Abstract
The compartmentalization of plasma membrane proteins has a key role in regulation of lymphocyte activation and development of immunity. We found that the proline-rich tyrosine kinase-2 (PYK-2/RAFTK) colocalized with the microtubule-organizing center (MTOC) at the trailing edge of migrating natural killer (NK) cells. When polyclonal NK cells bound to K562 targets, PYK-2 translocated to the area of NK–target cell interaction. The specificity of this process was assessed with NK cell clones bearing activatory or inhibitory forms of CD94/NKG2. The translocation of PYK-2, MTOC, and paxillin to the area of NK–target cell contact was regulated upon specific recognition of target cells through NK cell receptors, controlling target cell killing. Furthermore, parallel in vitro kinase assays showed that PYK-2 was activated in response to signals that specifically triggered its translocation and NK cell mediated cytotoxicity. The overexpression of both the wt and a dominant-negative mutant of PYK-2, but not ZAP-70 wt, prevented the specific translocation of the MTOC and paxillin, and blocked the cytotoxic response of NK cells. Our data indicate that subcellular compartmentalization of PYK-2 correlates with effective signal transduction. Furthermore, they also suggest an important role for PYK-2 on the assembly of the signaling complexes that regulate the cytotoxic response.
Keywords: CD94/NKG2, cytotoxicity, microtubule-organizing center, cytoskeletal proteins, HLA-E
Introduction
Cell compartmentalization and rearrangement of membrane and cytosolic proteins seem to be required for lymphocyte activation and the development of immune response (Dustin and Shaw 1999; Lanzavecchia et al. 1999; Sánchez-Madrid and del Pozo 1999; Serrador et al. 1999). In addition, recent studies on the molecular reorganization that occurs during the specific interaction of lymphocytes with antigen-presenting cells revealed that different molecules involved in T cell stimulation are compartmentalized, favoring the activation of these cells (Monks et al. 1997, Monks et al. 1998; Wülfing and Davis 1998; Xavier et al. 1998; Viola et al. 1999; reviewed in Dustin and Shaw 1999). Therefore, there is growing evidence that molecular compartmentalization through protein–protein or protein–lipid interactions may play an important role in regulating cell signaling (Mochly-Rosen 1995; Faux and Scott 1996; Pawson and Scott 1997).
Natural killer (NK) cell cytotoxic activity is modulated by positive and negative signals triggered by different membrane receptors. NK cell receptors specifically interact with molecular histocompatibility complex (MHC) class I on target cells and function either as inhibitory or activatory molecules. Thus, the human killer inhibitory receptors from the immunoglobulin superfamily or some members of another family of related Ig-like receptors specifically interact with different human leukocyte antigen (HLA) class I allotypes and suppress NK cell–mediated cytotoxicity (Moretta and Moretta 1997; Lanier 1998; Yokoyama 1998; Long 1999; López-Botet and Bellón 1999). In contrast, another set of molecules homologous to the killer inhibitory receptors triggers NK cell activity (Cosman et al. 1997; Samaridis and Colonna 1997). On the other hand, the CD94 C-type lectin NK cell receptor covalently assembles with distinct members of the NKG2 family, playing different functional roles. CD94/NKG2A constitutes an inhibitory receptor coupled to SHP tyrosine phosphatases through the immunoreceptor tyrosine-based inhibitory motifs (ITIM) bearing NKG2A subunit (Carretero et al. 1997, Carretero et al. 1998; Leibson 1997; Vivier and Daëron 1997; Le Drean et al. 1998). In contrast, the association of CD94 with the NKG2C protein, highly homologous to NKG2A but lacking ITIMs, forms a triggering receptor linked to DAP-12 (Houchins et al. 1997; Cantoni et al. 1998; Lanier et al. 1998a,Lanier et al. 1998b). Different signaling events such as the rise of intracellular Ca2+, activation of PLC-γ, and release of phosphoinositides, are involved in the activation signaling pathway that ultimately trig–gers the cytotoxic response, although the precise sequence of these biochemical events has not been fully defined (Daëron 1997; Lanier 1998).
Proline-rich tyrosine kinase 2 (PYK-2), also called RAFTK (related adhesion focal tyrosine kinase), CAK-β (cell adhesion kinase β), CADTK (cell adhesion–dependent tyrosine kinase), or focal adhesion kinase-2 (FAK2), is a member of the FAK non-receptor tyrosine kinase family that is expressed by different cell types, including neural and hematopoietic cells (Avraham et al. 1995; Earp et al. 1995; Lev et al. 1995; Sasaki et al. 1995; Herzog et al. 1996). PYK-2 shares significant sequence homology with FAK (60% identity in the central catalytic domain and 40% identity in both the COOH and NH2 termini) and does not contain SH2 or SH3 domains, but bears several binding sites for SH2/SH3-containing signaling proteins (Avraham et al. 1995; Lev et al. 1995). PYK-2 can be activated by a variety of stimuli that induce a rise in intracellular calcium concentrations (Lev et al. 1995). In addition, phorbol esters and agonists of G-protein–coupled receptors as well as signals triggered through integrins, may lead to tyrosine phosphorylation of PYK-2 (Lev et al. 1995; Dikic et al. 1996; Li et al. 1996). It has been reported that NK cells express PYK-2, but not FAK, and that β1 and β2 integrin outside-in signaling stimulates PYK-2 activation (Gismondi et al. 1997; Rodríguez-Fernández et al. 1999). Furthermore, it has been shown that PYK-2, together with Src, functions as a link between heterotrimeric G-protein–coupled receptors and the mitogen-activated protein (MAP) kinase signaling pathway (Dikic et al. 1996). Recent studies indicate that PYK-2 may be also involved in regulation of vesicular transport through its interaction with a new GTPase activating protein designated Pap (Andreev et al. 1999).
In this report, we found that changes in the subcellular localization of the tyrosine kinase PYK-2 occur in an NK cell receptor specifically regulated manner during the effector–target cell conjugate formation. The translocation of PYK-2 from the trailing edge of NK cells to the area of NK-target cell interaction correlated with its activation, suggesting that the compartmentalization of PYK-2 significantly contributes to both the signal transduction in NK cells and the microtubular rearrangements that occur during the cytotoxic process. In addition, overexpression of both a wt and a dominant-negative mutant of PYK-2, but not ZAP-70 wt, blocked the NK cell–mediated cytotoxic response, suggesting an additional role for PYK-2 in the assembly of the signaling complexes that participate in the cytotoxic response.
Materials and Methods
Cells
Interleukin-2 (IL-2)–cultured polyclonal NK cells were obtained essentially as described (Aramburu et al. 1990). In brief, peripheral blood lymphocytes were cultured with irradiated (5 Gy) RPMI 8866 lymphoblastoid cells for 6–9 d in RPMI 1640 supplemented with 10% FCS (complete medium), followed by a negative selection step using an anti-CD3 mAb and rabbit complement (Behring). The CD3− cells (<5% CD3+) were cultured with 50 IU/ml of rhIL-2 until use. We routinely obtained a cell population with >95% of CD56+ and CD16+ cells and <5% of CD3+, CD19+, or CD14+ cells. NK cell clones were established as described (Pérez-Villar et al. 1995). The inhibitory clones were selected by their high ability to kill .221 target cells, that was inhibited by HLA-E in .221-AEH cells. The selected activatory clones showed a low basal lysis of .221 target cells, thus allowing the detection of the lysis activation by HLA-E on .221-AEH target cells. All cell cultures were systematically phenotyped by flow cytometry analysis with a panel of NK receptor-specific mAbs, as described (Pérez-Villar et al. 1997). The NKL cell line, kindly provided by M. Robertson (Dana-Farber, Boston, MA; Robertson et al. 1996), was maintained in RPMI 1640 medium supplemented with 10% (vol/vol) heat-inactivated human AB serum, 2 mM glutamine, 1 mM sodium pyruvate, and 100 U/ml IL-2.
The HLA class I–deficient EBV-transformed B lymphoblastoid cell line 721.221 (.221) was grown in complete medium. The 721.221 cell line transfected with the AEH chimeric construct (.221-AEH), in which the HLA-E leader sequence is replaced by that of HLA-A2 was kindly provided by Dr. Geraghty (Lee et al. 1998).
Antibodies and Reagents
The anti-CD56 mAb (C218) was kindly provided by Dr. A. Moretta (Istituto Nazionale per la Ricerca sul Cancro e Centro Biotecnologie Avanzate, University of Genova, Genova, Italy). The anti-CD94 (HP-3B1) and the anti–HLA-class I (HP-1F7) mAbs have been described (Pérez-Villar et al. 1995). PYK-2 affinity-purified anti-peptide polyclonal antibodies C-19 and N-19, as well as the C-19 antibody cognate blocking peptide were from Santa Cruz Biotechnology, Inc. The anti–γ-tubulin and anti-talin were purchased from Sigma-Aldrich. The anti-paxillin mAb was from Transduction Laboratories, and the anti-Tyr(P) 4G10 was from Santa Cruz. The anti-moesin 38/87 was kindly provided by Dr. R. Schwartz-Albiez (German Cancer Research Center, Heidelberg, Germany) and has been described (Lankes et al. 1988). The anti-p37 15B6 mAb was kindly provided by Dr. R. Blasco (Instituto Nacional de Investigaciones Agrarias, Madrid). The anti-ZAP-70 701 polyclonal antibody was generously provided by Dr. B. Alarcón (Centro de Biología Molecular “Severo Ochoa”, Madrid). γ-[32P]ATP (4,000 Ci/mmol) was from ICN, and protein A–agarose and protein G–agarose were from Boehringer Mannheim. Enhanced chemiluminescence (ECL) reagents were from Amersham, and all other reagents used were of the purest grade available.
Immunofluorescence Analysis
NK cells (5 × 105) were added to coverslips coated with 30 μg/ml fibronectin (FN) in flat-bottomed, 24-well plates (Costar Corp.) in a final volume of 500 μl complete medium and allowed to settle for 30 min at 37°C in a 5% CO2 atmosphere. Then, cells were either fixed and permeabilized with 0.5% Triton, 2% formaldehyde, PBS or fixed in 2% formaldehyde, and then permeabilized in 0.1% saponin, 1% BSA, 0.005% NaN3, PBS, and stained with appropriate antibodies as previously described (Nieto et al. 1998).
