Abstract
T cell receptor (TCR)-driven activation of helper T cells induces a rapid polarization of their cytoskeleton towards bound antigen presenting cells (APCs). We have identified the Fyn- and SLP-76–associated protein Fyb/SLAP as a new ligand for Ena/ vasodilator-stimulated phosphoprotein (VASP) homology 1 (EVH1) domains. Upon TCR engagement, Fyb/SLAP localizes at the interface between T cells and anti-CD3–coated beads, where Evl, a member of the Ena/VASP family, Wiskott-Aldrich syndrome protein (WASP) and the Arp2/3 complex are also found. In addition, Fyb/SLAP is restricted to lamellipodia of spreading platelets. In activated T cells, Fyb/SLAP associates with Ena/VASP family proteins and is present within biochemical complexes containing WASP, Nck, and SLP-76. Inhibition of binding between Fyb/SLAP and Ena/VASP proteins or WASP and the Arp2/3 complex impairs TCR-dependent actin rearrangement, suggesting that these interactions play a key role in linking T cell signaling to remodeling of the actin cytoskeleton.
Keywords: Evl, EVH1, WASP, Nck, actin cytoskeleton
Introduction
T cells play a central role in immune responses mounted against pathogens and cancer. Dramatic cytoskeletal reorganization during T cell activation is a prerequisite for a specific immune response (for references see Penninger and Crabtree 1999). Upon binding to an antigen-presenting cell (APC), the cytoskeleton of a T cell rapidly polarizes. The accumulation of actin in a tight collar at the T cell–APC interface is thought to stabilize a continuous contact between T cells and APCs (Ryser et al. 1982; Valitutti et al. 1995). The formation of this tight contact is accompanied by the reorientation of the microtubule-organizing center towards the contact site to ensure a polarized release of cytokines or cytotoxic factors (Geiger et al. 1982; Kupfer et al. 1987, Kupfer et al. 1991). Although actin remodeling is thought to be essential for T cell activation (see Penninger and Crabtree 1999), it is not known how T cell receptor (TCR) signaling is linked to the rearrangement of the actin cytoskeleton.
Many of the signaling events that occur in T cells in response to TCR ligation have been described. TCR engagement induces the activation of tyrosine kinases (Fyn, Lck, and ZAP-70), leading to the phosphorylation of several components of the T cell signal transduction pathway (for reviews see Weiss and Littman 1994; Clements et al. 1999). Among the phosphorylated proteins are the 36–38 kD adaptor protein linker for activation of T cells (LAT) (Zhang et al. 1998a), SH2 domain–containing leukocyte protein of 76 kD (SLP-76) (Jackman et al. 1995), and a protein of 120–130 kD named Fyn-binding protein (Fyb) or SLP-76–associated protein of 130 kD (SLAP-130), which was originally identified independently as an SH2-binding protein of Fyn and SLP-76, respectively (Da Silva et al. 1997; Musci et al. 1997). In addition, phosphorylated SLP-76 binds to the SH2 domain of Vav (Tuosto et al. 1996), a guanine nucleotide exchange factor for Rho GTPases (Olson et al. 1996; Crespo et al. 1997; Han et al. 1997). Although Fyb/SLAP has been shown to function in TCR-dependent changes in gene expression, such as upregulation of IL-2, its precise role in T cell activation has not been determined (Da Silva et al. 1997; Musci et al. 1997; Raab et al. 1999).
Impaired T cell function is a hallmark of the Wiskott-Aldrich syndrome, which is also characterized by thrombocytopenia, eczema, and abnormally shaped platelets (for references see Nonoyama and Ochs 1998). T cells from Wiskott-Aldrich syndrome patients show characteristic cytoskeletal defects, which are due to mutations in the gene coding for Wiskott-Aldrich syndrome protein (WASP) (Derry et al. 1994). WASP is also linked to the actin cytoskeleton through binding to the Arp2/3 complex (Machesky and Insall 1998), which localizes to sites of actin assembly such as the lamellipodia or the actin tails of Listeria monocytogenes (Kelleher et al. 1995; Machesky et al. 1997; Welch et al. 1997a,Welch et al. 1997b). In vitro, this complex promotes actin nucleation, which is enhanced by the Listeria monocytogenes virulence factor ActA (Welch et al. 1998), the sole protein of this intracellular pathogen that is required for the initiation of actin polymerization at the bacterial surface leading to intracellular motility (Domann et al. 1992; Kocks et al. 1992). In mammalian cells, overexpression of COOH-terminal fragments of WASP family proteins leads to delocalization of the Arp2/3 complex, resulting in the complete loss of lamellipodia and stress fibers (Machesky and Insall 1998). Moreover, ectopic expression of Scar1, a member of the WASP family, in cells completely blocks Listeria-induced actin tail formation (May et al. 1999). These results suggest that the Arp2/3 complex as well as WASP family proteins act in concert to regulate the dynamics of the actin cytoskeleton.
The actin-based motility of Listeria monocytogenes is currently one of the best model systems for dissecting actin dynamics. Among the proteins thought to play a critical role in Listeria motility as well as in cellular processes requiring dynamic actin rearrangement are those of the Ena/vasodilator-stimulated phosphoprotein (VASP) family (Chakraborty et al. 1995; Gertler et al. 1996; Aszódi et al. 1999; Lanier et al. 1999; Laurent et al. 1999). By binding to a specific proline-rich motif (E/DFPPPPXDEE) repeated fourfold within ActA, the Ena/VASP homology 1 (EVH1) domain of VASP and Mena targets these proteins to the bacterial surface (Gertler et al. 1996; Niebuhr et al. 1997). Related EVH1-binding sites are also present in the focal contact proteins zyxin (Sadler et al. 1992; Gertler et al. 1996; Macalma et al. 1996) and vinculin (Brindle et al. 1996; Reinhard et al. 1996). Several lines of evidence suggest that proteins of the Ena/VASP family function as regulators of the actin cytoskeleton. First, Listeria monocytogenes require Ena/VASP proteins for efficient motility. Listeria expressing mutated versions of ActA, which lack EVH1-binding sites, fail to recruit Ena/VASP family proteins and move at a reduced speed (Smith et al. 1996; Niebuhr et al. 1997). In addition, in cell-free extracts the presence of Ena/VASP proteins enhances Listeria motility (Loisel et al. 1999). Second, VASP binds in vitro to F-actin through the EVH2 domain (Reinhard et al. 1992; Bachmann et al. 1999; Hüttelmaier et al. 1999; Laurent et al. 1999). Third, VASP localizes at the front of spreading lamellipodia (Rottner et al. 1999). Fourth, expression of the neuronal-specific isoform of Mena induces the formation of actin-rich cell surface projections in fibroblasts. Furthermore, Mena is highly concentrated at the distal tips of growth cone filopodia, and genetic analyses indicate that Mena and its Drosophila homologue Ena are required for axon guidance (Gertler et al. 1996; Lanier et al. 1999; Wills et al. 1999). Moreover, VASP and Mena are ligands for profilin (Reinhard et al. 1995; Gertler et al. 1996; Kang et al. 1997), an actin monomer binding protein that, under favorable conditions, can stimulate the polymerization of actin (Pantaloni and Carlier 1993). A physiological role for this interaction is supported by genetic evidence indicating that Mena and profilin function in concert during the actin-driven process of neurulation (Lanier et al. 1999).
In this report, we describe the identification and characterization of Fyb/SLAP as a new ligand for the EVH1 domain of Ena/VASP proteins. In contrast to other known EVH1 ligands, Fyb/SLAP localizes exclusively to the lamellipodia of spreading platelets. Fyb/SLAP is concentrated at the contact sites between Jurkat T cells with anti-CD3–coated beads, where it colocalizes with F-actin, Ena/VASP proteins, Vav, WASP, and the Arp2/3 complex. Inhibition of the binding between Ena/VASP family proteins and Fyb/SLAP or between WASP and the Arp2/3 complex abolishes actin remodeling upon TCR ligation. We propose a model in which Fyb/SLAP, Ena/VASP proteins, and the Arp2/3 complex participate in a regulated complex that links TCR signaling to actin remodeling during T cell activation.
Materials and Methods
Molecular Cloning and Sequence Analysis
Screening of a mouse embryo (d16) expression library λExlox (Novagen) was carried out using polyclonal antibodies raised against the synthetic peptide SFEFPPPPTDEELRL derived from ActA (Niebuhr et al. 1997). The expressed sequence tag (EST) clone (IMAGE clone ID 221953; RZPD IMAGp998F02441) was obtained from the Resource Centre of the German Human Genome Project (RZPD), Berlin, Germany. Complete RNA was purified from HL60 cells using Trizol reagent (GIBCO BRL) according to the manufacturer's instructions. Reverse transcription (RT)-PCR was carried out using the First strand synthesis kit according to the provided instructions (Amersham Pharmacia Biotech). Sequencing and polymerase chain reactions were performed according to standard procedures.
Fusion Proteins and Antibody Production
Fragments encoding amino acids 1–339 (Fyb/SLAP I), 341–598 (Fyb/SLAP III), 548–783 (Fyb/SLAP IX) of Fyb/SLAP1 (numbering according to Da Silva et al. 1997; Musci et al. 1997), and the NH2 terminus of murine WASP (amino acids 1–256; Derry et al. 1995) were generated by PCR and cloned into the pGEX-2TK, pGEX-6P1, or pGEX-5X3 vectors (Amersham Pharmacia Biotech). Fusion proteins were purified on glutathione–Sepharose (Amersham Pharmacia Biotech) or glutathione-agarose (Pierce). Immobilized Fyb/SLAP III glutathione S-transferase (GST) was digested with thrombin (Amersham Pharmacia Biotech) and used to raise the polyclonal rabbit antiserum #51 (Eurogentec Bel S.A.), which was affinity-purified using Fyb/SLAP III GST Sepharose. The antiserum #81 was raised against the synthetic peptide 656-LKGKDDRKKSIREKPKV-672 derived from Fyb/SLAP2 (see Fig. 1 D) and affinity-purified using the same peptide immobilized on EAH-Sepharose (Amersham Pharmacia Biotech). The Arp3 polyclonal antibody B7I was raised against the synthetic peptide 342-(C)TVDARLKLSEELSGGRLKPK-361 derived from bovine Arp3 sequence (sequence data available from EMBL/GenBank/DDBJ under accession no. P32391) and affinity-purified using the same peptide immobilized on EAH-Sepharose (Pharmacia). The proteins Fyb/SLAP I, III, IX GST prepared from pGEX-6P1 constructs, digested with Prescission™ protease (Pharmacia), and GST-WASP were used to raise mAbs as described previously (Niebuhr et al. 1998). A detailed analysis of the mAbs against Fyb/SLAP will be presented elsewhere (Krause, M., and J. Wehland, manuscript in preparation).
Figure 1.
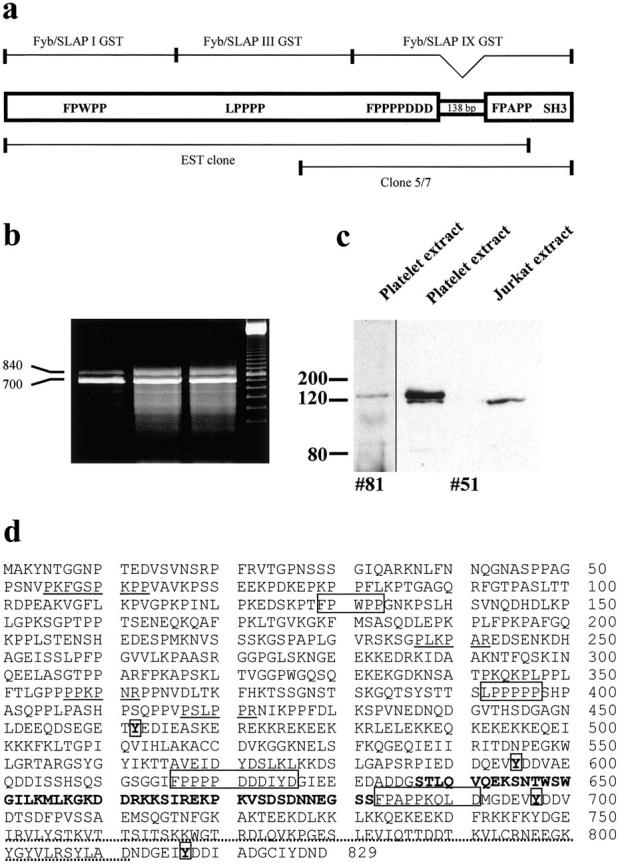
(a) Schematic representation of the full-length Fyb/SLAP2, the EST clone (IMAGE clone ID 221953; RZPD IMAGp998F02441), the murine clone 5/7, and the three GST fusion proteins encoding for different regions of Fyb/SLAP2 (Fyb/SLAP I, III, and IX). Potential EVH1 domain–binding sites, SH3-like domain, and the 138-bp insertion are also shown. (b) RT-PCR of the COOH terminus of Fyb/SLAP as obtained from HL60 cells shows two bands running at ∼700 and 840 bp (lanes 1–3, RT-PCRs of three independent experiments; lane 4, 100 bp marker). (c) Immunodetection of Fyb/SLAP in extracts of human platelets and Jurkat T cells. The affinity-purified polyclonal antibody #51 specifically reacts with a single band on Jurkat T cells lysate and with two bands on platelet extract, whereas the polyclonal antibody #81 reacts with a single band on platelet extract. (d) Amino acid sequence and structural motifs of Fyb/SLAP2. Single letter amino acids sequence of Fyb/SLAP2. Boxes represent potential EVH1 domain–binding sites, stretch of bold-faced amino acids represents the insertion not present in Fyb/SLAP1. Potential SH3-binding regions (underlined), potential tyrosine phosphorylation sites (bold and boxed), and the SH3-like domain (dotted) are shown. The sequence of Fyb/SLAP2 is available from EMBL/GenBank/DDBJ under accession no. AF198052.
