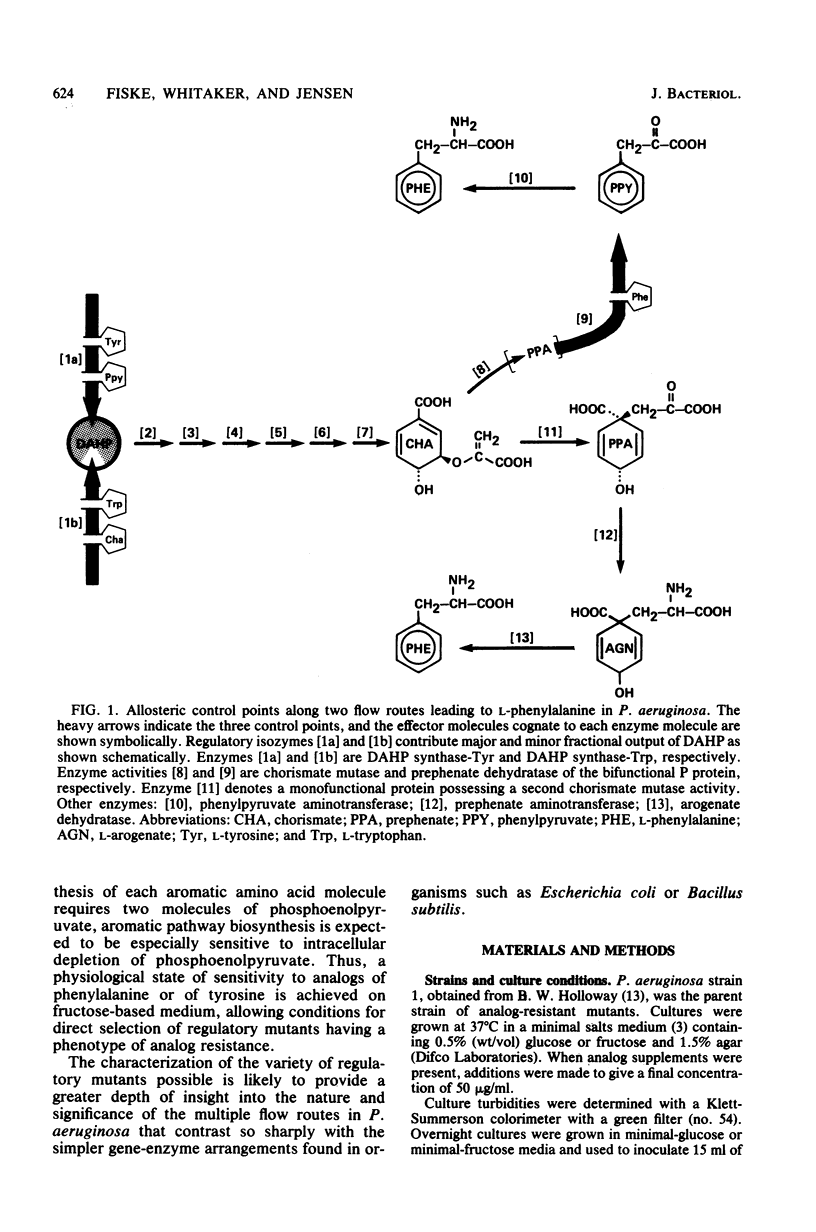
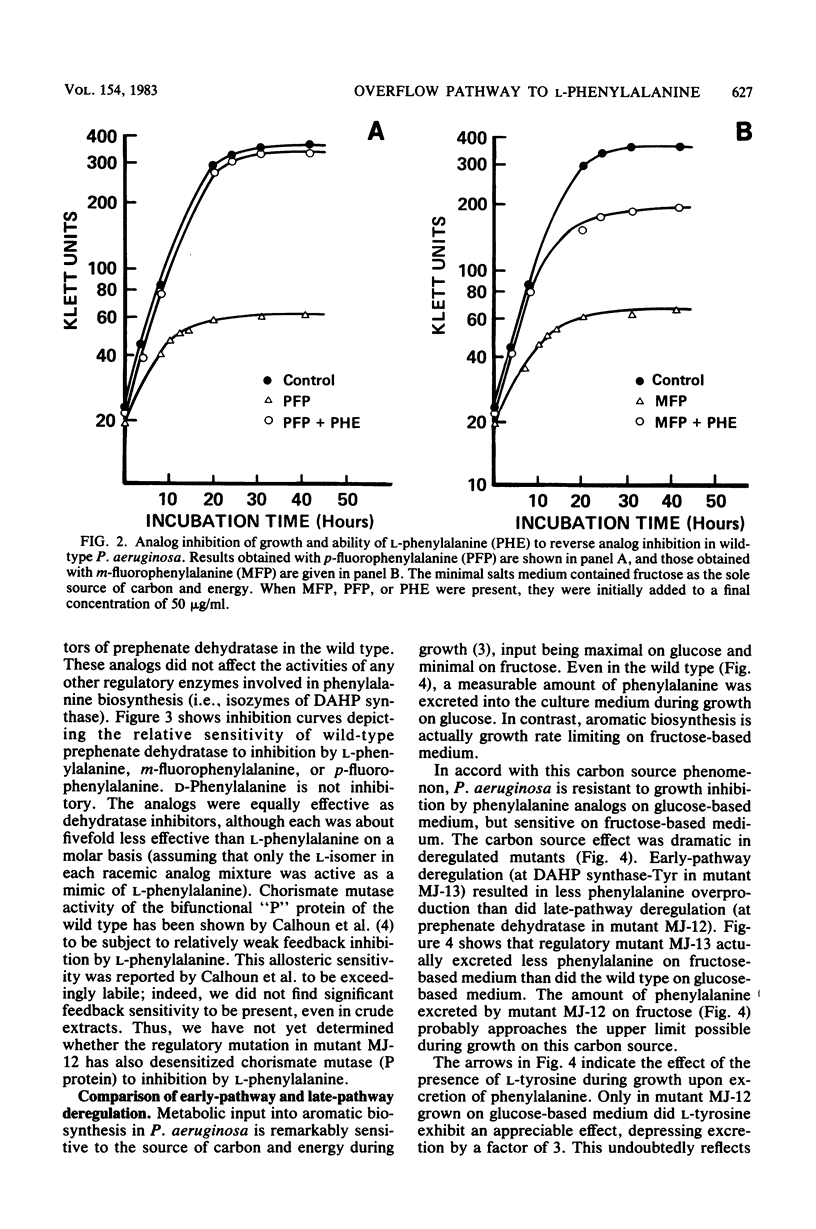
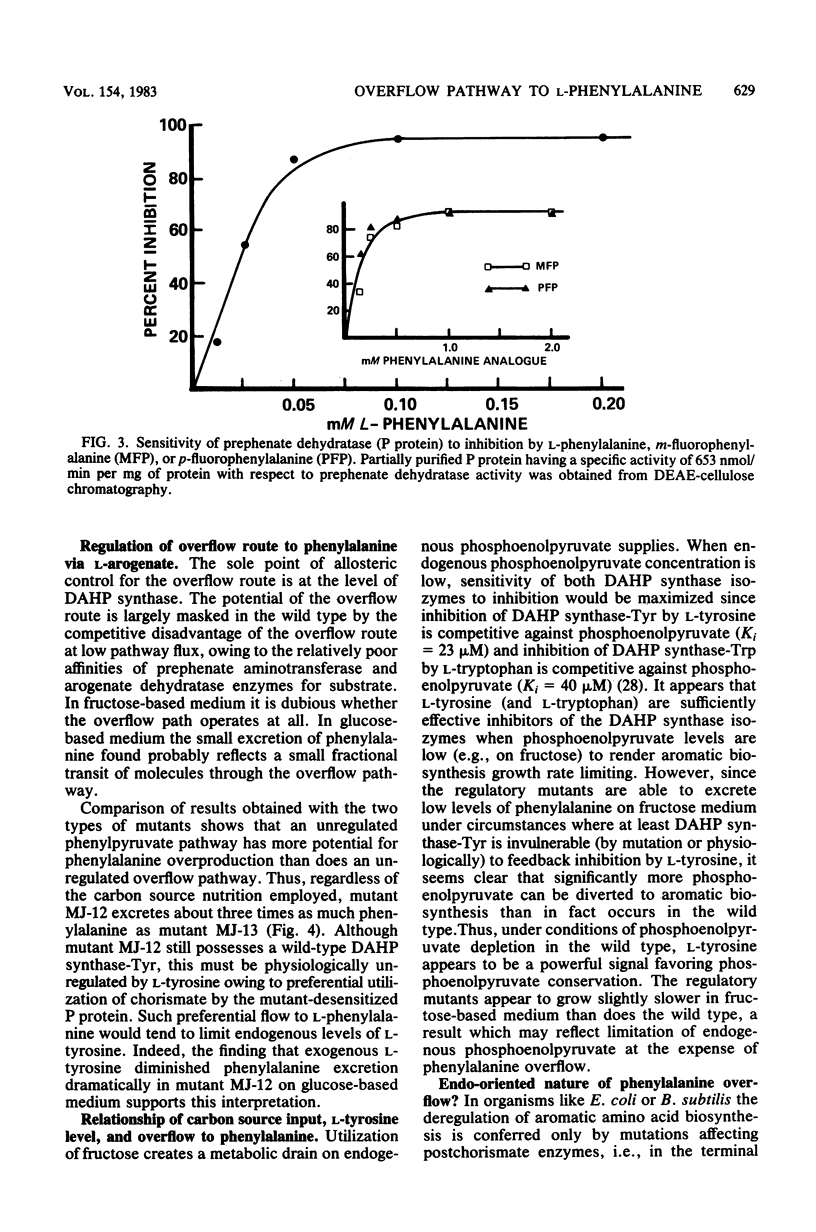
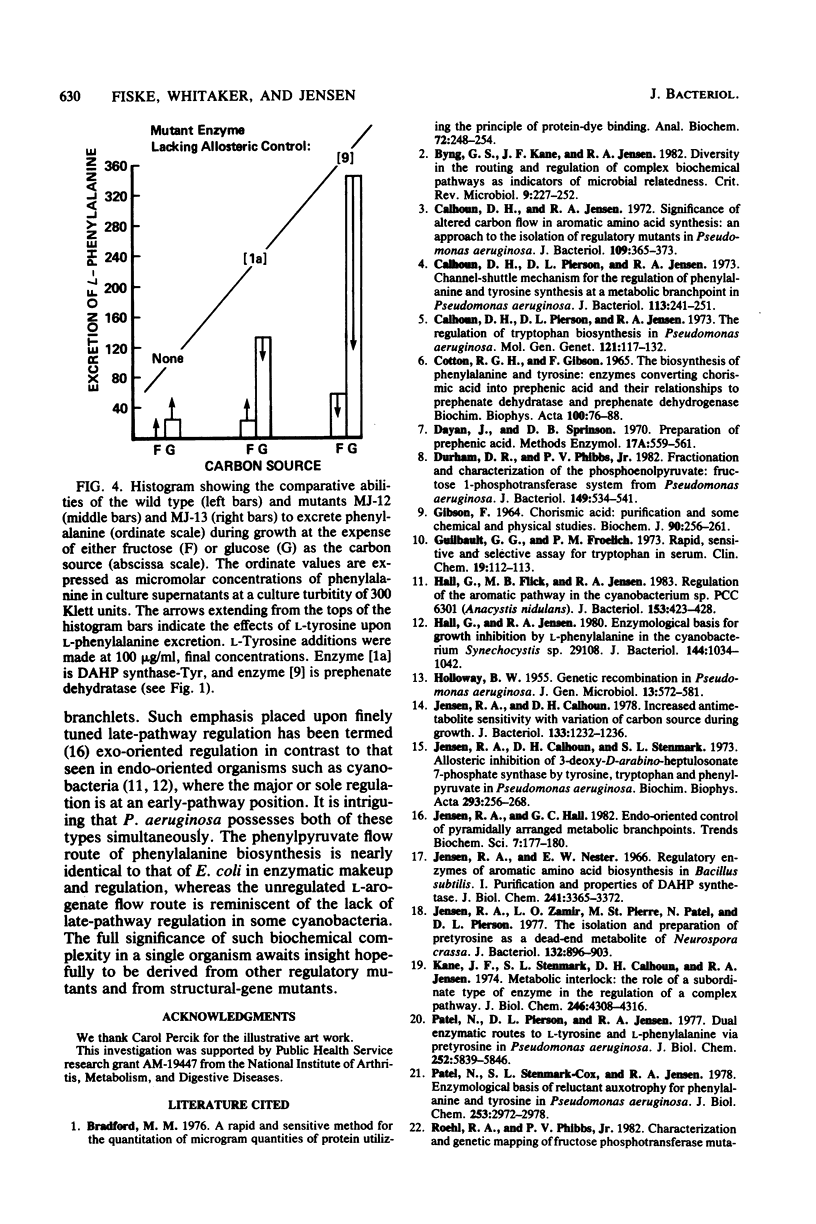
Abstract
Pseudomonas aeruginosa is representative of a large group of pseudomonad bacteria that possess coexisting alternative pathways to L-phenylalanine (as well as to L-tyrosine). These multiple flow routes to aromatic end products apparently account for the inordinate resistance of P. aeruginosa to end product analogs. Manipulation of carbon source nutrition produced a physiological state of sensitivity to p-fluorophenylalanine and m-fluorophenylalanine, each a specific antimetabolite of L-phenylalanine. Analog-resistant mutants obtained fell into two classes. One type lacked feedback sensitivity of prephenate dehydratase and was the most dramatic excretor of L-phenylalanine. The presence of L-tyrosine curbed phenylalanine excretion to one-third, a finding explained by potent early-pathway regulation of 3-deoxy-D-arabinoheptulosonate 7-phosphate (DAHP) synthase-Tyr (a DAHP synthase subject to allosteric inhibition by L-tyrosine). The second class of regulatory mutants possessed a completely feedback-resistant DAHP synthase-Tyr, the major species (greater than 90%) of two isozymes. Deregulation of DAHP synthase-Tyr resulted in the escape of most chorismate molecules produced into an unregulated overflow route consisting of chorismate mutase (monofunctional), prephenate aminotransferase, and arogenate dehydratase. In the wild type the operation of the overflow pathway is restrained by factors that restrict early-pathway flux. These factors include the highly potent feedback control of DAHP synthase isozymes