For studies of cell conjugates, 1 × 105 target cells were added to coverslips coated with 30 μg/ml FN in flat-bottomed, 24-well plates (Costar Corp.) in a final volume of 500 μl complete medium and allowed to settle 30 min at 37°C in a 5% CO2 atmosphere. Nonadherent cells were aspirated and 1 × 105 NK cells in 500 μl complete medium were then added and incubated for 1 h at 37°C in a 5% CO2 atmosphere. Cells were then fixed and permeabilized as previously indicated, stained with the indicated antibodies, and analyzed using a Nikon Labophot-2 photomicroscope with a 100× oil immersion objective. Confocal microscopy was performed as described (Rodríguez-Fernández et al. 1999) using a MRC-1024 Confocal Laser Scanning System (Bio-Rad Labs.).
Cytotoxic Assays
Microcytotoxicity chromium (51Cr) release assays were performed as described (Pérez-Villar et al. 1997), using 721.221 and .221-AEH target cells. The antagonistic effect of anti-CD94 mAb (for effectors) or anti-HLA mAb (for targets) was tested by preincubating cells for 10 min before performing the cytotoxicity assay. The percent of specific lysis was calculated (Aramburu et al. 1990) and only those experiments with a spontaneous release under 20% of the maximum 51Cr release were considered.
Cell Lysates and Immunoprecipitation Assays
The NKL cell line and the target cell lines (.221 and .221-AEH) were washed twice with RPMI 1640. Experiments were initiated by mixing 107 effector cells and 2 × 106 of each target cells (.221 or .221-AEH) in a final volume of 200 μl. When indicated, target cells were previously fixed in 1% paraformaldehyde and washed extensively before incubating with NK cells. Cells were spun, incubated at 37°C for the indicated times, and lysed in 1 ml of ice-cold lysis buffer (10 mM Tris-HCl, pH 7.65, 5 mM EDTA, 150 mM NaCl, 30 mM sodium pyrophosphate, 50 mM NaF, 2 mM sodium orthovanadate, 1% Triton X-100, 50 μg/ml aprotinin, 50 μg/ml leupeptin, 5 μg/ml pepstatin, and 1 mM phenylmethylsulfonyl fluoride). Cell lysates were clarified by centrifugation at 14,000 rpm for 10 min, and supernatants were immunoprecipitated at 4°C overnight with protein G–agarose coupled to goat polyclonal anti-PYK-2 (C-19) antibodies. Immunoprecipitates were washed three times with lysis buffer, and either used for in vitro kinase assays or Western blot (see below).
In Vitro Kinase Assay
This assay was performed as described (Rodríguez-Fernández and Rozengurt 1998). Briefly, immunoprecipitates were washed three times in lysis buffer and twice with kinase buffer (20 mM Hepes, 3 mM MnCl2, pH 7.35), pellets dissolved in 40 μl of kinase buffer, and reactions initiated by adding 10 μCi of γ-[32P]ATP. The reactions were carried out at 30°C for 15 min, and stopped by transferring to ice and adding 10 mM EDTA. Pellets were then washed in lysis buffer containing 10 mM EDTA, extracted for 5 min at 95°C in 2× SDS-PAGE sample buffer and analyzed by SDS-PAGE. Autoradiograms were processed using an AGFA Studio ScanIIsi scanner and bands were quantified using the Biorad Multi-Analyst Software.
Western Blot
After SDS-PAGE, immunoprecipitated proteins were transferred to Immobilon membranes using a Bio-Rad SD Transblot. Membranes were then blocked with 3% nonfat dried milk in PBS, pH 7.2, and incubated for 2 h RT with anti–PYK-2 (C-19 or N-19) polyclonal antibodies at a 1:500 dilution, anti PTyr 4G10 mAb at 1 μg/ml, and anti-p37 15B6 polyclonal antibody at a 1:50 dilution in PBS containing 3% nonfat dried milk. Bound antibodies were detected with horseradish peroxidase conjugate secondary antibodies, followed by visualization by ECL reagents.
Recombinant Vaccinia Viruses and Vaccinia Virus Infection of NKL Cell Line and NK Clones
To generate recombinant vaccinia viruses encoding the wt and the kinase dead form of PYK-2, the full-length PYK-2 and PYK-2 K457A (PYK-2 K-M) cDNAs (Li et al. 1996) were subcloned as a HindIII-XbaI fragment into the pRB21 vector (kindly provided by Dr. J.A. Melero, Instituto de Biología Fundamental, Instituto de Salud Carlos III, Madrid). The full-length ZAP-70 wt cDNA in EcoRI site of pSV.10.1 (kindly provided by Dr. B. Alarcon) was subcloned in EcoRI site of pRB21. Then, the relegated pRB21 vector and the constructs containing ZAP-70 wt, PYK-2 wt and PYK-2 (K-M) in the correct orientation were inserted into the defective vRB12 strain of vaccinia virus (Blasco and Moss 1995) by homologous recombination. Selection of recombinants was carried out by plaque formation as described (Blasco and Moss 1995). Viruses were then cloned, purified, and standard viral plaque assays on CV1 cells were used to determine the titer of each of the recombinants. To generate PYK-2-EGFP fusion proteins, PYK-2 cDNAs were subcloned as a HindIII-XbaI fragment into the pEGFPC3 vector (CLONTECH Laboratories, Inc.), and then the NheI-XbaI fragment subcloned in the pRB21 vector. ZAP-70-EGFP (kindly provided by Dr. B. Alarcón) was subcloned as an EcoRI-XbaI fragment into the pRB21 vector. As described before, viruses encoding EGFP, EGFP-ZAP-70 wt, EGFP-PYK2 wt, and EGFP-PYK-2 (K-M) fusion proteins were generated.
NKL cell line and NK clones were infected by recombinant vaccinia viruses essentially as described previously (Wagtmann et al. 1995). In brief, NKL cells and NK clones were washed three times with infection medium (Iscove's, 0.5% BSA, 1× nonessential amino acids, 2 mM l-glutamine) at room temperature and resuspended at 106 cells/ml in infection medium. Cell suspensions were given either no virus or recombinant vaccinia virus (control pRB21, ZAP-70 wt, PYK-2 wt, PYK-2 (K-M), or the EGFP fusion proteins) at 20 pfu/cell and incubated 2 h at 37°C with occasional stirring. After one wash in complete RPMI, the vaccinia-infected or uninfected control cells were simultaneously plated for standard 51Cr-release killing assays, for Western blot, and for immunofluorescence analysis as described. The level of infection was monitored by measuring the expression of the viral envelope p37 protein in the infected cells by Western blot.
Results
PYK-2 Colocalizes with the MTOC in Migrating NK Cells
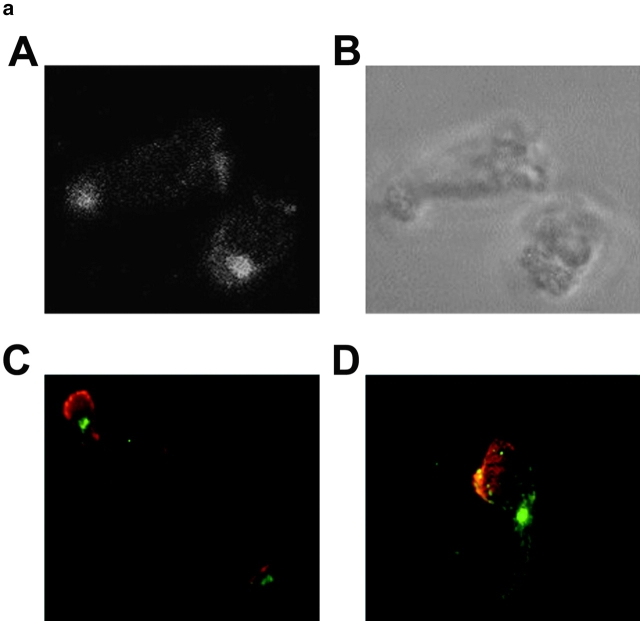
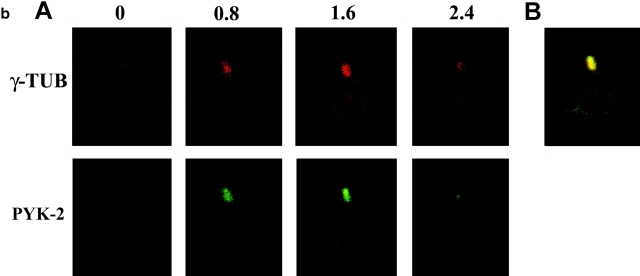
IL-2–activated NK lymphocytes show a high degree of cell polarization, in accordance with their high locomotive capability (Somersalo 1996–1997; Nieto et al. 1998). Immunofluorescence studies were performed to determine the cytoplasmic distribution of PYK-2 in IL-2–activated NK cells adhered and migrating onto FN. Confocal microscopy analysis revealed that PYK-2 is highly concentrated in a specific location in the cytoplasm of both rounded and polarized NK cells adhered to FN (Fig. 1 a, A and B). In polarized NK cells, the majority of PYK-2 was concentrated at the uropod, the cytoplasmic projection developed at the rear of polarized cells. Double staining of cells for PYK-2 and moesin, a member of the ezrin-radixin-moesin family of proteins that connects the cell membrane to the actin-cytoskeletal network and that marks the cytoplasmic membrane of the uropod (Serrador et al. 1997), confirmed that PYK-2 localizes at this protrusion of polarized NK cells (Fig. 1 a, C). A small fraction of PYK-2 was also observed at the advancing front of some NK cells (Fig. 1 a, A) colocalizing with talin, a cytoskeletal protein involved in the formation of integrin-mediated cell adhesion contacts with substratum (Fig. 1 a, D). As the uropod is the site of microtubule retraction (Ratner et al. 1997; Serrador et al. 1997), we performed immunofluorescence studies to determine the localization of MTOC in migrating, polarized NK cells. Confocal sections of cells double-stained for γ-tubulin, a microtubule protein that is a highly conserved component of the MTOC (Stearns et al. 1991; Zheng et al. 1991), and for PYK-2, demonstrated that this tyrosine kinase colocalizes with γ-tubulin, and thus with the MTOC, at the uropod of polarized NK cells adhered to FN (Fig. 1 b).
Figure 1.
PYK-2 is localized at the uropod projection of NK cells adhered to fibronectin. (a) Confocal section of PYK-2 immune staining of NK cells migrating on FN (A). The same section under brightfield illumination is shown in B. Epifluorescence images of NK cells double-stained for PYK-2 (green) and moesin (red, C), and PYK-2 (green) and talin (red, D). Control cells stained with goat serum or secondary antibody alone did not show any specific staining, and preincubation of the anti-PYK-2 antiserum with its specific blocking peptide prevented staining of NK cells (not shown). (b, A) Confocal serial sections of polarized NK cells adhered to FN and double-stained for γ-tubulin (red, upper panels, γ-TUB) and PYK-2 (green, lower panels, PYK-2). Sections were taken every 0.8 μm from the substratum (0) in the z-axis. b, B shows a merged image of double-labeled cells demonstrating the colocalization of γ-tubulin and PYK-2 at the uropod of NK cells.
