anxiety tests may reflect methodological variations in the duration of stress exposure and in the time points post-stress at which behavioral tests are conducted (Kim & Han, 2006). Many of the studies described above compare behavior of repeatedly-stressed groups to unstressed controls while we compared behavior of repeatedly-stressed rats to acutely-stressed rats. A single restraint reliably increases anxiety-related behavior in the EPM 24 hours later compared to unstressed controls (Mendonca & Guimaraes, 1998; Calvo & Volosin, 2001). Whether CRH in the mPFC regulates anxiety-related behavior in animals without any prior stress experience remains to be seen.
Although we did not assess CRH receptor binding and number in the mPFC in these experiments, the existing literature does suggest that CRH receptors can change after both acute and repeated stress in a number of brain regions, and that the direction of these changes is region-specific. For example, Makino et al (1995) reported that both acute (2hr) and repeated immobilization stress (2hr daily for 14 days) increased CRH receptor mRNA in the PVN, but decreased it in the anterior pituitary, and did not affect CRH receptor mRNA in the BLA. In another study, repeated social stress decreased the number of binding sites in the anterior pituitary and hippocampus, but increased the number of binding sites in the central and lateral amygdala as well as in various cortical regions including the frontal cortex and cingulate cortex (Fuchs & Flugge, 1995). Based on these studies, it appears that CRH receptor binding does not always remain stable with stress, and like other cortical regions, it is possible that the prelimbic and infralimbic mPFC might also display an increase in the number of CRH binding sites after repeated stress. However, even if there were changes in CRH receptor binding or number in the mPFC in our acutely- versus repeatedly-stressed groups, these changes were either not sufficient or relevant for producing differential HPA responses or anxiety-related behavior in acutely-versus repeatedly-stressed animals.
The lack of repeated stress-specific effects of CRH receptor manipulation in the mPFC, particularly with respect to HPA activity, were unexpected. Some evidence exists for a role for the mPFC in specifically regulating HPA responses in repeatedly-stressed rats. Lesions of the mPFC decrease restraint-induced corticosterone responses in repeatedly-restrained rats without affecting responses in acutely-restrained rats (Sullivan and Gratton, 1999). Furthermore, acute tail shock elicits a two-fold greater increase in extracellular norepinephrine in the mPFC of repeatedly cold-stressed rats compared to controls (Finlay et al, 1997). Glutamate in the PFC may also play a role in the neurochemical response to repeated stress since extracellular levels of glutamate in the mPFC decrease with repeated exposure to tail pinch (Bagley and Moghaddam, 1997). Additionally, the mPFC receives substantial input from the paraventricular thalamus (Pinto et al, 2003; Jaferi & Bhatnagar, 2006), a midline thalamic nucleus whose posterior division has been demonstrated to regulate HPA activity and anxiety-related behavior particularly in repeatedly-stressed animals (Bhatnagar et al, 2000, 2002, 2003). Therefore, it is possible that the mPFC receives information specific to the repeated stress state and integrates this information about prior experience with the appropriate behavioral and neuroendocrine outputs, but does this independent of CRH receptor activity.
In sum, the central findings of the present studies are that CRH receptor blockade in the mPFC inhibits HPA responses to both acute and repeated stress, and that CRH injections into the mPFC increase anxiety-related behavior in the elevated plus maze in both acutely- and repeatedly-stressed animals. These findings suggest that CRH acting at the mPFC increases stress-induced HPA activity and anxiety-related behavior regardless of prior repeated stress experience. The relevant sources of CRH to the mPFC remain to be determined. CRH inputs to the mPFC are known to derive from the laterodorsal tegmental nucleus of the pons, which contains cells that are co-localized for CRH and acetylcholinesterase (Crawley et al, 1985), as well as CRH-1 receptors and choline acetyltransferase (Sauvage & Steckler, 2001). Since cholinergic inputs to the mPFC influence attentional performance (Dalley et al, 2004), interactions between CRH and acetylcholine might influence attentional processes in stressed animals. Another possible source of CRH inputs to the mPFC is the locus coeruleus, an afferent site of the mPFC which contains an abundance of CRH-containing cell bodies (Swanson et al, 1983; Van Bockstaele et al, 1996), and plays substantial roles in behavioral responses to stressful stimuli (Smagin et al, 1996).
Additionally, intra-mPFC sources of CRH cannot be ruled out. In conclusion, the present experiments demonstrate an excitatory role for CRH receptor activation in the mPFC on stress-induced HPA activity and anxiety-related behavior regardless of whether or not animals have been exposed to prior repeated stress.
4. Methods
Animals
All experiments used adult male Sprague-Dawley rats supplied by Charles River (Wilmington, MA). Body weights ranged from 220–250g upon arrival at the animal housing facilities at the Department of Psychology, University of Michigan (Experiments 1a, 2) or at the Joseph Stokes Research Institute at Children’s Hospital of Philadelphia (Experiment 1b). Rats were individually housed in polypropylene tub cages lined with bedding material, and were allowed ad libitum access to rat chow and water. They were maintained on a 12 hr light/dark schedule (lights on at 07:00 h), and all experiments took place during the trough of the diurnal rhythm, starting at 10am. Animals were briefly handled the day before experiments were conducted. All experiments were approved by the University Committee on Use and Care of Animals at the University of Michigan (Experiments 1a, 2) and by the Institutional Animal Care and Use Committee at the Joseph Stokes Research Institute (Experiment 1b).