Biochemistry
The extracts of Jurkat E6-1 cells were prepared in two different ways. For the binding assays, Jurkat E6-1 cells were stimulated for 1 min with 0.2 mM Na3VO4, 8 mM H2O2 (pervanadate), which mimics the effect of TCR ligation in Jurkat cells (Secrist et al. 1993; Motto et al. 1994). Jurkat RIPA lysates were prepared in ice-cold RIPA buffer (25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, and 0.1% SDS) supplemented with 1 mM PMSF. Immunoprecipitations were carried out according to standard protocols, using the VASP mAb IE226C2 (Abel et al. 1996) and the WASP antiserum Fus3 (Symons et al. 1996). As negative controls, anti-ActA mAbs (358C1) (Niebuhr et al. 1993) and a rabbit nonimmune serum were used. For immunoprecipitation, 0.2 ml of Jurkat RIPA lysate (derived from 1.6 × 107 cells) was used.
For dissection of the complex Jurkat E6-1 cells were starved overnight in RPMI 1640 (GIBCO BRL) supplemented with 1% (vol/vol) FCS and 2 mM l-glutamine (GIBCO BRL). The next day, the Jurkat cells were washed two times with ice-cold RPMI 1640 media without supplements and resuspended at 5 × 107 cells/ml in ice-cold DME/Hepes (GIBCO BRL). OKT3 anti-CD3 antibodies (CRL-8001; ATCC) were added to a final concentration of 3 μg/ml for stimulation, or no antibodies as control, and cells were kept on ice for 15 min. After quick pelleting, stimulated and control cells were resuspended and incubated in DME/Hepes containing 15 μg/ml goat anti–mouse IgG antibodies at 37°C for 2 min. Jurkat NP-40 lysates were prepared at 1 × 108 cells/ml in ice-cold NP-40 lysis buffer (1% [vol/vol] NP-40, 150 mm NaCl, 10 mM Tris-Cl, pH 7.8) supplemented with 60 μg/ml chymostatin, 10 μg/ml pepstatin, 5 μg/ml leupeptin, 2 μg/ml aprotinin, 2 mM Pefabloc (Roche), 1 mM Na3VO4, and 10 mM NaF. For immunoprecipitations, affinity columns were prepared with the WASP mAb 67B4, and as control, with the anti-ActA 358C1 mAbs (Niebuhr et al. 1993) using CNBr–Sepharose (Pharmacia). Immunoprecipations were carried out according to standard protocols, using 0.2 ml of Jurkat NP-40 lysates derived from 2 × 107 cells. Immunoprecipitates were resolved by SDS-PAGE. As positive control, Jurkat E6-1 lysates were used. Blots were probed with the following antibodies to: Fyb/SLAP #51, Nck (Transduction Labs), SLP-76 (Transduction Labs), zyxin 164D4 (Krause et al., manuscript in preparation), and phosphotyrosine 4G10 (Upstate Biotechnology), and processed using ECL or ECL plus enhanced chemiluminescence detection kits (Amersham).
Protein Overlay Assays
35S-labeled full-length VASP and the 80-kD isoform of Mena were prepared in vitro using the TNT™ coupled transcription–translation system (Promega). The cDNA of the ActA repeats (amino acids 241–423; numbering as in Domann et al. 1992) was amplified by colony PCR from L. monocytogenes strain EGD and cloned into the pGEX-6P1 vector (Amersham Pharmacia Biotech). The murine clone 5/7 was cloned in the pGEX-6P1 vector (Amersham Pharmacia Biotech). Protein overlay assays were done as described previously (Chakraborty et al. 1995; Niebuhr et al. 1997). Spot synthesis was performed according to Frank 1992. Membranes were analyzed using a PhosphorImager (Molecular Dynamics).
Cell Culture and Fluorescence Microscopy
Platelets were obtained from the local blood bank, diluted in 10 mM Hepes, pH 7.4, 5 mM KCl, 145 mM NaCl, 10 mM glucose, 1 mM MgCl2, 1 mM CaCl2, and placed on glass coverslips. Platelets were allowed to settle and spread for 30 min in a humid chamber at 37°C. Jurkat E6-1 (TIB 152; ATCC) were grown in RPMI 1640 (GIBCO BRL), supplemented with 10% FCS and 2 mM l-glutamine (GIBCO BRL). For the bead assay, goat anti–mouse IgG dynabeads M-450 (Dynal) were coated with anti-CD3 mAb TR66 (Lanzavecchia and Scheidegger 1987). Coated beads and Jurkat cells were washed with and resuspended in DME/Hepes (GIBCO BRL) supplemented with 1% FCS. They were then mixed and incubated on ice for 30 min followed by 4 min at 37°C. Cells were allowed to attach on poly-l-lysine–coated coverslips for 2 min on ice, fixed with 3% (wt/vol) paraformaldehyde in cytoskeleton buffer, pH 7.0, and permeabilized with 0.1% (vol/vol) Triton X-100 in cytoskeleton buffer, pH 7.0. The zone of contact between Jurkat T cells and anti-CD3–coated beads was evaluated by measuring the length of the circular arc of contact according to the formula α×(π/180)×r, where α is the angle of the circular sector (in degrees) and r is the radius of the bead (in μm). The radius of the bead is 2.25 μm.
Fixation and immunofluorescence microscopy were done as already described (May et al. 1999) using the following antibodies: Fyb/SLAP #51 and 155E8, vinculin hvin1 (Sigma Chemical Co.), VASP IE226C2 (Abel et al. 1996), WASP (Fus3; Symons et al. 1996), Vav (mAb Vav-30; Sattler et al. 1995), Ena/VASP-like (Evl) (mAb 84H1; Lanier et al. 1999), zyxin 164D4 (Krause et al., manuscript in preparation), and Arp3 B7I. CY3-phalloidin was a kind gift of Dr. H. Faulstich (Heidelberg). Fluorescently-labeled secondary antibodies were purchased from Dianova. The mAb 9E10 against the myc tag was purchased from ATCC (clone no. CRL-1729). Image analysis was carried out as already described (May et al. 1999).
Green Fluorescent Protein (GFP) Constructs and Transfection Procedures
The cDNA of the ActA repeats (amino acids 241–423; numbering as in Domann et al. 1992) was amplified by colony PCR from L. monocytogenes strain EGD. The cDNA of the mutated ActA repeats was kindly provided by Dr. Susanne Pistor (Gesellschaft für Biotechnologische Forschung, Braunschweig, Germany). The wild-type and mutant repeats were tagged with pEGFP-N1 (Clontech). The myc-tagged ScarW and ScarWA were kindly provided by Dr. Laura M. Machesky (University of Birmingham, Birmingham, UK). Transfection in HeLa cells was performed with FuGENE (Boehringer Mannheim) according to manufacturer's instructions. Jurkat E6-1 cells (1 × 107 cells resuspended in DME/Hepes) were transfected with 40 μg of cDNA by electroporation using a Gene Pulser II (BioRad) with the following settings: voltage, 0.25 kV; capacitance, 950 μF.
Scanning EM
For scanning EM, Jurkat T cells were transiently transfected with GFP-ActA repeats or GFP-ActA repeats F > A and sorted using a FACS sorter (FACS Vantage; Becton Dickinson). This approach was necessary to obtain a cell population expressing equivalent levels of GFP-tagged proteins, and facilitated the analysis of the cells by scanning EM. Jurkat T cells expressing moderate to high levels of GFP-ActA repeats or GFP-ActA repeats F > A were used for analysis. Sorted cells were returned to the incubator and allowed to recover overnight. After the bead assay, Jurkat T cells were fixed with 3% paraformaldehyde in cytoskeleton buffer, pH 7.0, for 30 min on ice, then washed with the cytoskeleton buffer. Cells were postfixed with 2.5% glutaraldehyde in cacodylate buffer (0.1 M sodium cacodylate, 0.09 M sucrose, 10 mM MgCl2, 10 mM CaCl2, pH 7.2) for 2 h at room temperature. After washing with cacodylate buffer, the cells were dehydrated through a graded series of ethanol, processed by critical-point drying, and gold coated. Samples were examined with a digital scanning electron microscope (DSM 982 Gemini; Zeiss) using a working distance of 2–4 mm and acceleration voltage of 5 Kv. EM images were processed using Photoshop 5.0 (Adobe Systems, Inc.).
Results
Molecular Cloning of Fyb/SLAP as a Novel EVH1 Ligand
We observed previously that polyclonal antibodies raised against the EVH1-binding motif of ActA reacted with vinculin, zyxin, and several unidentified bands on Western blots of HeLa cell lysates (Niebuhr et al. 1997). In an attempt to identify novel EVH1-binding proteins, we screened a mouse embryonic expression library with these antibodies and obtained 15 positive clones. One of those (clone 5/7) bound to the 32P-labeled GST-EVH1 domain of Mena (Niebuhr et al. 1997) in a blot overlay assay (data not shown). Screening of the EST database with the sequence of the 5/7 partial clone revealed a human EST clone (IMAGE clone ID 221953; RZPD IMAGp998F02441) that contained a 2.3-kb cDNA encoding an unknown protein (Fig. 1 a). During the characterization of this protein, two identical protein sequences, termed Fyb (Da Silva et al. 1997) or SLAP-130 (Musci et al. 1997), were reported. The EST clone was identical to the published sequence of Fyb/SLAP except for an additional insertion of 131 bp and a frame shift that leads to an earlier termination of translation. Three independent RT-PCRs of the COOH terminus of Fyb/SLAP showed two bands of 700 and 840 bp, indicating that two isoforms of Fyb/SLAP, at least in the human myeloid cell line HL60, exist (Fig. 1 b). The sequences of these fragments indicated that the longer isoform contains an insertion of 138 bp and has the same COOH terminus as Fyb/SLAP. The 700-bp fragment corresponds to the published sequence. A full-length Fyb/SLAP clone without the 138-bp insertion was obtained by RT-PCR and termed Fyb/SLAP1. The full-length cDNA clone of the longer Fyb/SLAP isoform was amplified by PCR using the EST clone and the 840-bp RT-PCR fragment as templates, and was termed Fyb/SLAP2 (Fig. 1 a). Very recently, the murine homologue of Fyb/SLAP 2 has been reported (Veale et al. 1999).
Domain Structure and Isoforms of Fyb/SLAP
We have recently characterized the core sequence of the EVH1-binding site as F/Y/L/WPPPP (Niebuhr et al. 1997). Fyb/SLAP harbors four similar motifs (Fig. 1, a and d). However, only two of them contain the adjacent acidic residues that are essential for binding to EVH1 domains as shown previously for the analogous motifs of ActA (Niebuhr et al. 1997; Carl et al. 1999).
Two highly charged regions are also present within Fyb/SLAP (Fig. 1 d; amino acids 451–500 and 673–700). Interestingly, the first highly charged sequence shows a striking similarity with part of the NH2-terminal sequence of the L. monocytogenes protein ActA, which is involved in the actin recruitment by Listeria (Fig. 2 a) (Pistor et al. 1995; Lasa et al. 1995, Lasa et al. 1997). The region following the first highly charged region of Fyb/SLAP (amino acids 566-581) shows high homology with the known G-actin binding site of thymosin β4 (Van Troys et al. 1996), the human villin headpiece (Friederich et al. 1992), the putative G-actin binding site of the dematin headpiece (Van Troys et al. 1996), and a potential G-actin binding site of Ena/VASP proteins (Gertler et al. 1996) (Fig. 2 b). The 46–amino acid insertion in Fyb/SLAP2 is highly charged and shows no homology with known motifs. The COOH terminus harbors an SH3-like domain (Fig. 1 d) (Da Silva et al. 1997; Musci et al. 1997).