PYK-2 Is Translocated in NK Cells to the Area of Cell-to-Cell Contact during Specific Recognition of Target Cells
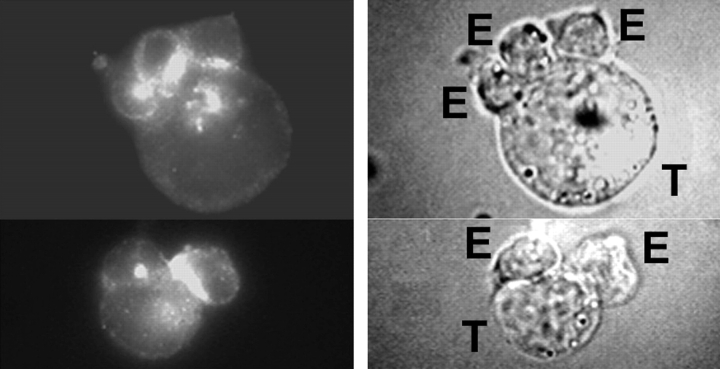
To assess whether PYK-2 is translocated during the killing process, we investigated the cellular localization of PYK-2 in NK–target cell conjugates. In a first set of experiments, K562 cells were used as targets since these cells do not express classical MHC class I molecules and are efficiently killed by IL-2–activated polyclonal NK cells. As it has been described (Nieto et al. 1998), asymmetrical binding of NK cells to target cells through the NK cell leading edge was observed, with the uropod projecting away from the area of cell–cell contact (Fig. 2). When NK–K562 cell conjugates were stained with anti–PYK-2 polyclonal antibodies, it was found that this kinase concentrated in a specific domain of the effector cells that faces the site of adhesion with the target cell (Fig. 2). In addition, in some effector cells, PYK-2 localized to cell-to-cell contact areas. In contrast, no staining for PYK-2 was detected at the cell uropod, developed at the opposite site of the contacting region. Therefore, PYK-2 is translocated from the rear of the cell to the area of cell contact during effector–target cell conjugate formation.
Figure 2.
PYK-2 is localized at the intercellular contact area of NK–K562 target cell conjugates. Effector–target cell conjugates of polyclonal NK cells (E) and K562 target cells (T) were stained for PYK-2. Cells were photographed under epifluorescence conditions (left) or phase contrast illumination (right).
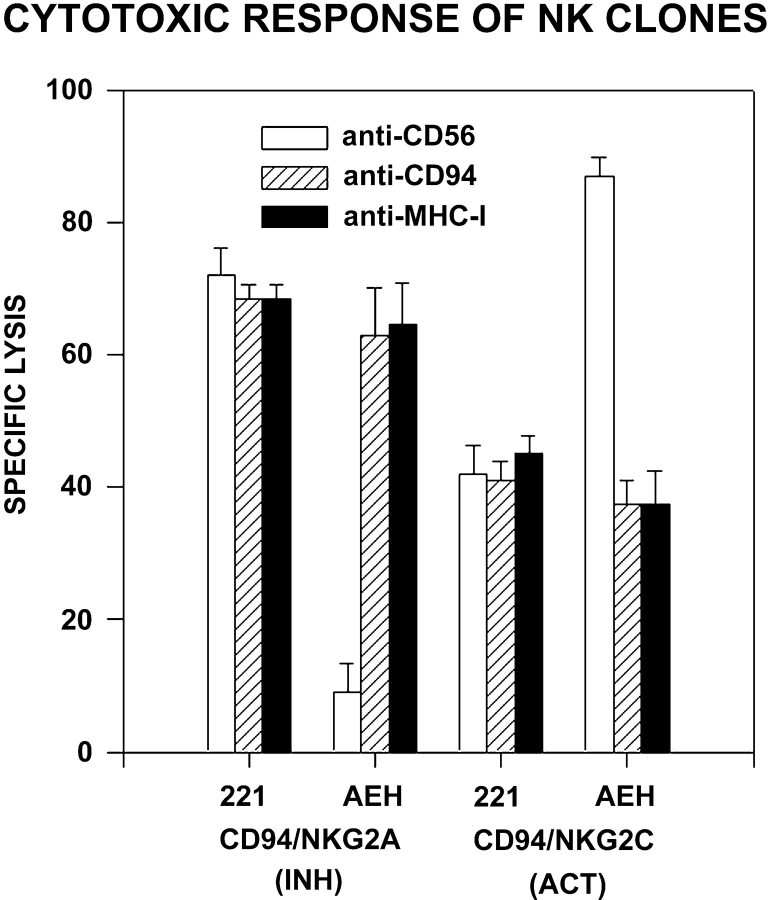
The triggering receptors for lysis of K562 cells have not been defined yet. To ascertain whether the translocation of PYK-2 in NK cells was related to the recognition of target cells through specific receptors, the positioning of PYK-2 was determined during the interaction of distinct NK cell clones with target cells that show a different sensitivity to be killed. To this end, we have used two different types of NK cell clones that express CD94/NKG2C or CD94/NKG2A heterodimers that interact with HLA-E. Fig. 3 shows the cytotoxic activity of NKG2A- and NKG2C-bearing NK cell clones against target cells expressing (.221-AEH) or not (.221) HLA-E. NKG2A+ cell clones showed an efficient cytotoxicity against .221 cells, but not to .221-AEH. The protection conferred by HLA-E was reversed by blocking mAbs against both CD94 and MHC I molecules, demonstrating that the inhibitory signal of cytotoxicity was induced through the specific interaction of these molecules. In contrast, the lytic ability of the NK clones that express the CD94/NKG2C is enhanced by .221-AEH HLA-E–bearing cells (Borrego et al. 1998; Braud et al. 1998; Lee et al. 1998; Llano et al. 1998). Hence, we selected some activatory clones whose basal lysis against .221 was low, thus allowing the detection of the enhancing effect by HLA-E (Fig. 3). In this case, mAbs against CD94 and MHC I molecules blocked the activation signal induced by HLA-E (Fig. 3).
Figure 3.
Cytotoxic response of NKG2A+ and NKG2C+ NK cell clones against .221 or .221-AEH cells. NK cell clones whose killing activity is inhibited (NKG2A+) or activated (NKG2C+) by HLA-E were tested in a 51Cr-release assay against .221 (HLA-E−) or .221-AEH (HLA-E+) target cells. This figure also shows the effect of anti-CD94 HP3B1, anti–MHC class I 1F7, and anti-CD56 C218 (control) mAbs, on the cytotoxic response. Representative data from three independent experiments are shown as arithmetic mean ± SE.
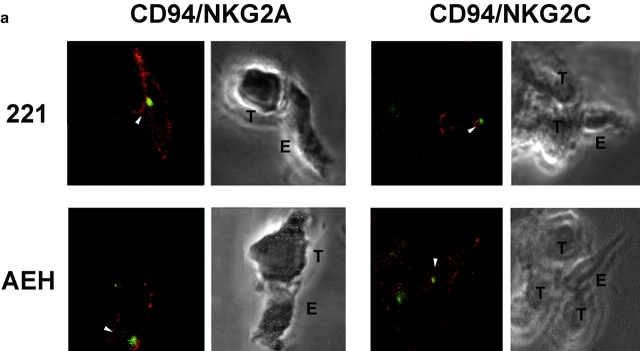
NKG2A+ and NKG2C+ cell clones were allowed to interact with .221 or .221-AEH target cells and then PYK-2 was stained in effector–target cell conjugates, and analyzed by confocal microscopy. In conjugates of inhibitory NKG2A+ cell clones with HLA-E–bearing target cells (.221-AEH), PYK-2 was clearly located at the cell uropod. In contrast, PYK-2 was found facing the area of cell–cell interaction in NK cells when .221 target cells did not express HLA-E (Fig. 4 a, left panels, and Table ). The correlation between the triggering of cytotoxic activity and translocation of PYK-2 was further confirmed in reciprocal experiments using activatory NKG2C-expressing clones. In this case, PYK-2 was mainly localized at the NK cell uropod in cell conjugates formed with .221 target cells, whereas it was translocated to the cell-to-cell contact area when cytotoxicity was triggered by recognition of HLA-E on .221-AEH cells (Fig. 4 a, right panels, and Table ).
Figure 4.
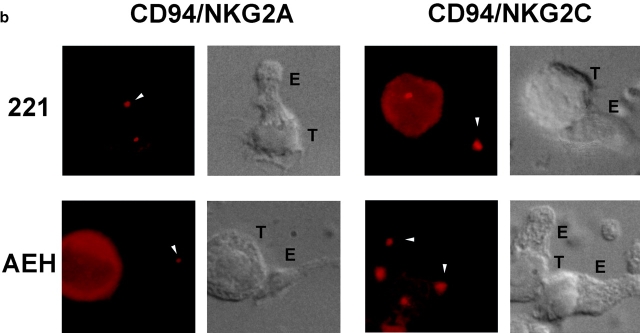
PYK-2 and the MTOC are translocated to the area of cell–cell contact of NK cells with target cells during specific recognition and cytotoxicity. (a) Confocal sections showing PYK-2 localization on NK cell clones (E) which are inhibited (CD94/NKG2A), or activated (CD94/NKG2C) by HLA-E, and that were interacting with HLA-E− (.221) or HLA-E+ (AEH) target cells (T). Cell–cell conjugates were stained for PYK-2 (green) and CD94 (red) and analyzed using confocal microscopy. Confocal sections under brightfield illumination are shown for each conjugate. Arrowheads show the localization of PYK-2 on effector cells. (b) MTOC localization on NK cell clones that are inhibited (CD94/NKG2A), or activated (CD94/NKG2C) by HLA-E, and that were interacting with HLA-E− (.221) or HLA-E+ (AEH) target cells. Cell conjugates stained for γ-tubulin were photographed under epifluorescence (red) and brightfield conditions using a Nomarski 60× objective. T and E indicate target and effector cells, respectively, while arrowheads point to the MTOC on effector cells.
Table 1.
