Abstract
Cadherins and integrins must function in a coordinated manner to effectively mediate the cellular interactions essential for development. We hypothesized that exchange of proteins associated with their cytoplasmic domains may play a role in coordinating function. To test this idea, we used Trojan peptides to introduce into cells and tissues peptide sequences designed to compete for the interaction of specific effectors with the cytoplasmic domain of N-cadherin, and assayed their effect on cadherin- and integrin-mediated adhesion and neurite outgrowth. We show that a peptide mimicking the juxtamembrane (JMP) region of the cytoplasmic domain of N-cadherin results in inhibition of N-cadherin and β1-integrin function. The effect of JMP on β1-integrin function depends on the expression of N-cadherin and is independent of transcription or translation. Treatment of cells with JMP results in the release of the nonreceptor tyrosine kinase Fer from the cadherin complex and its accumulation in the integrin complex. A peptide that mimics the first coiled-coil domain of Fer prevents Fer accumulation in the integrin complex and reverses the inhibitory effect of JMP. These findings suggest a new mechanism through which N-cadherin and β1-integrins are coordinately regulated: loss of an effector from the cytoplasmic domain of N-cadherin and gain of that effector by the β1-integrin complex.
Keywords: cadherin, integrin, Trojan peptides, adhesion, neurite outgrowth
Introduction
The temporal and spatial regulation of cell–cell and cell–substrate adhesion molecules is thought to direct tissue formation, axon guidance, cell migration, and tumor cell metastasis. Type I cadherins and the β1-integrins play a decisive role in all of these processes (Varner and Cheresh 1996; Beauvais-Jouneau, 1997; Semb and Christofori 1998; Vleminckx and Kemler 1999). Type I cadherins function as cis-homodimers (Brieher et al. 1996; Yap et al. 1997a, Yap et al. 1998; Takeda et al. 1999) to mediate homophilic cell–cell adhesion, forming adherens junctions (Marrs and Nelson 1996; Yap et al. 1997b). Strong adhesion formation by cadherins depends on their association with the actin-containing cytoskeleton, a connection mediated by α- and β- or γ-catenin (Aberle et al. 1996; Wheelock et al. 1996). Other potential regulatory molecules are associated either directly or indirectly with the cytoplasmic domain: p120ctn (Reynolds et al. 1994; Ohkubo and Ozawa 1999; Thoreson et al. 2000); the nonreceptor protein tyrosine kinase Fer (Kim and Wong 1995; Rosato et al. 1998); Shc (Xu et al. 1997; Xu and Carpenter 1999); and the nonreceptor tyrosine phosphatase PTP1B (Balsamo et al. 1996, Balsamo et al. 1998). Integrins function as heterodimers, mediating adhesion between cells and the extracellular matrix (Hynes 1992; Cheresh 1993). Integrin function also depends on association with the actin-containing cytoskeleton, forming well defined focal adhesion complexes at points where actin fibers are anchored (Critchley et al. 1999). The activity of integrins and their association with actin is mediated and regulated by an extensive set of structural and regulatory proteins associated directly or indirectly with the cytoplasmic domain of the β1 subunit (Aplin et al. 1998; Hemler 1998; Schoenwaelder and Burridge 1999).
It is apparent that the activity of these two receptor systems, and possibly many others, must be coordinated in time and space for the proper development of tissue architecture, and many of the regulatory proteins associated with the cytoplasmic domain of these two adhesion receptors are candidate cross-regulatory molecules. Although there is a growing body of literature documenting coordinate regulation between cadherin- and integrin-mediated adhesion systems, little is known about the levels or mechanisms through which such coordination takes place (Hodivala and Watt 1994; Finnemann et al. 1995; Monier-Gavelle and Duband, 1995, Monier-Gavelle and Duband 1997; Zu et al., 1996; Huttenlocher et al. 1998; Lu et al. 1998; Wu et al. 1998).
We speculated that coordinate regulation of cadherin and integrin function during development might be effected by the exchange of regulatory molecules associated with the cytoplasmic domain of these receptors. Thus, we sought to create reagents that have the potential to interfere specifically with the binding of such effectors to the cytoplasmic domain of cadherin. Two regions of the cytoplasmic domain of cadherin have been shown to be functionally important: a juxtamembrane region that acts as a dominant negative, blocking cadherin function when introduced into Xenopus blastomeres (Kintner 1992) or eye primordia (Riehl et al. 1996); and the β-catenin–binding region, which has been defined by cadherin deletions that result in the loss of β-catenin binding (Nagafuchi and Takeichi 1989; Stappert and Kemler 1994; Jou et al. 1995). Here, we show that peptides mimicking these two regions of N-cadherin attached to a cell permeation sequence enter cells and disrupt adhesion and neurite outgrowth. The peptide mimicking the juxtamembrane region (JMP) causes inhibition of both cadherin and integrin function, whereas the peptide mimicking the β-catenin–binding region causes inhibition of only cadherin function. We further show that inhibition of integrin function by JMP requires the presence of cadherin and the complex of proteins associated with its cytoplasmic domain. Finally, inhibition of integrin function is accompanied by a loss of the nonreceptor protein tyrosine kinase Fer from the cadherin complex of proteins and its concomitant appearance in the integrin complex of proteins, and blocking the association of Fer with the integrin complex reverses the effect of JMP on integrin function.
Materials and Methods
Antibodies
The anti–N-cadherin antibody NCD-2 (Hatta and Takeichi 1986) was purified from hybridoma cultures as described previously (Balsamo et al. 1991). The anti–β1-integrin antibodies are JG22, a mouse monoclonal IgG (Greve and Gottlieb 1982; Tomaselli et al. 1986), and a commercial anti–β1-integrin antibody (Transduction Laboratories). The anti-FAK antibody was a monoclonal mouse IgG from Chemicon International, Inc. Anti-Fer antiserum, a rabbit polyclonal antibody, was a gift from Dr. Nora Heisterkamp (University of Southern California, Los Angeles, CA). Anti-p120ctn, anti–β-catenin, anti-PTP1B, anti-p130cas, and antiphosphotyrosine (PY20) were products of Transduction Laboratories. Antiactin was from Boehringer-Mannheim. HRP-conjugated secondary antibodies were from Cappel Laboratories (ICN Biomedicals). The secondary antibodies conjugated to magnetic beads, used in immunoprecipitations, were from PerSeptive Diagnostics.
Other Materials
N-Cadherin, which was used as a substrate for neurite outgrowth, was purified from embryonic chick brains as described previously (Bixby and Zhang 1990; Balsamo et al. 1991). The laminin and fibronectin, which were used as substrates for neurite outgrowth or adhesion, were from GIBCO-BRL. For preparation of recombinant N-cadherin cytoplasmic domain and recombinant β-catenin, the cDNA sequence for the cytoplasmic domain of N-cadherin or the full-length cDNA for β-catenin were cloned into pGEX, expressed as glutathione-S-transferase fusion proteins, and purified on glutathione-4S-Sepharose columns (Pharmacia Biotech).
Preparation of Synthetic Peptides
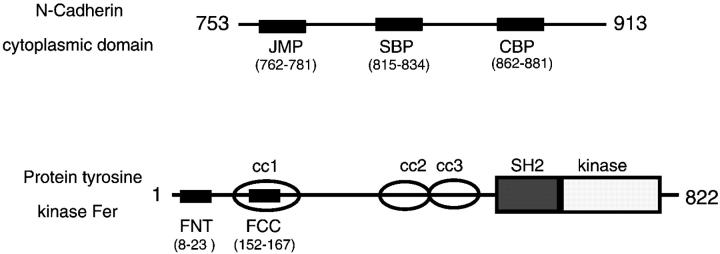
The following peptides from the N-cadherin cytoplasmic domain and the nonreceptor tyrosine kinase Fer were synthesized as fusions with the antennapedia homeodomain cell permeation sequence (Derossi et al. 1994; Prochiantz 1996) and purified to >90% by HPLC (Genemed Biotechnologies, Inc.; see Fig. 1): control antennapedia peptide (COP), RQIKIWFQNRRMKWKK; catenin binding peptide (CBP), RQIKIWFQNRRMKWKKSLLVFDYEGSGSTAGSLSSL; juxtamembrane peptide (JMP), RQIKIWFQNRRMKWKKRQAKQLLIDPEDDVRDNILK; Shc binding region peptide (SBP), RQIKIWFQNRRMKWKKRRLDERPIHAEPQYPVRSAA; Fer coiled-coil domain peptide (FCC), RQIKIWFQNRRMKWKKEKYKEALAKGKETEKA; and Fer NH2-terminal peptide (FNT), RQIKIWFQNRRMKWKKKNSHEAVLKLQDWELR. All peptides were dissolved in sterile deionized water to a concentration of 10–30 mg/ml and stored in small aliquots at −80°C. Peptide activity was assessed in neurite outgrowth and adhesion assays din a range of 0.2–50 μM. Biotinylation of peptides was carried out using Sulfo-NHS-biotin according to the manufacturer's instructions (Pierce Chemical Co.).