Figure 2.
(a) Alignment of protein sequences of the NH2 terminus of ActA (from L. monocytogenes), iActA (from L. ivanovii), and Fyb/SLAP. Identical amino acids are indicated with bold letters, homologous amino acids are underlined. Amino acids are numbered according to Domann et al. 1992 for ActA and to Kreft et al. 1995 for iActA. (b) Alignment of the G-actin binding sites of thymosin β4, villin, dematin, the potential G-actin binding site of Mena, and Fyb/SLAP. Amino acids involved in the binding to G-actin are indicated with bold letters. Homologous amino acids are underlined.
To analyze Fyb/SLAP in more detail, a polyclonal antiserum (#51) was raised against and affinity-purified with a recombinant Fyb/SLAP fragment covering the central domain common to both isoforms (Fyb/SLAP III GST; Fig. 1 a). This antibody specifically reacted with a 120-kD band in Jurkat T cell extracts and with two bands of 120 and 130 kD in extracts of human platelets (Fig. 1 c). Moreover, this antiserum did not react with extracts of nonhematopoietic cell lines such as HeLa (data not shown). An additional antiserum (#81) was raised against a synthetic peptide derived from the unique sequence of Fyb/SLAP2. On immunoblots of human platelet extracts these polyclonal antibodies reacted with a 130-kD band (the few bands detected below the 130-kD band most probably represent degradation products) (Fig. 1 c), indicating that the doublet detected with the antiserum #51 is indeed due to the insertion present in the Fyb/SLAP2 isoform.
Fyb/SLAP Is a New EVH1-binding Protein
Since Fyb/SLAP harbors four potential EVH1-binding sites, we sought to determine which of these binding sites interacts with VASP and Mena using a ligand overlay assay. The COOH terminus of Fyb/SLAP (Fyb/SLAP IX GST; Fig. 1 a), which harbors two potential binding sites, interacted with in vitro translated 35S-labeled VASP, whereas the central part of the protein (Fyb/SLAP III GST; Fig. 1 a) and its NH2 terminus (Fyb/SLAP I GST; Fig. 1 a) did not (Fig. 3 a). The same results were obtained using in vitro translated 35S-labeled Mena (data not shown). Thus, the COOH terminus of Fyb/SLAP interacts directly with VASP and Mena in vitro. To map the binding site more precisely, we performed a ligand overlay assay on scans of arrayed peptides covering the complete Fyb/SLAP1 sequence and the COOH terminus of Fyb/SLAP2 (Fig. 3 b) as recently described for the identification of the EVH1-binding sites of ActA (Niebuhr et al. 1997). As shown in Fig. 3 c, the binding site for the EVH1 domain corresponds to the motif 616-FPPPPDDDI-624 (Fig. 1 d). To determine if Fyb/SLAP and VASP associate in vivo, we prepared immunoprecipitates of VASP from lysates of activated human Jurkat T cells. The VASP immunoprecipitates contained Fyb/SLAP (Fig. 3 d). These data indicate that endogenous VASP and Fyb/SLAP are present in a protein complex within hematopoietic cells.
Figure 3.
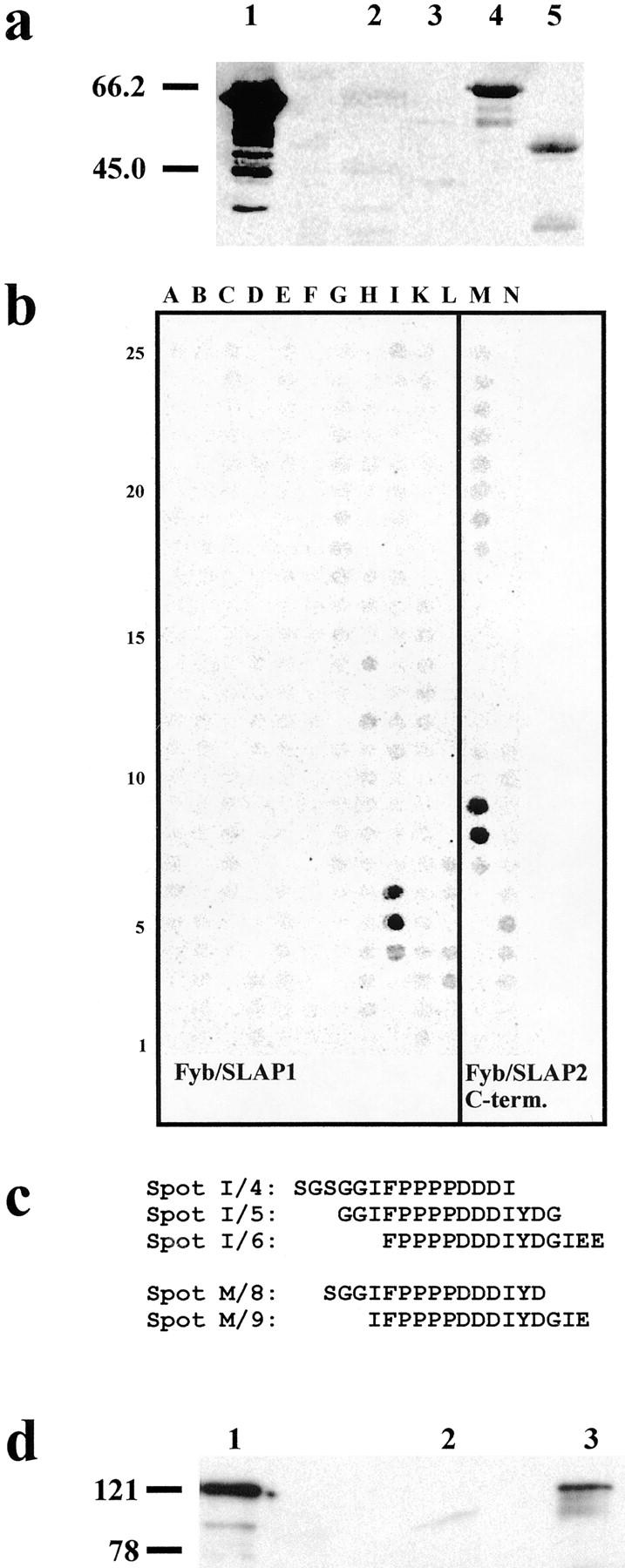
(a) Blot overlay assay of in vitro translated 35S-labeled VASP on GST fusion proteins of Fyb/SLAP and the murine clone 5/7. (lane 1) GST-ActA proline-rich repeats (positive control), (lane 2) Fyb/SLAP I GST, (lane 3) Fyb/SLAP III GST, (lane 4) Fyb/SLAP IX GST, and (lane 5) murine clone 5/7 GST. Note that bands representing degradation products are visible in lanes 1, 4, and 5. (b) Peptide spot analysis of the full-length Fyb/SLAP1 and of the COOH terminus of Fyb/SLAP2. Peptides were synthesized on a cellulose membrane and incubated with 35S-labeled VASP. Positive spots were detected with a PhosphorImager. (c) Single letter amino acid sequences of the peptides corresponding to the positive spots shown in b. (d) Coimmunoprecipitation of Fyb/SLAP and VASP from a lysate of Jurkat T cells. Extracts of activated Jurkat T cells were incubated with mAbs against VASP. Immune complexes were collected using anti–mouse IgG agarose. Bound proteins were separated by SDS-PAGE, blotted, and probed with the affinity-purified antibody #51 to Fyb/SLAP. (lane 1) Lysate of activated Jurkat T cells (positive control), (lane 2) proteins immunoprecipitated using the mAb (358C1) raised against a bacterial protein, and (lane 3) proteins immunoprecipitated using anti-VASP specific mAbs. Molecular weight markers are indicated on the left in a and d.
Fyb/SLAP Localizes to Sites of High Actin Dynamics
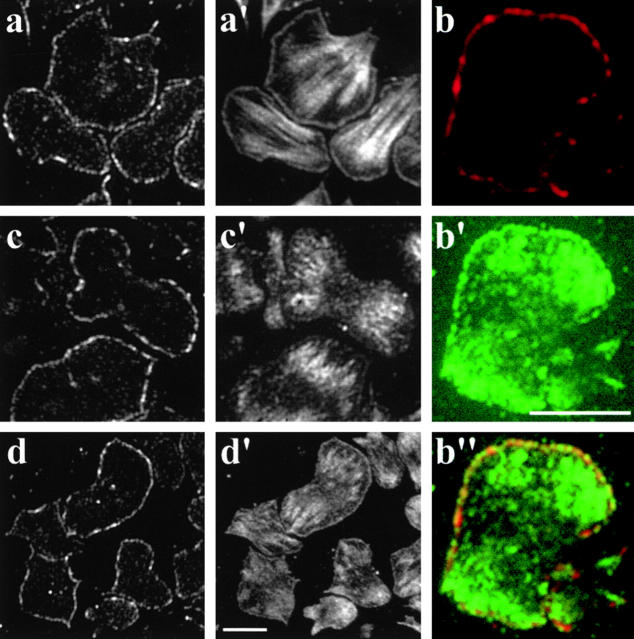
EVH1-binding motifs have been found in the cytoskeletal proteins zyxin and vinculin as well as in the Listeria protein ActA (Brindle et al. 1996; Reinhard et al. 1996; Niebuhr et al. 1997). These motifs are required for the proper subcellular targeting of Ena/VASP family proteins. Therefore, we analyzed the distribution of Fyb/SLAP in hematopoietic cells. In glass-activated spreading, human platelets Fyb/SLAP localized exclusively to lamellipodia (Fig. 4 a) where, in part, it overlapped with VASP (Fig. 4, b–b′′), which in addition, was also detectable in focal adhesions as shown previously (Reinhard et al. 1992). In contrast to Fyb/SLAP, zyxin (for references see Beckerle 1997) and vinculin (for references see Jockusch et al. 1995) were predominantly detected in focal contacts (Fig. 4c, Fig. c′, d, and d′), although we also found vinculin in lamellipodia as described previously (Takubo et al. 1998). The same distribution of Fyb/SLAP in spreading platelets was also obtained using the mAb 155E8 raised against Fyb/SLAP1 (data not shown).
Figure 4.
Fluorescence micrographs showing the localization of Fyb/SLAP and cytoskeletal proteins in human platelets. Platelets were allowed to attach and spread on glass coverslips, fixed, and double stained for Fyb/SLAP (a–d), actin (a′), VASP (b′), zyxin (c′), or vinculin (d′). (b′′) Merged picture of Fyb/SLAP (b) and VASP (b′). Bars, 5 μm.
Since Fyb/SLAP is a component of the TCR signaling complex, we examined the distribution of Fyb/SLAP in activated T cells. Jurkat T cells become polarized towards anti-CD3–coated beads and reorganize their actin cytoskeleton as a dense F-actin collar. Furthermore, F-actin–rich pseudopods protrude from the T cell surface to increase the contact area at the beads–T cells attachment sites. This approach mimics the actin reorganization taking place during T cell–APC interaction and permits the precise analysis of typical actin remodeling of T cells (Lowin-Kropf et al. 1998). We analyzed Jurkat T cells that bound to one bead. In addition, only T cell–bead conjugates in which both the T cell and the bead were in the same plane of focus were considered suitable for analysis. This enabled us to unambiguously identify the actin collar and achieve consistent results from different bead assays. Interestingly, Fyb/SLAP accumulated at the T cell–beads attachment sites where it colocalized with F-actin (Fig. 5 a′). Since Evl is the member of the Ena/VASP family proteins that is most enriched in hematopoietic cells (Lanier et al. 1999), we considered its localization in T cells as representative of the Ena/VASP proteins in general. Evl localized to the contact sites between T cells and beads (Fig. 5 b′). In addition, WASP, the Arp2/3 complex, e.g., Arp3, and Vav also localized at the T cell–bead interface (Fig. 5c′–e′), whereas vinculin and zyxin did not accumulate at these contact sites (Fig. 5f′ and g′).
Figure 5.
Distribution of Fyb/SLAP in activated T cells. Jurkat T cells were incubated with anti-CD3–coated beads (a′, asterisk) and allowed to settle on glass coverslips. T cells were then double stained for F-actin (a–g), Fyb/SLAP (a′), Evl (b′), WASP (c′), Arp3 (d′), Vav (e′), vinculin (f′), or zyxin (g′). Fyb/SLAP is enriched at the interface between the activated T cell and the anti-CD3–coated bead (a′, arrow). F-actin also colocalizes with Evl, WASP, Arp3, and Vav (arrows in b′, c′, d′, and e′ respectively). In contrast, vinculin and zyxin do not accumulate at the interface between T cells and beads (e′ and f′). Note that beads are visible in the panels e′–g′ due to the binding of the secondary goat anti–mouse antibody to the mAbs against CD3 used to coat the beads. Bar, 5 μm.
