Abstract
Phosphatidylethanolamine (PE) is a major membrane phospholipid that is mainly localized in the inner leaflet of the plasma membrane. We previously demonstrated that PE was exposed on the cell surface of the cleavage furrow during cytokinesis. Immobilization of cell surface PE by a PE-binding peptide inhibited disassembly of the contractile ring components, including myosin II and radixin, resulting in formation of a long cytoplasmic bridge between the daughter cells. This blockade of contractile ring disassembly was reversed by removal of the surface-bound peptide, suggesting that the PE exposure plays a crucial role in cytokinesis. To further examine the role of PE in cytokinesis, we established a mutant cell line with a specific decrease in the cellular PE level. On the culture condition in which the cell surface PE level was significantly reduced, the mutant ceased cell growth in cytokinesis, and the contractile ring remained in the cleavage furrow. Addition of PE or ethanolamine, a precursor of PE synthesis, restored the cell surface PE on the cleavage furrow and normal cytokinesis. These findings provide the first evidence that PE is required for completion of cytokinesis in mammalian cells, and suggest that redistribution of PE on the cleavage furrow may contribute to regulation of contractile ring disassembly.
Keywords: actin, cell division, phospholipid asymmetry, phospholipid-binding peptide, Chinese hamster ovary cell mutant
Introduction
The function of cytokinesis, the division of one cell into two, is to create a membranous barrier between the two daughter cells (Fishkind and Wang 1995; Glotzer 1997). The interaction of actin filaments with bipolar myosin II filaments applies tension to the membrane to form a cleavage furrow, which gradually deepens until it encounters the narrow remains of the mitotic spindle between the two nuclei. During the final stage of cytokinesis, both the microtubules and contractile ring at the cleavage furrow disassemble, which is followed by the fusion of opposing plasma membranes and cell separation. In the embryonic divisions of animal cells, new membrane is inserted behind the leading edge of the invaginating furrow, which may be achieved by the fusion of vesicular membrane with plasma membrane (Bluemink and deLaat 1973; Drechsel et al. 1997). Since membrane fusion requires the merging of lipid bilayers of the membranes (Zimmerberg et al. 1993), it is likely that the coordinated changes in the cytoskeleton and membrane lipids are essential for achieving successful cell division.
It is well established that phospholipids in biological membranes are distributed asymmetrically between the inner and outer leaflets of the lipid bilayer (Schroit and Zwaal; 1991; Zachowski 1993). In many eukaryotic plasma membranes, aminophospholipids, such as phosphatidylethanolamine (PE) and phosphatidylserine (PS), reside in the inner leaflet, whereas choline-containing phospholipids, such as phosphatidylcholine and sphingomyelin, are localized mainly in the outer leaflet. An increase in intracellular Ca2+ due to cell activation, cell injury, or apoptosis causes a rapid bidirectional movement of phospholipids, resulting in exposure of PE and PS on the cell surface (Schroit and Zwaal 1991; Emoto et al. 1997). The exposure of PS on the cell surface was shown to play a role in cell–cell interaction (Nishikawa et al. 1990; Fadok et al. 1992) and provide a surface for initiation of blood coagulation (Mann et al. 1998; Comfurius et al. 1994). It was suggested that the transmembrane movement of phospholipids may regulate the dynamic movement of membranes, such as vesicle fusion and fission (Devaux 1991), but no experimental evidence has been provided.
To study the molecular motion and the cellular functions of membrane phospholipids, we have established various phospholipid-binding probes that include mAbs against: phosphatidylcholine (Nam et al. 1990); PS (Umeda et al. 1989); and phosphatidylinositol 4,5-bisphosphate (Miyazawa et al. 1988); a tetracyclic polypeptide of 19 amino acids (Ro09-0198) that binds specifically to PE (Aoki et al. 1994); and lysenin, a sphingomyelin-specific binding protein derived from the earthworm (Yamaji et al. 1998). These probes have provided useful tools to study cellular localization (Miyazawa et al. 1988; Fujimoto et al. 1996; Boronenkov et al. 1998) and function (Stekhoven et al. 1994; Igarashi et al. 1995; Bogdanov et al. 1999) of phospholipids. Using the PE-binding peptide (Ro) conjugated to streptavidin (SA) (SA-Ro), we demonstrated that PE was exposed on the cell surface of the cleavage furrow as a result of enhanced transbilayer movement of the phospholipids at the final stage of cytokinesis (Emoto et al. 1996). Furthermore, addition of SA-Ro to the dividing cells prevented cytokinesis, which suggests that PE plays a role in completion of cytokinesis.
In this study, we examined in detail the effects of SA-Ro on the movement of the contractile ring in synchronized CHO cells. To further examine the role of PE in cytokinesis, we established and analyzed a mutant CHO cell line defective in PE biosynthesis (Emoto et al. 1999). The results obtained from both approaches clearly show that PE is required for proper progression of cytokinesis. In addition, our findings suggest that redistribution of PE on the cleavage furrow membrane may play a critical role in controlling the disassembly of the contractile ring.
Materials and Methods
Materials
Lipids were purchased from Avanti Polar Lipids, Inc. Ro peptide was kindly provided by H. Ishitsuka (Nippon Roch Research Center, Japan). Ro peptide was biotinylated and then conjugated to SA as described previously (Emoto et al. 1996). For the ethanolamine-supplementation experiment, 100 ml of newborn calf serum was dialyzed three times against 2 liters of phosphate-buffered saline for 12 h and filter-sterilized. Other chemicals were from Sigma-Aldrich.
Isolation of CHO Mutants Resistant to Ro Peptide-induced Cytolysis
CHO-K1 fibroblasts were obtained from the American Type Culture Collection (CCL 61). R-41, a CHO-K1–derived mutant cell line, was established as a variant resistant to Ro peptide as described (Emoto et al. 1999). In brief, for mutagenesis, exponentially growing cells were treated with 400 μg/ml ethyl methanesulfonate in growth medium at 37°C for 16 h, followed by incubation at 33°C for 3 d. The mutagenized cell colonies were replicated onto polyester discs for screening of mutant cells exhibiting a low binding activity to Ro peptide. The polyester discs were incubated for 24 h in growth medium at 39.5°C, washed twice with Ham's F-12 medium, and then incubated with 125I-labeled SA-Ro peptide complex (125I–SA-Ro) for 1 h at 39.5°C. The radioactivities bound to the colonies were analyzed by a Fuji bioimage analyzer, and a mutant exhibiting a low binding activity was isolated. The mutant cells were mutagenized again and cell monolayers were incubated in Ham's F-12 medium containing 5 μM Ro peptide at 39.5°C for 1 h, washed with Ham's F-12 medium, and cultured in the growth medium at 33°C for 20 d. Colonies of surviving cells were cloned and further purified by limiting dilution. CHO-K1 and R-41 mutant cells were cultured in Ham's F-12 medium supplemented with 10% newborn calf serum, penicillin G (100 U/ml), and streptomycin sulfate (100 μg/ml) in a 5% CO2/95% air incubator at 37°C. For ethanolamine-deficient culture, cells were incubated in Ham's F-12 medium supplemented with 10% dialyzed newborn calf serum at 39.5°C for indicated periods.
Incubation of Mitotic Cells with PE-binding Peptide
Mitotic cell populations were isolated from monolayer cultures grown in 850-cm2 plastic roller bottles (Falcon). In brief, each bottle was seeded with 8.8 × 106 cells in 100 ml growth medium. After incubation at 37°C for 48 h, the culture medium was replaced with growth medium containing 0.04 μg/ml nocodazole. The cells were incubated for 2 h at 37°C, and prometaphase cells were isolated by shaking.
Prometaphase cells (2 × 105 cells) were incubated with 100 μg/ml Ro peptide-SA complex in 1 ml of 0.5% BSA-Ham's F12 medium containing 1% Neutridoma (Boehringer) at 37°C for various periods. The effects on cell division were quantified by counting the number of the cells that exhibited either a cytoplasmic bridge or a dumbbell-shaped cytokinetic form per field of view, one field containing ∼100 cells. Time-lapse observation was performed in a Zeiss cell incubation system and with a Zeiss Axiovert 135 inverted microscope equipped with a 20× Plan-Apochromat objective.
For the release of Ro peptide-SA complex from the cell membrane, the peptide-treated cells were washed three times with Ham's F12 medium containing 10 μM PE liposomes (PE/PC = 1:1) and 1% Neutridoma, and further incubated in the same medium at 37°C.