by end products as well as the strikingly variable abilities of different carbon source nutrients to supply the aromatic pathway with beginning substrates. Even in the wild type, where all allosteric regulation in intact, some phenylalanine overflow was found on glucose-based medium, but not on fructose-based medium. This carbon source-dependent difference was much more exaggerated in each class of regulatory mutants.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Byng G. S., Kane J. F., Jensen R. A. Diversity in the routing and regulation of complex biochemical pathways as indicators of microbial relatedness. Crit Rev Microbiol. 1982 May;9(4):227–252. doi: 10.3109/10408418209104491. [DOI] [PubMed] [Google Scholar]
- COTTON R. G., GIBSON F. THE BIOSYNTHESIS OF PHENYLALANINE AND TYROSINE; ENZYMES CONVERTING CHORISMIC ACID INTO PREPHENIC ACID AND THEIR RELATIONSHIPS TO PREPHENATE DEHYDRATASE AND PREPHENATE DEHYDROGENASE. Biochim Biophys Acta. 1965 Apr 12;100:76–88. doi: 10.1016/0304-4165(65)90429-0. [DOI] [PubMed] [Google Scholar]
- Calhoun D. H., Jensen R. A. Significance of altered carbon flow in aromatic amino acid synthesis: an approach to the isolation of regulatory mutants in Pseudomonas aeruginosa. J Bacteriol. 1972 Jan;109(1):365–372. doi: 10.1128/jb.109.1.365-372.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun D. H., Pierson D. L., Jensen R. A. Channel-shuttle mechanism for the regulation of phenylalanine and tyrosine synthesis at a metabolic branch point in Pseudomonas aeruginosa. J Bacteriol. 1973 Jan;113(1):241–251. doi: 10.1128/jb.113.1.241-251.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun D. H., Pierson D. L., Jensen R. A. The regulation of tryptophan biosynthesis in Pseudomonas aeruginosa. Mol Gen Genet. 1973 Mar 1;121(2):117–132. doi: 10.1007/BF00277526. [DOI] [PubMed] [Google Scholar]