Ena/VASP Family Proteins Are Essential for the Remodeling of the Actin Cytoskeleton upon TCR Ligation
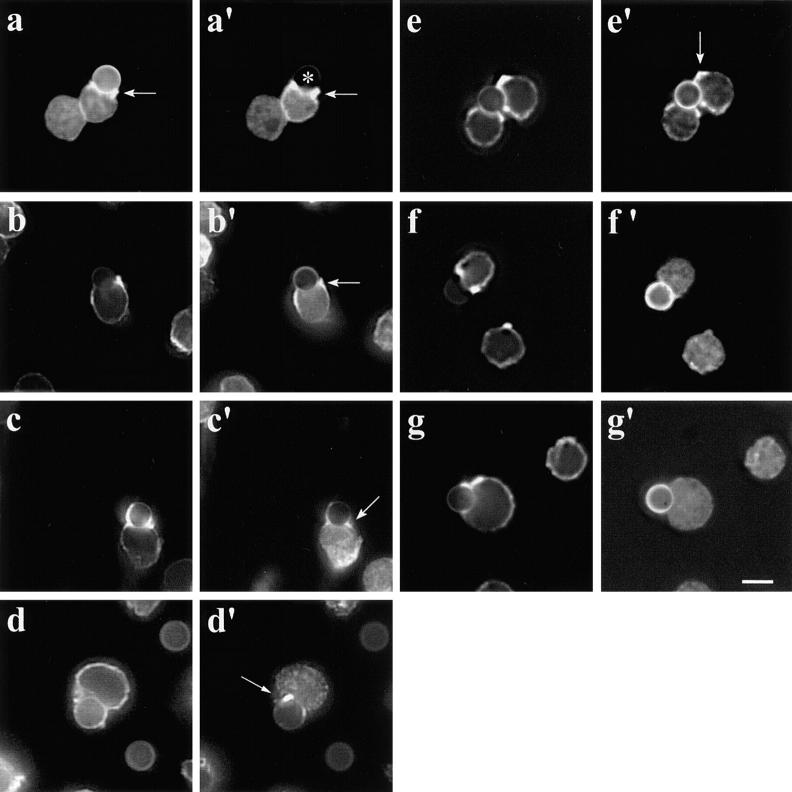
We sought to determine whether recruitment of Ena/VASP proteins is required for actin remodeling of activated T cells. We reasoned that disrupting the interaction between EVH1 domain and its ligands would neutralize the function of Ena/VASP proteins by preventing their proper subcellular targeting and association with natural ligands. The affinities of the Ena/VASP family proteins for the EVH1-binding sites of ActA are higher than those for their natural ligands (Niebuhr et al. 1997). Microinjection of a peptide containing an ActA EVH1-binding motif (FEFPPPPTDEELRL) displaces Ena/VASP proteins from the focal adhesions, whereas a mutated peptide (FEAPPPPTDEELRL) has no effect (Pistor et al. 1995; Gertler et al. 1996; Niebuhr et al. 1997). To overcome the difficulty in microinjecting T cells and to allow a rapid identification of transfected cells, the four EVH1-binding sites of ActA (ActA repeats) were tagged with the GFP (GFP-ActA repeats). As control, a GFP-tagged mutated variant of the four EVH1 domain–binding sites in which the phenylalanine residues preceding the four prolines of each repeat have been mutated to alanine was used (GFP-ActA repeats F > A). As a positive control for this approach, HeLa cells were transfected with GFP-ActA repeats. Focal adhesions in the GFP-ActA repeats expressing cells were obviously devoid of VASP, whereas cells transfected with GFP-ActA repeat F > A showed typical robust labeling of focal adhesions with VASP (Fig. 6, a and b′). The GFP-ActA repeats were then introduced into Jurkat T cells to test the effect of displacing Ena/VASP proteins on TCR-driven actin rearrangement. Jurkat T cells expressing the GFP-ActA repeats bound to anti-CD3–coated beads but did not form the typical actin collar at the T cell–bead interface (Fig. 6c and Fig. c′). In control untransfected cells, the length of the contact zone was 5.38 μm (SD = 0.98 μm, n = 32), whereas in T cells transfected with the GFP-ActA repeats the zone of contact was significantly reduced (3.84 ± 0.95 μm, n = 46). As a consequence of the impaired actin remodeling, the F-actin accumulation was undetectable at this contact site. In contrast, cells expressing the GFP-ActA repeats F > A exhibited normal F-actin accumulation (Fig. 6d and Fig. d′) as well as length of the contact zone (5.16 ± 1.33 μm, n = 30) at the T cell–bead interface. To investigate whether or not these effects on actin accumulation and length of the contact zone were accompanied by clear morphologic effects, we decided to analyze the interaction between T cells and beads by scanning EM in more detail. We found that T cells expressing GFP-ActA repeats simply contacted but did not surround CD3-coated beads (Fig. 7 b), whereas control T cells or cells expressing GFP-ActA repeats F > A almost completely surrounded the beads with sheet-like extensions (Fig. 7, a and c). Thus, we conclude that proteins of the Ena/VASP family are essential for actin remodeling upon T cell stimulation. We also analyzed the distribution of Evl, Fyb/SLAP, WASP, and the Arp2/3 complex in stimulated T cells. In Jurkat T cells transfected with the GFP-ActA repeats, Evl did not localize at the T cell–bead interface (Fig. 6 e), whereas the distribution of Fyb/SLAP, WASP, and the Arp2/3 complex was unaffected (Fig. 6, f–h). The localization of these proteins at the T cell–bead interface was unaffected in T cells transfected with GFP-ActA repeats F > A (data not shown).
Figure 6.
(a–b′) Displacement of Ena/VASP family proteins by the proline-rich repeats of ActA. HeLa cells were transfected with GFP-ActA repeats (a and a′) or with the mutant GFP-ActA repeats F > A (b and b′), fixed, and labeled with antibodies to VASP (a and b, VASP labeling; a′ and b′, GFP signal). In untransfected cells, VASP localizes to focal adhesions (a, arrows). In cells expressing the GFP-ActA repeats (a, asterisks), VASP is displaced from the focal adhesions. In contrast, cells transfected with the mutated ActA repeats (for instance, asterisks in b) show a normal VASP localization (b, arrows). (c–h′) Inhibition of F-actin accumulation at the interface between Jurkat T cells and anti-CD3–coated beads. Jurkat T cells were transfected with GFP-ActA repeats (c and c′) or with the mutant GFP-ActA repeats F > A (d and d′). Note the lack of actin accumulation at the T cell–bead interface and the reduced zone of contact between T cell and bead (c, arrow). Such actin accumulation is not affected in Jurkat T cells that were transfected with the mutated ActA repeats (d, arrow; c and d, fluorescent phalloidin; c′ and d′, GFP signal). (e–h′) The localization of Evl but not that of Fyb/SLAP, WASP, and the Arp2/3 complex at the T cell–bead interface is affected by the proline-rich repeats of ActA. Jurkat T cells were transfected with GFP-ActA repeats, incubated with anti-CD3–coated beads, fixed, and labeled with antibodies to Evl (e), Fyb/SLAP (f), WASP (g), and Arp3 (h) (e′–h′, GFP signal). In cells expressing the GFP-ActA repeats, Evl is displaced from the T cell–bead contact sites (e, arrow). In contrast, the localization of Fyb/SLAP, WASP, and the Arp2/3 complex is unaffected. Bars: 10 μm (a–b′); and 5 μm (c–h′).
Figure 7.
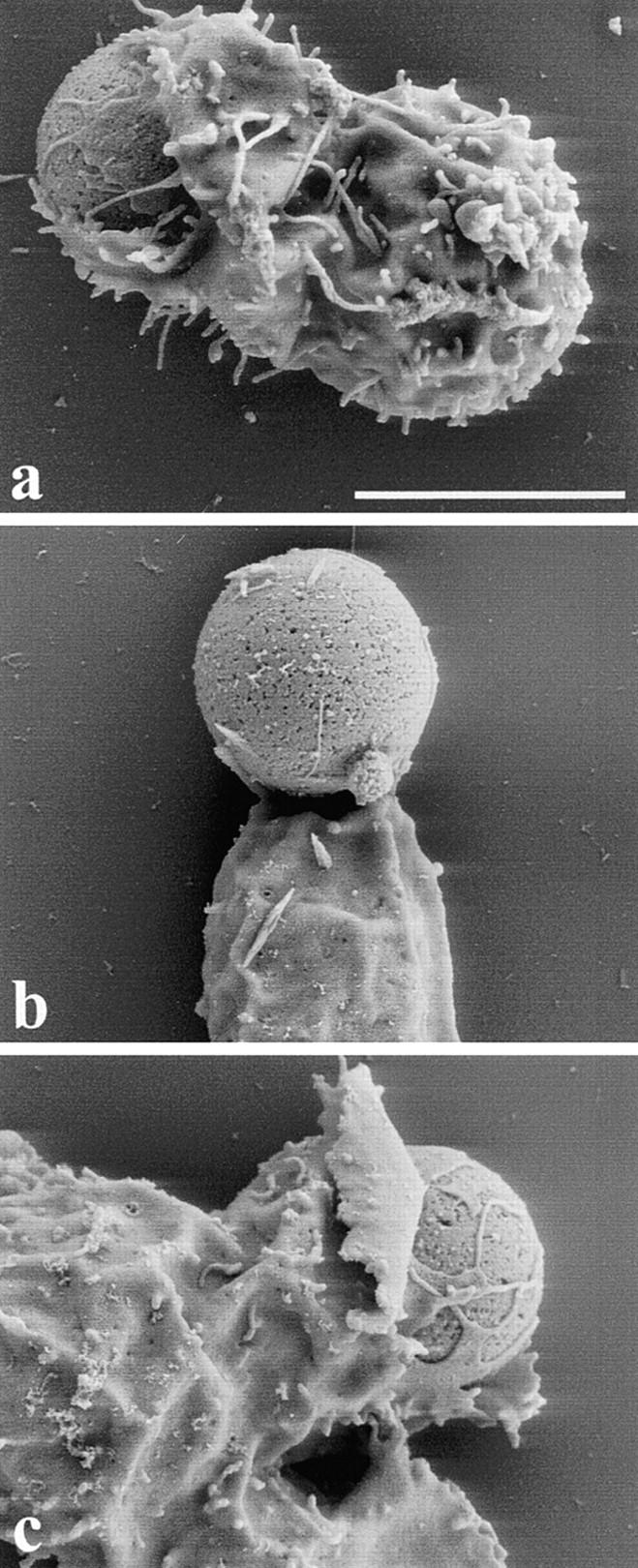
Scanning EM analysis of the interaction between Jurkat T cell and anti-CD3–coated beads. Untransfected Jurkat T cells (a), T cells transfected with GFP-ActA repeats (b), and T cells transfected with GFP-ActA repeats F > A (c) were incubated with anti-CD3–coated beads, fixed, and processed for scanning EM. Jurkat T cells, which expressed GFP-ActA repeats, formed a very loose contact with the bead. In contrast, control T cells or cells which expressed the mutated GFP-ActA repeats extended sheet-like protrusions that almost completely surrounded the beads. Bar, 5 μm.