Frequency of Translocation of PYK-2 and MTOC in NK Cells Interacting with Target Cells
| PYK-2 | MTOC | |||
|---|---|---|---|---|
| CD94/NKG2A(INH) | CD94/NKG2C(ACT) | CD94/NKG2A(INH) | CD94/NKG2C(ACT) | |
| .221 | 78.7 ± 13.5 | 32.6 ± 8.9 | 75.5 ± 12.8 | 30.1 ± 7.5 |
| AEH | 23.1 ± 8.3 | 81.2 ± 15.4 | 19.2 ± 7.4 | 82.7 ± 17.9 |
NK cells bearing the activatory (CD94/NKG2C), or inhibitory (CD94/NKG2A) receptors for HLA-E were allowed to interact with .221 (HLA-E−) or .221-AEH (HLA-E+) target cells on FN-coated plates. After 1 h, cell conjugates were fixed, and stained for PYK-2 or γ-tubulin. Translocation of PYK-2 or MTOC was measured in more than 100 conjugates in three independent experiments. Results correspond to the arithmetic mean ± SD.
Changes in the Distribution of Cytoskeletal Components during NK Cell Receptor–mediated Effector–Target Cell Interaction
It has been described that the MTOC of cytotoxic T lymphocytes is translocated to face the area of cell adhesion with the target cell during the cytotoxicity process (Kupfer and Singer 1989). Therefore, it was of interest to analyze the effect of NK cell–specific cytotoxic triggering in the cellular positioning of some cytoskeletal components. We then characterized the MTOC translocation triggered by CD94/NKG2-specific recognition. In accordance with their cytotoxic responses, the translocation of the MTOC observed when NKG2A+ clones interacted with .221 targets was inhibited when killing was prevented by HLA-E in .221-AEH cells (Fig. 4 b, left panels, and Table ). Conversely, an efficient translocation of the MTOC was observed on CD94/NKG2C NK cell clones interacting with .221-AEH target cells. The frequency of MTOC translocation was very low when these clones interacted with .221 target cells (Fig. 4 b, right panels, and Table ). Thus, the triggering of cytotoxic activity in these clones correlated with the translocation of MTOC to the area of interaction with sensitive target cells.
It has been recently reported that PYK-2 is constitutively associated to paxillin in NK cells (Gismondi et al. 1997). Accordingly, we found that paxillin colocalized with PYK-2 at the site of MTOC, and that both paxillin and PYK-2 were translocated to the cell–cell contact area when NK cell clones were bound to sensitive, but not to resistant target cells (Fig. 5). In contrast, no changes in the subcellular localization of other cytoskeletal components such as moesin, localized at the NK cell uropod, or talin, which participates in integrin-mediated cell adhesion and localizes at cell-to-cell boundaries areas, were observed upon triggering of killing (Fig. 5). These results demonstrate that PYK-2 and paxillin are specifically translocated, correlating with microtubular rearrangements, to the area of NK cell–target cell interaction in response to NK cell receptor–mediated specific signals that modulate cytotoxicity.
Figure 5.
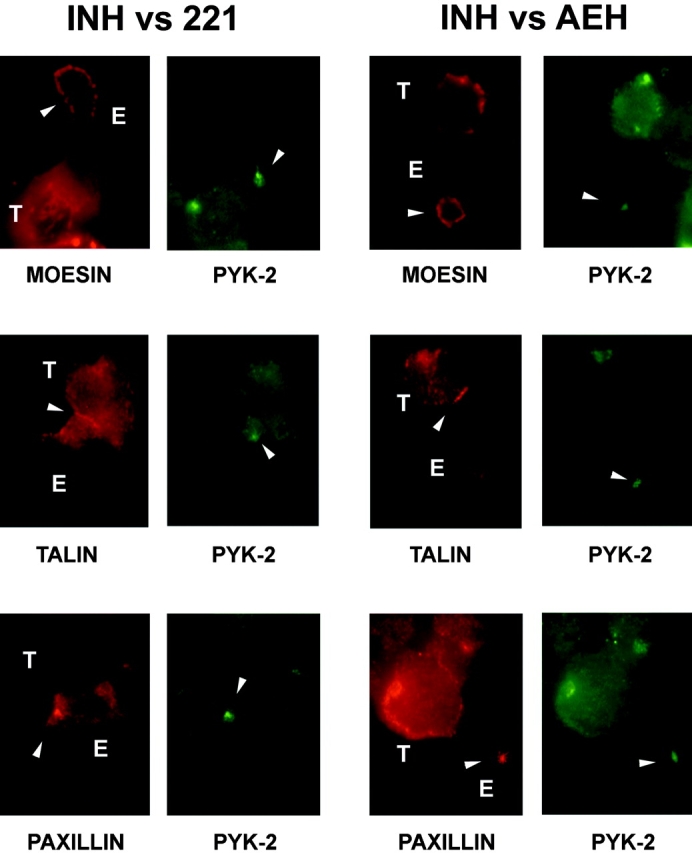
Subcellular localization of different cytoskeletal proteins in NK/target cell conjugates. Cytoplasmic localization of PYK-2 (green) and the cytoskeletal proteins moesin, talin, and paxillin (red) in cell conjugates formed by NK cell clones (E) which are inhibited by HLA-E+ (INH-AEH) but not by HLA-E− (INH-.221) target cells (T). Arrowheads indicate staining of the protein indicated at each panel.
PYK-2 Activation during Cytotoxic Activity of NK Cells
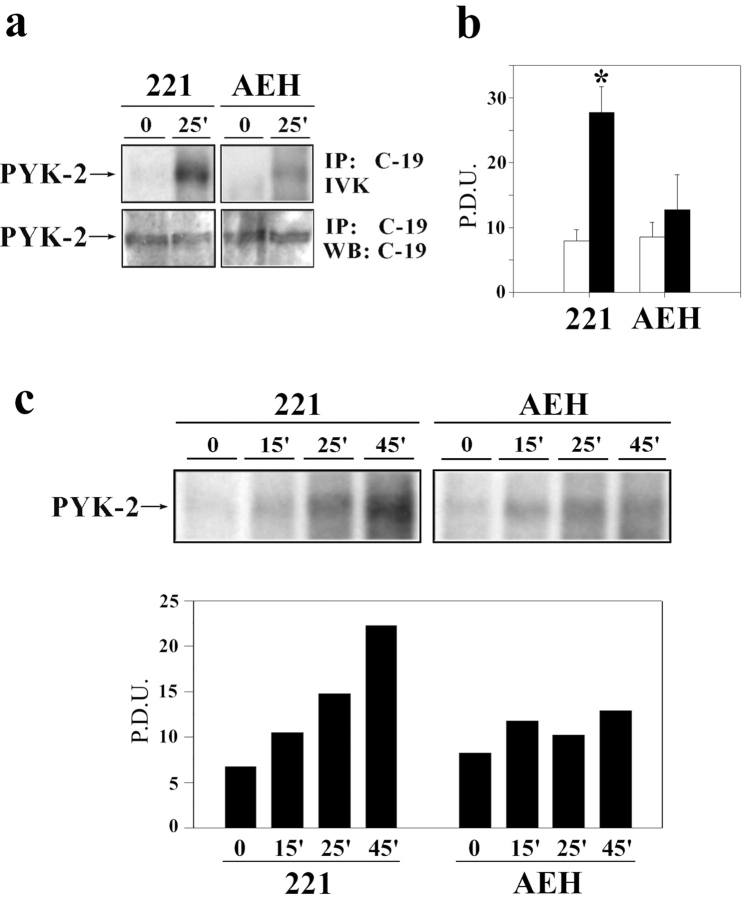
The selective translocation of PYK-2 during target cell killing suggests that this tyrosine kinase is activated as a consequence of the signals involved in the triggering of cytotoxicity. To assess this possibility, we analyzed the activation of PYK-2 during killing by an in vitro kinase assay using as effector the CD94/NKG2A expressing NKL cell line. The cytotoxic response and the translocation of the MTOC and PYK-2 in this cell line are similar to the CD94/NKG2A clones described above, and they are also inhibited upon recognition of HLA-E (data not shown). To analyze the kinase activity of PYK-2 during cytotoxicity, this kinase was immunoprecipitated from cell lysates of mixtures of NKL cells with either .221 wild-type cells or cells expressing HLA-E (.221-AEH). Immunoprecipitates were incubated with γ-[32P]ATP and analyzed by SDS-PAGE. The results obtained revealed a statistically significant difference between the activation state of PYK-2 in the two distinct effector–target cell conjugates (Fig. 6, a and b). Correlating with the triggering of cytotoxic activity (65.7 ± 5.6% specific lysis), PYK-2 was clearly activated when NKL cells interacted with wild-type–sensitive .221 cells. In contrast, a significant lower change in the activation state of PYK-2 was observed when NKL cells interacted with .221-AEH cells, which trigger an inhibitory signal resulting in a lower cytotoxic activity (27.5 ± 3.8% specific lysis). Immunoblotting with anti–PYK-2 C-19 antibody immunoprecipitates carried out in parallel verified that similar amounts of PYK-2 were recovered in each case (Fig. 6, a and b). Kinase activity was not detected when the PYK-2 antibody was preincubated with the immunizing peptide before the addition to the lysates or when the lysates were immunoprecipitated with a nonspecific goat serum (results not shown). Kinetics studies showed that the increase in the kinase activity of PYK-2 during cytotoxicity reached a maximum from 25 to 45 min (Fig. 6 c). The possible contribution of PYK-2 protein from target cells was ruled out when the same experiments performed with fixed target cells rendered similar results (data not shown). Fixation of target cells did not affect their capability to induce translocation of PYK-2 or MTOC on NK cells (data not shown). These results indicate that PYK-2 is activated in response to NK cell receptor-driven specific signals that lead to NK cell–mediated cytotoxicity.
Figure 6.
PYK-2 activation during the cytotoxic response. (a) NKL cells bearing the inhibitory CD94/NKG2A receptor were allowed to interact with HLA-E− (.221) or HLA-E+ (AEH) target cells for 25 min and then, half of the lysate was used to immunoprecipitate PYK-2 with the C-19 antibody and to perform an in vitro kinase reaction, and the other half to quantify the levels of PYK-2 by Western blot. Arrows indicate bands corresponding to PYK-2. (b) Statistical analysis of PYK-2 activation. The results of in vitro kinase assays performed as indicated in panel a are expressed as arithmetic mean ± SE of intensity expressed in pixel density units (P.D.U.) and represent three independent experiments. *, P < 0.05 compared to the other times and treatments (Student's t test). (c) Kinetics of the phosphorylating activity of PYK-2. NKL cells bearing the inhibitory CD94/NKG2A receptor were allowed to form conjugates with .221 or .221-AEH cells for different periods of time. Then, an in vitro kinase reaction was performed in cell lysates, as described in Materials and Methods. A representative experiment out of three is shown in the upper panel, whereas the mean intensity in P.D.U. of this experiment is depicted in the lower panel.
