Abstract
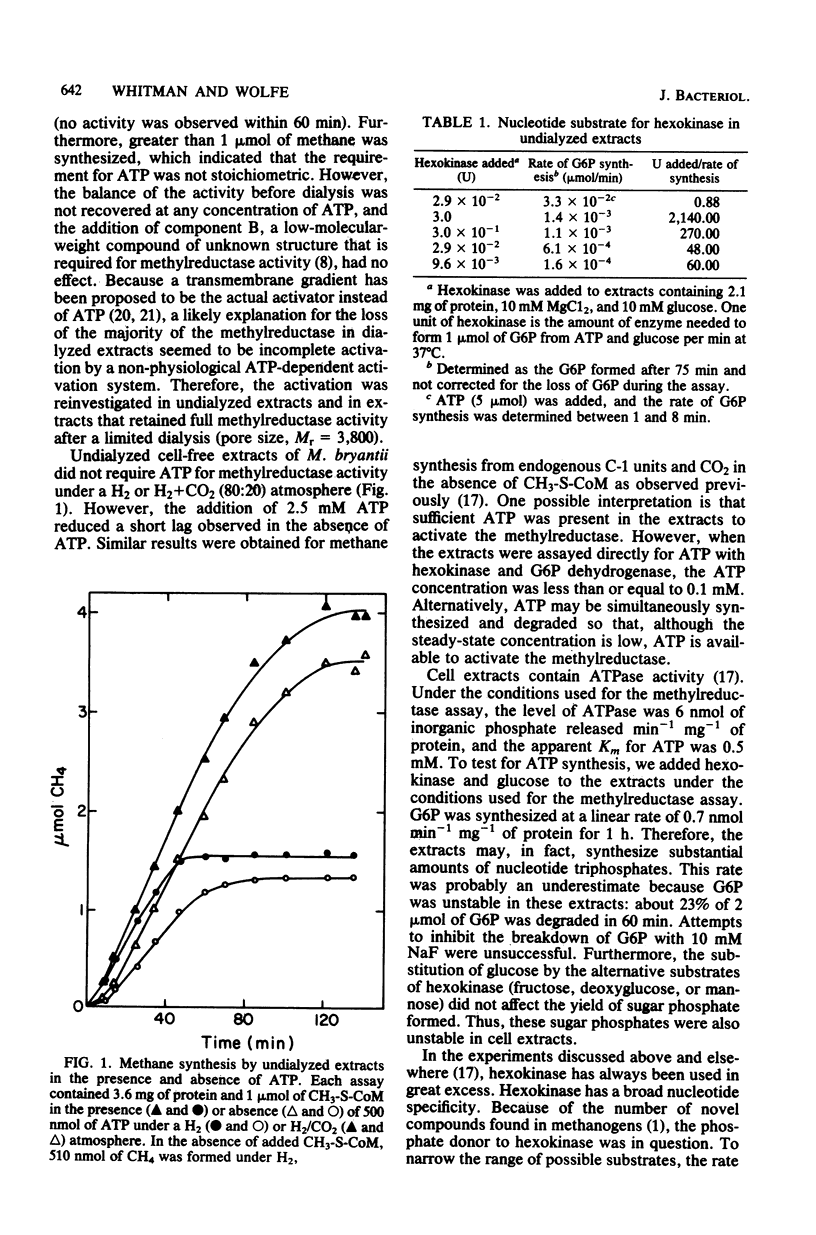
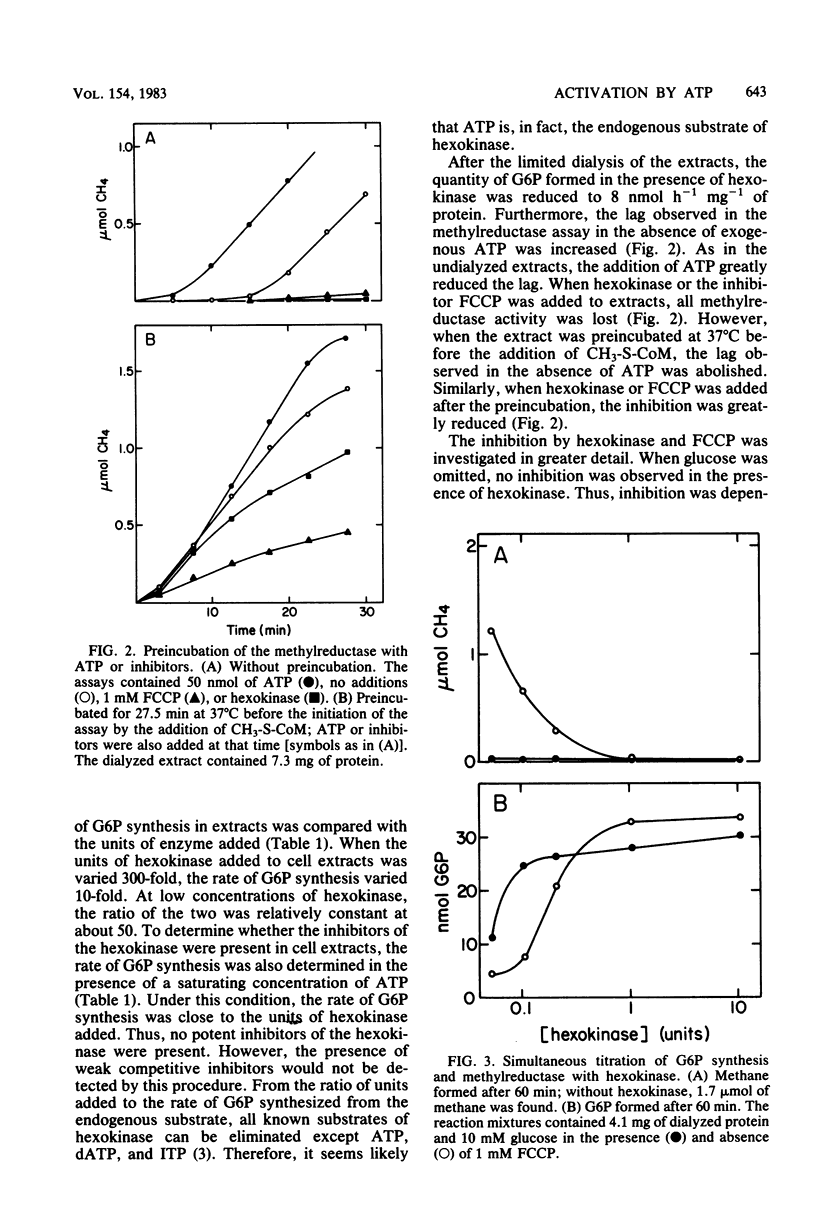
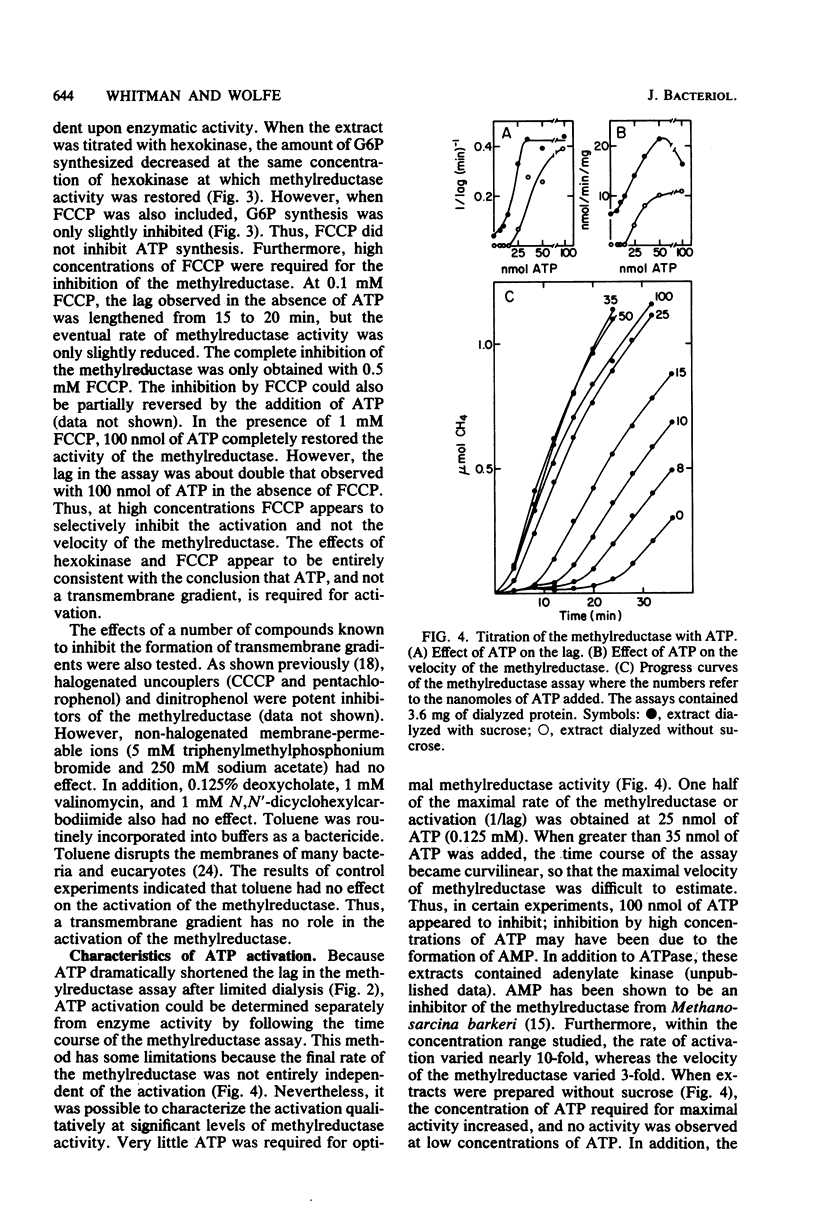
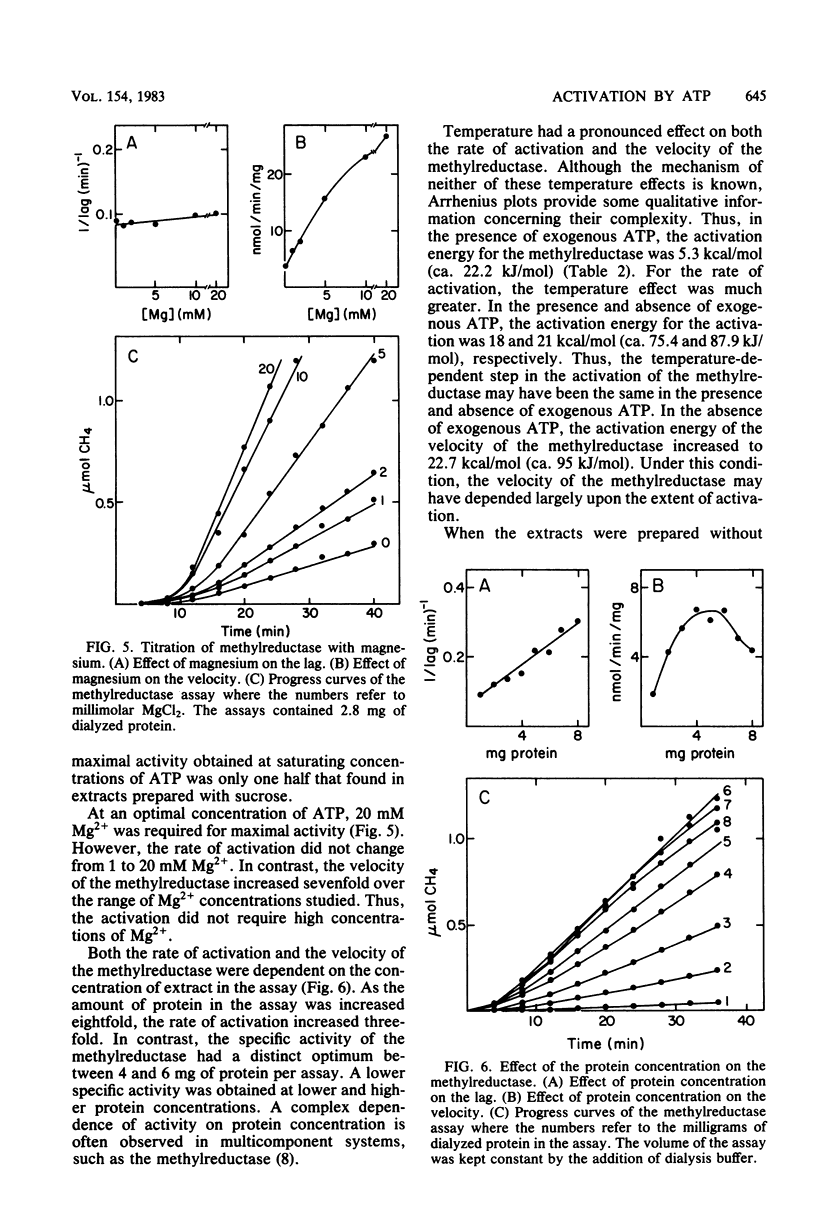
The methylreductase of Methanobacterium bryantii required ATP for activity. There was sufficient ATP synthesis in extracts to account for the observed activity. Hexokinase inhibited the methylreductase by competing for endogenously synthesized ATP. The uncoupler, carbonyl cyanide p-trifluoromethyoxyphenyl hydrazone, inhibited only at concentrations greater than 0.5 mM, and detergents and non-halogenated membrane-permeable-ions did not inhibit. Thus, membrane proton gradients are not important in activation. In addition, maximal activation was obtained with less than 0.25 mM ATP, was inhibited by beta, gamma-imido ATP, and was strongly temperature dependent. The activated state was very unstable, having a half-life of 5 to 15 min. After gel filtration at 5 degrees C, the methylreductase retained partial activity for a short time in the absence of ATP. These observations indicate that activation involves the modification of a protein or protein-bound cofactor of the methylreductase system.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doddema H. J., Hutten T. J., van der Drift C., Vogels G. D. ATP hydrolysis and synthesis by the membrane-bound ATP synthetase complex of Methanobacterium thermoautotrophicum. J Bacteriol. 