Figure 1.
Diagram showing the relative positions of the sequences used to generate cell permeable peptide competitors. N-Cadherin is numbered from the beginning of the signal sequence. The model of the nonreceptor protein tyrosine kinase Fer is based on Craig et al. 1999. CC1, CC2, and CC3, coiled-coil domains 1, 2, and 3; SH2, src homology 2 domain; kinase, tyrosine kinase catalytic domain.
Preparation of Retina Explants and Cell Culture
Neural retina explants were prepared from 7-d chick embryos (E7). Intact retinas were whole mounted onto black filters (Millipore) with the vitreous surface facing up, and cut with a tissue sectioner to squares of ∼0.09 mm2. Retina sections were resuspended in culture medium (F12 supplemented with 5 mM Hepes, pH 7.2, 0.6% glucose, 5 μg/ml insulin, 100 μg/ml transferrin, 5 ng/ml sodium selenite, and 5 mg/ml gentamycin) and plated on coverslips coated with the appropriate substrate. 2 h later, nonadherent sections were removed by washing. Single cells were prepared from chick retina as described previously (Balsamo et al. 1995). PC12 cells were cultured in DME supplemented with 5% FBS, 0.5% horse serum and antibiotics, and 100 μg/ml penicillin and streptomycin. L cells expressing N-cadherin (LN cells) were prepared by transfecting the full-length N-cadherin cDNA into L cells as previously described (Balsamo et al. 1998).
Neurite Outgrowth and Cell Adhesion Assays
Neural retina explants were cultured overnight in the presence of 2 μM peptides. Neurite outgrowth was visualized using phase optics. Neurite length was quantitatively assessed in sparse single cell cultures of E7 neural retina to prevent neurite fasciculation. Peptides were added 2 h after cell plating. After overnight culture, cells were fixed with 4% paraformaldehyde for 20 min at room temperature. The longest neurite per cell was measured using a Zeiss microscope equipped with a CCD camera attached to the Metamorph image analysis system (Universal Imaging Corp.). For each assay condition, the length of neurites from >100 cells bearing neurites longer than two cell diameters were categorized into three groups: 0–49, 50–99, and >100 μm.
Cell adhesion assays were carried out as described (Balsamo et al. 1996, Balsamo et al. 1998; Arregui et al. 1998). After a starvation period of 2 h for PC12 cells or overnight for L and LN cells, cells were resuspended by a brief treatment with 0.03% trypsin in HBSGK containing 1 mM Ca2+. Trypsin treatment was terminated by adding the trypsin inhibitor AESBF at 1 mM (Calbiochem). Cells in suspension were incubated for 2 h (retina cells, PC12 cells) or 6 h (L, LN cells) with peptides in DME-Hepes before plating onto the indicated substrates. For assays evaluating the effect of sequential addition of peptides, cells were incubated for 1 h with the first peptide (2 μM) before addition of the second (2 μM). Cell adhesion was evaluated after 45 min.
Substrates for neurite outgrowth and adhesion were prepared by coating glass coverslips (neurite outgrowth) or 96-well plates (adhesion) with poly-l-lysine for 2 h at room temperature, followed by the appropriate substrate (cadherin, 5 μg/ml; NCD-2, 50 μg/ml; laminin, 20 μg/ml; fibronectin, 20 μg/ml).
Peptide Penetration and Assays for Cytotoxicity
To assess peptide penetration into cells and tissue explants, biotinylated peptides were incubated with explants for 2, 4, and 16 h at a concentration of 2 μM. The explants were fixed, permeabilized with 0.5% Triton X-100 (for 10 min), and reacted with FITC-streptavidin. Fluorescent cells in different layers of the explant were examined using a confocal microscope.
To determine cytotoxicity, retina explants were incubated overnight in the presence of peptides (0.2, 2, 4, 10, and 50 μM). The medium was replaced by Hepes-buffered saline with added glucose and potassium (HBSGK: 20 mM Hepes, pH 7.2, 150 mM NaCl, 2 mM glucose, and 3 mM KCl) containing 1:500 dilution of the green fluorescent SYTO10 and the red fluorescent DEAD Red (Molecular Probes). After 15 min, explants were washed twice, fixed with paraformaldehyde, and observed using a fluorescence microscope. The fluorescence of SYTO10 and DEAD Red was analyzed using FITC and rhodamine filters, respectively.
Binding of Peptides to Recombinant Cadherin and β-Catenin
The antennapedia fusion peptides were biotinylated using sulfo-NHS-biotin (Pierce Chemical Co.). 4 μg of each peptide, or inactivated biotinylation reaction mix alone, were incubated overnight with 10 μg of recombinant cadherin cytoplasmic domain or β-catenin in 200 μl PBS, at 4°C. The reaction mixture was loaded into streptavidin-coated wells blocked with BSA. Protein bound to biotinylated peptide was detected using an antibody to the cytoplasmic domain of cadherin or a monoclonal anti–β-catenin antibody and the appropriate HRP-conjugated second antibody. Triplicate wells were measured for each peptide.
In Vitro Competition by CBP for Cadherin–β-Catenin Interaction
Neural retina homogenates were prepared in mild lysis buffer (MLB: 20 mM Tris, pH 7.4, 150 mM NaCl, 1% NP-40, 1 mM Na2VO4, 50 mM NaF, 1 mM AESBF, 10 μg/ml DNase, and 5 μg/ml each leupeptin, pepstatin, and aprotinin). The lysates were cleared by centrifugation at 14,000 g, and aliquots of the supernatant were incubated with increasing concentrations of JMP, CBP, or SBP for 2 h at 37°C with mixing. The lysates were immunoprecipitated with the anticadherin antibody NCD-2, the immunoprecipitates collected with anti-rat IgG conjugated to magnetic beads, fractionated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and probed with anti–β-catenin antibody and anticadherin antibody NCD-2. The density of the resulting bands was measured and the ratio of β-catenin to cadherin was determined.
Immunoprecipitation and Immunoblot Analysis
For analysis of the proteins associated with N-cadherin, cells were homogenized in MLB, the lysates were centrifuged at 14,000 g and the supernatants immunoprecipitated for 4 h at 4°C with the anti–N-cadherin antibody NCD-2. The NCD-2 immunoprecipitates were collected using goat anti–rat IgG conjugated to magnetic beads, and the supernatants were further immunoprecipitated with polyclonal anti–β-catenin antibody (Balsamo et al. 1995, Balsamo et al. 1998). Alternatively, to analyze protein associated with β1-integrin, cell lysates were immunoprecipitated with anti-FAK antibody. The immunoprecipitates were fractionated by SDS-PAGE and the Western transfers were probed with the indicated antibodies.
For analysis of tyrosine phosphorylation of p130cas, cells were lysed in RIPA buffer (10 mM Tris, pH 7.4, 150 mM NaCl, 1% NP-40, 0.1% SDS, 0.1% sodium deoxycholate, 50 mM NaF, 1 mM PMSF, 5 μg/ml leupeptin, and 1 mM Na2VO4), immunoprecipitated with anti-p130cas antibody, fractionated by SDS-PAGE, and Western transfers were probed with PY20.