Overexpression of PYK-2 in NK Cells Inhibits Cytotoxicity and Translocation of MTOC and Paxillin
To further assess the role of PYK-2 in the cytotoxic response, we used a vaccinia virus system to overexpress the wt and a kinase dead mutant of PYK-2 (PYK-2 KM) in the NKL cell line. Immunoblotting analyses with anti–PYK-2 confirmed the overexpression of the wt and the kinase dead mutant of PYK-2 (Fig. 7 a). As expected, overexpression of PYK-2 wt resulted in a substantial increase in the PYK-2 autokinase activity in immunoprecipitates from lysates of NKL-infected cells. In contrast, PYK-2 immunoprecipitates from cells infected with the kinase dead mutant PYK2 K-M did not show any kinase activity and, instead, it caused a significant inhibition of the basal kinase activity of endogenous PYK-2 (Fig. 7 a), as previously described (Sieg et al. 1998; Kumar et al. 1999). No significant effect on the kinase activity of PYK-2 was observed when cells were infected with the control VV pRB21 at the same pfu/cell. Equivalence of the level of infection between the vaccinia viruses containing different constructs was assessed by analysis of the expression of the viral p37 protein (Fig. 7 a).
Figure 7.
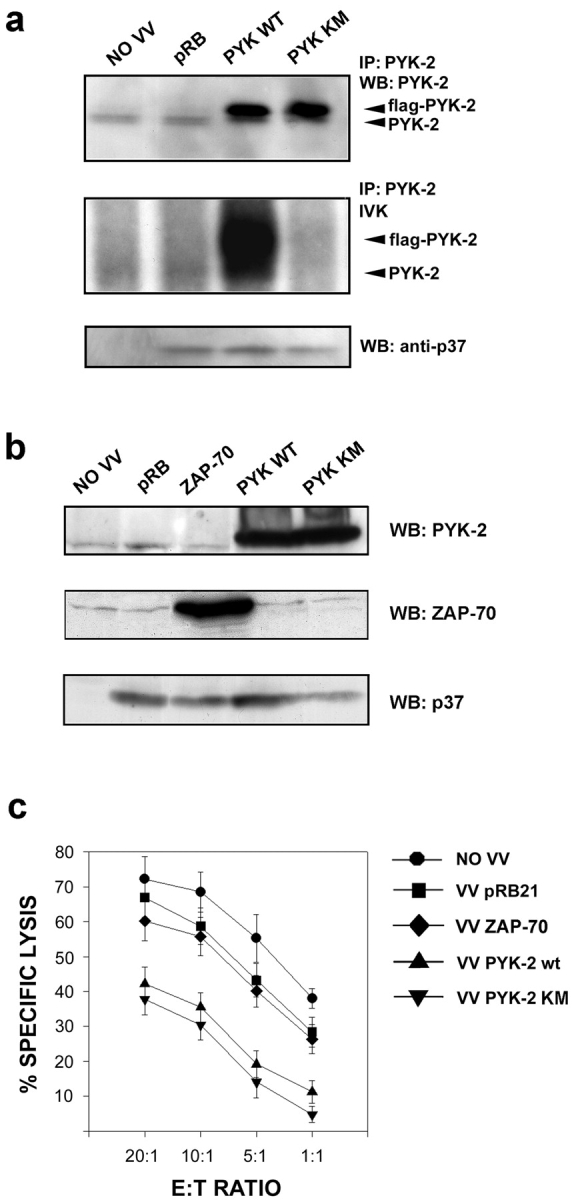
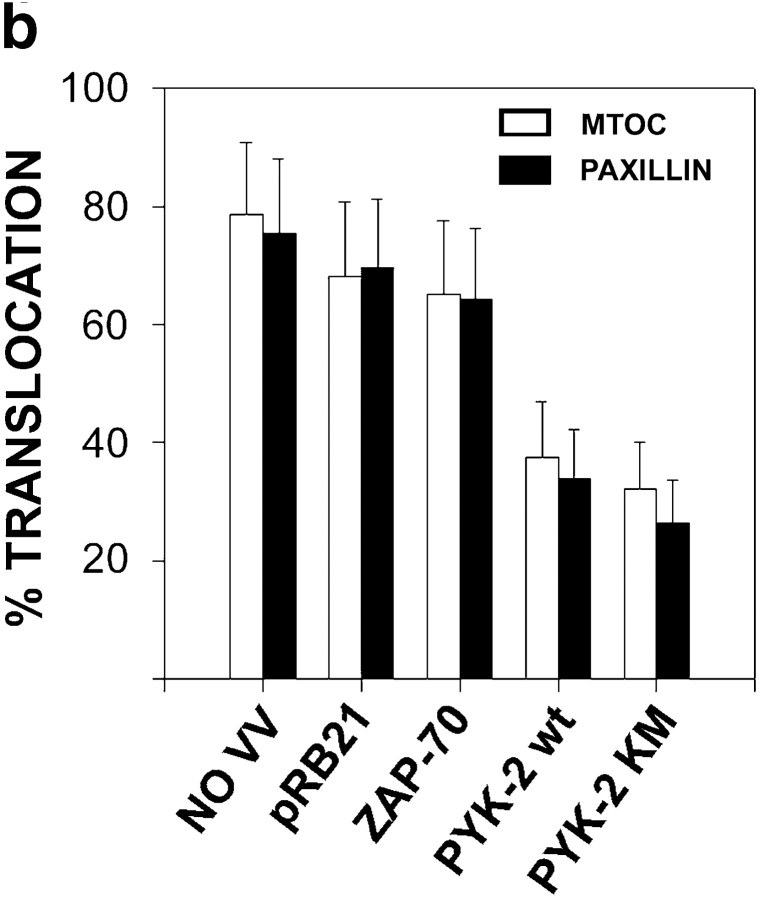
Overexpression of PYK-2 wt and PYK-2 (K-M) inhibits NK cells cytotoxic response. (a) Lysates from NKL cells uninfected (NO VV), infected with control vaccinia virus (pRB), infected with PYK-2 wt (PYK WT), or infected with PYK-2 (K-M) (PYK KM) were immunoprecipitated (IP) with C-19 anti–PYK-2 polyclonal antibody, analyzed by Western blotting (WB) with anti–PYK-2 polyclonal antibody, and parallel samples were assayed for in vitro autokinase activity. Whole cell lysates of these samples were analyzed by Western blotting with anti-p37 15B6 mAb. (b) Lysates from NKL cells uninfected (NO VV), infected with control vaccinia virus (pRB), infected with ZAP-70 (ZAP-70), infected with PYK-2 wt (PYK WT), or infected with PYK-2 (K-M) (PYK KM) were analyzed by Western blotting (WB) with C-19 anti–PYK-2 polyclonal antibody, 701 anti–ZAP-70 polyclonal antibody, and 15B6 anti-p37 mAb. (c) NKL cells uninfected (NO VV), infected with control VV (VVpRB21), infected with ZAP-70 (VV ZAP-70), infected with PYK-2 wt (VV PYK-2 wt), or infected with PYK-2 (K-M) (VV PYK-2 KM) were tested in a 51Cr-release assay against their sensitive target .221. Representative data from three independent experiments are shown as the mean percent specific lysis versus E/T ratio. The inhibition of cytotoxic response by overexpression of PYK-2 is significant (P < 0.05, Student's t test) for all E/T ratios tested.
To assess the specificity of the effect of PYK-2 overexpression on NK cell function, we generated ZAP-70 wt viruses overexpressing an equivalent level of this tyrosine kinase, compared to the overexpression of PYK-2 (Fig. 7 b). The ZAP-70 kinase has been previously described not to affect cytotoxicity (Brumbaugh et al. 1997). We then analyzed the cytotoxic activity of the NKL infected with the different vaccinia viruses against the .221-sensitive target. Interestingly, the killing was inhibited by the overexpression of both the wt and the kinase dead PYK-2, as compared to the slight effect of the infection with control pRB21 vaccinia virus and the tyrosine-kinase ZAP-70 (Fig. 7 c). In addition, the overexpression of another kinase, the serine-threonine kinase PAK-1, did not affect cytotoxicity, further confirming the specificity of the effect of PYK-2 on NK cell–mediated killing (data not shown).
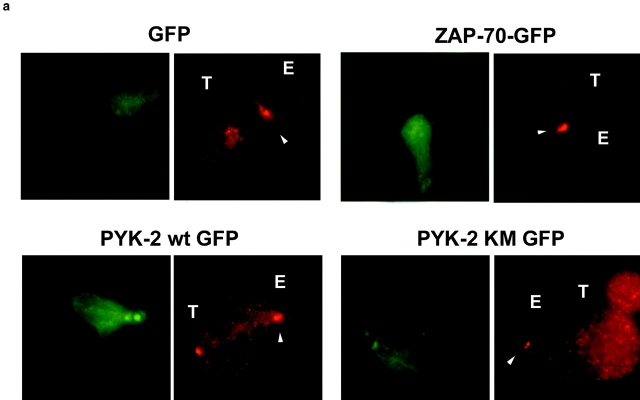
To explore the mechanism by which overexpression of PYK-2 inhibits the cytotoxic activity, we studied the subcellular localization of the different forms of PYK-2-EGFP fusion proteins and its influence on MTOC translocation. Vaccinia viruses were used to overexpress PYK-2 wt-EGFP, PYK-2 (K-M)-EGFP, ZAP-70-EGFP, and EGFP fusion proteins. Inhibitory clones (CD94/NKG2A) were infected and allowed to interact with .221 target cells, subcellular localization of MTOC, and paxillin studied by immunofluorescence analysis (Fig. 8 a). PYK-2 wt-EGFP and (K-M)-EGFP showed a broad distribution throughout the cell cytoplasm, with a higher concentration colocalizing with the MTOC and paxillin, whereas EGFP and ZAP-70-EGFP were found uniformly distributed in the cell cytoplasm (Fig. 8 a, and not shown). As expected, efficient translocation of the MTOC to the area of contact with target cells was observed on cells overexpressing EGFP or ZAP-70-EGFP, and only a slight reduction on the frequency of MTOC translocation was observed as compared to uninfected cells (Fig. 8, a and b). Interestingly, overexpression of both the fusion proteins PYK-2 wt-EGFP and PYK-2 (K-M)-EGFP inhibited the translocation of MTOC and paxillin in response to the sensitive .221 target cell (Fig. 8, a and b). Given the well-known interactions of PYK-2 with several regulatory molecules (Gismondi et al. 1997; Schlaepfer et al. 1999), these results suggest that PYK-2 has an important additional role as a scaffolding protein, whose overexpression deregulates the balance of signaling complexes that control the killing response, probably by interfering with the microtubular rearrangements involved in the translocation and orientation of the secretory apparatus.