Intracerebral cannula implantation & drug injection
Rats were anesthetized with a mixture of ketamine, xylazine and acepromazine (77: 1.5: 1.5 mg/ml given i.m. at 0.1 ml/100g body weight) and placed in a stereotaxic apparatus with the skull flat, and the tooth bar at −3.3mm. Bilateral guide cannula (22 gauge) were lowered into the mPFC according to the following coordinates from bregma: AP: +3.2mm, ML: ±0.8mm, DV: −3.0mm. The selection of these coordinates was based on previous studies using cannula in the mPFC (Baldwin et al, 2002; Capriles et al, 2003). Cannulae were then cemented into place using dental cement anchored by skull screws. Dummy cannulae were used to close the guide cannulae. At the time of experimentation, the dummy cannula was removed and replaced with an injector cannula connected to PE tubing attached to a Hamilton syringe. Drug or vehicle was injected over a 1 minute period, with the needle remaining in place for another one minute before removal. The injection volume that we used (0.5ul) has previously been used for microinjection of a variety of drugs into the prelimbic and infralimbic mPFC (Morency et al, 1987; Amat et al, 2005). While every drug may have a distinct solubility and distribution when injected locally, it is of interest that this 0.5ul volume has been demonstrated by Shah & Treit (2004) to be limited to the mPFC when microinjecting the benzodiazepine, midazolam. Specifically, the authors used the Chicago blue stain to estimate the extent of diffusion of intra-mPFC infusions when using dorsal-ventral coordinates similar to our own. They illustrated that their infusions were primarily limited to the ventral prelimbic and infralimbic subregions of the mPFC.
Repeated stress paradigm
We used a repeated restraint paradigm in order to examine habituation of HPA activity in experiment 1 and anxiety-related behavior in experiment 2. Restraint, which is considered to be a psychological or processive stressor (Herman et al, 1996), consisted of placing rats in a ventilated, cylindrical plexiglass tube for 30 min. Rats in acute stress groups were exposed to a single 30 min restraint. Rats in repeated stress groups were exposed to 8 consecutive days of 30 min restraint per day. We, and other groups, have observed habituation of ACTH and corticosterone responses following 30min of restraint per day for 8 days and up to14 days (Viau and Sawchenko, 2002; Jaferi et al, 2003; Jaferi & Bhatnagar, 2006).
Experiment 1: Effect of CRH receptor blockade in the mPFC on HPA responses to acute or repeated restraint
In Experiment 1a, we injected vehicle or 50ng of D-Phe-CRH bilaterally into the mPFC. This dose has previously been used by others to examine the role of CRH receptors in stress-induced reinstatement of cocaine-seeking (Erb et al, 2001), relapse to alcohol (Le et al, 2002), behavioral consequences of uncontrollable stress (Hammack et al, 2002), and stress-induced c-fos expression (Funk et al, 2003) after intracerebral administration. We found that 50ng of D-Phe-CRH had significant effects on corticosterone, but not on ACTH. Furthermore, the 50ng dose did not differentially affect responses to acute versus repeated restraint. Therefore, we subsequently tested 100ng D-Phe-CRH in Experiment 1b, a dose previously used for injections of D-Phe-CRH into the lateral septum to study stress-induced freezing (Bakshi et al, 2002). The following methods for Experiment 1a and 1b are identical with the exception of the dose of D-Phe-CRH used.
After 5–7 days of recovery from stereotaxic implantation of cannula in the mPFC, half of all rats were randomly assigned to either 1 or 8 days of 30 min restraint/day. On day 1 or day 8, rats received intra-mPFC injections of 0.5ul of D-Phe-CRH or vehicle 30 min prior to restraint and blood collection as described below. The 30 min post drug-injection time point has previously been used to examine footshock-induced defensive withdrawal as well as footshock-induced reinstatement of cocaine-seeking 30 min following local or intracerebrovetricular injection of D-Phe-CRH (Erb & Stewart, 2001; Bruijnzeel et al, 2001). Blood was collected according to the procedure described below. The final group sizes were n= 7–15 per time point for Experiment 1a, and n= 7–11 per time point for Experiment 1b. The n’s represent the final n’s after omission of rats with placements of cannula outside of the mPFC. To obtain the final n’s, Experiments 1a and 1b were each run in two separate batches. Placement criteria are described below.
Blood sampling procedure
In experiment 1, we collected blood samples on day 1 or 8 of restraint for assessment of HPA activity. 30 minutes after an intra-mPFC injection of drug or vehicle, each animal was placed in the restrainer and blood samples were taken from the tail vein at 0, 15 and 30 min during restraint. After collection of the 30 min samples, animals were removed from their restrainers and replaced in their home cages. At 60 min, blood samples were collected again and animals subsequently returned to their home cages. All samples were collected within 60 seconds of opening the cage in order to ensure that ACTH and corticosterone levels in plasma do not rise in response to the restraint itself (Akana et al, 1996). This sampling method of tail nicking, used by other groups as well, produces little reactivity in the animal and results in consistent basal and stress levels of ACTH and corticosterone (Bhatnagar & Dallman, 1998; Bhatnagar et al, 2000; Vahl et al, 2005).