The Arp2/3 Complex Is Required for the Reorganization of the Actin Cytoskeleton in T Cells
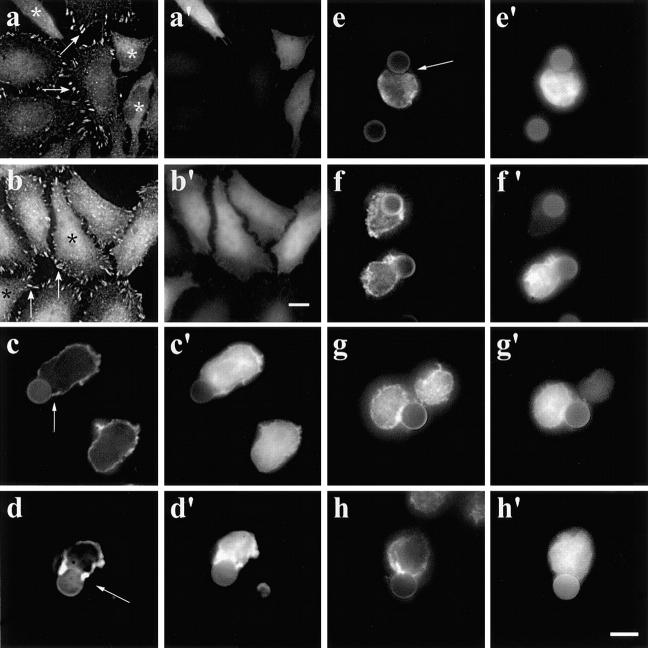
Based on our observation that WASP and the Arp2/3 complex localize to the attachment sites between T cells and anti-CD3–coated beads, and given that WASP family proteins directly interact with the Arp2/3 complex, we predicted that the Arp2/3 complex is involved in actin dynamics in T cells. The COOH-terminal domain of Scar, ScarWA, is similar to the COOH-terminal region of WASP and disrupts the binding of WASP family proteins to the Arp2/3 complex when expressed in mammalian cells (Machesky and Insall 1998). Overexpression of ScarWA, which binds to G-actin and the p21Arc subunit of the Arp2/3 complex, delocalizes the Arp2/3 complex and causes the loss of lamellipodia and stress fibers in Swiss3T3 fibroblasts and J774 macrophages. In addition, ScarWA inhibits the Listeria-induced actin tail formation in infected cells as well as in mouse brain extract. In contrast, ScarW, which binds to G-actin only, has no effect (Machesky and Insall 1998; May et al. 1999). To investigate the role of the Arp2/3 complex in the reorganization of the T cell actin cytoskeleton in response to TCR engagement, we overexpressed myc-tagged Scar1 fragments in T cells. In Jurkat T cells transfected with ScarWA, F-actin accumulation at the T cell–bead interface was not detectable, whereas it was not affected in Jurkat T cells transfected with ScarW (Fig. 8, a–b′). Moreover, the length of the contact zone between the beads and Jurkat T cells was significantly reduced (3.16 ± 0.78 μm, n = 39), whereas in ScarW-transfected cells the size of the contact zone was comparable to that of control cells (5.00 ± 1.08 μm, n = 22). The expression of ScarWA affected the distribution of the Arp2/3 complex, which was no longer detectable at the T cell–bead interface (Fig. 8 f), whereas Evl, Fyb/SLAP, and WASP still accumulated at this site (Fig. 8, c–e). As expected, the expression of ScarW did not affect the localization of these proteins (data not shown). These data indicate that in addition to the Ena/VASP proteins, the Arp2/3 complex is essential for the reorganization of the actin cytoskeleton taking place during T cell activation.
Figure 8.
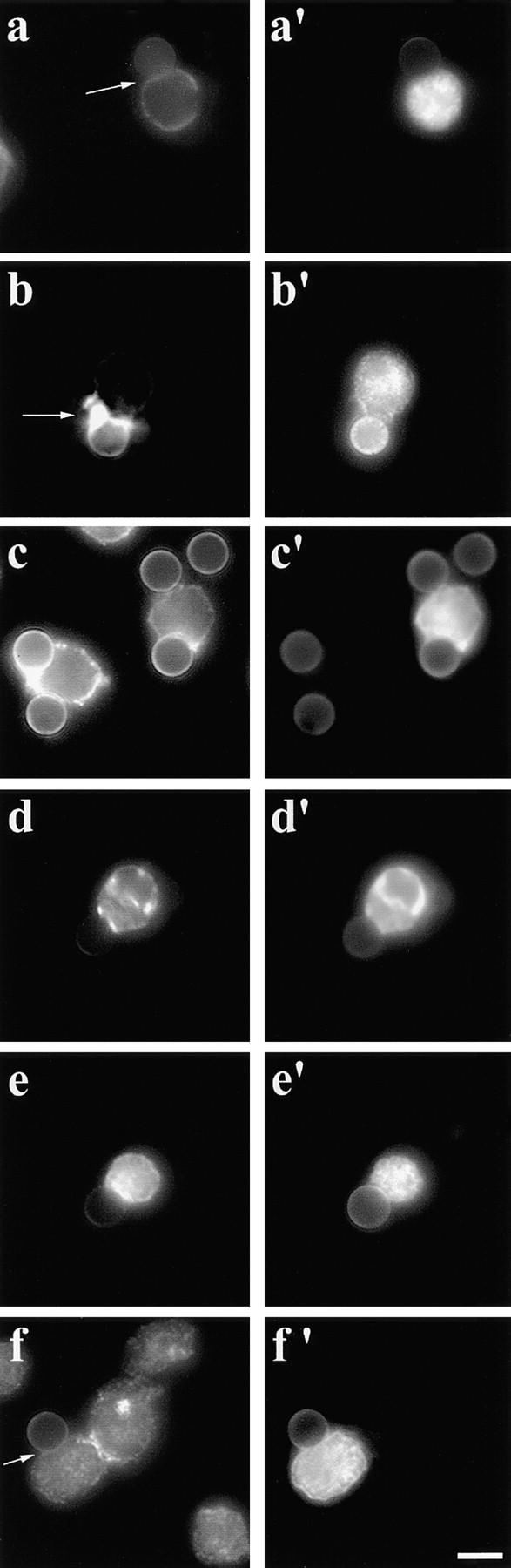
(a–b′) Delocalization of the Arp2/3 complex by the COOH terminus of Scar1 inhibits the actin reorganization at the T cell–bead interface. Jurkat T cells were transfected with myc-tagged ScarWA (a and a′) or ScarW (b and b′), incubated with anti-CD3–coated beads, fixed, and stained with fluorescent phalloidin and anti-myc antibodies. In Jurkat T cells, which expressed ScarWA, the actin accumulation at the T cell–bead interface is impaired (a, arrow), whereas T cells transfected with ScarW show a normal actin rearrangement (b, arrow; a and b, fluorescent phalloidin; a′ and b′, myc tag labeling). (c–f′) In T cells expressing ScarWA, Evl, Fyb/SLAP, and WASP accumulated at the T cell–bead interface. Jurkat T cells were transfected with myc-tagged ScarWA, incubated with anti-CD3–coated beads, fixed, and stained with antibodies to Evl (c), Fyb/SLAP (d), WASP (e), Arp3 (f), and myc tag (c′–f′). Note that the Arp2/3 complex did not localize at the T cell–bead interface. Bar, 5 μm.
Fyb/SLAP, SLP-76, Nck, and WASP Are Constituents of a Complex in T Cells
Since Fyb/SLAP and WASP localized to the contact site between activated Jurkat T cells and anti-CD3–coated beads, we speculated that both proteins might be constituents of a complex in activated Jurkat T cells. This possibility is consistent with recent findings indicating that Fyb/SLAP binds to the SH2 domain of SLP-76 (Da Silva et al. 1997; Musci et al. 1997), which forms a ternary complex with Vav and Nck (Bubeck Wardenburg et al. 1998). In addition, Rivero-Lezcano et al. 1995 show that the SH3 domain of Nck binds to WASP. To test this hypothesis, immunoprecipitates from extracts of activated Jurkat T cells were prepared using an antiserum against WASP. Western blot analysis demonstrated that the immunoprecipitates contained Fyb/SLAP, indicating that it is present in a protein complex with WASP in vivo (Fig. 9 a).
Figure 9.
(a) Fyb/SLAP coimmunoprecipitates with WASP. Lysates of activated Jurkat T cells were incubated with the WASP-specific antiserum Fus3. Immune complexes were collected using protein A–agarose. Bound proteins were resolved by SDS-PAGE, blotted, and probed with polyclonal antibodies #51 to Fyb/SLAP. (lane 1) Cell extract of activated Jurkat T cells; (lane 2) immunoprecipitation with a nonimmune serum; and (lane 3) immunoprecipitation using a WASP-specific antiserum. (b) Fyb/SLAP, SLP-76, Nck, and WASP form a multiprotein complex in Jurkat T cells. Lysates from nonstimulated (NST) or stimulated (ST) Jurkat T cells were immunoprecipitated using the WASP mAb #67B4, resolved by SDS-PAGE, blotted, and probed with antibodies to Fyb/SLAP, SLP-76, and Nck. As negative control, lysates were immunoprecipitated using an antibody to a bacterial protein. To exclude nonspecific trapping, WASP immunoprecipitated from lysates of activated T cells were probed using a zyxin mAb. (c) The stimulation of Jurkat T cells results in the association of additional tyrosine-phosphorylated proteins with the complex. WASP immunoprecipitates from lysates of nonstimulated (NST) or stimulated (ST) Jurkat T cells were resolved by SDS-PAGE and probed with an antibody to phosphotyrosine. Note the appearance of three additional bands in stimulated T cells. hc, heavy chain; lc, light chain. Molecular weight markers are indicated on the left in (a and b) and on the right in (c).
To further investigate the composition of this complex, we prepared immunoprecipitates from Jurkat T cell extracts using the WASP mAb 67B4. This antibody proved to be more suitable than the polyclonal to WASP for immunoprecipitation studies. Immunoprecipitates prepared from anti-CD3 stimulated Jurkat T cells were analyzed using antibodies to Fyb/SLAP, SLP-76, and Nck. We identified these three proteins in WASP immunoprecipitates, indicating that they each are present in complexes that contain WASP (Fig. 9 b). Since Fyb/SLAP binds directly to SLP-76 (Da Silva et al. 1997; Musci et al. 1997) and SLP-76 associates with Vav and Nck (Bubeck Wardenburg et al. 1998), all of these molecules may be associated with WASP in a single multiprotein complex. To rule out any unspecific trapping, we probed the same immunoprecipitates with the mAb to zyxin, which is not detectable at the interface between Jurkat T cells and beads. As expected, zyxin is not immunoprecipitated with WASP, proving the specificity of our approach. As negative controls, we prepared immunoprecipitates using an antibody to a bacterial protein; these immunoprecipitates contained neither Fyb/SLAP, SLP-76, nor Nck (Fig. 9 b). To investigate whether the complex composed of Fyb/SLAP, SLP-76, Nck, and WASP formed upon activation of T cells, T cell lysates from nonstimulated cells were immunoprecipitated with the WASP mAb and probed with antibodies to Fyb/SLAP, SLP-76, and Nck. These proteins could be detected in these precipitates, indicating that they also form a complex in nonstimulated T cells (Fig. 9 b). However, the relative amount of the complex in stimulated T cells seemed to be higher than that in nonstimulated cells, suggesting that the formation of such complex is, in part, stimulation-dependent. Tyrosine phosphorylation is a key step during T cell activation. At least two of the proteins involved in the complex, Fyb/SLAP and SLP-76, are tyrosine phosphorylated in response to T cell stimulation (Clements et al. 1999). Therefore, we analyzed the tyrosine phosphorylation pattern of WASP immunoprecipitates from Jurkat T cells. WASP immunoprecipitates of nonstimulated T cell lysates probed with an antibody to phosphotyrosine revealed three prominent bands running at ∼33, 76, and 95 kD (Fig. 9 c). They were also detected in WASP immunoprecipitates of stimulated T cell lysates with three additional bands running at ∼36, 47 and 70 kD (Fig. 9 c). Given that Nck and SLP-76 are phosphorylated in T cells, the bands running at 47 and 76 kD may correspond to these proteins. Taken together, these data show that Fyb/SLAP, SLP-76, Nck, and WASP form a complex in T cells and that the formation of this complex is enhanced upon stimulation of T cells.
Discussion
Fyb/SLAP Is a Novel EVH1-binding Protein
We have identified Fyb/SLAP as a new EVH1 domain–binding protein. Fyb/SLAP harbors four putative EVH1-binding motifs, though only one of them binds EVH1 domains in vitro. This functional EVH1-binding site harbors the acidic amino acid residues, which we have demonstrated recently to be essential for efficient binding to EVH1 domains (Niebuhr et al. 1997; Carl et al. 1999). Whether or not the other candidate EVH1-binding sites in Fyb/SLAP are important for function(s) in vivo remains to be determined.
EVH1 domains have emerged recently as important mediators of the subcellular localization of Ena/VASP family proteins, which are thought to be potential regulators of actin dynamics (Chakraborty et al. 1995; Gertler et al. 1996; Laurent et al. 1999; Carl et al. 1999). Given that EVH1-binding proteins recruit Ena/VASP family proteins to focal contacts (Gertler et al. 1996; for references see Beckerle 1997), and based on our observations showing the restricted localization of Fyb/SLAP to lamellipodia of spreading platelets and at the interface between T cell and anti-CD3–coated beads, we suggest that Fyb/SLAP recruits Ena/VASP family proteins to sites where remodeling of the actin cytoskeleton is required in platelets and T cells. However, we cannot exclude that other as-yet unidentified proteins harboring EVH1-binding sites are involved in this process.