Fluorescence Microscopy
Cells were fixed with 3.7% (wt/vol) formaldehyde in PBS for 30 min at room temperature, followed by a 5-min permeabilization with 0.1% Triton X-100 in PBS. The cells were then blocked with PBS containing 10% (wt/vol) goat serum for 30 min at 25°C and reacted with the first antibodies for 16 h at 4°C. Rat antiyeast tubulin mAb (ICN), mouse antivimentin mAb (ICN), rabbit antimyosin II polyclonal antibody (Biomedical Tech.) and rabbit anti-ERM protein polyclonal antibody (kindly provided by Dr. S. Tsukita, Kyoto University, Japan) were diluted to 1:100. Then the cells were washed with PBS and incubated with rhodamine-conjugated goat anti–rat IgG or FITC-conjugated goat anti–rabbit IgG (Cappel) diluted to 1:100 for 1 h at 37°C. For F-actin and DNA staining, cells were incubated with 40 ng/ml TRITC-phalloidin (Sigma-Aldrich) and 0.2 μg/ml 4′,6′-diamidino-2-phenylindole (DAPI; Molecular Probe) in PBS at 25°C for 1 h, washed, and photographed. Fluorescence microscopy was performed using a Zeiss Axioplan microscope equipped with Planneofluar 40× and 100× objective. Photographs were taken on Fuji NEOPAN 400 film.
Flow Cytometry
Cells were incubated in Ham's F-12 medium containing 10% dialyzed newborn calf serum supplemented with or without 20 μM ethanolamine at 39.5°C for 3 d. Cells were then trypsinized and fixed in cold 70% ethanol. Cell cycle parameters were determined by propidium iodide labeling of nuclear DNA. The labeled cells were analyzed on a flow cytometer, FACScan (Becton Dickinson).
Lipid Analysis
Cells were seeded at 5 × 105 cells per 100 diameter dish and were then incubated in normal growth medium at 39.5°C for 3 d. To create ethanolamine deficiency, cells were cultured in Ham's F-12 medium supplemented with 10% dialyzed newborn calf serum in the presence or absence of either 30 μM PE or 20 μM ethanolamine. After washing twice with PBS, the cells were harvested by scraping, and the cellular lipids were extracted from the harvested cells (Bligh and Dyer 1959). Phospholipids were separated by two-dimensional thin-layer chromatography. Solvent systems used for chromatography were as follows: first dimension, chloroform/methanol/acetic acid, 65/2510 (vol/vol); second dimension, chloroform/methanol/formic acid, 65/25/10 (vol/vol). Phospholipid phosphorus was chemically determined as described (Zhou and Arthur 1992).
Results
Inhibition of Contractile Ring Disassembly by SA-Ro
To study the role of PE in cytokinesis, we examined the effects of Ro peptide, a PE-binding peptide, on cytokinesis. Time-lapse observations revealed that SA-Ro–treated cells formed cleavage furrows normally, and fully contracted to produce daughter cells, but the cells could not part and remained connected by a cytoplasmic bridge (Fig. 1 a). Approximately 60% (57.5 ± 7.3%, n = 7) of the cells formed a long intracellular cytoplasmic bridge between daughter cells. These cells remained connected up to 16 h after the initiation of cytokinesis. No significant effect on cytokinesis was detected using other phospholipid-specific probes, including annexin V that binds to PS (Koopman et al. 1994), and antiphospholipid mAbs specific for either PS (Umeda et al. 1989), phosphatidylcholine (Nam et al. 1990), or phosphatidylinositol-4,5-bisphosphate (Miyazawa et al. 1988; data not shown). These results confirm our previous data obtained with cell suspensions (Emoto et al. 1996), and suggest that PE plays a significant role in the late stage of cytokinesis.
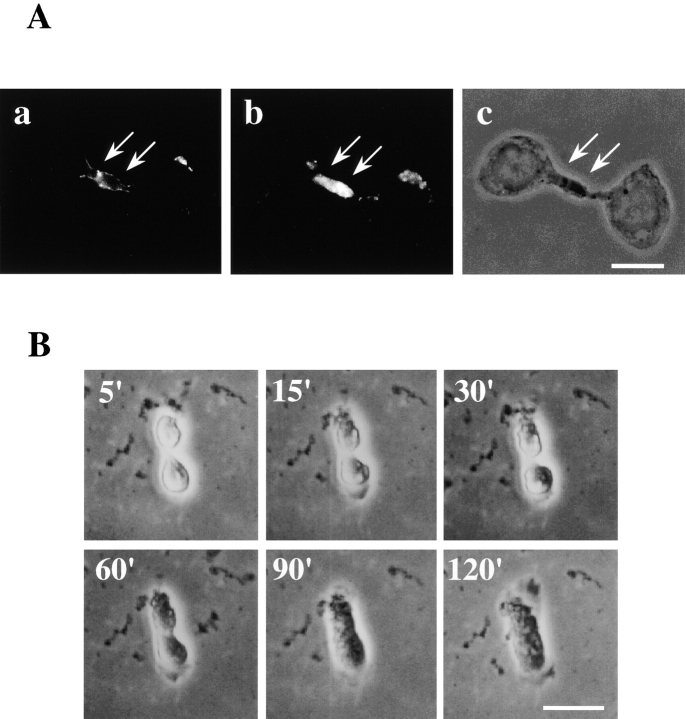
Figure 1.
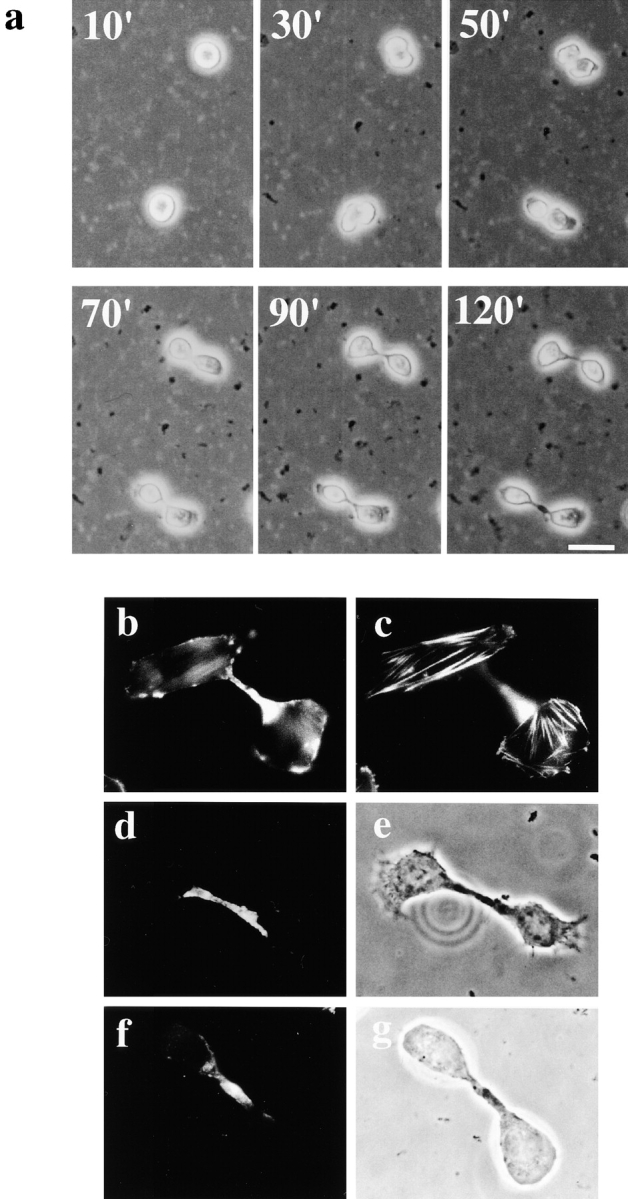
SA-Ro treatment blocks disassembly of the contractile ring. a, Time-lapse observation of cytokinesis of cells treated with SA-Ro. The time shown in the photograph represents time points from the initiation of cell division (min). Bar, 30 μm. b and c, CHO cells synchronized in prometaphase were incubated with 50 μg/ml SA-Ro for 2 h, and then fixed and stained with rhodamine-phalloidin. b, Shows the focal plane of the cytoplasmic bridge where actin bundles remains concentrated. c, Shows the focal plane of the bottom layer of the daughter cells where stress fibers are seen. d–g, Distribution of myosin II (d) and radixin (f) in SA-Ro–treated cells. Phase-contrast of each specimen was shown in e and g, respectively. Bar, 10 μm.
Staining with phalloidin showed that most of SA-Ro–treated cells contained actin filament bundles in the cytoplasmic bridge even 2 h after the beginning of cytokinesis, although new stress fibers and nuclear envelopes already appeared in the daughter cells (Fig. 1b and Fig. c). Immunofluorescence staining revealed that other components of the contractile ring, such as myosin II (Schroeder 1973; Mittal et al. 1987) and radixin (Sato et al. 1991; Tsukita et al. 1997), also remained in the cytoplasmic bridge (Fig. 1, d–g). Other cytoskeletal components, such as microtubules (Wgeatly and Wang, 1996) and intermediate filaments (Inagaki et al. 1996), had disappeared from the cytoplasmic bridge (data not shown). These results indicate that SA-Ro treatment does not affect the formation and contraction of the contractile ring, nor the rearrangement of microtubules and intermediate filaments, but specifically blocks the disassembly of the contractile ring.