Experiment 2: Effect of CRH administration in the mPFC on anxiety-related behavior following acute or repeated restraint
We examined whether bilateral injections of CRH in the mPFC alter anxiety-related behavior in the elevated plus maze in rats that are exposed to either 1 or 8 days of restraint. Intra-mPFC administration of CRH is only expected to activate the CRH-1 receptor subtype since the mPFC reportedly contains CRH-1, but not CRH-2, receptors (Chalmers et al, 1995; Radulovic et al, 1998; Van Pett et al, 2000). In addition, CRH has a relatively low affinity for the CRH-2 receptor (Bale & Vale, 2004). Before injecting CRH in acutely versus repeatedly restrained rats in this experiment, we first conducted a pilot study to determine a dose of CRH that would alter behavior in the EPM after acute restraint when injected into the mPFC (n =5–6; data not shown). We injected CRH (20ng, 200ng) or vehicle into the mPFC 30 min prior to testing. The selection of these doses was based on previous studies from our lab that injected CRH into the basolateral amygdala to examine behavior in the EPM and from others who have injected similar doses of CRH into the amygdala and examined behavioral activation and grooming in an open field (Wiersma et al, 1998; Daniels et al, 2004). We found that 20ng of CRH decreased the number of open arm entries (0.3 ± 0.2) compared to vehicle (2.8 ± 1.2) as well as the time spent in the open arms (2.7 sec ± 1.2) compared to vehicle (29.6 sec ± 10.7). For rats receiving 200ng, the number of open arm entries (2.4 + 1.3) and the time spent in the open arms (30.8 + 11.0) was similar to vehicle-treated rats. Therefore, we chose the 20ng dose of CRH in this experiment. It was surprising that a higher dose of CRH had no substantial effect on EPM behavior, but the lower dose did. CRH generally produces dose-dependent increases in anxiety-related behaviors when microinjected into limbic regions such as the BNST (Sahuque et al, 2006) or periaqueductal grey (Martins et al, 1997). However, very little is known about CRH receptor activation specifically in cortical regions and its effects on behavior. Perhaps, in the mPFC, CRH receptor activation above a certain level activates other mechanisms that serve to bring stress-induced anxiety levels back to normal. To fully characterize these potential dose-dependent effects on behavior, future studies would need to employ a range of doses with a larger number of animals than was used in our pilot study.
After 5 days of recovery from stereotaxic implantation of cannulae in the mPFC, half of all rats were randomly assigned to either 1 (acute stress rats) or 8 days of 30 min restraint/day (repeated stress rats). On day 2 or day 9, rats received 0.5ul of 20ng CRH or vehicle into the mPFC at 30 min prior to EPM testing (n=6–8). The n’s represent the final n’s after omission of rats with placements of cannula outside of the mPFC. To obtain the final n’s, experiments were run in two separate batches.
Behavior in the Elevated plus maze
In Experiment 2, we conducted testing approximately 24 hours following 1st or 8th restraint. Evaluation of responses on the EPM 24 hours after restraint is a commonly used test of stress-induced anxiety-like behavior in rodents (Martijena et al, 1997; Mendonca & Guimaraes, 1998; Calvo and Volosin, 2001). We selected the EPM as our test of anxiety-related behavior because of its documented utility in measuring stress-induced anxiety following exposure to various stressors including restraint (Martijena et al, 1997; Mendonca & Guimaraes, 1998; Calvo and Volosin, 2001; Korte & De Boer, 2003) and because it does not require lengthy training, the use of noxious stimuli such as electric shock, or manipulation of appetitive behaviors. Furthermore, the EPM has been pharmacologically validated, as a test of anxiety in rodents (Pellow et al, 1985; Pellow & File, 1986). Rats confined to the open arms show significantly more behavioral and physiological indices of anxiety than rats confined to the closed arms as shown by increased freezing, immobility, defecation as well as increased corticosterone secretion (Pellow et al, 1985). Rats also spend more time in the closed arms than the open arms and enter them more frequently than the open arms regardless of factors relating to illumination level or novelty (Pellow et al, 1985). This aviodance of the open arms likely reflects the rodent’s innate aversion to open spaces. These pharmacological, behavioral and physiological studies strongly suggest that the behavior measured in the EPM is a valid measure of anxiety-related behavior in rodents. The plus-shaped apparatus consisted of two open and two closed arms with an open roof, arranged such that the two arms of each type are opposite to each other. At the onset of testing, each rat was placed onto the central area of the plus maze facing one of the closed arms. Behavior for each rat was recorded for 10 minutes by a video camera facing the closed arm. The maze was cleaned with a bleach/water solution after each rat was tested. The following EPM behaviors were later scored by two experimenters, one of which was blind to drug/stress condition of the animal being analyzed: 1. total number of open arm entries (counted when all four paws are placed in the open arm), 2. time spent in open arms (with all four paws in open arms)/total time, 3. time spent in closed arms (with all four paws in closed arms)/total time.