Results
Peptides Containing a Cell Permeation Sequence Fused to Sequences Mimicking Specific Regions of the N-Cadherin Cytoplasmic Domain Enter Cells and Are Nontoxic
To introduce peptide competitors into living cells, amino acid sequences mimicking several domains of the cytoplasmic region of N-cadherin were fused to the 16–amino acid, cell permeable peptide derived from the third helix of the antennapedia homeodomain (Derossi et al. 1994; Prochiantz 1996; Hall et al. 1996; see Fig. 1 for diagram and Materials and Methods for peptide sequences). These sequences correspond to 20 amino acids in the juxtamembrane region of N-cadherin (JMP) shown to act as a dominant negative when introduced into Xenopus blastomeres (Kintner 1992); 20 amino acids in the catenin binding region (CBP) (Nagafuchi and Takeichi 1989; Stappert and Kemler 1994; Jou et al. 1995) and 20 amino acids in the central region close to the binding region for the adaptor protein Shc (SBP) (Xu et al. 1997; Xu and Carpenter 1999).
To establish that the antennapedia fusion peptides are indeed able to penetrate cells, we incubated E7 chick neural retina explants of ∼1 mm2 with each of the fusion peptides covalently linked to biotin. The explants were incubated with fluorescein-conjugated streptavidin and peptide penetration was assessed in confocal, planar images through the explant as well as cross-sectional reconstructions. All peptides penetrate equally well (Fig. 2 shows penetrance of CBP as an example). In addition, the antennapedia fusion peptides are not toxic to cells, as determined by assessing cell death using a SYTO/10 DEAD Red (Fig. 2, live cells are labeled green and dead cells are red). Very few cells are dead after 24 h of incubation of the explant either in the absence or presence of antennapedia peptides, and those that do appear dead are at the edge of the explant and may have been damaged during dissection and initial placement of the explant.
Figure 2.
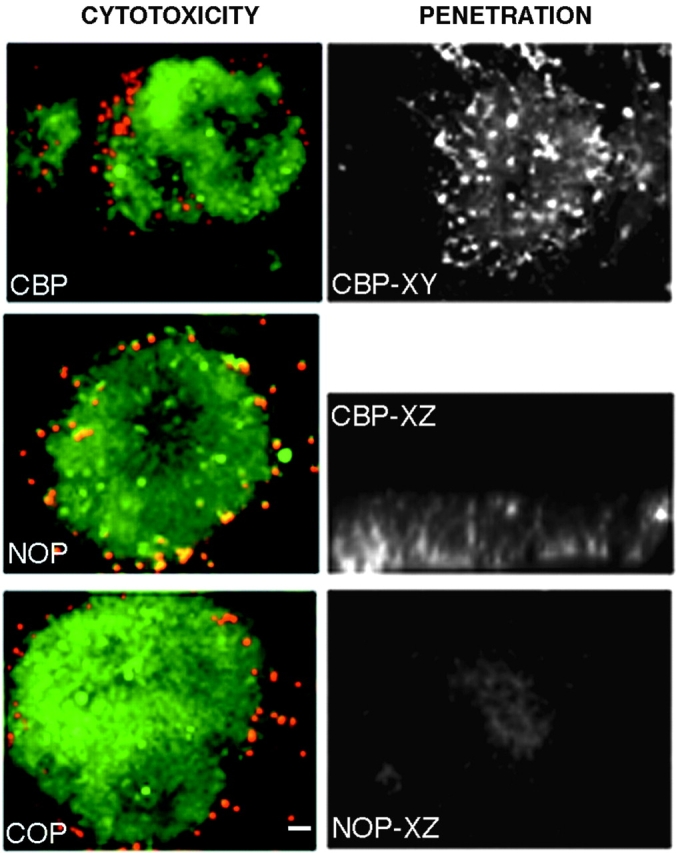
Toxicity and penetration of fusion peptides. Left panels show live (green) and dead (red) cells in the presence of CBP (top), NOP (no peptide, middle) or COP (bottom). Right panels show a retina explant incubated with biotinylated CBP and detected with fluorescein-conjugated avidin. XY and XZ are confocal sections of the explant showing fluorescent cells throughout the explant. Lower right panel shows background fluorescence in a control where peptide incubation is omitted. Bar, ∼20 μm.
JMP and CBP Have Unique and Specific Effects on N-Cadherin and Integrin-mediated Neurite Outgrowth and Adhesion
To determine the effect of the cell permeable peptides on N-cadherin– or integrin-mediated neurite outgrowth, explants from 7-d embryonic chick retinas were plated on N-cadherin (or anti–N-cadherin antibody NCD-2) or laminin in the presence of JMP, CBP, SBP, antennapedia peptide alone (COP), or no peptides (NOP). Treatment with either JMP or CBP results in a dramatic reduction of neurite outgrowth on cadherin substrates (Fig. 3). However, when explants are plated on laminin, where neurite extension depends on β1-integrin function and is inhibited by the function blocking anti–β1-integrin antibody JG22 (Greve and Gottlieb 1982; Tomaselli et al. 1986; not shown), numerous long neurites develop in the presence of CBP, but only a few short neurites develop in the presence of JMP (Fig. 3). In contrast, neither SBP (not shown) nor COP (Fig. 3) have any effect on either N-cadherin or laminin substrates. Thus, while JMP was designed to mimic the cadherin juxtamembrane sequence, it nevertheless influences integrin-mediated neurite outgrowth.
Figure 3.
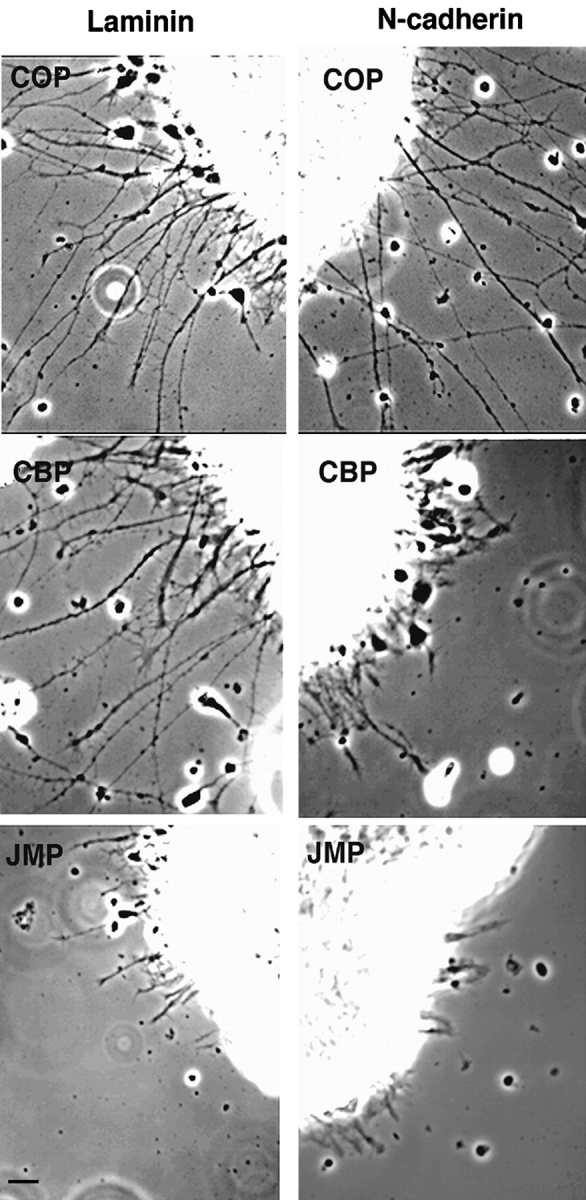
Effect of peptides on neurite outgrowth from explants of E7 neural retina. Phase-contrast of retina explants incubated in the presence of control peptide (COP), catenin-binding peptide (CBP), or the juxtamembrane peptide (JMP) and plated on laminin or N-cadherin. Bar, ∼20 μm.