Fyb/SLAP Is a Constituent of a Complex that Links TCR Signaling to Reorganization of the Actin Cytoskeleton
Engagement of the TCR leads to the formation of actin-supported signaling, which serves to coordinate downstream events such as proliferation and secretion of cytokines. Several lines of evidence support a role of WASP in TCR-dependent rearrangement of the actin cytoskeleton. Antigen-receptor induced capping is severely impaired in WASP knockout T cells (Snapper et al. 1998). Furthermore, actin reorganization in response to CD3-mediated stimulation is inhibited in human Wiskott-Aldrich syndrome T cells (Gallego et al. 1997). We showed that Fyb/SLAP and WASP localize to the interface between T cell and anti-CD3–coated beads and Fyb/SLAP, SLP-76, and Nck can be identified in WASP immunoprecipitates. In activated T cells, Nck, Vav, and SLP-76 form a trimolecular complex (Bubeck Wardenburg et al. 1998). Moreover, SLP-76 interacts with Fyb/SLAP (Da Silva et al. 1997; Musci et al. 1997), whereas Nck binds directly to WASP (Rivero-Lezcano et al. 1995). Therefore, our data are consistent with the possibility that Fyb/SLAP interacts through a molecular chain with WASP (Fig. 10 a, red highlighted proteins).
Figure 10.
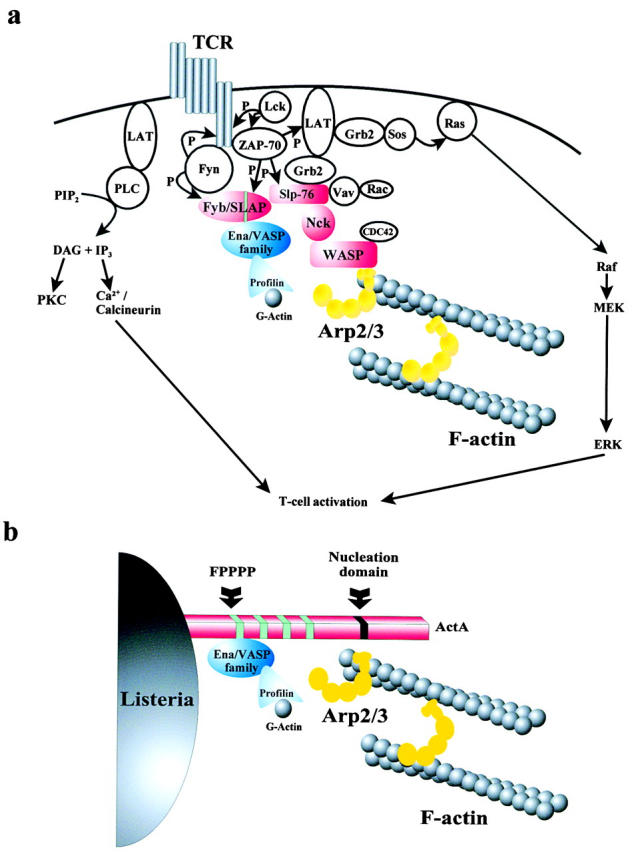
(a) Model describing intermolecular interactions and signal transduction pathways leading to the cytoskeletal remodeling and T cell activation upon TCR ligation (adapted from Koretzky 1997). Ena/VASP family proteins and WASP are linked through a molecular chain formed by Fyb/SLAP, SLP-76, and Nck (red highlighted proteins) to the T cell–bead interface (see text for further details). The green line in the Fyb/SLAP molecule indicates the EVH1 domain–binding site. (b) Schematic representation of the proteins involved in the actin-based motility of Listeria monocytogenes showing the recruitment of Ena/VASP family proteins and Arp2/3 complex to the bacterial surface protein ActA.
Previous investigations using the Listeria model system have clearly demonstrated that at least two host cell components are required for an efficient actin tail formation: Ena/VASP family proteins (Smith et al. 1996; Niebuhr et al. 1997; Laurent et al. 1999) and Arp2/3 complex (Welch et al. 1998; Loisel et al. 1999; May et al. 1999). In this work we show that both components are also essential for an efficient actin rearrangement in activated T cells. We propose a model in which a complex composed of Fyb/SLAP, SLP-76, Nck, and WASP brings together Ena/VASP family proteins and the Arp2/3 complex, and suggest that this macromolecular assembly links TCR signaling to the remodeling of the actin cytoskeleton (Fig. 10 a). Our hypothesis is consistent with the following observations. First, a signaling pathway similar to the TCR-signaling cascade mediates collagen-induced activation of platelets. In addition, immunoprecipitation studies demonstrated the association of SLP-76 with Fyb/SLAP, Vav, Fyn, Lyn, and the FcR γ-chain in platelets stimulated with a collagen-related peptide (Watson and Gibbins 1998; Gross et al. 1999), suggesting that hematopoietic cells may use a common signal transduction pathway to link cell activation to the reorganization of the actin cytoskeleton. Second, vaccinia virus, which subverts the actin polymerization machinery to move inside infected cells, recruits Nck and N-WASP at the site of actin assembly, indicating that the signal transduction pathway involving Nck and N-WASP plays a key role in this process (Frischknecht et al. 1999).
How Is the Activity of the Complex Regulated?
The cytoskeletal reorganization occurring in response to antigen-receptor engagement requires the coordination of the functions of the proteins involved in this process (Fig. 10 a, red highlighted proteins). T cell activation results in the formation of a complex between Fyb/SLAP and SLP-76, and we have demonstrated that this complex also contains WASP and Nck. Although we favor a model in which Fyb/SLAP, SLP-76, Nck, and WASP form a complex upon T cell activation, our data show that this complex, although at low levels, is also present in nonstimulated T cells. This result can be explained in at least two ways. First, because Jurkat T cells and not primary T cells were used for this study, it is not unlikely that Jurkat T cells are in a state of basal activation, which results in the formation of small amounts of the complex. This is consistent with previous findings that basal levels of tyrosine phosphorylation of Fyb/SLAP could be observed in nonstimulated Jurkat T cells resulting in binding to SLP-76. This interaction was found to increase upon T cell stimulation (Musci et al. 1997). Second, the complex present in nonstimulated T cells might be in an inactive state, which upon TCR engagement, might be converted into an active state. This idea is consistent with our observation that upon T cell activation, additional tyrosine-phosphorylated proteins are recruited to the complex. Although our data do not permit us to prove that the complex present in nonstimulated T cells is activated upon TCR signaling, we clearly show that T cell activation results in the enhancement of the complex formation.
The complex may be regulated at different levels. First, because in T cells Fyb/SLAP also interacts with VASP, it is possible that a link between Ena/VASP proteins and WASP–Arp2/3 is formed upon T cell activation, resulting in actin cup formation. This possibility is consistent with our observations showing that inhibition of the binding of Ena/VASP to Fyb/SLAP or Arp2/3 complex to WASP leads to inhibition of actin cup formation.
Second, the binding of WASP family proteins to the Arp2/3 complex activates its actin nucleation activity (Machesky et al. 1999; Rohatgi et al. 1999; Winter et al. 1999; Yarar et al. 1999). Moreover, given that WASP family proteins interact with Cdc42, it is possible that the association of WASP with the Arp2/3 complex, or its actin nucleation activity is regulated by Cdc42.
Third, the complex might be recruited to the engaged TCR. In support of this idea are the following findings: the activated TCR is recruited to pp36/LAT (Xavier et al. 1998; Zhang et al. 1998b). In addition, LAT is found in immunoprecipitates of SLP-76 only in activated T cells (Motto et al. 1996). Moreover, we observed the appearance of a 36-kD band in WASP immunoprecipitates of activated T cells.
Fourth, the complex might be regulated through the association with additional proteins. We consistently found an additional phosphorylated protein of ∼70 kD in WASP immunoprecipitates of activated T cells, which might represent the tyrosine kinases ZAP-70 or Fyn-R (Da Silva and Rudd 1993).
The Ena/VASP Enigma
This and other reports (Niebuhr et al. 1997; Rottner et al. 1999; Loisel et al. 1999) clearly show that Ena/VASP proteins act as positive regulators during remodeling of the actin cytoskeleton. However, Aszódi et al. 1999 recently showed that VASP-null platelets aggregate faster than wild-type platelets. Furthermore, we have observed that Ena/VASP proteins appear to retard cell motility in Rat2 fibroblasts and in cells derived from Mena/VASP double knockout mice (Bear, J.E., I. Libova, J.J. Loureiro, J. Wehland, and F.B. Gertler, manuscript submitted for publication; Bear, J.E., R. Fässler, and F.B. Gertler, unpublished observations). Although in both reports the Ena/VASP proteins are interpreted as having a negative regulatory function, in neither case was a direct assay of actin remodelling performed. However, it is likely that Ena/VASP proteins play a variety of roles depending on cell type, subcellular compartment, interacting proteins, or the regulatory pathway in which they are involved. Future studies will certainly help us to better understand the function of this important protein family.
Conclusions
Spatial and temporal segregation of signal transduction molecules induces a cascade of events culminating in the activation and proliferation of lymphocytes. Focused reorganization of the actin cytoskeleton at the T cell/APC contact site is essential for successful T cell activation. Despite a wealth of knowledge on TCR signaling, it is only partially understood how the signals originating from an engaged TCR are linked to the rearrangement of the actin cytoskeleton. Here we provide evidence that proteins of the Ena/VASP family and the WASP–Arp2/3 complex are recruited to complexes that are targeted to sites of actin reorganization in activated T cells. This scenario is reminiscent of the actin tail formation induced by Listeria monocytogenes, which utilizes a deregulated system in which the ActA protein recruits Ena/VASP proteins and the Arp2/3 complex to the bacterial surface to generate new actin filaments for propelling the bacteria forward (Fig. 10 b). Therefore, we speculate that upon TCR ligation, T cells induce the regulated assembly of a complex that results in the juxtaposition of Ena/VASP proteins and the Arp2/3 complex, thereby inducing temporally and spatially restricted actin nucleation and assembly. To our knowledge this is the first demonstration of a direct connection between external TCR signaling and some of the key players involved in actin remodeling. The specific insights we have presented into the molecular basis of the formation of the actin collar at the T cell–APC interface may prove to be useful for the development of new treatments of inflammational and other disorders of the immune system. We suggest that similar complexes, whose functions are subjected to different regulatory pathways, are involved in actin-based processes such as lamellipodia and filopodia formation.
Acknowledgments
The authors thank Dr. Susanne Pistor (Gesellschaft für Biotechnologische Forschung [GBF], Braunschweig, Germany) for the ActA F > A mutant and the Arp3 antibody; Dr. Jonathan M.J. Derry (Immunex Corp., Seattle, WA) for the generous gift of the anti-WASP antiserum Fus3; Dr. Ronald Frank (GBF) for peptide scans; Dr. Laura M. Machesky (University of Birmingham, Birmingham, UK) for the Scar1 constructs; Dr. Siegfried Weiss (GBF) for the anti-CD3 mAb (TR66); Dr. Ravi Salgia (Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA) for anti–Vav-30 mAbs; and Dr. H. Faulstich (Max Planck Institute, Heidelberg, Germany) for the kind gift of CY-3 phalloidin. We are grateful to Dr. Thomas Harder (Basel Institute for Immunology, Basel, Switzerland) for advice on the bead assay and to Dr. Uwe D. Carl (GBF) for discussion. We thank Petra Hagendorff, Ellruth Müller, and Maria Höxter for technical assistance. We also thank the Resource Centre of the German Human Genome Project at the Max-Planck-Institute for Molecular Genetics (Berlin, Germany) for providing the EST clones (IMAGE clone ID 221953; IMAGp998F02441).
J. Wehland is supported by the Deutsche Forschungsgemeinschaft (WE 2047/5-1) and the Fonds der Chemischen Industrie. F.B. Gertler is supported by the National Institutes of Health grant GM58801.
Footnotes
Abbreviations used in this paper: APC, antigen-presenting cell; EST, expressed sequence tag; EVH1, Ena/VASP homology 1; Evl, Ena/VASP-like; Fyb, Fyn-binding protein; GFP, green fluorescent protein; GST, glutathione S-transferase; RT, reverse transcription; SLAP, SLP-76–associated protein; SLP-76, SH2 domain–containing leukocyte protein of 76 kD; TCR, T cell receptor; VASP, vasodilator-stimulated phosphoprotein; WASP, Wiskott-Aldrich syndrome protein.