1978 Oct;136(1):19–23. doi: 10.1128/jb.136.1.19-23.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doddema H. J., van der Drift C., Vogels G. D., Veenhuis M. Chemiosmotic coupling in Methanobacterium thermoautotrophicum: hydrogen-dependent adenosine 5'-triphosphate synthesis by subcellular particles. J Bacteriol. 1979 Dec;140(3):1081–1089. doi: 10.1128/jb.140.3.1081-1089.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellefson W. L., Wolfe R. S. Role of component C in the methylreductase system of Methanobacterium. J Biol Chem. 1980 Sep 25;255(18):8388–8389. [PubMed] [Google Scholar]
- Gunsalus R. P., Wolfe R. S. ATP activation and properties of the methyl coenzyme M reductase system in Methanobacterium thermoautotrophicum. J Bacteriol. 1978 Sep;135(3):851–857. doi: 10.1128/jb.135.3.851-857.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus R. P., Wolfe R. S. Methyl coenzyme M reductase from Methanobacterium thermoautotrophicum. Resolution and properties of the components. J Biol Chem. 1980 Mar 10;255(5):1891–1895. [PubMed] [Google Scholar]
- Kaback H. R., Reeves J. P., Short S. A., Lombardi F. J. Mechanisms of active transport in isolated bacterial membrane vesicles. 18. The mechanism of action of carbonylcyanide m-chlorophenylhydrazone. Arch Biochem Biophys. 1974 Jan;160(1):215–222. doi: 10.1016/s0003-9861(74)80028-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lanyi J. K., MacDonald R. E. Existence of electrogenic hydrogen ion/sodium ion antiport in Halobacterium halobium cell envelope vesicles. Biochemistry. 1976 Oct 19;15(21):4608–4614. doi: 10.1021/bi00666a010. [DOI] [PubMed] [Google Scholar]
- McBride B. C., Wolfe R. S. A new coenzyme of methyl transfer, coenzyme M. Biochemistry. 1971 Jun 8;10(12):2317–2324. doi: 10.1021/bi00788a022. [DOI] [PubMed] [Google Scholar]
- McFadden B. A. Autotrophic CO2 assimilation and the evolution of ribulose diphosphate carboxylase. Bacteriol Rev. 1973 Sep;37(3):289–319. doi: 10.1128/br.37.3.289-319.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountfort D. O. Effect of adenosine 5'-monophosphate on adenosine 5'-triphosphate activation of methyl coenzyme M methylreductase in cell extracts of Methanosarcina barkeri. J Bacteriol. 1980 Aug;143(2):1039–1041. doi: 10.1128/jb.143.2.1039-1041.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountfort D. O. Evidence from ATP synthesis driven by a proton gradient in Methanosarcina barkeri. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1346–1351. doi: 10.1016/0006-291x(78)91151-8. [DOI] [PubMed] [Google Scholar]