Incubation of SA-Ro–treated Cells with PE Liposome Leads to Disassembly of Contractile Ring
To further analyze the effect of Ro peptide on cytokinesis, we examined the distribution of SA-Ro bound to the arrested cell membrane. SA-Ro bound predominantly to the surface of the cytoplasmic bridge (Fig. 2 A). In addition, the actin filaments concentrated in the cytoplasmic bridge colocalized just underneath the plasma membrane to which the SA-Ro bound (Fig. 2 A). These results, together with our previous finding that PE is predominantly exposed on the cleavage furrow surface during late telophase (Emoto et al. 1996), imply that the peptide binding to the PE exposed on the cleavage furrow surface causes inhibition of contractile ring disassembly. To examine this possibility, we attempted to remove SA-Ro from the cell surface of the SA-Ro–treated cells. When SA-Ro–treated cells were incubated in tissue culture medium containing 10 μM PE liposomes, the contraction in the cytoplasmic bridge was gradually released and eventually the daughter cells fused with each other to form binucleated cells within 2 h (Fig. 2 B). No significant binding of SA-Ro to the binucleated cells was observed, which suggests that the cell-surface bound SA-Ro was absorbed by the PE liposomes (Fig. 2 C). The contractile ring remaining in the cytoplasmic bridge disappeared and stress fibers were formed throughout the binucleated cells (Fig. 2 C). Myosin II and radixin that had been concentrated in the cytoplasmic bridge translocated to cytoplasm in the binucleated cells (Fig. 2 C). These results demonstrate that the inhibition of contractile ring disassembly is reversible upon removal of the surface-bound SA-Ro, and suggest that exposure of PE on the cleavage furrow membrane plays a crucial role in the regulation of contractile ring disassembly.
Figure 2.
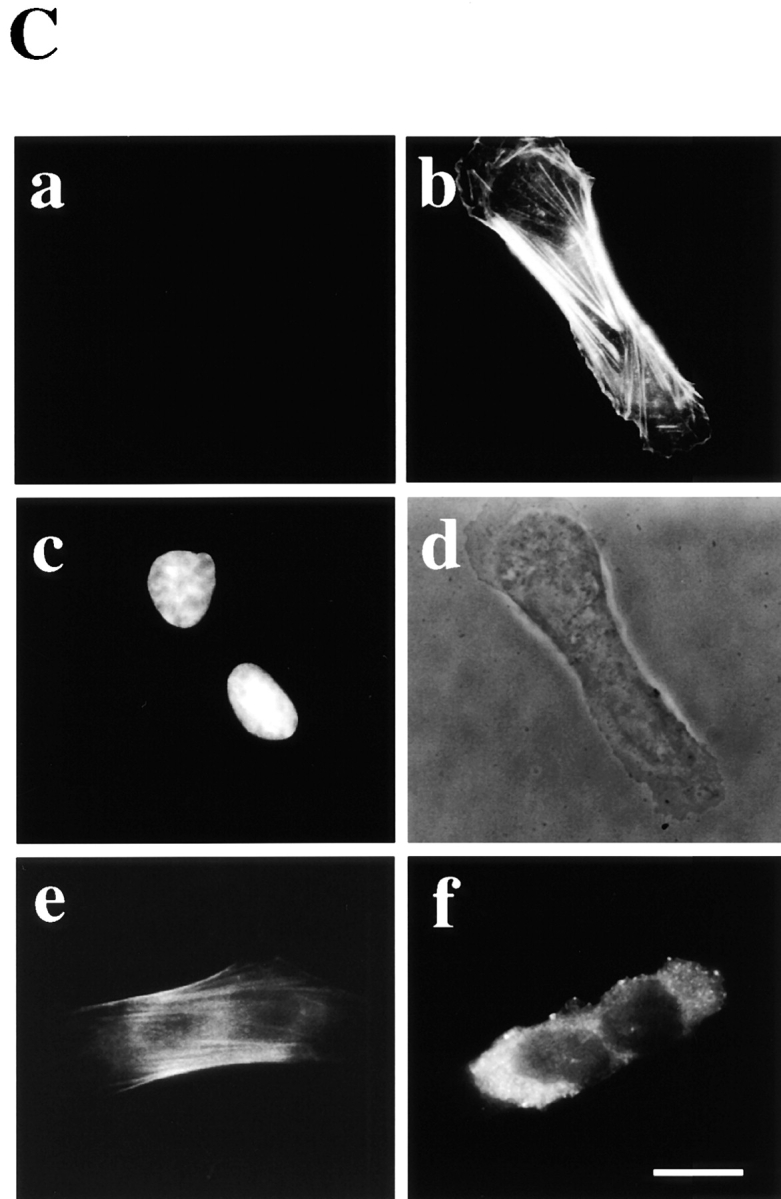
Incubation of the arrested cells with PE liposomes leads to disassembly of the contractile ring. A, Colocalization of SA-Ro bound to the cell surface of PE (a) and actin filaments remaining in the cytoplasmic bridge (b). Cells were incubated with 50 μg/ml SA-Ro for 2 h, and then fixed and double-labeled with either anti-SA antibody (a) or rhodamine-phalloidin (b). The photograph shows the focal plane of the cytoplasmic bridge where actin bundles remain concentrated. Phase-contrast of the specimen is shown in c. The arrows indicate the cytoplasmic bridge. B, Time-lapse observations of the arrested cells incubated in the presence of 10 μM PE liposomes. The time shown in the photograph represents the time points after initiation of the incubation (min). C, The arrested cells were incubated in the presence of 10 μM PE liposomes, and then fixed and triple-stained with anti-SA antibody (a), rhodamine-phalloidin (b), and DAPI (c). Phase-contrast of the same specimen is shown in (d). e and f, The arrested cells were incubated with 10 μM PE liposomes, and then fixed and stained with antimyosin II antibody (e) and antiradixin antibody (f). Bars, 10 μm.
Cell Growth of a CHO Mutant Line with a Specific Lesion in PE Biosynthesis
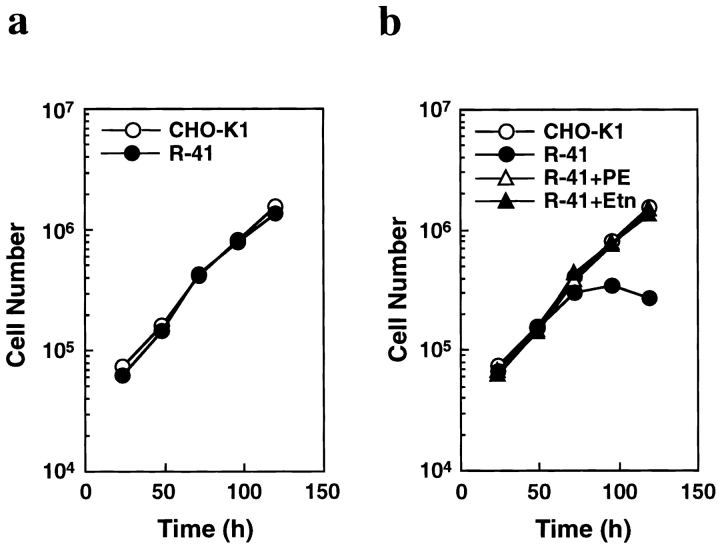
To further study the role of PE in cytokinesis, we established a CHO mutant cell line with a specific defect in PE biosynthesis. The mutant, designated as R-41, was isolated as a variant resistant to the cytotoxicity of Ro peptide (Emoto et al. 1999). When the mutant cells were cultured in normal growth medium containing 10% newborn calf serum for three days, cellular PE content was about half that of the wild-type CHO-K1 cells, whereas the levels of other phospholipids, including PS, were not significantly changed (Table ). In these circumstances, the mutant cells grew normally (Fig. 3 a). PE in mammalian cells is produced by three distinct pathways: the PS decarboxylase pathway, the CDP–ethanolamine pathway, and the phospholipid base–exchange pathway (Kent 1995). We have demonstrated that R-41 mutant cells have a specific defect in the PS decarboxylation pathway, but were not affected in the CDP-ethanolamine pathway (Emoto et al. 1999). Thus, we next examined if exogenous ethanolamine derived from newborn calf serum contributes to the restoration of PE biosynthesis in R-41 mutant cells using dialyzed newborn calf serum. CHO-K1 cells grew exponentially in the medium supplemented with 10% dialyzed newborn serum, whereas the mutant cells ceased growing (Fig. 3 b). When either PE or ethanolamine was added to the medium at the concentration of 30 or 20 μM, respectively, the mutant cells were able to grow normally for five days (Fig. 3 b). Other phospholipids, such as PS and phosphatidylcholine, failed to restore normal growth of the mutant cells (data not shown). The PE level of the mutant cells grown in the medium with the dialyzed serum for three days was reduced to approximately five percent of total cellular phospholipids, whereas the addition of PE or ethanolamine to the medium restored a normal PE level in the mutant cells (Table ). These results indicate that the growth of R-41 mutant cells is dependent on exogenous PE or its precursor and provide the first evidence that a certain level of PE is essential for cell growth in CHO cells.
Table 1.