Figure 8.
Overexpression of PYK-2 wt-EGFP and PYK-2 (K-M)-EGFP prevents the translocation of MTOC and paxillin during specific target recognition. (a) NK inhibitory clones were infected with control VV-pRB21-EGFP (EGFP) (upper left panels), ZAP-70-EGFP (upper right panels), PYK-2 wt-EGFP (lower left panels), and PYK-2 (K-M)-EGFP (lower right panels). NK cell conjugates with the sensitive .221 target were studied by immunofluorescence. EGFP green fluorescence shows the infected NK cells (left panels). The red fluorescence corresponds to γ-tubulin (MTOC) staining (right panels). T and E indicate target and effector cells, respectively, while arrowheads point to the MTOC on effector cells. (b) Quantification of the translocation of MTOC and paxillin in conjugates formed by a green (infected) NK cell from inhibitory clones and its sensitive target .221. Translocation of MTOC or paxillin was measured in >100 conjugates in three independent experiments. Results correspond to the arithmetic mean ± SD.
Discussion
We report herein the changes in the subcellular localization of the tyrosine kinase PYK-2 in NK cells and its activation during the effector–target cell conjugate formation and the triggering of cytotoxic activity. In addition, we provide evidence on the role of PYK-2 in the regulation of cytoskeletal rearrangements, required for NK cell–mediated killing activity. It is now becoming evident that in addition to other regulatory elements such as phosphorylation or aggregation of molecules, protein compartmentalization provides an additional modulatory mechanism in the cell (Mochly-Rosen 1995; Faux and Scott 1996; Pawson and Scott 1997). Subcellular positioning of signaling molecules can be accomplished by their association with cytoskeletal scaffolds or, as it has been recently demonstrated, by their preferential localization into specific cell membrane domains (Dustin and Shaw 1999). The selective confining of proteins into specific cell domains has been shown to be of particular importance during the immune response (reviewed in Dustin and Shaw 1999; Lanzavecchia et al. 1999; Penninger and Crabtree 1999; Serrador et al. 1999). During the T cell response, the concentration of several proteins, including the T cell receptor molecules, into distinct segregated domains, is necessary to accomplish T cell activation. Interestingly, the protein kinase C-θ and Src-kinase family members Lck and Fyn colocalize with the T cell receptor complex at the area of cell–cell contact during antigen presentation (Monks et al. 1997, Monks et al. 1998). Altogether these observations suggest that protein compartmentalization, via recruitment and inclusion of activating signaling molecules and exclusion of potential negative regulators, may provide a unique restricted subcellular microenvironment that promotes optimal signal transduction. By this way, the cytoskeleton can directly regulate, both temporally and spatially, the molecular dynamics of signaling pathways.
The localization of PYK-2 at the site of cell contact during conjugate formation could be explained by its association with the MTOC, but such phenomenon would also imply the topological restricted regulation of signaling events at the site of intercellular contacts such as the aforementioned vesicle secretion or cell adhesion. In this regard, it has been shown that PYK-2 is involved in the outside-in signaling mediated by integrins, which are involved in NK cell adhesion to their targets, and act as costimulatory receptors during NK cell function (Astier et al. 1997; Gismondi et al. 1997; Ma et al. 1997). In addition, it is worth mentioning that a small fraction of PYK-2 is located at the advancing front of some polarized NK cells, the site that nucleates cell adhesion. Furthermore, PYK-2 was observed predominantly concentrated in the area of cell adhesion in polyclonal NK-target cell conjugates. The translocation of PYK-2 to the site of contact with target cell upon the triggering of cytotoxic activity suggests that this enzyme participates in the signaling mediated by integrins during NK cell activation. In agreement with this possibility, we found that PYK-2 colocalizes with paxillin at the site of MTOC and that both proteins are translocated to the cell–cell contact area of NK–target cell–specific conjugates during the cytotoxic process. In this regard, it has been recently reported the constitutive association of PYK-2 with paxillin, which has been largely implicated in the formation of adhesion structures (Burridge et al. 1997; Gismondi et al. 1997).
However, several pieces of evidence indicate that integrin signaling does not seem to account by itself for the activation and translocation of PYK-2 observed after the triggering of cytotoxic activity by sensitive target cells. First, the interaction of NK cells with FN through β1 integrins does not induce the translocation of PYK-2 and the MTOC. Second, we found adhesion of NK cells, likely mediated by β2 integrins, to both sensitive and resistant target cells, but the translocation and activation of PYK-2 was only induced by targets that elicited NK cell cytotoxicity. Furthermore, PYK-2 translocation was induced upon specific recognition of MHC class I molecules by the activatory NK cell receptor CD94/NKG2C. In addition, we found that the signaling through the inhibitory receptor CD94/NKG2A, specifically inhibited both PYK-2 activation and translocation. Therefore, our data demonstrate that the translocation and activation of this enzyme is closely associated with triggering of the cascade of signaling events that induce NK cell cytotoxicity and that, therefore, is specifically regulated by MHC receptors. In agreement with this point, it has been reported that engagement of the T cell receptor triggers PYK-2 activation (Berg and Ostergaard 1997; Ganju et al. 1997; Qian et al. 1997).
The compartmentalization of PYK-2 may imply several important issues concerning the function of PYK-2 in NK cells. First, the colocalization of PYK-2 with the MTOC suggests a role for this tyrosine kinase or its associated proteins in phosphorylating different cytoskeletal elements as well as other effector-associated proteins upon the triggering of cytotoxic activity. In this regard, different protein targets for PYK-2 have been recently described, including a GTPase-activating protein designated Pap. Pap exhibits a strong GTPase-activating protein activity towards different small GTPases of the Arf family, and has been implicated in the control of vesicular trafficking (Andreev et al. 1999; Lakkakorpi et al. 1999; Lev et al. 1999). Polarized vesicle secretion has been described to occur during the killing response of lymphocytes (Kupfer and Singer 1989).
In an attempt to further assess the involvement of PYK-2 in the cytotoxic activity of NK cells, we used a vaccinia virus system overexpressing PYK-2 wt and a kinase dead mutant PYK-2 (K-M). The infection with vaccinia viruses has been used previously to test the function of individual receptor molecules in NK cells and the involvement of the tyrosine phosphatase SHP-1 in KIR-mediated signaling (Wagtmann et al. 1995; Binstadt et al. 1996). Our results showing the inhibitory effect of overexpressing either the wt or a kinase dead PYK-2 in the NK killing response are unexpected, but are consistent with an additional role of PYK-2 as a scaffolding protein. This putative role of PYK-2 could not be overcome by the kinase activity of PYK-2 wt. Since proteins such as paxillin, p130Cas, and Graf bind to PYK-2 in a phosphorylation- and kinase-independent manner (Gismondi et al. 1997), it is feasible that PYK-2 may indeed function as a linker protein in the intracellular signal transduction pathways activated by the NK-sensitive target cell interaction, which would be deregulated upon its overexpression, thus resulting in nonfunctional signaling complexes. This possible role of PYK-2 as an adapter molecule, independently of its kinase activity has also been suggested for FAK to explain the effects of its overexpression on the motility of CHO cells (Cary et al. 1996). Our finding on the prevention of the rearrangement of microtubules and the translocation of MTOC upon the overexpression of PYK-2 supports this view. However, the issue of the possible function of the kinase activity of PYK-2 during cytotoxicity, which may play a role since PYK-2 becomes activated as a consequence of activatory signals, remains uncertain. In this regard, a recent report described that overexpression of wild-type SHP-1 blocks tyrosine phosphorylation of PYK-2 (Kumar et al. 1999). This tyrosine phosphatase has been involved in KIR-mediated inhibition of cytotoxicity (Binstadt et al. 1996), and it would be feasible that this inhibitory effect via SHP-1 phosphatases on cytotoxicity could be mediated by PYK-2 inhibition. In addition, the kinase activity of PYK-2 could be important for its adapter role, since the association of important kinases which would be involved in cytotoxicity, such as Src, is dependent on the autophosphorylation of the Tyr402 of PYK-2 (Schlaepfer et al. 1999). The precise mechanism of action of PYK-2 in NK cell cytotoxicity deserves further research.
In summary, our data indicate that the specific recognition of target cell by NK cell receptors directs the translocation and activation of PYK-2, paxillin, and MTOC from the uropod to the cell–cell contact area. In addition, PYK-2 regulates NK cell cytotoxic activity. These findings further support the important role of cell compartmentalization in the key functions of immune cells.
Note Added in Proof. In a recent paper, another group has also shown a functional role for PYK-2 in NK cell cytotoxicity (Gismondi, A., J. Jacobelli, F. Mainiero, R. Paolini, M. Piccoli, L. Frati, and A. Santoni. 2000. Functional role for proline-rich tyrosine kinase 2 in NK-cell–mediated natural cytotoxicity. J. Immunol. 164:2272–2276.
Acknowledgments
We thank M.A. Ollacarizqueta and E. de la Rosa for technical assistance with confocal microscopy; Dr. R. Gónzalez-Amaro and Dr. H.M. van Santen for critical reading of the manuscript; and Dr. B. García-Barreno and Dr. T. Delgado for their assistance with the vaccinia virus system.
This work was supported by grant SAF99/0034-C01, SAF98-0006, and 2FD97-0680-C02-02 from the Ministerio de Educación y Cultura; grant 08.1/0011/97 (to F. Sánchez-Madrid), 08/005/97 (to M. López-Botet), and 08.1/0015/97 (to C. Cabañas) from the Comunidad Autónoma de Madrid; and grant SAF98/0080 (to C. Cabañas) and fellowships from the Fondo de Investigaciones Sanitarias BAE 97/5089 (to M. Nieto). J.L. Rodriguez-Fernández was supported by a “Contrato de Reincorporación” associated to grants PB94-0231, SAF98/0080, and SAF98/0006 awarded by the “Ministerio Español de Educación y Cultura”.
Footnotes
D. Sancho and M. Nieto contributed equally to this article.
Dr. Nieto's current address is IGBMC, BP163, 67404 Illkirch Cédex, CU de Strasbourg, France.