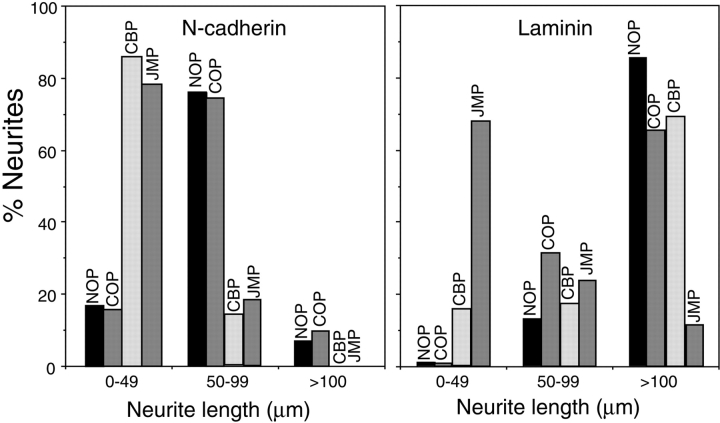
To quantify the results, we cultured E7 retina cells on N-cadherin or laminin-coated slides and determined the length of neurites after 20 h in the presence of JMP, CBP, and COP, or in the absence of peptide (NOP). When cells are plated on N-cadherin, in the absence of peptide or in the presence of control peptides, ∼80% of neurites are between 50 and 99 μm in length (Fig. 4). In contrast, in the presence of JMP or CBP, only ∼20% of the neurites fall in this category (Fig. 4). When cells are plated on laminin, CBP has no discernible effect; however, treatment with JMP reduces the number of neurites in the >100 μm class to ∼10% (Fig. 4).
Figure 4.
Quantitative determination of neurite outgrowth by single E7 cells in the presence and absence of peptides. Data are expressed as the percent of neurites in each of three length categories. More than 100 cells bearing neurites were counted for each treatment. NOP, no peptide.
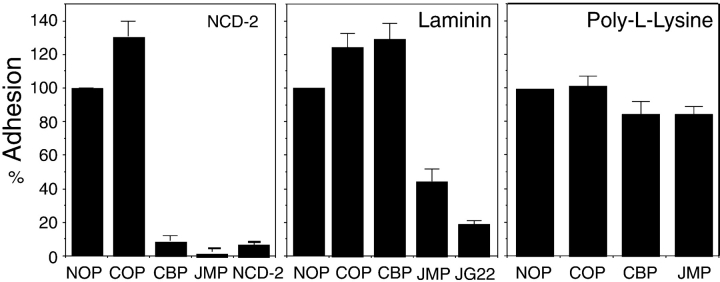
Consistent with the effect of JMP and CBP on neurite outgrowth from explants or single cells, N-cadherin–mediated adhesion of E7 retina cells is inhibited by JMP and CBP, whereas only JMP inhibits integrin-mediated adhesion (Fig. 5). That adhesion is indeed mediated by N-cadherin or β1-integrin is demonstrated by the ability of the function blocking antibodies NCD-2 (Hatta and Takeichi 1986) and JG22 (Greve and Gottlieb 1982; Tomaselli et al. 1986) to specifically inhibit adhesion to N-cadherin or laminin, respectively (Fig. 5). JMP also inhibits integrin-mediated adhesion among PC12 cells, which differ from retina cells in expressing distinct α chains, in combination with β1-integrin, as receptors for laminin (see Fig. 8 D), indicating that the effect of JMP is specific for β1-integrin, independent of the attached α chain (Tomaselli et al. 1990; de Curtis and Reichardt 1993). Additionally, treatment of cells with cycloheximide or actinomycin D, to specifically inhibit protein or RNA synthesis, respectively, has no effect on the inhibition of cadherin or β1-integrin function by JMP (not shown). And finally, adhesion to poly-l-lysine, which does not involve cadherins or integrins, is unaffected by CBP or JMP (Fig. 5).
Figure 5.
The effect of peptides on cadherin- and integrin-mediated cell adhesion by E7 retina cells. Cells preincubated in the absence or presence of peptides were plated on the indicated substrates, the wells were washed, and adherent cells were counted. Values for each condition represent the percentage of attached cells as compared with controls lacking peptide. Each bar is the average of three measurements, and the error bars represent the SD. The substrate is indicated in the top right of each panel: NCD-2, anti–N-cadherin antibody; LM, laminin; and PL, poly-l-lysine.
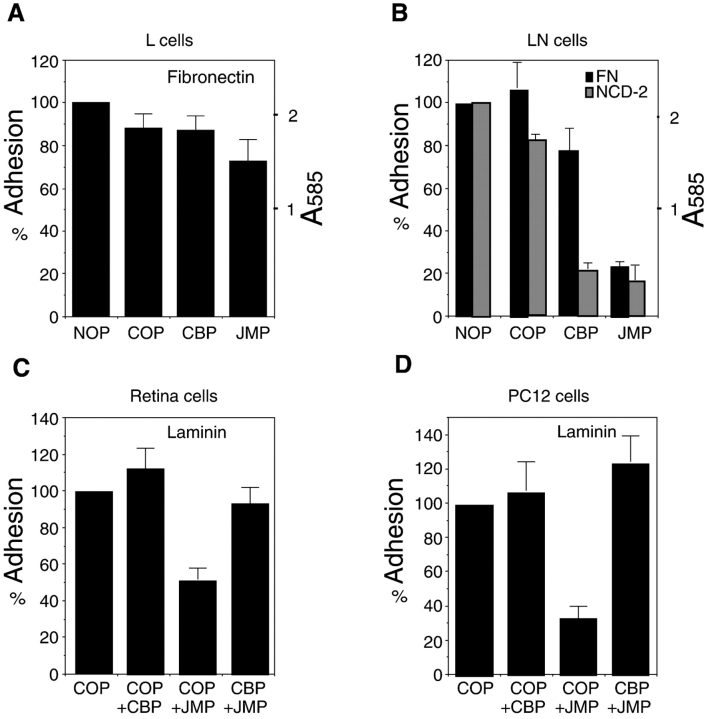
Figure 8.
The effect of peptides on adhesion of L-cells (A), L-cells constitutively expressing N-cadherin (LN-cells, B), E7 retina cells (C), and PC-12 cells (D). The substrate is indicated. A585 reflects the number of adherent cells in A and B. In C and D, the indicated peptide pairs were added sequentially with a 1-h incubation following the addition of the first peptide. Each bar represents the average of three measurements and error bars represent the SD.
To determine the effect of COP, CBP, and JMP on cell-surface expression, N-cadherin and β1-integrin were immunoprecipitated from cells on which cell-surface proteins were biotinylated. Additionally, cells were incubated with anti–β1-integrin antibody, followed by fluorescein-conjugated second antibody, and the amount of cell-bound fluorescence was determined by flow cytometry. No difference in cell-surface N-cadherin or β1-integrin is seen by either technique in the presence of any of the peptides (not shown).