Phospholipid Compositions of CHO-K1 and R-41 Mutant Cells Cultured in Normal Growth Medium
| Percentage of total phospholipids | ||||||
|---|---|---|---|---|---|---|
| Strain | PE | PS | PC | SM | PI | Others |
| CHO-K1 | 17.6 ± 1.6 | 5.5 ± 0.8 | 58.9 ± 3.8 | 7.8 ± 1.1 | 6.8 ± 0.8 | 3.4 ± 0.4 |
| R-41 | 9.0 ± 0.9 | 5.9 ± 0.5 | 68.8 ± 4.3 | 8.9 ± 1.3 | 5.9 ± 1.2 | 2.1 ± 0.3 |
Cells were incubated in Ham's F-12 medium containing 10% newborn calf serum at 39.5°C for 3 d. Phospholipids were extracted, separated, and quantified. PE, Phosphatidylethanolamine; PS, phosphatidylserine; PC, phosphatidylcholine; SM, sphingomyelin; PI, phosphatidylinositol. Others include phosphatidylglycerol, phosphatidic acid, and cardiolipin. Data shown are the mean ± SD for three independent experiments.
Figure 3.
The growth of R-41 mutant cells is dependent on PE or its precursor. a, CHO-K1 and R-41 mutant cells were cultured in normal medium containing 10% newborn calf serum at 39.5°C. b, CHO-K1 and R-41 mutant cells were cultured in ethanolamine-deficient medium at 39.5°C (CHO-K1, R-41), and R-41 mutant cells were cultured in either 30 μM PE (R-41+PE) or 20 μM ethanolamine-supplemented medium (R-41+Etn) at 39.5°C.
Table 2.
Phospholipid Compositions of CHO-K1 and R-41 Mutant Cells Cultured in Ethanolamine-deficient Medium
| Percentage of total phospholipids | ||||||
|---|---|---|---|---|---|---|
| Strain | PE | PS | PC | SM | PI | Others |
| CHO-K1 | 12.6 ± 1.3 | 5.4 ± 0.6 | 61.2 ± 5.2 | 12.1 ± 0.8 | 5.1 ± 0.6 | 3.5 ± 0.4 |
| R-41 | 5.6 ± 0.8 | 4.6 ± 0.4 | 70.4 ± 6.1 | 11.8 ± 1.2 | 5.5 ± 0.8 | 2.1 ± 0.6 |
| R-41 + PE | 14.7 ± 1.3 | 5.7 ± 0.6 | 60.4 ± 4.2 | 10.3 ± 1.2 | 5.7 ± 0.9 | 2.7 ± 0.2 |
| R-41 + Etn | 15.8 ± 0.9 | 5.5 ± 0.4 | 59.7 ± 3.9 | 10.1 ± 0.9 | 5.9 ± 1.1 | 3.1 ± 0.3 |
Cells were incubated in Ham's F-12 medium containing 10% dialyzed newborn calf serum supplemented with or without either 30 μM PE (R-41 + PE) or 20 μM ethanolamine (R-41 + Etn) at 39.5°C for 3 d. Phospholipids were extracted, separated, and quantified. PE, Phosphatidylethanolamine; PS, phosphatidylserine; PC, phosphatidylcholine; SM, sphingomyelin; PI, phosphatidylinositol. Others include phosphatidylglycerol, phosphatidic acid, and cardiolipin. Data shown are the mean ± SD for three independent experiments.
R-41 Mutant Cells Have a Defect in Cytokinesis
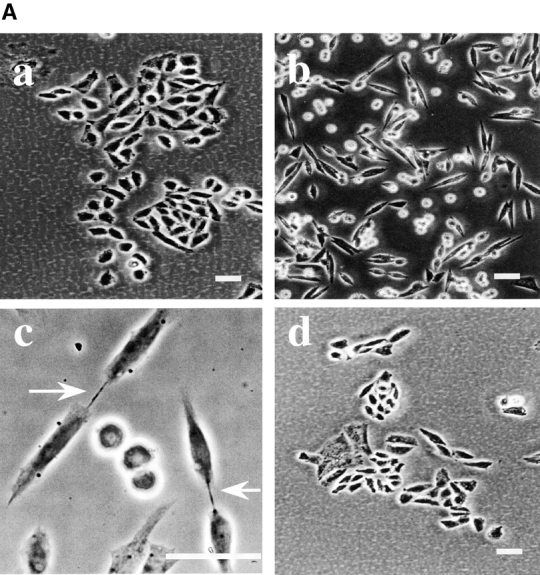
Although R-41 mutant cells grew well in normal medium, the morphology of the mutant cells was different from that of wild-type CHO cells in several respects. The population of round-shaped cells increased in the mutant cells, compared with the wild-type cells (Fig. 4 A). In addition, ∼20% of the mutant cells had a cytoplasmic bridge between the two daughter cells (Fig. 4 A, arrow), which is quite similar to SA-Ro–treated cells (Fig. 1 a). These mutant cells remained connected about four hours after the initiation of cytokinesis. The cytoplasmic bridge was eventually resolved by traction between the daughter cells. These phenotypes were no longer observed in the mutant cells cultured in the ethanolamine-supplemented medium (Fig. 4 A).
Figure 4.
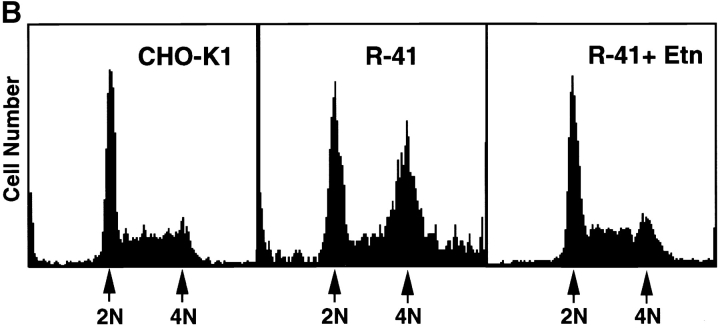
R-41 mutant cells have a defect in cytokinesis. A, Morphology of CHO-K1 (a) and R-41 mutant cells (b–d) cultured in normal medium. CHO-K1 and R-41 mutant cells were cultured in normal medium containing 10% newborn calf serum supplemented with (d) or without (a–c) 20 μM ethanolamine at 39.5°C for 3 d. B, Flow cytometric analyses of the DNA contents. CHO-K1 and mutant cells were cultured in ethanolamine-deficient medium supplemented with (R-41+Etn) or without (CHO-K1, R-41) 20 μM ethanolamine at 39.5°C for 4 d. CHO-K1: G1, 41.6%; S, 49.1%; G2/M, 9.3%. R-41: G1, 38.9%; S, 16.3%; G2/M, 44.8%. R-41+Etn: G1, 38.2%; S, 50.5%; G2/M, 11.3%. Bars, 30 μm.
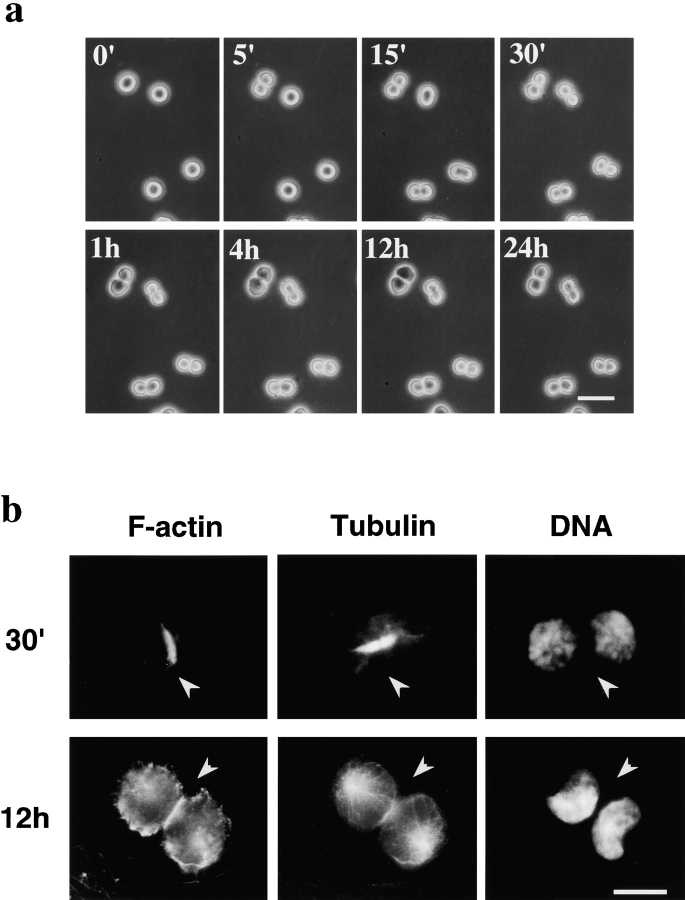
A more severe defect in cell division was observed in R-41 mutant cells cultured in the ethanolamine-deficient medium. Flow cytometric analyses showed that 41.6, 49.1, and 9.3% of CHO-K1 cells growing in the ethanolamine-deficient medium for three days were in G1, S, and G2/M phases, respectively (Fig. 4 B). In contrast, 44.8% of R-41 mutant cells growing in the ethanolamine-deficient medium were in G2/M phases (Fig. 4 B). No significant difference was observed in the proportion of G1, S, and G2/M phases between CHO-K1 cells growing in the ethanolamine-deficient medium and the mutant cells growing in the ethanolamine-supplemented medium (Fig. 4 B), suggesting that PE is required for G2/M transition. Time-lapse observation of cell division of synchronized R-41 mutant cells cultivated in the ethanolamine-deficient medium revealed that early events of cytokinesis, such as formation and contraction of the contractile ring proceeded normally, but cell division was blocked at the final stage of cleavage (Fig. 5 a). This arrest of cell division was not observed in the mutant cells cultured in the ethanolamine-supplemented medium (data not shown). These results indicate R-41 mutant cells are defective in the late stage of cytokinesis.