Confirmation of cannula placements
After completion of the experiment, brains were collected, post-fixed in 4% formalin and sliced coronally at 30um on a cryostat and mounted onto Superfrost Plus slides. The exact placement of the cannulae was confirmed by staining sections with cresyl violet and visualizing the tips of the cannulae. Representative cannulae placements are shown in Figure 1. Any animals whose cannulae placements were found to be outside of the mPFC were excluded from the analysis. More specifically, animals were deemed to have inappropriate placements if one or both of the cannula were located outside of the dorsal mPFC (anterior cingulate and prelimbic subregion) and ventral mPFC (infralimbic subregion) as outlined by the atlas of Paxinos and Watson (1997). Out of the rats that were included in the analysis, 65% had both cannula placed in the dorsal mPFC in Experiment 1a, 100% had both cannula in the dorsal mPFC in Experiment 1b and 93% had both cannula in the dorsal mPFC in Experiment 2. The rest had both cannula in the infralimbic subregion, or one cannula in dorsal mPFC and the other in infralimbic mPFC. Given the likely diffusion of the drug into dorsal and ventral regions of the mPFC, it is reasonable to conclude that the observed drug effects are due to actions in both dorsal and ventral subregions of the mPFC.
ACTH and Corticosterone Radioimmunoassays
Blood was collected in microcentrifuge tubes containing10ul of sodium EDTA and kept on ice until centrifuged. After centrifugation, the plasma was aliquoted and kept frozen at −20° Celsius until assay. Plasma ACTH was measured by using a specific antiserum generously donated by Dr. William Engeland (Univ. of Minnesota) at a final dilution of 1:120,000, and [I125]ACTH as tracer (Diasorin, Stillwater, MN). The minimum level of detection of the assay is 10 pg/ml. Plasma corticosterone was measured using a kit from MP Biomedicals (Irvine, CA) and its minimum detection level is 0.625 ug/dl.
Drugs
In experiment 1, [DPhe12, Nle21,38, CalphaMe Leu37] r/h CRH(12–41) (D-Phe-CRH) (Bachem) was dissolved in 0.9% saline. In experiment 2, CRH (Sigma) was dissolved in 0.9% saline. 0.9% saline served as the vehicle for all experiments. D-Phe-CRH, CRH and vehicle solutions were prepared the day before intra-mPFC injections.
Statistical Analyses
Data were analyzed using analysis of variance (ANOVA). In Experiment 1, Stress Group (acute or repeated stress) × Drug Treatment (vehicle or D-Phe-CRH, either 50ng or 100ng) ANOVAs were performed at each time point of blood sampling. Due to missing samples at certain time points because of insufficient blood collection or problems in the assays, we were unable to perform repeated measures ANOVAs in this experiment. In Experiment 2, Stress Group (acute or repeated stress) × Drug Treatment (vehicle or CRH) ANOVAs were carried out for behavior in the EPM. The significance levels in all tests were set at p≤0.05.
Acknowledgments
This work was supported by NIMH 5F31MH069071 to AJ and NIMH 067651 to SB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamec RE, McKay D. Amygdala kindling, anxiety, and corticotrophin releasing factor (CRF) Physiol Behav. 1993;54(3):423–31. doi: 10.1016/0031-9384(93)90230-d. [DOI] [PubMed] [Google Scholar]
- Akana SF, Chu A, Soriano L, Dallman MF. Corticosterone exerts site-specific and state-dependent effects in prefrontal cortex and amygdala on regulation of adrenocorticotropic hormone, insulin and fat depots. J Neuroendocrinol. 2001;13(7):627–37. doi: 10.1046/j.1365-2826.2001.00676.x. [DOI] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nature Neurscience. 2005;8(3):365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Bagley, Moghaddam Temporal dynamics of glutamate efflux in the prefrontal cortex and in the hippocampus following repeated stress: effects of pretreatment with saline or diazepam. Neuroscience. 1997;77(1):65–73. doi: 10.1016/s0306-4522(96)00435-6. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Smith-Roe S, Newman SM, Grigoriadis DE, Kalin NH. Reduction of stress-induced behavior by antagonism of corticotropin-releasing hormone 2 (CRH2) receptors in lateral septum or CRH1 receptors in amygdala. Journal of Neuroscience. 2002;22(7):2926–2935. doi: 10.1523/JNEUROSCI.22-07-02926.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin HA, Rassnick S, Rivier J, Koob GF, Britton KT. CRF antagonist reverses the “anxiogenic” response to ethanol withdrawal in the rat. Psychopharmacology (Berl) 1991;103(2):227–32. doi: 10.1007/BF02244208. [DOI] [PubMed] [Google Scholar]