- Durham D. R., Phibbs P. V., Jr Fractionation and characterization of the phosphoenolpyruvate: fructose 1-phosphotransferase system from Pseudomonas aeruginosa. J Bacteriol. 1982 Feb;149(2):534–541. doi: 10.1128/jb.149.2.534-541.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson F. Chorismic acid: purification and some chemical and physical studies. Biochem J. 1964 Feb;90(2):256–261. doi: 10.1042/bj0900256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLOWAY B. W. Genetic recombination in Pseudomonas aeruginosa. J Gen Microbiol. 1955 Dec;13(3):572–581. doi: 10.1099/00221287-13-3-572. [DOI] [PubMed] [Google Scholar]
- Hall G. C., Flick M. B., Jensen R. A. Regulation of the aromatic pathway in the cyanobacterium Synechococcus sp. strain Pcc6301 (Anacystis nidulans). J Bacteriol. 1983 Jan;153(1):423–428. doi: 10.1128/jb.153.1.423-428.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall G. C., Jensen R. A. Enzymological basis for growth inhibition by L-phenylalanine in the cyanobacterium Synechocystis sp. 29108. J Bacteriol. 1980 Dec;144(3):1034–1042. doi: 10.1128/jb.144.3.1034-1042.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A., Calhoun D. H. Increased antimetabolite sensitivity with variation of carbon source during growth. J Bacteriol. 1978 Mar;133(3):1232–1236. doi: 10.1128/jb.133.3.1232-1236.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A., Calhoun D. H., Stenmark S. L. Allosteric inhibition of 3-deoxy-D-arabino-heptulosonate 7-phosphate synthetase by tyrosine, tryptophan and phenylpyruvate in Pseudomonas aeruginosa. Biochim Biophys Acta. 1973 Jan 12;293(1):256–268. doi: 10.1016/0005-2744(73)90398-7. [DOI] [PubMed] [Google Scholar]