Figure 5.
R-41 mutant cells have a defect in the disassembly of the contractile ring. a, Time-lapse observation of the cell division of R-41 mutant cells cultured in ethanolamine-deficient medium. R-41 mutant cells cultured in ethanolamine-deficient medium for 3 d were synchronized in mitotic phase by nocodazole. The synchronized cells were collected, washed, and then further incubated in the ethanolamine-deficient medium. The time indicates the time point after the initiation of the incubation of synchronized cells. Bar, 30 μm. b, Localization of actin filaments and microtubules in R-41 mutant cells during cytokinesis. The synchronized mutant cells were incubated for 30 min (30') or 12 h in ethanolamine-deficient medium. The cells were then fixed and labeled with rhodamine-phalloidin (for F-actin), antitubulin antibody, or DAPI (for DNA). Bar, 10 μm. The arrowheads indicate the cleavage furrow.
30 min after the initiation of cytokinesis in R-41 cells, furrowing and chromosome separation proceeded normally and arrays of microtubules and the contractile ring were clearly seen between the poles of two daughter cells (Fig. 5 b). Actin filaments were concentrated at the cleavage furrow even 12 h after the initiation of cytokinesis, although microtubule rearrangement and nuclear division proceeded normally to show typical features of cells in G1 phase (Fig. 5 b). These observations suggest that disassembly of the contractile ring is affected in R-41 mutant cells.
Exposure of PE on the Surface of R-41 Mutant Cells during Cytokinesis
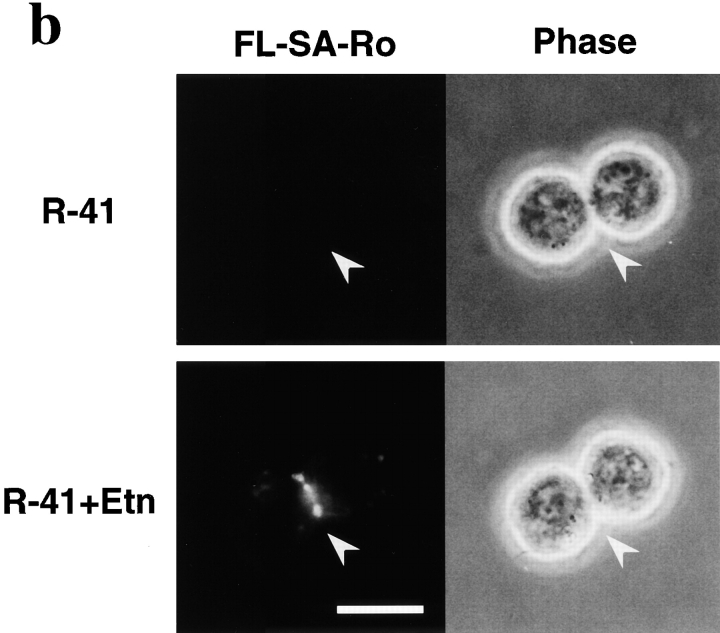
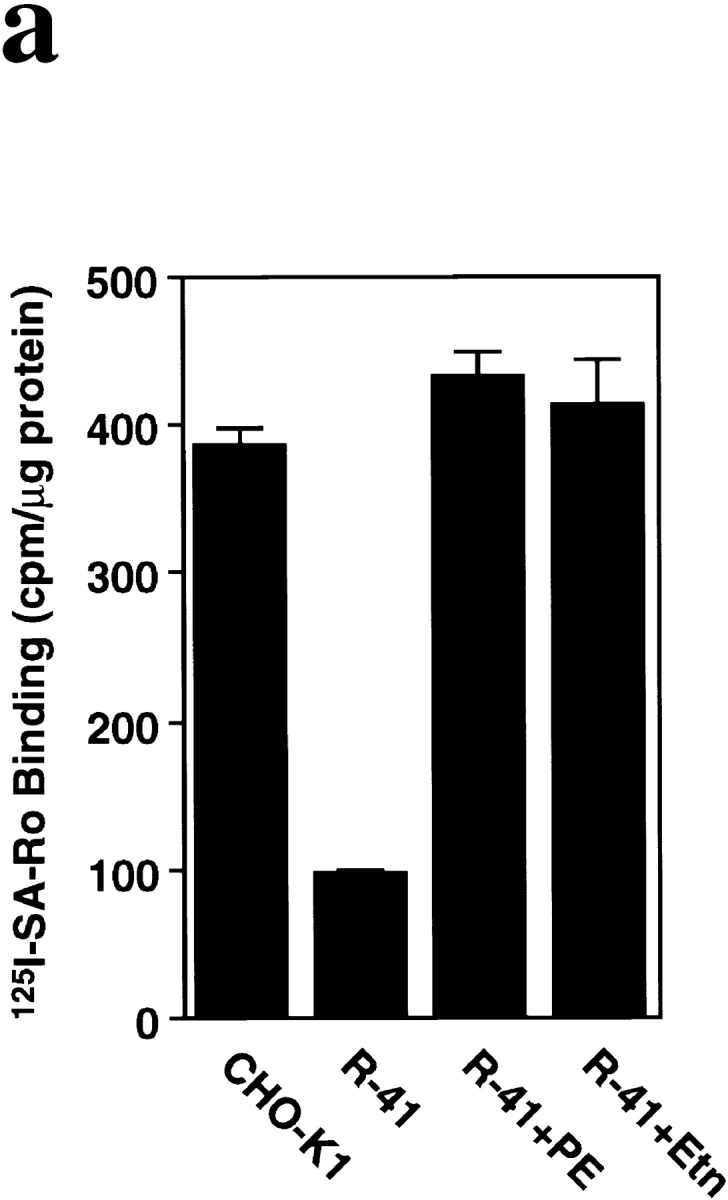
Studies using the PE-binding peptide suggested that PE exposure on the cleavage furrow membrane contributes to regulation of contractile ring disassembly. Thus, we next examined surface exposure of PE in R-41 mutant cells cultured in the either ethanolamine-deficient or ethanolamine-supplemented medium. When the mutant cells were cultured in the ethanolamine-deficient medium for three days, binding of Ro peptide coupled with 125I-SA-Ro to the cell surface was decreased to one-fourth, compared with that of the parent cells, suggesting that the cell surface PE content was markedly reduced in the mutant cells (Fig. 6 a). Addition of 30 μM PE or 20 μM ethanolamine to the culture medium restored 125I-SA-Ro binding to the mutant cells to that of wild-type level (Fig. 6 a). Next, surface exposure of PE during cytokinesis was examined using the PE-binding peptide coupled with fluorescein-labeled SA (FL–SA-Ro; Emoto et al. 1996). Although no significant exposure of PE was detected in R-41 mutant cells cultured in the ethanolamine-deficient medium, intense staining of the cleavage furrow membrane was observed in ∼60% of the mutant cells cultured in the ethanolamine-supplemented medium (Fig. 6 b). These results demonstrate a good correlation between PE exposure on the cleavage furrow membrane and successful cytokinesis, and provide further support for the proposal that redistribution of PE on the cleavage furrow membrane may play a crucial role in completion of cytokinesis.
Figure 6.
Exposure of PE on the surface of R-41 mutant cells. a, Binding of 125I-SA-Ro to the plasma membrane. CHO-K1 and R-41 mutant cells were incubated in ethanolamine-deficient medium without (CHO-K1, R-41) or with either 30 μM PE (R-41+PE) or 20 μM ethanolamine (R-41+Etn) at 39.5°C for 3 d. Cells were incubated with 125I-SA-Ro (30,000 cpm/ml) on ice for 30 min. The cells were then washed and their radioactivity was measured. The results show the mean ± SEM (n = 3). b, Exposure of PE on the cleavage furrow membrane of R-41 mutant cells. R-41 mutant cells were incubated in ethanolamine-deficient medium with (R-41+Etn) or without (R-41) 20 μM ethanolamine at 39.5°C for 3 d and then synchronized in mitotic phase. The synchronized cells were collected, washed, and then further incubated for 30 min. The cells were washed and stained with FL–SA-Ro for 30 min on ice, then washed and photographed. Bar, 10 μm. The arrowheads indicate the cleavage furrow.