- Baldwin AE, Sadeghian K, Kelley AE. Appetitive instrumental learning requires coincident activation of NMDA and dopamine D1 receptors within the medial prefrontal cortex. J Neurosci. 2002;22(3):1063–71. doi: 10.1523/JNEUROSCI.22-03-01063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–57. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Meaney MJ. Hypothalamic-pituitary-adrenal function in chronic intermittently cold-stressed neonatally handled and non handled rats. J Neuroendocrinol. 1995;7:97–108. doi: 10.1111/j.1365-2826.1995.tb00672.x. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Dallman MF. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84 (4):1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Viau V, Chu A, Soriano L, Meijer OC, Dallman MF. A cholecystokinin-mediated pathway to the paraventricular thalamus is recruited in chronically stressed rats and regulates hypothalamic-pituitary-adrenal function. Journal of Neuroscience. 2000;20 (14):5564–5573. doi: 10.1523/JNEUROSCI.20-14-05564.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S, Huber R, Nowak N, Trotter P. Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. Journal of Neuroendocrinology. 2002;14:403–410. doi: 10.1046/j.0007-1331.2002.00792.x. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Huber R, Lazar E, Pych L, Vining C. Chronic stress alters behavior in the conditioned defensive burying test: role of the posterior paraventricular thalamus. Pharmacol Biochem Behav. 2003;76(2):343–9. doi: 10.1016/j.pbb.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20(11):4320–4. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruuijnzeel AW, Stam R, Wiegant VM. Effect of a benzodiazepine receptor agonist and corticotropin-releasing hormone receptor antagonists on long-term foot-shock-induced increase in defensive withdrawal behavior. Psychopharmacology (Berl) 2001;158(2):132–9. doi: 10.1007/s002130100863. [DOI] [PubMed] [Google Scholar]
- Calvo N, Volosin M. Glucocorticoid and mineralocorticoid receptors are involved in the facilitation of anxiety-like response induced by restraint. Neuroendocrinology. 2001;73(4):261–71. doi: 10.1159/000054643. [DOI] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress-and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2003;168(1–2):66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Chadda R, Devaud LL. Differential effects of mild repeated restraint stress on behaviors and GABA(A) receptors in male and female rats. Pharmacol Biochem Behav. 2005;81(4):854–63. doi: 10.1016/j.pbb.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15(10):6340–50. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde F, Maire-Lepoivre E, Audinat E, Crepel F. Afferent connections of the medial frontal cortex of the rat. II. Cortical and subcortical afferents. J Comp Neurol. 1995;352(4):567–93. doi: 10.1002/cne.903520407. [DOI] [PubMed] [Google Scholar]
- Crane JW, Ebner K, Day TA. Medial prefrontal cortex suppression of the hypothalamic-pituitary-adrenal axis response to a physical stressor, systemic delivery of interleukin-1beta. Eur J Neurosci. 2003;17(7):1473–81. doi: 10.1046/j.1460-9568.2003.02568.x. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Olschowka JA, Diz DI, Jacobowitz DM. Behavioral investigation of the coexistence of substance P, corticotropin releasing factor, and acetylcholinesterase in lateral dorsal tegmental neurons projecting to the medial frontal cortex of the rat. Peptides. 1985;6(5):891–901. doi: 10.1016/0196-9781(85)90320-1. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Helmreich DL, Watson SJ. Fos expression in forebrain afferents to the hypothalamic paraventricular nucleus following swim stress. J Comp Neurol. 1996;368(1):88–99. doi: 10.1002/(SICI)1096-9861(19960422)368:1<88::AID-CNE6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Theobald DE, Bouger P, Chudasama Y, Cardinal RN, Robbins TW. Cortical cholinergic function and deficits in visual attentional performance in rats following 192 IgG-saporin-induced lesions of the medial prefrontal cortex. Cereb Cortex. 2004;14(8):922–32. doi: 10.1093/cercor/bhh052. [DOI] [PubMed] [Google Scholar]
- Daniels WM, Richter L, Stein DJ. The effects of repeated intra-amygdala CRF injections on rat behavior and HPA axis function after stress. Metab Brain Dis. 2004;19(1–2):15–23. doi: 10.1023/b:mebr.0000027413.42946.61. [DOI] [PubMed] [Google Scholar]
- Deak T, Nguyen KT, Ehrlich A, Watkins L, Spencer R, Maier S, Licinio J, Wong M, Chrousos G, Webster E, Gold P. The impact of the nonpeptide corticotropin releasing hormone antagonist antalarmin on behavioral and endocrine responses to stress. Endocrinology. 1999;140:79–86. doi: 10.1210/endo.140.1.6415. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13(9):3839–47. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Rev. 2001;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, File SE. Corticotropin-releasing factor has an anxiogenic action in the social interaction test. Horm Behav. 1987;21(2):193–202. doi: 10.1016/0018-506x(87)90044-4. [DOI] [PubMed] [Google Scholar]
- Edwards AV, Jones CT. Autonomic control of adrenal function. J Anat. 1993;183 (Pt 2):291–307. [PMC free article] [PubMed] [Google Scholar]
- Engeland WC. Functional innervation of the adrenal cortex by the splanchnic nerve. Horm Metab Res. 1998;30(6–7):311–4. doi: 10.1055/s-2007-978890. [DOI] [PubMed] [Google Scholar]
- Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci. 2001;19(20):RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2001;158(4):360–5. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Espejo EF. Selective dopamine depletion within the medial prefrontal cortex induces anxiogenic-like effects in rats placed on the elevated plus maze. Brain Res. 1997;762(1–2):281–4. doi: 10.1016/s0006-8993(97)00593-3. [DOI] [PubMed] [Google Scholar]