- Jensen R. A., Nester E. W. Regulatory enzymes of aromatic amino acid biosynthesis in Bacillus subtilis. I. Purification and properties of 3-deoxy-D-arabino-heptulosonate 7-phosphate synthetase. J Biol Chem. 1966 Jul 25;241(14):3365–3372. [PubMed] [Google Scholar]
- Jensen R. A., Zamir L., Saint Pierre M., Patel N., Pierson D. L. Isolation and preparation of pretyrosine, accumulated as a dead-end metabolite by Neurospora crassa. J Bacteriol. 1977 Dec;132(3):896–903. doi: 10.1128/jb.132.3.896-903.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane J. F., Stenmark S. L., Calhoun D. H., Jensen R. A. Metabolic interlock. The role of the subordinate type of enzyme in the regulation of a complex pathway. J Biol Chem. 1971 Jul 10;246(13):4308–4316. [PubMed] [Google Scholar]
- Patel N., Pierson D. L., Jensen R. A. Dual enzymatic routes to L-tyrosine and L-phenylalanine via pretyrosine in Pseudomonas aeruginosa. J Biol Chem. 1977 Aug 25;252(16):5839–5846. [PubMed] [Google Scholar]
- Patel N., Stenmark-Cox S. L., Jensen R. A. Enzymological basis of reluctant auxotrophy for phenylalanine and tyrosine in Pseudomonas aeruginosa. J Biol Chem. 1978 May 10;253(9):2972–2978. [PubMed] [Google Scholar]
- Roehl R. A., Phibbs P. V., Jr Characterization and genetic mapping of fructose phosphotransferase mutations in Pseudomonas aeruginosa. J Bacteriol. 1982 Mar;149(3):897–905. doi: 10.1128/jb.149.3.897-905.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer M. H., Baumann P., Baumann L., Berman S. M., Cánovas J. L., Berman R. H. Pathways of D-fructose catabolism in species of Pseudomonas. Arch Microbiol. 1977 Feb 4;112(1):49–55. doi: 10.1007/BF00446653. [DOI] [PubMed] [Google Scholar]