Discussion
Since cytokinesis is the process that partitions the cell membrane of one cell to form two cells, coordinated movements between the plasma membrane and cytoskeleton networks are required for proper progression of cytokinesis. In this study, we demonstrate that PE exposed on the surface of cleavage furrow plays a pivotal role in regulating contractile ring disassembly, which allows the plasma membrane to separate into two membranes. We employed two different approaches: manipulation of cell surface PE by the PE-binding peptide, and the establishment of a mutant cell line specifically defective in PE biosynthesis. Among the various phospholipid-binding probes, only the PE-binding peptide bound specifically to the surface of cleavage furrow and inhibited contractile ring disassembly beneath the plasma membrane to which the peptide bound (Fig. 3). Neither change in the transbilayer distribution of other membrane phospholipids nor any effect on cytokinesis was observed using other phospholipid-specific probes, suggesting that PE plays a specific role in cytokinesis (Fig. 1). Since SA-Ro is accessible to only those PE molecules on the outer leaflet of the plasma membrane (Aoki et al. 1994; Emoto et al. 1996), it is likely that immobilization of the cell surface PE by SA-Ro prevents disassembly of the contractile ring. Furthermore, the blockage of contractile ring disassembly was reversed by removal of the surface-bound SA-Ro using PE liposomes (Fig. 2). These observations suggest that PE molecules on the cell surface, rather than those on other cellular membranes, including the inner leaflet of the plasma membrane, play a role in contractile ring disassembly. It remains unclear why the peptide-treated cells collapse into binucleated cells after addition of PE liposome. The molecules transiently activated to accomplish the final membrane separation may be either inactive or released from the cleavage furrow membrane of the peptide-treated cells, since the daughter cells have already entered G1 phase.
To further confirm the role of PE in cytokinesis, we established a CHO cell mutant line (R-41) that was defective in PE biosynthesis, and analyzed its cytokinetic process. When R-41 mutant cells were cultured in normal medium, the cells exhibited a partial cytokinetic defect in which the late telophase of cytokinesis was extensively prolonged (Fig. 4 A). Further decrease in the PE level of the mutant cells, by culturing them in ethanolamine-deficient medium, blocked the contractile ring disassembly without affecting the initial formation of the contractile ring or its contraction, resulting in cell division arrest in late telophase (Fig. 5). In this culture condition, no significant exposure of PE on the cleavage furrow surface was detected by the FL–SA-Ro in the mutant cells (Fig. 6). Addition of PE or ethanolamine, a precursor of PE, restored cellular PE levels and normal cell division (Fig. 5 and Fig. 6). Although several CHO mutant cell lines that have specific defects in phospholipid biosynthesis, such as phosphatidylcholine and sphingomyelin, have been established, no significant defect in cytokinesis was observed in these mutant cell lines (Cui et al. 1996; Nishijima et al. 1997; Hanada et al. 1998). Thus, it is likely that the cytokinetic arrest of R-41 mutant cells in ethanolamine-deficient culture medium is due to a PE-specific functional defect in cytokinesis, rather than nonspecific perturbation of membrane structure resulting from a change in the lipid composition.
PE is produced by three distinct pathways (Kent 1995; Voelker 1997). A substantial amount of PE is synthesized through the PS decarboxylase pathway, in which PS produced in the ER is transported to mitochondria and then decarboxylated by an inner mitochondrial membrane enzyme, PS decarboxylase. PE is also made from ethanolamine via the CDP–ethanolamine pathway, the last step of which occurs on endoplasmic membranes. In a quantitatively minor pathway, PE can also be synthesized by the phospholipid base–exchange pathway in which free ethanolamine is exchanged with base moiety of preexisting phospholipids. Although the cellular PE level in R-41 mutant cells was decreased to about half that of the parent cells, the SA-Ro binding to the mutant cells was reduced to one-fourth compared with that of the parent cells (Fig. 6), suggesting that the level of PE in the plasma membrane of the mutant cells decreased to a greater extent than the total cellular content of PE. Vance et al. 1991 metabolically labeled rat hepatocytes with either [3H]serine or [3H]ethanolamine, and showed that [3H]serine-labeled PE derived from the PS decarboxylase pathway appears on the cell surface much faster than [3H]ethanolamine-labeled PE, suggesting that PE made by the PS decarboxylase pathway is preferentially transported to the cell surface compared with ethanolamine-derived PE. We recently demonstrated that R-41 mutant cells have a specific defect in the transport of PS between the outer and inner mitochondrial membranes, resulting in reduced production of PE via the PS decarboxylation pathway (Emoto et al. 1999). This defect in PE production via the PS decarboxylase pathway in R-41 mutant cells may cause the significant reduction of PE content in the plasma membrane, and its exposure on the surface of the cleavage furrow. Restoration of the cell surface exposure of PE leads to normal disassembly of the contractile ring (Fig. 6 b), again suggesting that the redistribution of PE on the cleavage furrow play a critical role in regulating disassembly of the contractile ring.
Recently, it was shown that a mutant Escherichia coli strain that completely lacks PE has a specific defect in cytokinesis (Mileykovskaya et al. 1998). In E. coli, cell division is mediated by the formation of a septal ring, the FtsZ ring, which circumferentially invaginates the cell wall to cleave the cell into two (Bi and Luthenhaus 1991). In the PE-deficient strain, FtsZ protein and other essential division proteins are recruited to the division site, but the FtsZ ring failed to constrict (Mileykovskaya et al. 1998). This phenotype is not observed in the strains with specific defects in other phospholipid biosynthesis (Dowhan 1997). Thus, it is likely that PE is essential for cytoskeletal organization in the completion of cytokinesis in prokaryotic cells, as well as mammalian cells.
We can only speculate about the mechanism by which PE redistribution on the cleavage furrow membrane contributes to regulation of the contractile ring disassembly. One possibility is that a particular lipid domain is formed on the cleavage furrow membrane. It was previously reported that the lateral mobility of lipid probes in the cell membrane was significantly reduced during mitosis in cultured mammalian cells (deLaat et al. 1980). It was also shown that exchange of lipid probes between the cleavage furrow membrane and other membrane domains was restricted in dividing eggs (Tetteroo et al. 1984). These findings raised the possibility that some lipid domains are formed in the dividing cells. We recently found that the plasma membrane sphingomyelin, which is shown to be distributed on the cell surface uniformly in interphase cells (Yamaji et al. 1998), was excluded from the surface of the cytoplasmic bridge by staining with a sphingomyelin-specific binding protein, lysenin (data not shown). Our findings, together with the previous report that the amount of PE in the outer leaflet of plasma membrane of CHO cells increased threefold during cytokinesis (Kobayashi and Pagano 1989), suggest that a unique PE-rich lipid domain may be formed in the outer leaflet of the cleavage furrow membrane during cytokinesis. Many proteins, such as ERM (ezrin/radixin/moesin) proteins (Sato et al. 1991; Tsukita et al. 1997), septin family proteins (Kinoshita et al. 1997; Field and Kellogg 1999), and GTP-binding protein Rho and its targets (Hall 1998; Madaule et al. 1998), that play crucial roles in contractile ring reorganization, are known to be recruited to the cleavage furrow, but it is still unclear how the disassembly of these proteins is spatially regulated. It has been shown that certain structural features of the membrane bilayer play a role in modulating the membrane activity of various proteins (Yeagle 1989; Igarashi et al. 1995). In particular, PE-rich membranes tend to form a nonbilayer hexagonal structure that has been shown to regulate various membrane-bound enzymes, such as the calcium pomp (Yeagle and Sen 1986), protein kinase C (Bazzi et al. 1992), and phospholipase D (Nakamura et al. 1996). Thus, the PE-rich domain on the cleavage furrow may provide a specific milieu for activation or assembly of certain molecules regulating disassembly of the contractile ring. Aberrant organization of the PE-rich membrane domain by either the PE-binding peptide or a decrease in the surface PE level in the mutant cells may affect contractile ring disassembly, which prevents the final separation of daughter cells.
The redistribution of membrane PE molecules at the cleavage furrow also may be involved in bringing about membrane separation. Recent studies suggest that cone-shaped lipids, such as phophatidic acid and cholesterol, play a role in modulating the membrane curvature in vesicle formation (Schmidt et al. 1999; Thiele et al. 1999; Weigert et al. 1999). Since PE is a typical cone-shaped lipid, PE-rich surface membrane tends to exert bending fluctuations (Chernomordik et al. 1995). It was also suggested that PE-rich membranes tend to form nonbilayer intermediates that have been hypothesized to exist during the membrane fusion process (Verkleij et al. 1984; Ellens et al. 1989; Chernomordik et al. 1995). In fact, PE accelerates the generation of clathrin-coated buds on protein-free liposome (Takei et al. 1998). Therefore, it is conceivable that formation of the PE-rich membrane domain in the outer leaflet of the cleavage furrow membrane facilitates the inward bending of the plasma membrane, which may induce membrane fusion between vesicles and plasma membrane, as well as opposing plasma membranes.