JMP and CBP Are Targeted to Specific Components of the Cadherin Adhesion Complex
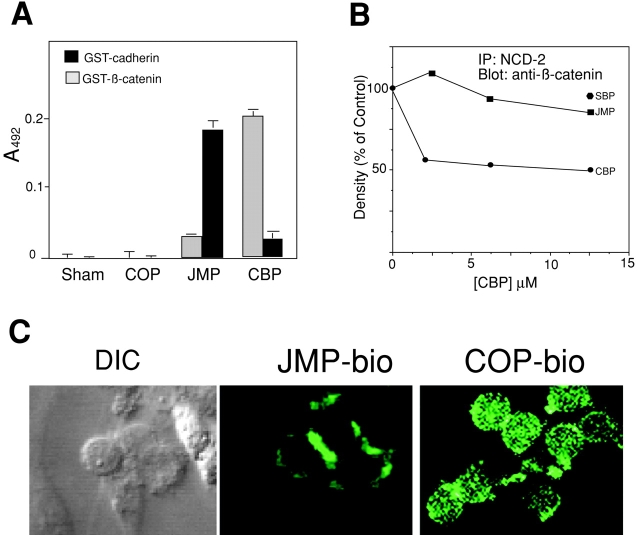
CBP and JMP, but not control peptides, have specific effects on cadherin and integrin function, presumably based on their ability to compete for specific protein–protein interactions at the cytoplasmic domain of cadherin. To further demonstrate the specificity of these effects, we examined the interaction of the peptides with their putative targets. The CBP sequence corresponds to the binding site for β-catenin and, indeed, CBP binds directly to recombinant β-catenin (Fig. 6 A). In addition, CBP competes for up to 50% of the N-cadherin–bound β-catenin in cell lysates, as determined by immunoprecipitation with NCD-2 and immunodetection of β-catenin (Fig. 6 B). In contrast, JMP and SBP have little effect, even at concentrations as high as 12.5 μM (Fig. 6 B). On the other hand, JMP binds in vitro to the cytoplasmic domain of N-cadherin (Fig. 6 A), and biotin-labeled JMP localizes to areas of cell–cell contact, where cadherin is enriched (Fig. 6 C). This suggests that the juxtamembrane region of cadherin may participate in homodimer formation through a direct interaction, which is consistent with reports suggesting that the juxtamembrane domain of cadherin is indeed involved in cis-homodimerization (Ozawa and Kemler 1998; Yap et al. 1998).
Figure 6.
Interaction of peptides with presumptive targets. (A) In vitro binding of peptides to the N-cadherin cytoplasmic domain and to β-catenin. 10 μg of recombinant GST-N-cadherin cytoplasmic domain or GST-β-catenin were incubated with 4 μg of biotinylated CBP or JMP. The amount of protein bound to immobilized peptide was determined as described in Materials and Methods. Each point is the average of three measurements and bars represent the SD. (B) The ability of CBP to disrupt the association of cadherin with β-catenin. The ratio of β-catenin to cadherin was measured in neutral detergent extracts in the presence of increasing concentrations of CBP and JMP, and reported as a percentage of the value in the absence of peptide. The results shown are one experiment representative of many. (C) Localization of JMP to regions of cell–cell contact. The left frame labeled DIC (differential interference contrast microscopy) and the middle frame labeled JMP-bio are the same field. The frame on the right shows the distribution of biotin-labeled control peptide (COP-bio). Individual cells are ∼8 μm in diameter.
Treatment of Cells with JMP or CBP Alters the Array of Proteins Associated with the Cytoplasmic Domain of N-Cadherin
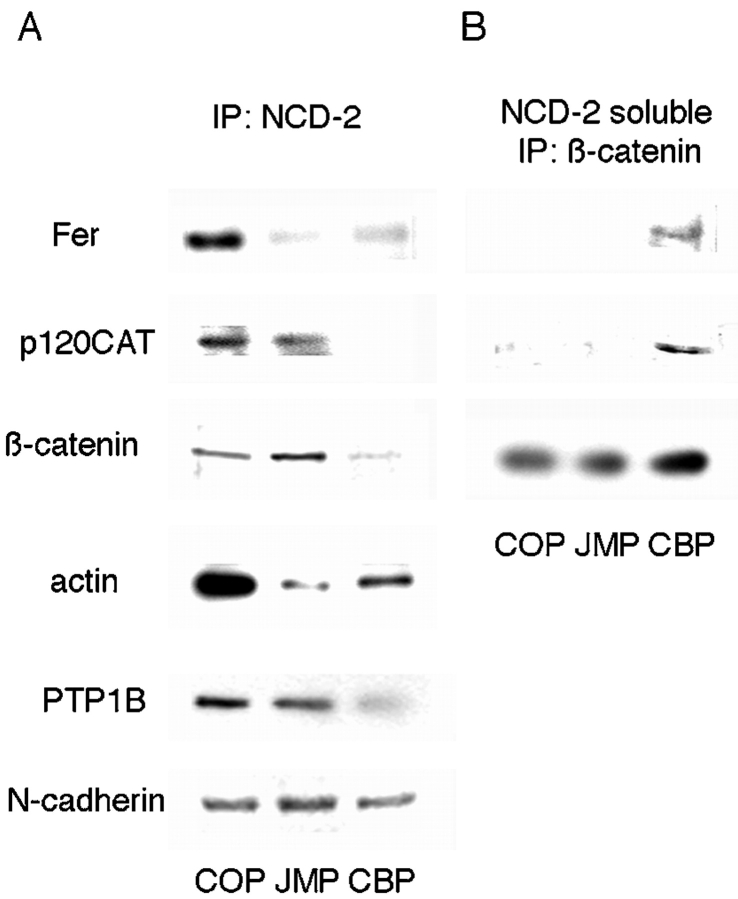
The targeting of CBP to β-catenin and its ability to compete for binding of β-catenin to cadherin in vitro, and the targeting of JMP to cadherin itself suggest that treatment of cells and tissues with these peptides disrupts interactions crucial to cadherin function. To examine the in situ effects of these fusion peptides, cells were pretreated with JMP, CBP, or a control peptide, and the array of polypeptides coimmunoprecipitated with anti–N-cadherin antibody was examined. Preincubation of cells with either CBP or JMP, but not control peptide, reduces the amount of actin immunoprecipitated with anti–N-cadherin antibody, suggesting that both peptides uncouple N-cadherin from its association with the actin-containing cytoskeleton (Fig. 7 A). The association of actin with the cytoplasmic domain of cadherin is mediated by β-catenin interacting with α-catenin, which in turn interacts with actin, either directly or through α-actinin (Wheelock et al. 1996). Binding of JMP does not alter the amount of β-catenin associated with N-cadherin (Fig. 7 A). On the other hand, CBP reduces the amount of β-catenin associated with cadherin (Fig. 7 A), as expected from results shown in Fig. 6. Thus, CBP uncouples cadherin from the cytoskeleton with a concomitant loss of function, probably through competition with the endogenous β-catenin binding site.
Figure 7.
The effect of peptides on the association of proteins with the cytoplasmic domain of N-cadherin (A) and cadherin-free pool of β-catenin (B). (A) Neutral detergent extracts of cells preincubated with peptides were immunoprecipitated with anti–N-cadherin antibody NCD-2, the immunoprecipitates were fractionated by SDS-PAGE, and Western transfers were blotted with the indicated antibodies. (B) The supernatants from the NCD-2 immunoprecipitations were immunoprecipitated with polyclonal anti–β-catenin antibody and processed as in A.
We also examined the distribution of p120ctn (Reynolds et al. 1994) and the nonreceptor tyrosine phosphatase PTP1B (Balsamo et al. 1996, Balsamo et al. 1998), which are bound directly to the cytoplasmic domain of cadherin, and the protein tyrosine kinase Fer, which is associated with p120ctn (Kim and Wong 1995) and possibly β-catenin (Rosato et al. 1998). Treatment with CBP reduces the amount of all three components precipitated with cadherin (Fig. 7 A). Additionally, after treatment with CBP, p120ctn and Fer are coimmunoprecipitated with β-catenin from the N-cadherin–free β-catenin pool (Fig. 7 B), indicating that β-catenin, Fer, and p120ctn are released as a complex. In contrast, binding of JMP directly to cadherin results in a reduction in the amount of Fer, leaving p120ctn and PTP1B largely unaffected (Fig. 7 A). Thus, JMP and CBP act through different mechanisms to differentially alter the association of effector proteins with the cytoplasmic domain of cadherin. The differences are noteworthy: competition for β-catenin results in the release of a complex of associated proteins including β-catenin, p120ctn, and Fer, suggesting that this set of bound components depends on β-catenin for stable association, in spite of the fact that at least one, p120ctn, binds directly to cadherin (Roura et al. 1999; Thoreson et al. 2000). In contrast, direct binding of JMP to cadherin specifically causes the release of Fer, other components remaining associated with cadherin. As Fer is not known to associate directly with cadherin, the binding of JMP appears to alter the association of Fer through a change in its binding partner(s), p120ctn and/or β-catenin. Thus, interactions among the components associated with cadherin are complex and mutually interdependent.