- Figueiredo HF, Bruestle A, Bodie B, Dolgas CM, Herman JP. The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. Eur J Neurosci. 2003;18(8):2357–64. doi: 10.1046/j.1460-9568.2003.02932.x. [DOI] [PubMed] [Google Scholar]
- Finlay JM, Jedema HP, Rabinovic AD, Mana MJ, Zigmond MJ, Sved AF. Impact of corticotropin-releasing hormone on extracellular norepinephrine in prefrontal cortex after chronic cold stress. J Neurochemistry. 1997;69:144–150. doi: 10.1046/j.1471-4159.1997.69010144.x. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Flugge G. Modulation of binding sites for corticotropin-releasing hormone by chronic psychosocial stress. Psychoneuroendocrinology. 1995;20(1):33–51. doi: 10.1016/0306-4530(94)e0006-u. [DOI] [PubMed] [Google Scholar]
- Funk D, Li Z, Shaham Y, Le AD. Effect of blockade of corticotropin-releasing factor receptors in the median raphe nucleus on stress-induced c-fos mRNA in the rat brain. Neuroscience. 2003;122(1):1–4. doi: 10.1016/j.neuroscience.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Garcia A, Marti O, Valles A, Dal-Zotto S, Armario A. Recovery of the hypothalamic-pituitary-adrenal response to stress. Effect of stress intensity, stress duration and previous stress exposure. Neuroendocrinology. 2000;72(2):114–125. doi: 10.1159/000054578. [DOI] [PubMed] [Google Scholar]
- Gonzalez LE, Rujano M, Tucci S, Paredes D, Silva E, Alba G, Hernandez L. Medial prefrontal transection enhances social interaction. I: behavioral studies. Brain Res. 2000;887(1):7–15. doi: 10.1016/s0006-8993(00)02931-0. [DOI] [PubMed] [Google Scholar]
- Gregus A, Wintink AJ, Davis AC, Kalynchuk LE. Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behavior in male rats. Behav Brain Res. 2005;156(1):105–14. doi: 10.1016/j.bbr.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Grissom N, Iyer V, Vining C, Bhatnagar S. The physical context of previous stress exposure modifies hypothalamic-pituitary-adrenal responses to a subsequent homotypic stress. Horm Behav. 2007;51(1):95–103. doi: 10.1016/j.yhbeh.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Habib KE, Weld KP, Rice KC, Pushkas J, Champoux M, Listwak S, Webster EL, Atkinson AJ, Schulkin J, Contoreggi C, Chrousos GP, McCann SM, Suomi SJ, Higley JD, Gold PW. Oral administration of a corticotropin-releasing hormone receptor antagonist significantly attenuates behavioral, neuroendocrine, and autonomic responses to stress in primates. Proc Natl Acad Sci. 2000;97(11):6079–84. doi: 10.1073/pnas.97.11.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Schmid MJ, LoPresti ML, Watkins LR, Maier SF. The role of corticotropin-releasing hormone in the dorsal raphe nucleus in mediating the behavioral consequences of uncontrollable stress. J Neurosci. 2002;22(3):1020–6. doi: 10.1523/JNEUROSCI.22-03-01020.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27(6):555–79. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Heinrichs S, Lapsansky J, Lovenberg T, DeSouza E, Chalmers D. Corticotropin releasing factor CRF-1 but not CRF-2 receptors mediate anxiogenic-like behavior. Regul Pept. 1997;71:15–21. doi: 10.1016/s0167-0115(97)01005-7. [DOI] [PubMed] [Google Scholar]
- Herman JP, Prewitt CM, Cullinan WE. Neuronal circuit regulation of the hypothalamo-pituitary-adrenocortical stress axis. Crit Rev Neurobiol. 1996;10(3–4):371–94. doi: 10.1615/critrevneurobiol.v10.i3-4.50. [DOI] [PubMed] [Google Scholar]
- Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. J Comp Neurol. 1991;308(2):249–76. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- Jaferi A, Nowak N, Bhatnagar S. Negative feedback functions in chronically stressed rats: role of posterior paraventricular thalamus. Physiology & Behavior. 2003;78:365–373. doi: 10.1016/s0031-9384(03)00014-3. [DOI] [PubMed] [Google Scholar]
- Jaferi A, Bhatnagar S. Corticosterone can act at the posterior paraventricular thalamus to inhibit hypothalamic-pituitary-adrenal activity in animals that habituate to repeated stress. Endocrinology. 2006;147(10):4917–30. doi: 10.1210/en.2005-1393. [DOI] [PubMed] [Google Scholar]
- Kim KS, Han PL. Optimization of chronic stress paradigms using anxiety- and depression-like behavioral parameters. J Neurosci Res. 2006;83(3):497–507. doi: 10.1002/jnr.20754. [DOI] [PubMed] [Google Scholar]
- Korte SM, De Boer SF. A robust animal model of state anxiety: fear-potentiated behaviour in the elevated plus-maze. Eur J Pharmacol. 2003;463(1–3):163–75. doi: 10.1016/s0014-2999(03)01279-2. [DOI] [PubMed] [Google Scholar]
- Lacroix L, Spinelli S, Heidbreder CA, Feldon J. Differential role of the medial and lateral prefrontal cortices in fear and anxiety. Behav Neurosci. 2000;114(6):1119–1130. doi: 10.1037//0735-7044.114.6.1119. [DOI] [PubMed] [Google Scholar]
- Landgraf R. Neuropeptides and anxiety-related behavior. Endocr J. 2001;48(5):517–33. doi: 10.1507/endocrj.48.517. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Fletcher PJ, Shaham Y. The role of corticotropin-releasing factor in the median raphe nucleus in relapse to alcohol. J Neurosci. 2002;22(18):7844–9. doi: 10.1523/JNEUROSCI.22-18-07844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HY, Sawchenko PE. Hypothalamic effector neurons and extended circuitries activated in “neurogenic” stress: a comparison of footshock effects exerted acutely, chronically, and in animals with controlled glucocorticoid levels. J Comp Neurol. 1998;6;393(2):244–66. [PubMed] [Google Scholar]