In summary, we have provided the first evidence that PE is essential for completion of cytokinesis. In addition, we propose that redistribution of PE on the cleavage furrow surface is involved in regulating the contractile ring disassembly. Elucidation of the molecular mechanism by which the membrane phospholipids regulate contractile ring disassembly may help us to understand the mechanism by which coordination between the cytoskeleton and plasma membrane is achieved in the final stage of cytokinesis.
Acknowledgments
We thank Drs. Masahiro Nishijima and Osamu Kuge (National Institute of Infectious Diseases, Tokyo, Japan) for helpful suggestions, Dr. Donald M. Marcus (Baylor College of Medicine, Houston, Texas) for helpful comments during preparation of the manuscript, and Dr. Keizo Inoue (Univ. of Tokyo) for encouragement.
This study was supported by Special Coordination Funds for Promoting Science and Technology from the Science and Technology Agency and by Grant-in-Aid for Scientific Research of Japan Society for the Promotion of Science.
Footnotes
Abbreviations used in this paper: DAPI, 4′,6′-diamidino-2-phenylindole; FL–SA-Ro, fluorescein-labeled SA-Ro; 125I–SA-Ro, 125I-labeled SA-Ro; PE, phosphatidylethanolamine; PS, phosphatidylserine; Ro peptide, Ro09-0198; SA, streptavidin; SA-Ro, streptavidin conjugated to biotinyl Ro peptide.
References
- Aoki Y., Uenaka T., Aoki J., Umeda M., Inoue K. A novel peptide probe for studying the transbilayer movement of phosphatidylethanolamine. J. Biochem. 1994;116:291–297. doi: 10.1093/oxfordjournals.jbchem.a124522. [DOI] [PubMed] [Google Scholar]
- Bazzi M.D., Youakin M.A., Nelsestuen G.L. Importance of phosphatidylethanolamine for association of protein kinase C and other cytoplasmic proteins with membranes. Biochemistry. 1992;31:1125–1134. doi: 10.1021/bi00119a022. [DOI] [PubMed] [Google Scholar]
- Bi E., Luthenhaus J. FtsZ ring structure associated with division in Escherichia coli . Nature. 1991;354:161–164. doi: 10.1038/354161a0. [DOI] [PubMed] [Google Scholar]
- Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bluemink J.G., deLaat S.W. New membrane formation during cytokinesis in normal and cytochalasin B-treated eggs of Xenopus laevis. I. Electron microscope observations. J. Cell Biol. 1973;59:89–108. doi: 10.1083/jcb.59.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanov M., Umeda M., Dowhan W. Phospholipid-assisted refolding of an integral membrane protein. Minimum structural features for phosphatidylethanolamine to act as a molecular chaperone. J. Biol. Chem. 1999;274:12339–12345. doi: 10.1074/jbc.274.18.12339. [DOI] [PubMed] [Google Scholar]
- Boronenkov I.V., Loijens J.C., Umeda M., Anderson R.A. Phosphoinositide signaling pathways in nuclei are associated with nuclear speckles containing premRNA processing factors. Mol. Biol. Cell. 1998;9:3547–3560. doi: 10.1091/mbc.9.12.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik L., Kozlov M.M., Zimmerberg J. Lipids in biological membrane fusion. J. Membrane Biol. 1995;146:1–14. doi: 10.1007/BF00232676. [DOI] [PubMed] [Google Scholar]
- Comfurius P., Smeets E.F., Willems G.M., Bevers E.M., Zwaal R.F.A. Assembly of the prothrombinase complex on the lipid vesicles dependent on the stereochemical configuration of polar head group of phosphatidylserine. Biochemistry. 1994;33:10319–10324. doi: 10.1021/bi00200a012. [DOI] [PubMed] [Google Scholar]
- Cui Z., Houweling M., Chen M.H., Record M., Chap H., Vance D.E., Terce F. A genetic defect in phosphatidylcholine biosynthesis triggers apoptosis in Chinese hamster ovary cells. J. Biol. Chem. 1996;271:14668–14671. doi: 10.1074/jbc.271.25.14668. [DOI] [PubMed] [Google Scholar]
- deLaat S.W., van der Saag P.T., Elson E.L., Schlessinger J. Lateral diffusion of membrane lipids and proteins during the cell cycle of neuroblastoma cells. Proc. Natl. Acad. Sci. USA. 1980;77:1526–1528. doi: 10.1073/pnas.77.3.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux P.F. Static and dynamic lipid asymmetry in cell membranes. Biochemistry. 1991;30:1163–1173. doi: 10.1021/bi00219a001. [DOI] [PubMed] [Google Scholar]
- Dowhan W. Molecular basis for membrane phospholipid diversitywhy are there so many lipids? Annu. Rev. Biochem. 1997;66:199–232. doi: 10.1146/annurev.biochem.66.1.199. [DOI] [PubMed] [Google Scholar]
- Drechsel D.N., Hyman A.A., Hall A., Glotzer M. A requirement for Rho and Cdc42 during cytokinesis in Xenopus embryos. Curr. Biol. 1997;7:12–23. doi: 10.1016/s0960-9822(06)00023-6. [DOI] [PubMed] [Google Scholar]
- Ellens H., Siegel D.P., Alford D., Yeagle P.L., Boni L., Lis L.J., Quinn P.J., Bentz J. Membrane fusion and inverted phases. Biochemistry. 1989;28:3692–3703. doi: 10.1021/bi00435a011. [DOI] [PubMed] [Google Scholar]
- Emoto K., Kobayashi T., Yamaji A., Aizawa H., Yahara I., Inoue K., Umeda M. Redistribution of phosphatidylethanolamine at the cleavage furrow of dividing cells during cytokinesis. Proc. Natl. Acad. Sci. USA. 1996;93:12867–12872. doi: 10.1073/pnas.93.23.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emoto K., Toyama-Sorimachi N., Karasuyama H., Inoue K., Umeda M. Exposure of phosphatidylethanolamine on the surface of apoptotic cells. Exp. Cell Res. 1997;232:430–434. doi: 10.1006/excr.1997.3521. [DOI] [PubMed] [Google Scholar]
- Emoto K., Kuge O., Nishijima M., Umeda M. Isolation of a Chinese hamster ovary cell mutant defective in intramitochondrial transport of phosphatidylserine. Proc. Natl. Acad. Sci. USA. 1999;96:12400–12405. doi: 10.1073/pnas.96.22.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok V.A., Voelker D.R., Campbell P.A., Cohen J.J., Bratton D.L., Henson P.M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophases. J. Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- Field C.M., Kellogg D. Septinscytoskeletal polymers or signalling GTPase? Trends Cell Biol. 1999;9:387–394. doi: 10.1016/s0962-8924(99)01632-3. [DOI] [PubMed] [Google Scholar]
- Fishkind D.J., Wang Y.-L. New horizons for cytokinesis. Curr. Opin. Cell Biol. 1995;7:23–31. doi: 10.1016/0955-0674(95)80041-7. [DOI] [PubMed] [Google Scholar]
- Fujimoto K., Umeda M., Fujimoto S. Transmembrane phospholipid distribution revealed by freeze-fracture replica labeling. J. Cell Sci. 1996;109:2453–2460. doi: 10.1242/jcs.109.10.2453. [DOI] [PubMed] [Google Scholar]
- Glotzer M. The mechanism and control of cytokinesis. Curr. Opin. Cell Biol. 1997;9:815–823. doi: 10.1016/s0955-0674(97)80082-8. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPase and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hanada K., Hara T., Fukasawa M., Yamaji A., Umeda M., Nishijima M. Mammalian cell mutants resistant to a sphingomyelin-directed cytolysin. J. Biol. Chem. 1998;273:33787–33794. doi: 10.1074/jbc.273.50.33787. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Kaneda M., Yamaji A., Saido T.C., Kikkawa U., Ono Y., Inoue K., Umeda M. A novel phosphatidylserine-binding peptide motif defined by an anti-idiotypic monoclonal antibodylocalization of phosphatidylserine-specific binding sites on protein kinase C and phosphatidylserine decarboxylase. J. Biol. Chem. 1995;270:29075–29078. doi: 10.1074/jbc.270.49.29075. [DOI] [PubMed] [Google Scholar]
- Inagaki M., Matsuoka Y., Tsujimura K., Ando S., Tokui T., Takahashi T., Inanaki N. Dynamic property of intermediate filamentsregulation of phosphorylation. Bioessays. 1996;18:481–487. [Google Scholar]
- Kent C. Eukaryotic phospholipid biosynthesis. Ann. Rev. Biochem. 1995;64:315–343. doi: 10.1146/annurev.bi.64.070195.001531. [DOI] [PubMed] [Google Scholar]