The Effect of JMP on Integrin Function Requires N-Cadherin and the Complex of Proteins Associated with the Cadherin Cytoplasmic Domain
A database search reveals no close similarity between the amino acid sequence of JMP and that of β1-integrin or any other integrin-associated peptide, suggesting that the effect of JMP on integrin function may occur through cadherin. To determine if this is indeed the case, we compared the effects of JMP on L cells, which express β1-integrins but not N-cadherin, and L-cells stably transfected with N-cadherin (Balsamo et al. 1998), which express both β1-integrins and N-cadherin. In this case, we used fibronectin as a substrate, since L cells adhere poorly to laminin. Both L cells and LN cells adhere equally well to fibronectin (see A585 values in Fig. 8A and Fig. B) and this adhesion is integrin-dependent, as >90% of attachment is inhibited by an arginine, glycine, aspartic acid–containing peptide (Arregui et al. 1998). However, similar concentrations of JMP inhibit LN cell adhesion to fibronectin but have little or no effect on L cell adhesion (Fig. 8A and Fig. B). CBP and control peptides have little or no effect on integrin-mediated adhesion of L-cells or LN-cells (Fig. 8A and Fig. B) but, as expected, CBP does inhibit cadherin-mediated adhesion of LN cells (Fig. 8 B). Thus, inhibition of integrin function by JMP requires the expression of cadherin. Furthermore, perturbation of the array of proteins associated with the cytoplasmic domain of cadherin by prior treatment with CBP, which causes the release of a complex of proteins containing β-catenin, p120ctn, and Fer, eliminates the effect of JMP on integrin-mediated adhesion. This is true for all three cell types tested: LN (not shown), E7 retina (Fig. 8 C), and PC12 (Fig. 8 D). Thus, JMP acts through N-cadherin to affect integrin function, and specific alterations in the cadherin complex induced by CBP abolish the effect of JMP on integrin function.
Inhibition of β1-Integrin Function by JMP Correlates with an Increase in Tyrosine Kinase Fer Associated with the Integrin Complex
Inhibition of integrin function depends on a specific configuration of effector molecules associated with the cytoplasmic domain of N-cadherin and is independent of protein and RNA synthesis. This suggests that one or more effectors, originally associated with the cytoplasmic domain of N-cadherin, may be released and directly affect integrin function. Of the proteins associated with the cytoplasmic domain of N-cadherin, only the tyrosine kinase Fer is specifically released on treatment of cells with JMP and is thus a candidate for cross-regulation. Therefore, we looked at the presence of Fer associated with the integrin complex. Neural retina cells were incubated with peptides, lysed in mild lysis buffer, and immunoprecipitated with anti-FAK antibody. After treatment with JMP, and concomitant with its release from the cadherin complex, there is an increase in Fer coimmunoprecipitated with FAK (Fig. 9 A) over control cells or cells treated with COP or CBP (Fig. 9 A). While Fer is also released from the cadherin cytoplasmic domain after treatment with CBP, it is in a complex with β-catenin and p120ctn and, thus, may be unable to translocate and/or bind to the integrin complex. In addition, we consistently see a decrease of 30–50% in the tyrosine phosphorylation of the integrin-associated adaptor protein p130cas after treatment of cells with JMP (Fig. 9 B). These data are in agreement with Rosato et al. 1998, correlating overexpression of Fer with inhibition of integrin function as well as hypophosphorylation of p130cas.
Figure 9.
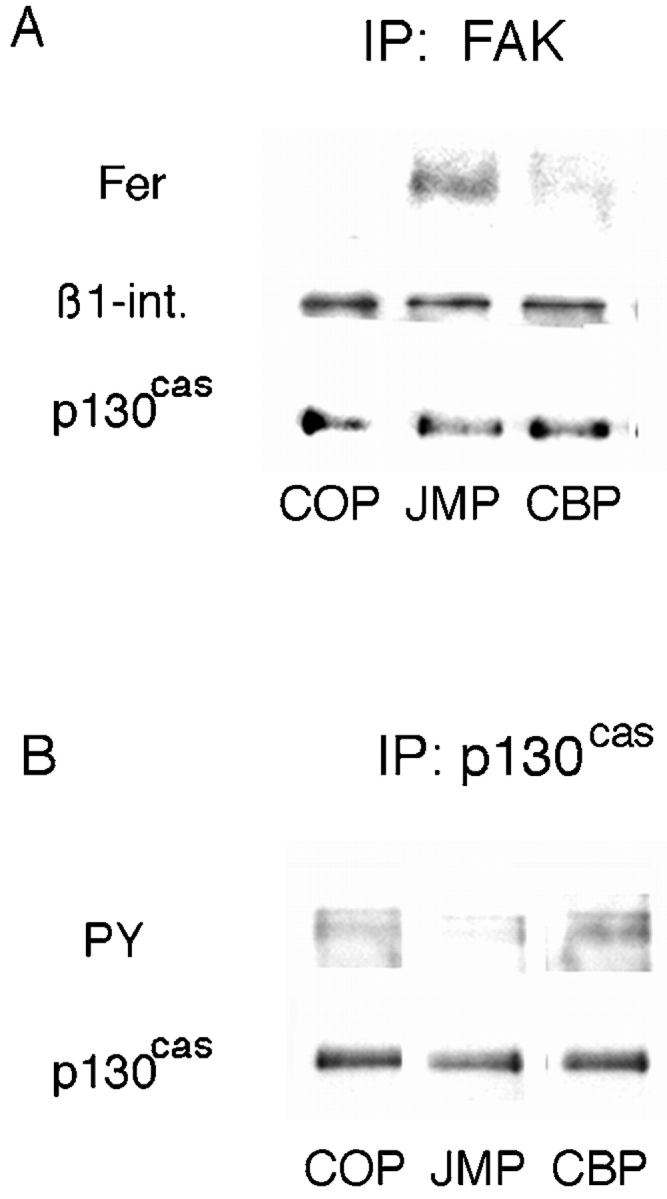
The effect of peptides on the association of Fer with the β1-integrin complex and phosphorylation of p130cas. (A) Neutral detergent extracts of E8 retina cells preincubated with peptides were immunoprecipitated with anti-FAK antibody, the precipitates were fractionated by SDS-PAGE, and Western transfers were blotted with anti-Fer, anti-p130cas, and anti–β1-integrin antibodies. (B) Cells preincubated with peptides were lysed with RIPA buffer, immunoprecipitated with anti-p130cas, and the precipitates were fractionated by SDS-PAGE. Western transfers were blotted with the antiphosphotyrosine mAb PY20 and anti-p130cas antibody.
A Peptide that Mimics a Sequence in the First Coiled-coil Domain in Fer Reverses the Effect of JMP on Integrin Function
The protein kinase Fer contains three presumptive coiled-coil domains in its NH2-terminal region, two of which, CC1 and CC2 are involved in oligomerization, that have the potential to mediate targeting (Craig et al. 1999). To definitively implicate Fer in the inhibition of integrin function by JMP, we attempted to interfere with targeting of Fer to the integrin complex using a cell permeable peptide that mimics a coiled-coiled domain. We hypothesized that if Fer were prevented from targeting to the integrin complex, JMP should lose its inhibitory effect on integrin function. Two Fer peptides were prepared: one mimicking the 16–amino acid sequence (amino acids 152–168) in coiled-coil domain 1 (FCC) and the second mimicking a 16–amino acid sequence at the NH2 terminus (FNT; Fig. 1). Both of these peptides show little sequence similarity to other members of the c-Fes family (Smithgall et al. 1998). Neural retina cells were plated on laminin-coated slides and allowed to adhere for 1 h. FCC, FNT, or control peptide (COP) were added and the cells were incubated for an additional hour before addition of JMP. The cells were incubated overnight and neurite growth was assessed. Cells incubated in the presence of FCC, before the addition of JMP, are able to extend neurites as well as control cells or cells treated with control peptides, whereas few neurites are seen on cells treated with JMP alone or JMP after COP or FNT (Fig. 10).
Figure 10.