Abbreviations used in this paper: EGFP, enhanced green fluorescent protein; FAK, Focal adhesion kinase; FN, fibronectin; HLA, human leukocyte antigen; IL-2, interleukin-2; ITIM, immunoreceptor tyrosine-based inhibitory motifs; MHC, molecular histocompatibility complex; MTOC, microtubule-organizing center; NK, natural killer; PYK-2, proline-rich tyrosine kinase 2; SHP, Src homology 2 domain containing protein tyrosine phosphatase.
References
- Andreev J., Simon J., Sabatini D.D., Kam J., Plowman G., Randazzo P.A., Schlessinger J. Identification of a new PYK-2 target protein with Arf-GAP activity. Mol. Cell. Biol. 1999;19:2338–2350. doi: 10.1128/mcb.19.3.2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramburu J., Balboa M.A., Ramírez A., Silva A., Acevedo A., Sánchez-Madrid F., de Landázuri M.O., López-Botet M. A novel functional cell surface dimer (Kp43) expressed by natural killer cells and T cell receptor-γδ T lymphocytes. Inhibition of IL-2 dependent proliferation by anti-Kp43 monoclonal antibody. J. Immunol. 1990;147:714–721. [PubMed] [Google Scholar]
- Astier A., Avraham H., Manie S.N., Groopman J., Canty T., Avraham S., Freedman A.S. The related adhesion focal tyrosine kinase is tyrosine phosphorylated after β1-integrin stimulation in B cells and binds to p130cas . J. Biol. Chem. 1997;272:228–232. doi: 10.1074/jbc.272.1.228. [DOI] [PubMed] [Google Scholar]
- Avraham S., London R., Fu Y., Ota S., Hiregowdara D., Li S., Jiang S., Pasztor L.M., White R.A., Groopman J.E., Avraham H. Identification and characterization of a novel related adhesion focal tyrosine kinase (RAFTK) from megakaryocytes and brain. J. Biol. Chem. 1995;270:27742–27751. doi: 10.1074/jbc.270.46.27742. [DOI] [PubMed] [Google Scholar]
- Berg N.N., Ostergaard H.L. T cell receptor engagement induces tyrosine phosphorylation of FAK and PYK-2 and their association with Lck. J. Immunol. 1997;159:1753–1757. [PubMed] [Google Scholar]
- Binstadt B.A., Brumbaugh K.M., Dick C.J., Scharenberg A.M., Williams B.L., Colonna M., Lanier L.L., Kinet J.P., Abraham R.T., Leibson P.J. Sequential involvement of Lck and SHP-1 with MHC-recognizing receptors on NK cells inhibits FcR-initiated tyrosine kinase activation. Immunity. 1996;5:629–638. doi: 10.1016/s1074-7613(00)80276-9. [DOI] [PubMed] [Google Scholar]
- Blasco R., Moss B. Selection of recombinant vaccinia viruses on the basis of plaque formation. Gene. 1995;158:157–162. doi: 10.1016/0378-1119(95)00149-z. [DOI] [PubMed] [Google Scholar]
- Borrego F., Ulbrecht M., Weiis E.H., Coligan J.E., Brooks A.G. Recognition of human histocompatibility leukocyte antigen (HLA)-E complexed with HLA class I signal sequence derived peptides by CD94/NKG2 confers protection from natural killer-cell mediated lysis. J. Exp. Med. 1998;187:813–818. doi: 10.1084/jem.187.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braud V.M., Allan D.S., O'Callaghan C.A., Soderstrom K., Dándrea A., Ogg G.S., Lazetic S., Young N.T., Bell J.I., Phillips J.H. HLA-E binds to natural killer cell receptors CD94/NKG2A, B, and C. Nature. 1998;391:795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- Brumbaugh K.M., Binstadt B.A., Billadeau D.D., Schoon R.A., Dick C.J., Ten R.M., Leibson P.J. Functional role for Syk tyrosine kinase in natural killer cell-mediated natural cytotoxicity. J. Exp. Med. 1997;186:1965–1974. doi: 10.1084/jem.186.12.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Chrzanowska-Wodnicka M., Zhong C. Focal adhesion assembly. Trends Cell Biol. 1997;7:342–347. doi: 10.1016/S0962-8924(97)01127-6. [DOI] [PubMed] [Google Scholar]
- Cantoni C., Biassoni R., Pende D., Sivori S., Accame L., Pareti L., Semenzato G., Moretta L., Moretta A., Bottino C. The activating form of CD94 receptor complexCD94 covalently associates with the Kp39 protein that represents the product of the NKG-2C gene. Eur. J. Immunol. 1998;28:327–338. doi: 10.1002/(SICI)1521-4141(199801)28:01<327::AID-IMMU327>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Carretero M., Cantoni C., Bellón T., Bottino C., Biassoni R., Rodriguez A., Pérez-Villar J.J., Moretta L., Moretta A., López-Botet M. The CD94 and NKG2-A C type lectins covalently assemble to form a natural killer cell inhibitory receptor for HLA class I molecules. Eur. J. Immunol. 1997;27:563–567. doi: 10.1002/eji.1830270230. [DOI] [PubMed] [Google Scholar]
- Carretero M., Palmieri G., Llano M., Tulio V., Santoni A., Geraghty D.E., López-Botet M. Specific engagement of the CD94/NKG2A killer inhibitory receptor by the HLA-E class Ib molecule induces SHP-1 phosphatase recruitment to tyrosine-phosphorylated NKG2Aevidence for receptor function in heterologous transfectants. Eur. J. Immunol. 1998;28:1280–1291. doi: 10.1002/(SICI)1521-4141(199804)28:04<1280::AID-IMMU1280>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Cary L.A., Chang J.F., Guan J.L. Stimulation of cell migration by overexpression of focal adhesion kinase and its association with Src and Fyn. J. Cell. Sci. 1996;108:1787–1794. doi: 10.1242/jcs.109.7.1787. [DOI] [PubMed] [Google Scholar]
- Cosman D., Fanger N., Borges L., Kubin M., Chin W., Peterson L., Hsu M.L. A novel immunoglobulin superfamily receptor for cellular and viral MHC class I molecules. Immunity. 1997;7:273–282. doi: 10.1016/s1074-7613(00)80529-4. [DOI] [PubMed] [Google Scholar]
- Daëron M. Fc receptor biology. Annu. Rev. Immunol. 1997;15:203–234. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- Dikic I., Tokiwa G., Lev S., Courtneidge S.A., Schlessinger J. A role for PYK-2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- Dustin M.L., Shaw A.S. Costimulationbuilding an immunological synapse. Science. 1999;283:649–650. doi: 10.1126/science.283.5402.649. [DOI] [PubMed] [Google Scholar]
- Earp H.S., Huckle W.R., Dawson T.L., Li X., Graves L.M., Dy R. Angiotensin II activates at least two tyrosine kinases in rat liver epithelial cells. J. Biol. Chem. 1995;270:28440–28447. doi: 10.1074/jbc.270.47.28440. [DOI] [PubMed] [Google Scholar]
- Faux M.C., Scott J.D. More on target with protein phosphorylationconferring specificity by location. Trends. Biochem. Sci. 1996;21:312–315. [PubMed] [Google Scholar]
- Ganju R.K., Hatch W.C., Avraham H., Ona M.A., Druker B., Avraham S., Groopman J.E. RAFTK, a novel member of the focal adhesion kinase family, is phosphorylated and associates with signaling molecules upon activation of mature lymphocytes. J. Exp. Med. 1997;185:1055–1063. doi: 10.1084/jem.185.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gismondi A., Bisogno L., Mainiero F., Palmieri G., Piccoli M., Frati L., Santoni A. Proline-rich tyrosine kinase-2 activation by β1 integrin fibronectin receptor cross-linking and association with paxillin in human natural killer cells. J. Immunol. 1997;159:4729–4736. [PubMed] [Google Scholar]
- Herzog H., Nicholl J., Hort Y.J., Sutherland G.R., Shine J. Molecular cloning and assignment of FAK-2, a novel human focal adhesion kinase to 8p11.2-p22 by nonisotopic in situ hybridization. Genomics. 1996;32:484–486. doi: 10.1006/geno.1996.0149. [DOI] [PubMed] [Google Scholar]
- Houchins J.P., Lanier L.L., Niemi E.C., Phillips J.H., Ryan J.C. Natural killer cell cytolytic activity is inhibited by NKG2-A and activated by NKG2-C. J. Immunol. 1997;158:3603–3609. [PubMed] [Google Scholar]
- Kumar S., Avraham S., Bharti A., Goyal J., Pandey P., Kharbanda S. Negative regulation of PYK-2/related adhesion focal tyrosine kinase signal transduction by hematopoietic tyrosine phosphatase SHPTP1. J. Biol. Chem. 1999;274:30657–30663. doi: 10.1074/jbc.274.43.30657. [DOI] [PubMed] [Google Scholar]
- Kupfer A., Singer S.J. Cell biology of cytotoxic and helper T cell functionsimmunofluorescence microscopy studies of single cells and cell couples. Annu. Rev. Immunol. 1989;7:309–337. doi: 10.1146/annurev.iy.07.040189.001521. [DOI] [PubMed] [Google Scholar]
- Lakkakorpi P.T., Nakamura I., Nagy R.M., Parsons J.T., Rodant G.A., Duong L.T. Stable association of PYK-2 and p130(Cas) in osteoclasts and their co-localization in the sealing zone. J. Biol. Chem. 1999;19:4900–4907. doi: 10.1074/jbc.274.8.4900. [DOI] [PubMed] [Google Scholar]
- Lanier L.L. NK cell receptors. Annu. Rev. Immunol. 1998;16:359–393. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- Lanier L.L., Corliss B.C., Wu J., Leong C., Philips J.H. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells Nature. 391 1998. 703 707a [DOI] [PubMed] [Google Scholar]
- Lanier L.L., Corliss B.C., Wu J., Philips J.H. Association of DAP12 with activating CD94/NKG2C NK cell receptors Immunity. 8 1998. 693 701b [DOI] [PubMed] [Google Scholar]
- Lankes W., Griesmacher A., Grünwald J., Schwartz-Albiez R., Keller R. A heparin-binding protein involved in inhibition of smooth muscle cell proliferation. Biochem. J. 1988;251:831–842. doi: 10.1042/bj2510831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A., Iezzi G., Viola A. From TCR engagement to T cell activationa kinetic view of T cell behavior. Cell. 1999;96:1–4. doi: 10.1016/s0092-8674(00)80952-6. [DOI] [PubMed] [Google Scholar]