- Portis A. R., Jr, Chon C. J., Mosbach A., Heldt H. W. Fructose-and sedoheptulosebisphosphatase. The sites of a possible control of CO2 fixation by lightdependent changes of the stromal Mg2+ concentration. Biochim Biophys Acta. 1977 Aug 10;461(2):313–325. doi: 10.1016/0005-2728(77)90181-5. [DOI] [PubMed] [Google Scholar]
- Roberton A. M., Wolfe R. S. Adenosine triphosphate pools in Methanobacterium. J Bacteriol. 1970 Apr;102(1):43–51. doi: 10.1128/jb.102.1.43-51.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson A. M., Wolfe R. S. ATP requirement for methanogenesis in cell extracts of methanobacterium strain M.o.H. Biochim Biophys Acta. 1969 Dec 30;192(3):420–429. doi: 10.1016/0304-4165(69)90391-2. [DOI] [PubMed] [Google Scholar]
- Romesser J. A., Balch W. E. Coenzyme M: preparation and assay. Methods Enzymol. 1980;67:545–552. doi: 10.1016/s0076-6879(80)67067-0. [DOI] [PubMed] [Google Scholar]
- Sauer F. D., Erfle J. D., Mahadevan S. Evidence for an internal electrochemical proton gradient in Methanobacterium thermoautotrophicum. J Biol Chem. 1981 Oct 10;256(19):9843–9848. [PubMed] [Google Scholar]
- Sauer F. D., Erfle J. D., Mahadevan S. Methane production by the membranous fraction of Methanobacterium thermoautotrophicum. Biochem J. 1980 Jul 15;190(1):177–182. doi: 10.1042/bj1900177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer F. D., Erfle J. D., Mahadevan S. Methane synthesis without the addition of adenosine triphosphate by cell membranes isolated from Methanobacterium ruminantium. Biochem J. 1979 Jan 15;178(1):165–172. doi: 10.1042/bj1780165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer F. D., Mahadevan S., Erfle J. D. Valinomycin inhibited methane synthesis in Methanobacterium thermoautotrophicum. Biochem Biophys Res Commun. 1980 Jul 31;95(2):715–721. doi: 10.1016/0006-291x(80)90844-x. [DOI] [PubMed] [Google Scholar]
- Serrano R., Gancedo J. M., Gancedo C. Assay of yeast enzymes in situ. A potential tool in regulation studies. Eur J Biochem. 1973 May 2;34(3):479–482. doi: 10.1111/j.1432-1033.1973.tb02783.x. [DOI] [PubMed] [Google Scholar]
- Taylor C. D., Wolfe R. S. Structure and methylation of coenzyme M(HSCH2CH2SO3). J Biol Chem. 1974 Aug 10;249(15):4879–4885. [PubMed] [Google Scholar]
- WOLIN E. A., WOLIN M. J., WOLFE R. S. FORMATION OF METHANE BY BACTERIAL EXTRACTS. J Biol Chem. 1963 Aug;238:2882–2886. [PubMed] [Google Scholar]
- WOLIN M. J., WOLIN E. A., WOLFE R. S. ATP-DEPENDENT FORMATION OF METHANE FROMMETHYLCOBALAMIN BY EXTRACTS OF METHANOBACILLUS OMELIANSKII. Biochem Biophys Res Commun. 1963 Aug 20;12:464–468. doi: 10.1016/0006-291x(63)90316-4. [DOI] [PubMed] [Google Scholar]