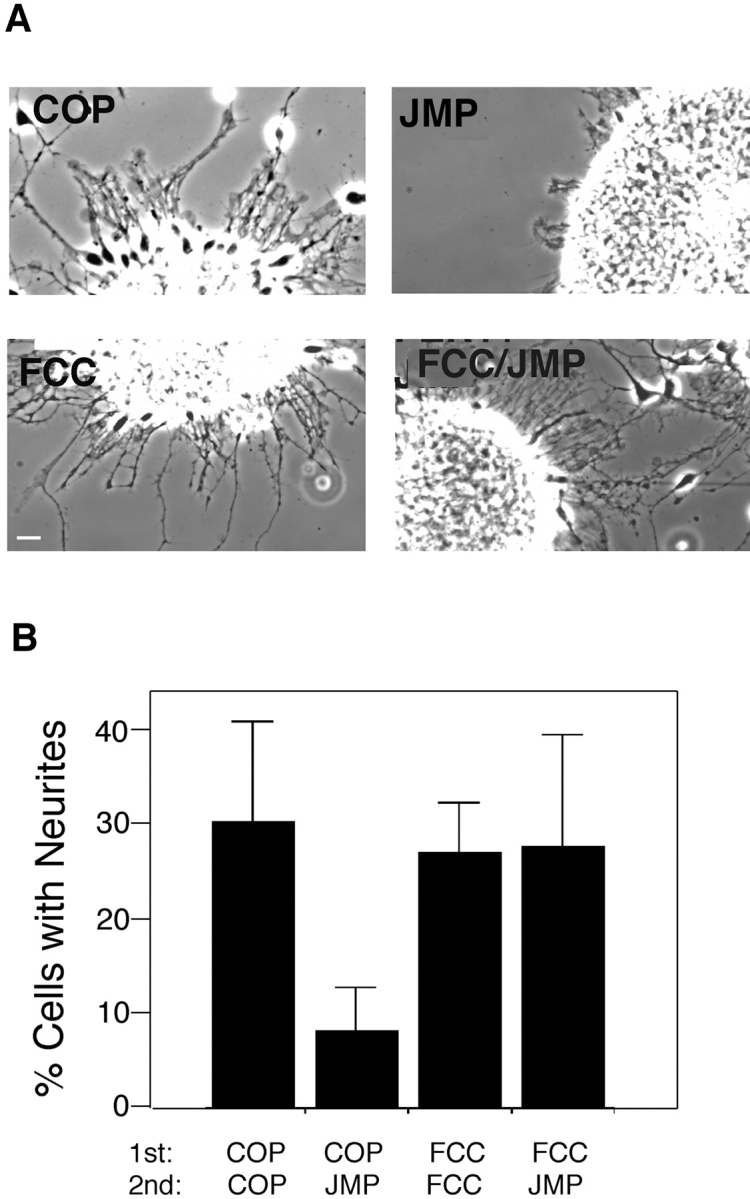
The effect of peptides mimicking specific regions of Fer on inhibition of integrin-mediated neurite outgrowth by JMP. (A) Phase-contrast views of neurite outgrowth from explants of E7 retina. (B) Quantitative determination of neurite outgrowth by single E7 cells after incubation with peptides. Over 100 cells in each treatment group were counted and all cells bearing neurites longer than two cell diameters were considered positive. Bar, ∼20 μm.
To determine if targeting of Fer to the integrin complex is disrupted in the presence of FCC as we predicted, cells were incubated for 1 h with FCC, FNT, or COP, followed by another hour in the presence of JMP. The cells were lysed in mild detergent and immunoprecipitated with anti-FAK antibody. As expected, Fer coprecipitates with FAK after treatment with JMP alone and FNT or COP followed by JMP (Fig. 11 A). However, when cells are treated with FCC before JMP, Fer is not found in the anti-FAK precipitate (Fig. 11 A). Indeed, FCC prevents targeting of Fer to the integrin complex and, thus, prevents the inhibitory effect of JMP on integrin function. In contrast, FCC has no effect on the association of Fer with the cadherin complex (Fig. 11 B) or on cadherin function (not shown).
Figure 11.
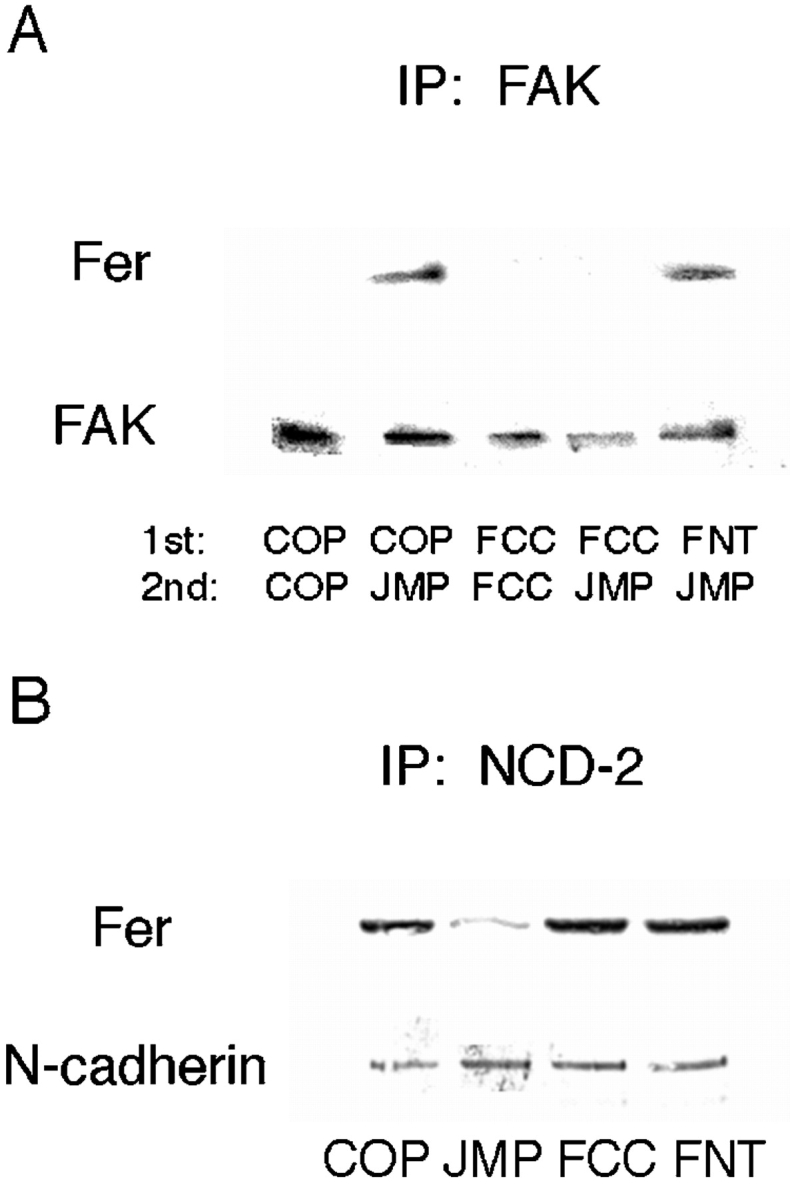
The effect of peptides mimicking specific regions of Fer on its association with the integrin and cadherin complex. (A) Peptide FCC reverses the effect of JMP on targeting of Fer to the integrin complex. The indicated peptide pairs were added to retina cells sequentially with a 1-h incubation after the addition of the first peptide, and a 1-h incubation after the second. The cells were homogenized in neutral detergent and immunoprecipitated with anti-FAK antibody. The immunoprecipitates were fractionated by SDS-PAGE, and Western transfers were blotted with anti-Fer and anti-FAK antibodies. (B) FCC does not affect the association of Fer with the cadherin complex. The indicated peptides were added to retina cells for 1 h, the cells were homogenized in neutral detergent and immunoprecipitated with anti–N-cadherin antibody NCD-2. The immunoprecipitates were fractionated by SDS-PAGE, and Western transfers were blotted with anti-Fer and anticadherin antibodies.