- Makino S, Schulkin J, Smith MA, Pacak K, Palkovits M, Gold PW. Regulation of corticotropin-releasing hormone receptor messenger ribonucleic acid in the rat brain and pituitary by glucocorticoids and stress. Endocrinology. 1995;136(10):4517–25. doi: 10.1210/endo.136.10.7664672. [DOI] [PubMed] [Google Scholar]
- Martijena ID, Calvo N, Volosin M, Molina VA. Prior exposure to a brief restraint session facilitates the occurrence of fear in response to a conflict situation: behavioral and neurochemical correlates. Brain Res. 1997;752(1–2):136–42. doi: 10.1016/s0006-8993(96)01465-5. [DOI] [PubMed] [Google Scholar]
- Martins AP, Marras RA, Guimaraes FS. Anxiogenic effect of corticotropin-releasing hormone in the dorsal periaqueductal grey. Neuroreport. 1997;8(16):3601–4. doi: 10.1097/00001756-199711100-00036. [DOI] [PubMed] [Google Scholar]
- McElroy JF, Ward KA, Zeller KL, Jones KW, Gilligan PJ, He L, Lelas S. The CRF(1) receptor antagonist DMP696 produces anxiolytic effects and inhibits the stress-induced hypothalamic-pituitary-adrenal axis activation without sedation or ataxia in rats. Psychopharmacology (Berl) 2002;165(1):86–92. doi: 10.1007/s00213-002-1239-3. [DOI] [PubMed] [Google Scholar]
- Melia KR, Ryabinin AE, Schroeder R, Bloom FE, Wilson MC. Induction and habituation of immediate early gene expression in rat brain by acute and repeated restraint stress. J Neurosci. 1994;14(10):5929–38. doi: 10.1523/JNEUROSCI.14-10-05929.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonca FH, Guimaraes FS. Intra-hippocampal administration of cycloheximide attenuates the restraint-induced exploratory deficit of an elevated plus maze. Behav Brain Res. 1998;91(1–2):207–11. doi: 10.1016/s0166-4328(97)00129-0. [DOI] [PubMed] [Google Scholar]
- Morency MA, Stewart RJ, Beninger RJ. Circling behavior following unilateral microinjections of cocaine into the medial prefrontal cortex: dopaminergic or local anesthetic effect? J Neurosci. 1987;7(3):812–8. doi: 10.1523/JNEUROSCI.07-03-00812.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci. 1995;109(4):681–8. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego, CA: Academic Press, Harcourt Brace; 1997. [Google Scholar]
- Pellow S, Chopin P, File S, Briley M. Validation of open:closed arm entries in an elevated plus maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav. 1986;24(3):525–9. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Gregus A, Boudreau D, Kalynchuk LE. Sex and repeated restraint stress interact to affect cat odor-induced defensive behavior in adult rats. Brain Res. 2004;1027(1–2):161–72. doi: 10.1016/j.brainres.2004.08.056. [DOI] [PubMed] [Google Scholar]
- Pinto A, Jankowski M, Sesack SR. Projections from the paraventricular nucleus of the thalamus to the rat prefrontal cortex and nucleus accumbens shell: ultrastructural characteristics and spatial relationships with dopamine afferents. J Comp Neurol. 2003;459(2):142–55. doi: 10.1002/cne.10596. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Gehlert DR. Inhibition of the amygdala: key to pathological states? Ann N Y Acad Sci. 2003;985:263–72. doi: 10.1111/j.1749-6632.2003.tb07087.x. [DOI] [PubMed] [Google Scholar]
- Radulovic J, Sydow S, Spiess J. Characterization of native corticotropin-releasing factor receptor type 1 (CRFR1) in the rat and mouse central nervous system. J Neurosci Res. 1998;54(4):507–21. doi: 10.1002/(SICI)1097-4547(19981115)54:4<507::AID-JNR8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME. The contribution of cholinergic and dopaminergic afferents in the rat prefrontal cortex to learning, memory and attention. Psychobiology. 2000;28:238–47. [Google Scholar]
- Rangel A, Gonzalez LE, Villarroel V, Hernandez L. Anxiolysis followed by anxiogenesis relates to coping and corticosterone after medial prefrontal cortical damage in rats. Brain Research. 2003;992:96–103. doi: 10.1016/j.brainres.2003.08.038. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306(5695):443–7. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rivier CL, Grigoriasdis DE, Rivier JE. Role of corticotropin-releasing factor receptors type 1 and 2 in modulating the rat adrenocorticotropin response to stressors. Endocrinology. 2003;144(6):2396–403. doi: 10.1210/en.2002-0117. [DOI] [PubMed] [Google Scholar]
- Roland BL, Sawchenko PE. Local origins of some GABAergic projections to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol. 1993;332(1):123–43. doi: 10.1002/cne.903320109. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McReynolds JR, McGaugh JL. The basolateral amygdala interacts with the medial prefrontal cortex in regulating glucocorticoid effects on working memory impairment. J Neurosci. 2004;24(6):1385–92. doi: 10.1523/JNEUROSCI.4664-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Markowitsch HJ. Convergence of basolateral amygdaloid and mediodorsal thalamic projections in different areas of the frontal cortex in the rat. Brain Res Bull. 1983;10(5):607–22. doi: 10.1016/0361-9230(83)90029-1. [DOI] [PubMed] [Google Scholar]