- Le Drean E., Vely F., Olcese L., Cambiaggi A., Guia S., Krystal G., Gervois N., Moretta A., Jotereau F., Vivier E. Inhibition of antigen-induced T cell response and antibody induced NK cell cytotoxicity by NKG2Aassociation of NKG2A with SHP-1 and SHP-2 protein-tyrosine phosphatases. Eur. J. Immunol. 1998;28:264–276. doi: 10.1002/(SICI)1521-4141(199801)28:01<264::AID-IMMU264>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Lee N., Llano M., Carretero M., Ishitani A., Navarro F., López-Botet M., Geraghty D. HLA-E is a major ligand for the natural killer inhibitor receptor CD94/NKG2A. Proc. Natl. Acad. Sci. USA. 1998;95:5119–5204. doi: 10.1073/pnas.95.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibson P.J. Signal transduction during natural killer cell activationinside the mind of a killer. Immunity. 1997;6:655–661. doi: 10.1016/s1074-7613(00)80441-0. [DOI] [PubMed] [Google Scholar]
- Lev S., Moreno H., Martinez R., Canoll P., Peles E., Musacchio J.M., Plowman G.D., Rudy B., Schlessinger J. Protein tyrosine kinase PYK-2 involved in Ca2+ induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- Lev S., Hernandez J., Martinez R., Chen A. Identification of a novel family of targets of PYK-2 related to Drosophila retinal degeneration B (rdgB) protein. Mol. Cell. Biol. 1999;19:2278–2288. doi: 10.1128/mcb.19.3.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Avraham H., Rogers R.A., Raja S., Avraham S. Characterization of RAFTK, a novel focal adhesion kinase, and its integrin-dependent phosphorylation and activation in megakaryocytes. Blood. 1996;88:417–428. [PubMed] [Google Scholar]
- Llano M., Lee N., Navarro F., García P., Albar J.P., Geragthy D.E., López-Botet M. HLA-E bound peptides influence recognition by inhibitory and triggering CD94/NKG2 receptorspreferential response to an HLA-G-derived nonamer. Eur. J. Immunol. 1998;28:2854–2863. doi: 10.1002/(SICI)1521-4141(199809)28:09<2854::AID-IMMU2854>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Long E.O. Regulation of immune responses through inhibitory receptors. Annu. Rev. Immunol. 1999;17:875–904. doi: 10.1146/annurev.immunol.17.1.875. [DOI] [PubMed] [Google Scholar]
- López-Botet M., Bellón T. Natural killer cell activation and inhibition by receptors for MHC class I. Curr. Opin. Immunol. 1999;11:301–307. doi: 10.1016/s0952-7915(99)80048-x. [DOI] [PubMed] [Google Scholar]
- Ma E.A., Lou O., Berg N.N., Ostergaard H.I. Cytotoxic T lymphocytes express a β3 integrin which can induce the phosphorylation of focal adhesion kinase and the related PYK-2. Eur. J. Immunol. 1997;27:329–335. doi: 10.1002/eji.1830270147. [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen D. Localization of protein kinases by anchoring proteinsa theme in signal transduction. Science. 1995;268:247–251. doi: 10.1126/science.7716516. [DOI] [PubMed] [Google Scholar]
- Monks C.R.F., Kupfer H., Tamir I., Barlow A., Kupfer A. Selective modulation of protein kinase C-θ during T-cell activation. Nature. 1997;385:83–86. doi: 10.1038/385083a0. [DOI] [PubMed] [Google Scholar]
- Monks C.R.F., Freiberg B.A., Kupfer H., Sciaky N., Kupfer A. Three dimensional segregation of supramolecular activation cell clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- Moretta A., Moretta L. HLA class I specific inhibitory receptors. Curr. Opin. Immunol. 1997;9:694–701. doi: 10.1016/s0952-7915(97)80051-9. [DOI] [PubMed] [Google Scholar]
- Nieto M., Navarro F., Perez-Villar J.J., del Pozo M.A., Gonzalez-Amaro R., Mellado M., Frade J.M., Martinez-Arca C., Lopez-Botet M., Sanchez-Madrid F. Roles of chemokines and receptor polarization in NK-target cell interactions. J. Immunol. 1998;161:3330–3339. [PubMed] [Google Scholar]
- Pawson T., Scott J.D. Signaling through scaffold, anchoring and adapter proteins. Science. 1997;278:2075. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- Penninger J.M., Crabtree G.R. The actin cytoskeleton and lymphocyte activation. Cell. 1999;96:9–12. doi: 10.1016/s0092-8674(00)80954-x. [DOI] [PubMed] [Google Scholar]
- Pérez-Villar J.J., Melero I., Rodríguez A., Carretero M., Aramburu J., Sivori S., Orengo A.M., Moretta A., López-Botet M. Functional ambivalence of the Kp43 (CD94) NK cell-associated surface antigen. J. Immunol. 1995;154:5779–5788. [PubMed] [Google Scholar]
- Pérez-Villar J.J., Melero I., Navarro F., Carretero M., Bellón T., Llano M., Colonna M., Geragthy D.E., López-Botet M. The CD94/NKG2A inhibitory receptor complex is involved in natural killer cell mediated recognition of cells expressing HLA-G1. J. Immunol. 1997;158:5736–5743. [PubMed] [Google Scholar]
- Qian D., Lev S., van Oers N.S.C., Dikic I., Schlessinger J., Weiss A. Tyrosine phosphorylation of PYK-2 is selectively regulated by Fyn during TCR signaling. J. Exp. Med. 1997;185:1253–1259. doi: 10.1084/jem.185.7.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner S., Sherrod S.W., Lichlyter D. Microtubule retraction into the uropod and its role in T cell polarization and motility. J. Immunol. 1997;159:1063–1067. [PubMed] [Google Scholar]
- Robertson M.J., Cochran K.J., Cameron C., Le J.M., Tantravahi R., Ritz J. Characterization of a cell line, NKL, derived from an aggressive human natural killer cell leukemia. Exp. Hematol. 1996;24:406–415. [PubMed] [Google Scholar]
- Rodríguez-Fernández J.L., Rozengurt E. Bombesin, vasopressin, lysophosphatidic acid, and sphingosylphosphorylcholine induce focal adhesion kinase activation in intact Swiss 3T3 cells. J. Biol. Chem. 1998;273:19321–19328. doi: 10.1074/jbc.273.30.19321. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Fernández J.L., Gómez M., Luque A., Hogg N., Sánchez-Madrid F., Cabañas C. The interaction of activated integrin lymphocyte function-associated antigen 1 with ligand intercellular adhesion molecule 1 induces activation and redistribution of focal adhesion kinase and proline-rich tyrosine kinase 2 in T lymphocytes. Mol. Biol. Cell. 1999;10:1891–1907. doi: 10.1091/mbc.10.6.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaridis J., Colonna M. Cloning of novel immunoglobulin superfamily receptors expressed on human myeloid and lymphoid cellsstructural evidence for new stimulatory and inhibitory pathways. Eur. J. Immunol. 1997;27:660–665. doi: 10.1002/eji.1830270313. [DOI] [PubMed] [Google Scholar]
- Sánchez-Madrid F., del Pozo M.A. Leukocyte polarization in cell migration and immune interactions. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:501–511. doi: 10.1093/emboj/18.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H., Nagura K., Ishino M., Tobioka H., Kotani K., Sasaki T. Cloning and characterization of cell adhesion kinase p, a novel protein tyrosine kinase of the focal adhesion kinase subfamily. J. Biol. Chem. 1995;270:21206–21219. doi: 10.1074/jbc.270.36.21206. [DOI] [PubMed] [Google Scholar]
- Schlaepfer D.D., Hauck C.R., Sieg D.J. Signaling through focal adhesion kinase. Prog. Biophys. Mol. Biol. 1999;71:435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- Serrador J.M., Alonso-Lebrero J.L., del Pozo M.A., Furthmayr H., Schwartz-Albiez R., Calvo J., Lozano F., Sánchez-Madrid F. Moesin interacts with the cytoplasmic region of intercellular adhesion molecule-3 and is redistributed to the uropod of T lymphocytes during cell polarization. J. Cell Biol. 1997;138:1409–1423. doi: 10.1083/jcb.138.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrador J.M., Nieto M., Sánchez-Madrid F. Cytoskeletal rearrangement during migration and activation of T lymphocytes. Trends Cell Biol. 1999;9:228–233. doi: 10.1016/s0962-8924(99)01553-6. [DOI] [PubMed] [Google Scholar]
- Sieg J.D., Ilic D., Jones K.C., Damsky C.H., Hunter T., Schlaepfer D.D. PYK-2 and Src family protein tyrosine kinases compensate for the loss of FAK in fibronectin-stimulated signaling events but PYK-2 does not fully function to enhance FAK− cell migration. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:5933–5947. doi: 10.1093/emboj/17.20.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somersalo K. Migratory functions of natural killer cells Nat. Immunol. 15 1996. 117 133–1997 [PubMed] [Google Scholar]
- Stearns T., Evans L., Kirschner M. γ-tubulin is a highly conserved component of the centrosome. Cell. 1991;65:825–836. doi: 10.1016/0092-8674(91)90390-k. [DOI] [PubMed] [Google Scholar]
- Viola A., Schroeder S., Sakakibara Y., Lanzavecchia A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 1999;283:680–682. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- Vivier E., Daëron M. Immunoreceptor tyrosine-based motifs. Immunol. Today. 1997;18:286–291. doi: 10.1016/s0167-5699(97)80025-4. [DOI] [PubMed] [Google Scholar]
- Wagtmann N., Rajagopalan S., Winter C.C., Peruzzi M., Long E.O. Killer cell inhibitory receptors specific for HLA-C and HLA-B identified by direct binding and by functional transfer. Immunity. 1995;3:801–809. doi: 10.1016/1074-7613(95)90069-1. [DOI] [PubMed] [Google Scholar]
- Wülfing C., Davis M.M. Receptor/cytoskeletal movement triggered by costimulation during T cell activation. Science. 1998;282:2266–2269. doi: 10.1126/science.282.5397.2266. [DOI] [PubMed] [Google Scholar]
- Xavier R., Brennan T., Li Q., McCormack C., Seed B. Membrane compartmentalization is required for efficient T cell activation. Immunity. 1998;8:723–732. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- Yokoyama W.M. Natural killer cell receptors. Curr. Opin. Immunol. 1998;10:298–305. doi: 10.1016/s0952-7915(98)80168-4. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Jung M.K., Oakley B.R. γ-tubulin is present in Drosophila melanogaster and homo sapiens and is associated with the centrosome. Cell. 1991;65:817–823. doi: 10.1016/0092-8674(91)90389-g. [DOI] [PubMed] [Google Scholar]