- Kinoshita M., Kumar S., Mizoguchi A., Ide C., Kinoshita A., Haraguchi T., Noda M. Nedd5, a mammalian septin, is a novel cytoskeletal component interacting with actin-based structures. Genes Dev. 1997;11:1535–1547. doi: 10.1101/gad.11.12.1535. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Pagano R.E. Lipid transport during mitosis. Alternative pathways for delivery of newly synthesized lipids to cell surface. J. Biol. Chem. 1989;264:5966–5973. [PubMed] [Google Scholar]
- Koopman G., Reutelingsperger C.P.M., Kuijten G.A.M., Keehne R.M.J., Pals S.T., vanOers M.H.J. Annexin V for flow cytometric detection of phosphatidylserine expression on B cell undergoing apoptosis. Blood. 1994;84:1415–1420. [PubMed] [Google Scholar]
- Madaule P., Eda M., Watanabe N., Fujisawa K., Matsuoka T., Bito H., Ishizaki T., Narumiya S. Role of citron kinase as a target of small GTPase Rho in cytokinesis. Nature. 1998;394:491–494. doi: 10.1038/28873. [DOI] [PubMed] [Google Scholar]
- Mann K.G., Jenny R.J., Krishnaswamy S. Cofactor proteins in the assembly and expression of blood clotting enzyme complex. Annu. Rev. Biochem. 1998;57:915–956. doi: 10.1146/annurev.bi.57.070188.004411. [DOI] [PubMed] [Google Scholar]
- Mileykovskaya E., Sun Q., Margolin W., Dowhan W. Localization and function of early cell division proteins in filamentous Escherichia coli cell lacking phosphatidylethanolamine. J. Bacteriol. 1998;180:4252–4257. doi: 10.1128/jb.180.16.4252-4257.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal B., Sanger J.M., Sanger J.W. Visualization of myosin in the cytoplasm, cleavage furrow, and mitotic spindle of human cells. J. Cell Biol. 1987;105:1753–1760. doi: 10.1083/jcb.105.4.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa A., Inoue H., Yoshioka T., Horikoshi T., Yanagisawa K., Umeda M., Inoue K. Production and characterization of monoclonal antibodies that bind to phosphatidylinositol 4,5-bisphosphate. Mol. Immunol. 1988;25:1025–1031. doi: 10.1016/0161-5890(88)90010-7. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Kiyohara Y., Jinnal H., Hitomi T., Ogino C., Yoshida K., Nishizuka Y. Mammalian phospholipase Dphosphatidylethanolamine as an essential component. Proc. Natl. Acad. Sci. USA. 1996;93:4300–4304. doi: 10.1073/pnas.93.9.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam K.S., Igarashi K., Umeda M., Inoue K. Production and characterization of monoclonal antibodies that specifically bind to phosphatidylcholine. Biochim. Biophys. Acta. 1990;1046:89–96. doi: 10.1016/0005-2760(90)90098-i. [DOI] [PubMed] [Google Scholar]
- Nishijima M., Kuge O., Hanada K. Mammalian cell mutants of membrane phospholipid biogenesis. Trends Cell Biol. 1997;7:324–329. doi: 10.1016/S0962-8924(97)01084-2. [DOI] [PubMed] [Google Scholar]
- Nishikawa K., Arai H., Inoue K. Scavenger receptor-mediated uptake and metabolism of lipid vesicles containing acidic phospholipids by mouse peritoneal macrophases. J. Biol. Chem. 1990;265:5226–5231. [PubMed] [Google Scholar]
- Sato N., Yonemura S., Obinata T., Tsukita S., Tsukita S. Radixin, a barbed endo-capping actin-modulating protein, is concentrated at cleavage furrow during cytokinesis. J. Cell Biol. 1991;113:321–330. doi: 10.1083/jcb.113.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Wolde M., Thiele C., Fest W., Kratzin H., Podtelejnikov A.V., Witke W., Huttner W.B., Soling H.-D. Endophilin I mediates synaptic vesicle formation by transfer of arachidonate to lysophosphatidic acid. Nature. 1999;401:133–141. doi: 10.1038/43613. [DOI] [PubMed] [Google Scholar]
- Schroeder T.E. Actin in dividing cells. Contractile ring filaments bind heavy meromyosin. Proc. Natl. Acad. Sci. USA. 1973;70:1688–1693. doi: 10.1073/pnas.70.6.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder T.E. The contractile ring and furrowing in dividing cells. Ann. NY Acad. Sci. 1990;582:78–87. doi: 10.1111/j.1749-6632.1990.tb21669.x. [DOI] [PubMed] [Google Scholar]
- Schroit A.J., Zwaal R.F.A. Transbilayer movement of phospholipids in red cell and platelet membranes. Biochim. Biophys. Acta. 1991;1071:313–329. doi: 10.1016/0304-4157(91)90019-s. [DOI] [PubMed] [Google Scholar]
- Stekhoven F.M., Tijmes J., Umeda M., Inoue K., DePont J.J. Monoclonal antibody to phosphatidylserine inhibits Na+/K+-ATPase activity. Biochim. Biophys. Acta. 1994;1194:155–165. doi: 10.1016/0005-2736(94)90215-1. [DOI] [PubMed] [Google Scholar]
- Takei K., Haucke V., Slepnev V., Farsad K., Salazar M., Chen H., Camilli P.D. Generation of coated intermediates of clathrin-mediated endocytosis on protein-free liposomes. Cell. 1998;94:131–141. doi: 10.1016/s0092-8674(00)81228-3. [DOI] [PubMed] [Google Scholar]
- Tetteroo P.A.T., Bluemink J.G., Dictus W.J.A.G., vanZoelen E.J.J., deLaat S.W. Lateral mobility of plasma membrane lipids in dividing Xenopus eggs. Dev. Biol. 1984;104:210–218. doi: 10.1016/0012-1606(84)90049-6. [DOI] [PubMed] [Google Scholar]
- Thiele C., Hannah M.J., Fahrenholz F., Muttner W.B. Cholesterol binds synaptophysin and is required for biogenesis of synaptic vesicles. Nature Cell Biol. 1999;2:42–49. doi: 10.1038/71366. [DOI] [PubMed] [Google Scholar]
- Tsukita S., Yonemura S., Tsukita S. ERM proteinshead-to-tail regulation of actin-plasma membrane interaction. Trends Biochem. Sci. 1997;22:53–58. doi: 10.1016/s0968-0004(96)10071-2. [DOI] [PubMed] [Google Scholar]
- Umeda M., Igarashi K., Nam K.S., Inoue K. Effective production of monoclonal antibodies against phosphatidylserinestereo-specific recognition of phosphatidylserine by monoclonal antibody. J. Immunol. 1989;143:2273–2279. [PubMed] [Google Scholar]
- Vance J.E., Aasman E.J., Szarka R. Brefeldin A does not inhibit the movement of phosphatidylethanolamine from its sites of synthesis to the cell surface. J. Biol. Chem. 1991;266:8241–8247. [PubMed] [Google Scholar]
- Verkleij A.J., Leunissen-Bijvelt J., de Kruijff B., Hope M., Cullis P.R. Non-bilayer structures in membrane fusion. Ciba Found. Symp. 1984;103:45–59. doi: 10.1002/9780470720844.ch4. [DOI] [PubMed] [Google Scholar]
- Voelker D.R. Lipid transport pathways in mammalian cells. Biochem. Biophys. Acta. 1997;1348:236–244. [Google Scholar]
- Weigert R., Silletta M.G., Spano S., Turacchio G., Cericola C., Colanzi A., Senatore S., Mancini R., Polishchuk E.V., Salmona M. CtBP/BARS induces fission of Golgi membranes by acylating lysophosphatidic acid. Nature. 1999;402:429–433. doi: 10.1038/46587. [DOI] [PubMed] [Google Scholar]
- Wgeatley S.P., Wang Y.L. Midzone microtubules are continuously required for cytokinesis in cultured epithelial cells. J. Cell Biol. 1996;135:981–989. doi: 10.1083/jcb.135.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji A., Sekizawa Y., Emoto K., Sakuraba H., Inoue K., Kobayashi H., Umeda M. Lysenin, a novel sphingomyelin-specific binding protein. J. Biol. Chem. 1998;273:5300–5306. doi: 10.1074/jbc.273.9.5300. [DOI] [PubMed] [Google Scholar]
- Yeagle P.L. Lipid regulation of cell membrane structure and function. FASEB J. 1989;3:1833–1842. [PubMed] [Google Scholar]
- Yeagle P.L., Sen A. Hydration and the lamellar to hexagonal II phase transition of phosphatidylethanolamine. Biochemistry. 1986;25:7518–7522. doi: 10.1021/bi00371a039. [DOI] [PubMed] [Google Scholar]
- Zachowski A. Phospholipids in animal eukaryotic membranestransverse asymmetry and movement. Biochem. J. 1993;294:1–14. doi: 10.1042/bj2940001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Arthur G. Improved procedures for determination of lipid phosphorus by malachite green. J. Lipid Res. 1992;33:1233–1236. [PubMed] [Google Scholar]
- Zimmerberg J., Vogel S.S., Chernomordik L.V. Mechanisms of membrane fusion. Annu. Rev. Biophys. Biomol. Struct. 1993;22:433–466. doi: 10.1146/annurev.bb.22.060193.002245. [DOI] [PubMed] [Google Scholar]