- Sahuque LL, Kullberg EF, Mcgeehan AJ, Kinder JR, Hicks MP, Blanton MG, Janak PH, Olive MF. Anxiogenic and aversive effects of corticotropin-releasing factor (CRF) in the bed nucleus of the stria terminalis in the rat: role of CRF receptor subtypes. Psychopharmacology (Berl) 2006;186(1):122–32. doi: 10.1007/s00213-006-0362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvage M, Steckler T. Detection of corticotropin-releasing hormone receptor 1 immunoreactivity in cholinergic, dopaminergic and noradrenergic neurons of the murine basal forebrain and brainstem nuclei--potential implication for arousal and attention. Neuroscience. 2001;104(3):643–52. doi: 10.1016/s0306-4522(01)00137-3. [DOI] [PubMed] [Google Scholar]
- Shah AA, Treit D. Excitotoxic lesions of the medial PFC attenuate fear responses in the EPM, social interaction and shock probe burying tests. Brain Research. 2003;969:183–194. doi: 10.1016/s0006-8993(03)02299-6. [DOI] [PubMed] [Google Scholar]
- Shah AA, Treit D. Infusions of midazolam into the medial prefrontal cortex produce anxiolytic effects in the elevated plus-maze and shock-probe burying tests. Brain Res. 2004;996(1):31–40. doi: 10.1016/j.brainres.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Shah AA, Sjovold T, Treit D. Inactivation of the medial prefrontal cortex with the GABAA receptor agonist muscimol increases open-arm activity in the elevated plus-maze and attenuates shock-probe burying in rats. Brain Res. 2004;1028(1):112–5. doi: 10.1016/j.brainres.2004.08.061. [DOI] [PubMed] [Google Scholar]
- Smagin GN, Harris RB, Ryan DH. Corticotropin-releasing factor receptor antagonist infused into the locus coeruleus attenuates immobilization stress-induced defensive withdrawal in rats. Neurosci Lett. 1996;220(3):167–70. doi: 10.1016/s0304-3940(96)13254-7. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Buller KM, Day TA. Medial prefrontal cortex control of the paraventricular hypothalamic nucleus response to psychological stress: possible role of the bed nucleus of the stria terminalis. J Comp Neurol. 2005;481(4):363–76. doi: 10.1002/cne.20376. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. Lateralized effects of mPFC lesions on neuroendocrine and autonomic stress responses in rats. Journal of Neuroscience. 1999;19(7):2834–2840. doi: 10.1523/JNEUROSCI.19-07-02834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. Prefrontal cortical regulation of hypothalamic-pituitary-adrenal function in the rat and implications for psychopathology: side matters. Psychoneuroendocrinology. 2002;27:99–114. doi: 10.1016/s0306-4530(01)00038-5. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Kalin NH, Vanden Burgt JA, Sherman JE. Corticotropin-releasing factor modulates defensive-withdrawal and exploratory behavior in rats. Behav Neurosci. 1989;103(3):648–54. doi: 10.1037//0735-7044.103.3.648. [DOI] [PubMed] [Google Scholar]
- Terreberry RR, Neafsey EJ. The rat medial frontal cortex projects directly to autonomic regions of the brainstem. Brain Res Bull. 1987;19(6):639–49. doi: 10.1016/0361-9230(87)90050-5. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Carlsson K, Ekman R, Heilig M. Behavioral and endocrine adaptation, and up-regulation of NPY expression in rat amygdala following repeated restraint stress. Neuroreport. 1999;10(14):3003–7. doi: 10.1097/00001756-199909290-00024. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Differential effects of discrete subarea-specific lesions of the rat medial prefrontal cortex on amphetamine- and cocaine-induced behavioural sensitization. Cereb Cortex. 2000;10(5):488–98. doi: 10.1093/cercor/10.5.488. [DOI] [PubMed] [Google Scholar]
- Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, D-Alessio DA, Herman JP. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab. 2005;289(5):E823–8. doi: 10.1152/ajpendo.00122.2005. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EE, Pickel VM. Enkephalin terminals form inhibitory-type synapses on neurons in the rat nucleus locus coeruleus that project to the medial prefrontal cortex. Neuroscience. 1996;71(2):429–42. doi: 10.1016/0306-4522(95)00432-7. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt J, Chan R, Li H, Arias C, Prins G, Perrin M, Vale W, Sawchenko P. Distribution of mRNAs Encoding CRF Receptors in Brain and Pituitary of Rat and Mouse. Journal of Comparative Neurology. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51(1):32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Viau V, Sawchenko PE. Hypophysiotropic neurons of the paraventricular nucleus respond in spatially, temporally, and phenotypically differentiated manners to acute vs. repeated restraint stress: rapid publication. J Comp Neurol. 2002;445(4):293–307. doi: 10.1002/cne.10178. [DOI] [PubMed] [Google Scholar]
- Wall PM, Messier C. U-69,593 microinjection in the infralimbic cortex reduces anxiety and enhances spontaneous alternation memory in mice. Brain Res. 2000;856(1–2):259–80. doi: 10.1016/s0006-8993(99)01990-3. [DOI] [PubMed] [Google Scholar]
- Wiersma A, Konsman JP, Knollema S, Bohus B, Koolhaas JM. Differential effects of CRH infusion into the central nucleus of the amygdala in the Roman high-avoidance and low-avoidance rats. Psychoneuroendocrinology. 1998;23(3):261–74. doi: 10.1016/s0306-4530(97)00098-x. [DOI] [PubMed] [Google Scholar]