References
- Abel K., Lingnau A., Niebuhr K., Wehland J., Walter U. Monoclonal antibodies against the focal adhesion protein VASP revealing epitopes involved in the interaction with two VASP binding proteins and VASP phosphorylation Eur. J. Cell Biol. 69Suppl. 421996. 39a [Google Scholar]
- Aszódi A., Pfeifer A., Ahmad M., Glauner M., Zhou X.-H., Ny L., Andersson K.-E., Kehrel B., Offermanns S., Fässler R. The vasodilator-stimulated phosphoprotein (VASP) is involved in cGMP- and cAMP-mediated inhibition of agonist induced platelet aggregation but is dispensable for smooth muscle function. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:37–48. doi: 10.1093/emboj/18.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann C., Fischer L., Walter U., Reinhard M. The EVH2 domain of the vasodilator-stimulated phosphoprotein mediates tetramerization, F-actin binding, and actin bundle formation. J. Biol. Chem. 1999;274:23549–23557. doi: 10.1074/jbc.274.33.23549. [DOI] [PubMed] [Google Scholar]
- Beckerle M.C. Zyxinzinc fingers at sites of cell adhesion. Bioessays. 1997;19:949–957. doi: 10.1002/bies.950191104. [DOI] [PubMed] [Google Scholar]
- Brindle N.P.J., Holt M.R., Davies J.E., Price C.J., Critchley D.R. The focal-adhesion vasodilator-stimulated phosphoprotein (VASP) binds to the proline-rich domain in vinculin. Biochem. J. 1996;318:753–757. doi: 10.1042/bj3180753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubeck Wardenburg J., Pappu R., Bu J.-Y., Mayer B., Chernoff J., Strauss D., Chan A.C. Regulation of PAK activation and the T-cell cytoskeleton by the linker protein SLP-76. Immunity. 1998;9:607–616. doi: 10.1016/s1074-7613(00)80658-5. [DOI] [PubMed] [Google Scholar]
- Carl U.D., Pollmann M., Orr E., Gertler F.B., Chakraborty T., Wehland J. Aromatic and basic residues within the EVH1 domain of VASP specify its interaction with proline-rich ligands. Curr. Biol. 1999;9:715–718. doi: 10.1016/s0960-9822(99)80315-7. [DOI] [PubMed] [Google Scholar]
- Chakraborty T., Ebel F., Domann E., Niebuhr K., Gerstel B., Pistor S., Temm-Grove C.J., Jockusch B.M., Reinhard M., Walter U., Wehland J. A focal adhesion factor directly linking intracellularly motile Listeria monocytogenes and Listeria ivanovii to the actin-based cytoskeleton of mammalian cells. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:1314–1321. doi: 10.1002/j.1460-2075.1995.tb07117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J.L., Boerth N.J., Lee J.R., Koretzky G.A. Integration of T cell receptor-dependent signaling pathways by adapter proteins. Annu. Rev. Immunol. 1999;17:89–108. doi: 10.1146/annurev.immunol.17.1.89. [DOI] [PubMed] [Google Scholar]
- Crespo P., Schuebel K.E., Ostrom A.A., Gutkind J.S., Bustelo X.R. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature. 1997;385:169–172. doi: 10.1038/385169a0. [DOI] [PubMed] [Google Scholar]
- Da Silva A.J., Rudd C.E. A 72-kilodalton fyn related polypeptide (p72fyn-R) binds to the antigen receptor/CD3 (TcR/CD3) complex. J. Biol. Chem. 1993;268:16537–16543. [PubMed] [Google Scholar]
- Da Silva A.J., Li Z., De Vera C., Canto E., Findell P., Rudd C.E. Cloning of a novel T-cell protein FYB that binds FYN and SH2-domain-containing leukocyte protein 76 and modulates interleukin 2 production. Proc. Natl. Acad. Sci. USA. 1997;94:7493–7498. doi: 10.1073/pnas.94.14.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry J.M., Ochs H.D., Francke U. Isolation of a novel gene mutated in Wiskott-Aldrich syndrome. Cell. 1994;78:635–644. doi: 10.1016/0092-8674(94)90528-2. [DOI] [PubMed] [Google Scholar]
- Derry J.M., Wiedemann P., Blair P., Wang Y., Kerns J.A., Lemahieu V., Godfrey V.L., Wilkinson J.E., Francke U. The mouse homolog of the Wiskott-Aldrich syndrome protein (WASP) gene is highly conserved and maps near the scurfy (sf) mutation on the X chromosome. Genomics. 1995;29:471–477. doi: 10.1006/geno.1995.9979. [DOI] [PubMed] [Google Scholar]
- Domann E., Wehland J., Rohde M., Pistor S., Hartl M., Goebel W., Leimeister-Wächter M., Wuenscher M., Chakraborty T. A novel bacterial virulence gene in Listeria monocytogenes required for host cell microfilament interaction with homology to the proline-rich region of vinculin. EMBO (Eur. Mol. Biol. Organ.) J. 1992;11:1981–1990. doi: 10.1002/j.1460-2075.1992.tb05252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank R. Spot synthesisan easy technique for the positionally addressable, parallel chemical synthesis on a membrane support. Tetrahedron. 1992;48:9217–9232. [Google Scholar]
- Friederich E., Vancompernolle K., Huet C., Goethals M., Finidori J., Vandekerckhove J., Louvard D. An actin-binding site containing a conserved motif of charged amino acid residues is essential for the morphogenic effect of villin. Cell. 1992;70:81–92. doi: 10.1016/0092-8674(92)90535-k. [DOI] [PubMed] [Google Scholar]
- Frischknecht F., Moreau V., Rottger S., Gonfloni S., Reckmann I., Superti-Furga G., Way M. Actin-based motility of vaccinia virus mimics receptor tyrosine kinase signalling. Nature. 1999;401:926–929. doi: 10.1038/44860. [DOI] [PubMed] [Google Scholar]
- Gallego M.D., Santamaría M., Peña J., Molina I.J. Defective actin reorganization and polymerization of Wiskott-Aldrich T-cells in response to CD3-mediated stimulation. Blood. 1997;90:3089–3097. [PubMed] [Google Scholar]
- Geiger B., Rosen D., Berke G. Spatial relationships of microtubule-organizing centers and the contact area of cytotoxic T-lymphocytes and target cells. J. Cell Biol. 1982;95:137–143. doi: 10.1083/jcb.95.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertler F.B., Niebuhr K., Reinhard M., Wehland J., Soriano P. Mena, a relative of VASP and Drosophila Enabled, is implicated in the control of microfilament dynamics. Cell. 1996;87:227–239. doi: 10.1016/s0092-8674(00)81341-0. [DOI] [PubMed] [Google Scholar]
- Gross B.S., Lee J.R., Clements J.L., Turner M., Tybulewcz V.L.J., Findell P.R., Koretzky G.A., Watson S.P. Tyrosine phosphorylation of SLP-76 is downstream of Syk following stimulation of the collagen receptor in platelets. J. Biol. Chem. 1999;274:5963–5971. doi: 10.1074/jbc.274.9.5963. [DOI] [PubMed] [Google Scholar]
- Han J., Das B., Wie W., Van Aelst L., Mosteller R.D., Khosravi-Far R., Westwick J.K., Der C.J., Broek D. Lck regulates Vav activation of members of the Rho family of GTPases. Mol. Cell. Biol. 1997;17:1346–1353. doi: 10.1128/mcb.17.3.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüttelmaier S., Harbeck B., Steffens O., Messerschmidt T., Illenberger S., Jockusch B.M. Characterization of the actin binding properties of the vasodilator-stimulated phosphoprotein VASP. FEBS Lett. 1999;451:68–74. doi: 10.1016/s0014-5793(99)00546-3. [DOI] [PubMed] [Google Scholar]
- Jackman J.K., Motto D.G., Sun Q., Tanemoto M., Turck C.W., Peltz G.A., Koretzky G.A., Findell P.R. Molecular cloning of SLP-76, a 76-kDa tyrosine phosphoprotein associated with Grb2 in T-cells. J. Biol. Chem. 1995;270:7029–7032. doi: 10.1074/jbc.270.13.7029. [DOI] [PubMed] [Google Scholar]
- Jockusch B.M., Bubeck P., Giehl K., Kroemker M., Moschner J., Rothkegel M., Rüdiger M., Schlüter K., Stanke G., Winkler J. The molecular architecture of focal adhesions. Annu. Rev. Cell Dev. Biol. 1995;11:379–416. doi: 10.1146/annurev.cb.11.110195.002115. [DOI] [PubMed] [Google Scholar]
- Kang F., Laine R.O., Bubb M.R., Southwick F.S., Purich D.L. Profilin interacts with the Gly-Pro-Pro-Pro-Pro-Pro sequences of vasodilator-stimulated phosphoprotein (VASP)implications for actin-based Listeria motility. Biochemistry. 1997;36:8384–8392. doi: 10.1021/bi970065n. [DOI] [PubMed] [Google Scholar]
- Kelleher J.F., Atkinson S.J., Pollard T.D. Sequences, structural models and subcellular localization of the actin-related proteins Arp2 and Arp3 from Acanthamoeba . J. Cell Biol. 1995;131:385–397. doi: 10.1083/jcb.131.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocks C., Gouin E., Tabouret M., Berche P., Ohayon H., Cossart P. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell. 1992;68:521–531. doi: 10.1016/0092-8674(92)90188-i. [DOI] [PubMed] [Google Scholar]
- Koretzky G.A. The role of Grb2-associated proteins in T-cell activation. Immunol. Today. 1997;18:401–406. doi: 10.1016/s0167-5699(97)01088-8. [DOI] [PubMed] [Google Scholar]
- Kreft J., Dumbsky M., Theiss S. The actin-polymerization protein from Listeria ivanovii is a large repeat protein which shows only limited amino acid sequence homology to ActA from Listeria monocytogenes . FEMS Microbiol. Lett. 1995;126:113–122. doi: 10.1111/j.1574-6968.1995.tb07403.x. [DOI] [PubMed] [Google Scholar]
- Kupfer A., Swain S.L., Singer S.J. The specific interaction of helper T cells and antigen-presenting B cells. J. Exp. Med. 1987;165:1565–1580. doi: 10.1084/jem.165.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A., Mosmann T.R., Kupfer H. Polarized expression of cytokines in cell conjugates of helper T-cells and splenic B cells. Proc. Natl. Acad. Sci. USA. 1991;88:775–779. doi: 10.1073/pnas.88.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L.M., Gates M.A., Witke W., Menzies S., Wehman A.M., Macklis J.D., Kwiatkowski D., Soriano P., Gertler F.B. Mena is required for neurulation and commissure formation. Neuron. 1999;22:313–325. doi: 10.1016/s0896-6273(00)81092-2. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A., Scheidegger D. The use of hybrid hybridomas to target human cytotoxic T lymphocytes. Eur. J. Immunol. 1987;17:105–111. doi: 10.1002/eji.1830170118. [DOI] [PubMed] [Google Scholar]
- Lasa I., David V., Gouin E., Marchand J.-B., Cossart P. The amino-terminal part of ActA is critical for the actin-based motility of Listeria monocytogenes; the central proline-rich region acts as a stimulator. Mol. Microbiol. 1995;18:425–436. doi: 10.1111/j.1365-2958.1995.mmi_18030425.x. [DOI] [PubMed] [Google Scholar]
- Lasa I., Gouin E., Goethals M., Vancompernolle K., David V., Vandekerckhove J., Cossart P. Identification of two regions in the N-terminal domain of ActA involved in the actin comet tail formation by Listeria monocytogenes . EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:1531–1540. doi: 10.1093/emboj/16.7.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent V., Loisel T.P., Harbeck B., Wehman A., Gröbe L., Jockusch B.M., Wehland J., Gertler F.B., Carlier M.-F. Role of proteins of the Ena/VASP family in actin-based motility of Listeria monocytogenes . J. Cell Biol. 1999;144:1245–1258. doi: 10.1083/jcb.144.6.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loisel T.P., Boujemaa R., Pantaloni D., Carlier M.F. Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature. 1999;401:613–616. doi: 10.1038/44183. [DOI] [PubMed] [Google Scholar]
- Lowin-Kropf B., Shapiro V.S., Weiss A. Cytoskeletal polarization of T-cells is regulated by an immunoreceptor tyrosine-based activation motif-dependent mechanism. J. Cell Biol. 1998;140:861–871. doi: 10.1083/jcb.140.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macalma T., Otte J., Hensler M.E., Bockholt S.M., Louis H.A., Kalff-Suske M., Grzeschik K.H., von der Ahe D., Beckerle M.C. Molecular characterization of human zyxin. J. Biol. Chem. 1996;271:31470–31478. doi: 10.1074/jbc.271.49.31470. [DOI] [PubMed] [Google Scholar]
- Machesky L.M., Insall R.H. Scar1 and the related Wiskott-Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr. Biol. 1998;8:1347–1356. doi: 10.1016/s0960-9822(98)00015-3. [DOI] [PubMed] [Google Scholar]