- Shapiro C. L., Jensen R. A., Wilson K. A., Bowen J. R. An assay for activity of arogenate dehydratase base upon the selective oxidation of arogenate. Anal Biochem. 1981 Jan 1;110(1):27–30. doi: 10.1016/0003-2697(81)90106-8. [DOI] [PubMed] [Google Scholar]
- WONG P. W., O'FLYNN M. E., INOUYE T. MICROMETHODS FOR MEASURING PHENYLALANINE AND TYROSINE IN SERUM. Clin Chem. 1964 Dec;10:1098–1104. [PubMed] [Google Scholar]
- Whitaker R. J., Byng G. S., Gherna R. L., Jensen R. A. Comparative allostery of 3-deoxy-D-arabino-heptulosonate 7-phosphate synthetase as an indicator of taxonomic relatedness in pseudomonad genera. J Bacteriol. 1981 Feb;145(2):752–759. doi: 10.1128/jb.145.2.752-759.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker R. J., Byng G. S., Gherna R. L., Jensen R. A. Diverse enzymological patterns of phenylalanine biosynthesis in pseudomonads are conserved in parallel with deoxyribonucleic acid homology groupings. J Bacteriol. 1981 Aug;147(2):526–534. doi: 10.1128/jb.147.2.526-534.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker R. J., Fiske M. J., Jensen R. A. Pseudomonas aeruginosa possesses two novel regulatory isozymes of 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase. J Biol Chem. 1982 Nov 10;257(21):12789–12794. [PubMed] [Google Scholar]
- Whitaker R. J., Gaines C. G., Jensen R. A. A multispecific quintet of aromatic aminotransferases that overlap different biochemical pathways in Pseudomonas aeruginosa. J Biol Chem. 1982 Nov 25;257(22):13550–13556. [PubMed] [Google Scholar]