- Machesky L.M., Reeves E., Wientjes F., Mattheyse F.J., Grogan A., Totty N.F., Burlingame A.L., Hsuan J.J., Segal A.W. Mammalian actin-related 2/3 complex localizes to regions of lamellipodial protrusion and is composed of evolutionary conserved proteins. Biochem. J. 1997;328:105–112. doi: 10.1042/bj3280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky L.M., Mullins R.D., Higgs H.N., Kaiser D.A., Blanchoin L., May R.C., Hall M.E., Pollard T.D. Scar, a WASp-related protein, activates nucleation of actin filaments by the Arp2/3 complex. Proc. Natl. Acad. Sci. USA. 1999;96:3739–3744. doi: 10.1073/pnas.96.7.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May R.C., Hall M.E., Higgs H.N., Pollard T.D., Chakraborty T., Wehland J., Machesky L.M., Sechi A.S. The Arp2/3 complex is essential for the actin-based motility of Listeria monocytogenes . Curr. Biol. 1999;9:759–762. doi: 10.1016/s0960-9822(99)80337-6. [DOI] [PubMed] [Google Scholar]
- Motto D.G., Ross S.E., Jackman J.K., Sun Q., Olson A.L., Findell P.R., Koretzky G.A. In vivo association of Grb2 with pp116, a substrate of the T cell antigen receptor-activated protein tyrosine kinase. J. Biol. Chem. 1994;269:21608–21613. [PubMed] [Google Scholar]
- Motto D.G., Ross S.E., Jun W., Hendricks-Taylor L.R., Koretzky G.A. Implication of the GRB2-associated phosphoprotein SLP-76 in T cell receptor-mediated interleukin 2 production. J. Exp. Med. 1996;183:1937–1943. doi: 10.1084/jem.183.4.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musci M.A., Hendricks-Taylor L.R., Motto D.G., Paskind M., Kamens J., Turck C.W., Koretzky G.A. Molecular cloning of SLAP-130, an SLP-76-associated substrate of the T cell antigen receptor-stimulated protein tyrosine kinases. J. Biol. Chem. 1997;272:11674–11677. doi: 10.1074/jbc.272.18.11674. [DOI] [PubMed] [Google Scholar]
- Niebuhr K., Chakraborty T., Rhode M., Gazlig T., Jansen B., Köllner P., Wehland J. Localization of the ActA polypeptide of Listeria monocytogenes in infected tissue culture cell linesActA is not associated with actin comets. Infect. Immun. 1993;61:2693–2802. doi: 10.1128/iai.61.7.2793-2802.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niebuhr K., Ebel F., Frank R., Reinhard M., Domann E., Carl U.D., Walter U., Gertler F.B., Wehland J., Chakraborty T. A novel proline-rich motif present in ActA of Listeria monocytogenes and cytoskeletal proteins is the ligand for the EVH1 domain, a protein module present in the Ena/VASP family. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:5433–5444. doi: 10.1093/emboj/16.17.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niebuhr K., Lingnau A., Frank R., Wehland J. Rapid procedures for preparing monoclonal antibodies and identifying their epitopes. In: Celis J., editor. Cell BiologyA Laboratory Handbook. Vol. 2. Academic Press; San Diego, CA: 1998. pp. 398–403. [Google Scholar]
- Nonoyama S., Ochs H.D. Characterization of the Wiskott-Aldrich syndrome protein and its role in the disease. Curr. Opin. Immun. 1998;10:407–412. doi: 10.1016/s0952-7915(98)80113-1. [DOI] [PubMed] [Google Scholar]
- Olson M.F., Pasteris N.G., Gorski J.L., Hall A. Faciogenital dysplasia protein (FGD1) and Vav, two related proteins required for normal embryonic development, are upstream regulators of Rho GTPases. Curr. Biol. 1996;6:1628–1633. doi: 10.1016/s0960-9822(02)70786-0. [DOI] [PubMed] [Google Scholar]
- Pantaloni D., Carlier M.F. How profilin promotes actin filament assembly in the presence of thymosin β4. Cell. 1993;75:1007–1014. doi: 10.1016/0092-8674(93)90544-z. [DOI] [PubMed] [Google Scholar]
- Penninger J.M., Crabtree G.R. The actin cytoskeleton and lymphocyte activation. Cell. 1999;96:9–12. doi: 10.1016/s0092-8674(00)80954-x. [DOI] [PubMed] [Google Scholar]
- Pistor S., Chakraborty T., Walter U., Wehland J. The bacterial actin nucleator protein ActA of Listeria monocytogenes contains multiple binding sites for host microfilament proteins. Curr. Biol. 1995;5:517–525. doi: 10.1016/s0960-9822(95)00104-7. [DOI] [PubMed] [Google Scholar]
- Raab M., Kang H., da Silva A., Zhu X., Rudd C.E. FYN-T-FYB-SLP-76 interactions define a T-cell receptor zeta/CD3-mediated tyrosine phosphorylation pathway that up-regulates interleukin 2 transcription in T-cells. J. Biol. Chem. 1999;274:21170–21179. doi: 10.1074/jbc.274.30.21170. [DOI] [PubMed] [Google Scholar]
- Reinhard M., Halbrügge M., Scheer U., Wiegand C., Jockusch B.M., Walter U. The 46/50 kDa phosphoprotein VASP purified from human platelets is a novel protein associated with actin filaments and focal contacts. EMBO (Eur. Mol. Biol. Organ.) J. 1992;11:2063–2070. doi: 10.1002/j.1460-2075.1992.tb05264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard M., Giehl K., Abel K., Haffner C., Jarchau T., Hoppe V., Jockusch B.M., Walter U. The proline-rich focal adhesion and microfilament protein VASP is a ligand for profilins. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:1583–1589. doi: 10.1002/j.1460-2075.1995.tb07146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard M., Rüdiger M., Jockusch B.M., Walter U. VASP interaction with vinculina recurring theme of interactions with proline-rich motifs. FEBS Lett. 1996;399:103–107. doi: 10.1016/s0014-5793(96)01295-1. [DOI] [PubMed] [Google Scholar]
- Rivero-Lezcano O.M., Marcilla A., Sameshima J.H., Robbins K.C. Wiskott-Aldrich syndrome protein physically associates with Nck through Src homology 3 domains. Mol. Cell. Biol. 1995;15:5725–5731. doi: 10.1128/mcb.15.10.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R., Ma L., Miki H., Lopez M., Kirchhausen T., Takenawa T., Kirschner M. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell. 1999;97:1–20. doi: 10.1016/s0092-8674(00)80732-1. [DOI] [PubMed] [Google Scholar]
- Rottner K., Behrendt B., Small J.V., Wehland J. VASP dynamics during lamellipodia protrusion. Nat. Cell Biol. 1999;1:321–322. doi: 10.1038/13040. [DOI] [PubMed] [Google Scholar]
- Ryser J.-E., Rungger-Brändle E., Chaponnier C., Gabbiani G., Vassalli P. The area of attachment of cytotoxic T lymphocytes to their target cells shows high motility and polarization of actin, but not myosin. J. Immun. 1982;128:1159–1162. [PubMed] [Google Scholar]
- Sadler I., Crawford A.W., Michelsen J.W., Beckerle M.C. Zyxin and cCRPtwo interactive LIM domain proteins associated with the cytoskeleton. J. Cell Biol. 1992;119:1573–1587. doi: 10.1083/jcb.119.6.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler M., Durstin M.A., Frank D.A., Okuda K., Kaushansky K., Salgia R., Griffin J.D. The thrombopoietin receptor c-MPL activates JAK2 and TYK2 tyrosine kinases. Exp. Hematol. 1995;23:1040–1048. [PubMed] [Google Scholar]
- Secrist J.P., Burns L.A., Karnitz L., Koretzky G.A., Abraham R.T. Stimulatory effects of the protein tyrosine phosphatase inhibitor, pervanadate, on T-cell activation events. J. Biol. Chem. 1993;268:5886–5893. [PubMed] [Google Scholar]
- Smith G.A., Theriot J.A., Portnoy D.A. The tandem repeat domain in the Listeria monocytogenes ActA protein controls the rate of actin-based motility, the percentage of moving bacteria, and the localization of vasodilator-stimulated phosphoprotein and profilin. J. Cell Biol. 1996;135:647–660. doi: 10.1083/jcb.135.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper S.B., Rosen F.S., Mizoguchi E., Cohen P., Khan W., Liu C.-H., Hagemann T.L., Kwan S.-P., Ferrini R., Davidson L. Wiskott-Aldrich syndrome protein-deficient mice reveal a role for WASP in T but not B cell activation. Immunity. 1998;9:81–91. doi: 10.1016/s1074-7613(00)80590-7. [DOI] [PubMed] [Google Scholar]
- Symons M., Derry J.M.J., Karlak B., Jiang S., Lemahieu V., McCormick F., Francke U., Abo A. Wiskott-Aldrich syndrome protein, a novel effector for the GTPase CDC42Hs, is implicated in actin polymerization. Cell. 1996;84:723–734. doi: 10.1016/s0092-8674(00)81050-8. [DOI] [PubMed] [Google Scholar]
- Takubo T., Hino M., Suzuki K., Tatsumi N. Localization of myosin, actin, alpha-actinin, tropomyosin, and vinculin in surface-activated, spreading human platelets. Biotech. Histochem. 1998;73:310–315. doi: 10.3109/10520299809141124. [DOI] [PubMed] [Google Scholar]
- Tuosto L., Michel F., Acuto O. p95vav associates with tyrosine-phosphorylated SLP-76 in antigen stimulated T cells. J. Exp. Med. 1996;184:1161–1166. doi: 10.1084/jem.184.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valitutti S., Dessing M., Aktories K., Gallati H., Lanzavecchia A. Sustained signaling leading to T-cell activation results from prolonged T-cell receptor occupancy. Role of T-cell actin cytoskeleton. J. Exp. Med. 1995;181:577–584. doi: 10.1084/jem.181.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Troys M., Dewitte D., Goethals M., Carlier M.-F., Vanderkerckhove J., Ampe C. The actin binding site of thymosin β4 mapped by mutational analysis. EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:201–210. [PMC free article] [PubMed] [Google Scholar]
- Veale M., Raab M., Li Z., da Silva A.J., Kraeft S.K., Weremowicz S., Morton C.C., Rudd C.E. Novel isoform of lymphoid adaptor FYN-T-binding protein (FYB-130) interacts with SLP-76 and up-regulates interleukin 2 production. J. Biol. Chem. 1999;274:28427–28435. doi: 10.1074/jbc.274.40.28427. [DOI] [PubMed] [Google Scholar]
- Watson S.P., Gibbins J. Collagen receptor signalling in plateletsextending the role of the ITAM. Immunol. Today. 1998;19:260–264. doi: 10.1016/s0167-5699(98)01267-5. [DOI] [PubMed] [Google Scholar]
- Weiss A., Littman D.R. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- Welch M.D., Iwamatsu A., Mitchison T.J. Actin polymerization is induced by Arp2/3 protein complex at the surface of Listeria monocytogenes Nature. 385 1997. 265 269a [DOI] [PubMed] [Google Scholar]
- Welch M.D., DePace A.H., Verma S., Iwamatsu A., Mitchison T.J. The human Arp2/3 complex is composed of evolutionarily conserved subunits and is localized to cellular regions of dynamic actin filament assembly J. Cell Biol. 138 1997. 375 384b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch M.D., Rosenblatt J., Skoble J., Portnoy D.A., Mitchison T.J. Interaction between the human Arp2/3 complex and Listeria monocytogenes ActA protein in actin filament nucleation. Science. 1998;281:105–108. doi: 10.1126/science.281.5373.105. [DOI] [PubMed] [Google Scholar]
- Wills Z., Bateman J., Korey C.A., Comer A., Van Vactor D. The tyrosine kinase Abl and its substrate enabled collaborate with the receptor phosphatase Dlar to control motor axon guidance. Neuron. 1999;22:301–312. doi: 10.1016/s0896-6273(00)81091-0. [DOI] [PubMed] [Google Scholar]
- Winter D., Lechler T., Li R. Activation of the yeast Arp2/3 complex by bee1p, a WASP-family protein. Curr. Biol. 1999;9:501–504. doi: 10.1016/s0960-9822(99)80218-8. [DOI] [PubMed] [Google Scholar]
- Xavier R., Brennan T., Li Q., McCormack C., Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:723–732. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- Yarar D., To W., Abo A., Welch M.D. The Wiskott-Aldrich syndrome protein directs actin-based motility by stimulating actin nucleation with the Arp2/3 complex. Curr. Biol. 1999;9:555–558. doi: 10.1016/s0960-9822(99)80243-7. [DOI] [PubMed] [Google Scholar]
- Zhang W., Sloan-Lancaster J., Kitchen J., Trible R.P., Samelson L.E. LATthe ZAP-70 tyrosine kinase substrate that links T-cell receptor to cellular activation Cell. 92 1998. 83 92a [DOI] [PubMed] [Google Scholar]
- Zhang W., Trible R.P., Samelson L.E. LAT palmitoylationits essential role in membrane microdomain targeting and tyrosine phosphorylation during T-cell activation Immunity. 9 1998. 239 246b [DOI] [PubMed] [Google Scholar]