Discussion
We have used cell permeable peptides containing the cell permeation antennapedia sequence (Derossi et al. 1994; Prochiantz 1996), fused to peptides mimicking regions of the cytoplasmic domain of N-cadherin, to specifically perturb protein–protein interactions essential to cadherin and integrin function. These peptides rapidly enter cells and are nontoxic. One peptide, CBP, mimics the cadherin binding site for β-catenin, which is conserved among all Type I cadherins. CBP is targeted to β-catenin, as shown by direct binding and dissolution of β-catenin–cadherin complex both in situ and in vitro. In situ, this peptide causes the release of a complex of proteins containing β-catenin, p120ctn, and Fer from the cytoplasmic domain of cadherin and loss of cadherin function. A second peptide, JMP, is based on the juxtamembrane region of cadherin, which is conserved in all N- and R-cadherins, and was shown to act as a dominant negative when introduced into Xenopus blastomeres (Kintner 1992). It binds directly to the cadherin cytoplasmic domain and localizes to sites of cell–cell association. This peptide causes release of the nonreceptor tyrosine kinase Fer from the N-cadherin complex and loss of N-cadherin and β1-integrin function. The loss of integrin function is accompanied by accumulation of Fer in the integrin complex. Furthermore, a cell permeable peptide containing a sequence mimicking a portion of the first coiled-coil domain of Fer inhibits the accumulation of Fer in the integrin complex, reversing the effect of Fer on integrin-mediated adhesion. Thus, Fer appears to be a critical component of an epigenetic pathway through which cadherin regulates integrin function.
This pathway is activated by the binding of the chondroitin sulfate proteoglycan neurocan to its receptor GalNAcPTase. This interaction results in the loss of cadherin and integrin function concomitant with the loss of Fer from the cadherin complex and its appearance in the integrin complex (Li et al. 2000). Neurocan is present in the developing central nervous system (Oohira et al. 1994; Meyer-Puttlitz et al. 1995; Fukuda et al. 1997; Matsui et al. 1998) and is prominent along axon tracts (Margolis et al. 1996). It is our working hypothesis that coordinate inactivation of cadherin and integrin at the edges of axon tracts is a major contributor to guidance.
What Is the Role of Fer in the Cadherin Complex?
We have previously reported that the presence of the nonreceptor protein tyrosine phosphatase PTP1B in the cadherin complex is essential for dephosphorylation of β-catenin and, therefore, N-cadherin function, and that binding of PTP1B to cadherin requires phosphorylation on tyrosine residues (Balsamo et al. 1996, Balsamo et al. 1998). We have also reported that hyperphosphorylation of β-catenin is not only correlated with the loss of PTP1B from the cadherin complex, but also with reduced tyrosine kinase activity associated with the cadherin complex (Balsamo et al. 1995). This loss of kinase activity from the cadherin complex may reflect the loss of Fer. Therefore, Fer may be required to phosphorylate PTP1B, targeting it to the cadherin complex. We are investigating this possibility. Additionally, overexpression of Fer has been shown to result in increased phosphorylation of p120ctn and β-catenin with concomitant dissolution of the cadherin–catenin complex, a state consistently correlated with loss of cadherin function (Daniel and Reynolds 1997; Lilien et al. 1997).
Fer may also play a role in cis-dimerization of cadherin through phosphorylation of p120ctn. cis-dimerization is required for strong adhesion (Brieher et al. 1996; Yap et al., 1997, Yap et al. 1998; Takeda et al. 1999) and the juxtamembrane region of cadherin has been implicated in regulation of cis-dimerization (Ozawa and Kemler 1998) and in binding of p120ctn (Thoreson et al. 2000). Ohkubo and Ozawa 1999 have reported that the presence of p120ctn interferes with homodimerization, whereas Thoreson et al. 2000 report that p120ctn is essential for the formation of strong adhesions. In fact, this apparent paradox may be a result of changes in the affinity of p120ctn for cadherin because of different states of p120ctn phosphorylation (Thoreson et al. 2000), possibly mediated by Fer. Indeed, increased phosphorylation on tyrosine residues has been reported to increase the affinity of p120ctn for cadherin (Roura et al. 1999). The direct binding of JMP to the cytoplasmic domain of N-cadherin suggests that this region mediates dimerization, whereas the immediate COOH-terminal sequences, through interaction with p120ctn, perform a regulatory function that may be modulated by Fer. The effect of JMP on N-cadherin–mediated neurite outgrowth and adhesion, thus, may be because of direct inhibition of dimerization, or indirectly a result of release of Fer and disruption of the phosphorylation of p120ctn. This suggests that Fer's role is in the formation and/or maintenance of cadherin dimers. In this same vein, Fer may be specifically recruited to cadherin dimers and any circumstances that inhibit dimerization might prevent binding of Fer, leaving it free to associate with integrin. However, this suggests a pool of free Fer, but this seems unlikely as integrin function would be continuously compromised. There is a pool of Fer associated with cortactin (Kim and Wong 1998) and it seems likely that Fer is sequestered by cortactin, p120ctn or other targets preventing its interaction with the β1-integrin complex.
If Fer is in fact bound to p120ctn as Kim and Wong 1995, Kim and Wong 1998 suggest, the ability of JMP to specifically effect its release suggests that interactions among the components associated with the cytoplasmic domain of cadherin are complex and interdependent. This complexity is also revealed by the effect of CBP. CBP competes for β-catenin binding, resulting in the release of a complex of proteins that includes p120ctn, β-catenin, and Fer. Indeed, a recent report (Huber et al. 1999) indicates that the cytoplasmic tail of E-cadherin folds upon binding of β-catenin, and that folding of the cytoplasmic tail may facilitate the binding of p120ctn. The converse may also hold; i.e., competition for β-catenin may cause the unfolding of the cytoplasmic tail affecting the binding of all components, resulting in their release as a complex. While these suggestions are speculative, they do raise interesting questions concerning interactions among the cadherin effectors and cadherin.
How Does Fer Affect Integrin Function?
The loss of integrin function on interaction of Fer is consistent with the loss of cell–substrate adhesion on overexpression of Fer (Rosato et al. 1998). Our observations and those of Rosato et al. 1998, that Fer-mediated loss of integrin function is correlated with a reduction in phosphorylation of p130cas are consistent with reports that phosphorylation of p130cas is correlated with assembly into focal adhesions (Nojima et al. 1995; Petch et al. 1995; Vuori and Ruoslahti 1995). p130cas is at the nexus of a subarray of effector molecules that regulate integrin function. It is an adaptor or docking protein with multiple interaction domains: an SH3 domain, multiple proline-rich regions, and critical tyrosine residues (Sakai et al. 1994). Both the tyrosine phosphatases PTP-PEST (Garton et al. 1997) and PTP1B (Liu et al. 1996), as well as the tyrosine kinase FAK (Polte and Hanks 1995) interact with p130cas through its SH3 domain, and it has been suggested that these components compete with each other to regulate the tyrosine phosphorylation of p130cas and, therefore, assembly of focal adhesions (Garton et al. 1997). Tyrosine phosphorylated p130cas also interacts with Crk through an SH2 domain (Matsuda and Kurata 1996). This interaction has two ramifications: it has the potential to protect p130cas from dephosphorylation (Birge et al. 1992) and appears to act through Rac to stimulate cell migration (Klemke et al. 1998). While the loss of focal adhesions correlates with the loss of tyrosine phosphorylation of p130cas, it is not clear how the presence of Fer in the integrin complex causes a reduction in p130cas phosphorylation. It is obvious that the effect of Fer is indirect; however, it is not clear through what partners Fer affects the dephosphorylation of p130cas. The simplest explanation is that phosphorylation of one of the p130cas' binding partners, PTP-PEST, PTP1B, FAK, or even Crk, alters their activity or their interaction with p130cas, changing the balance of phosphorylated tyrosine residues.
The Interaction of Fer with the Cadherin and Integrin Complexes
Fer has the potential to interact with multiple target sites. It has three coiled-coil domains (Craig et al. 1999), which have the potential to mediate multiple protein–protein interactions (Lupas 1996), and it has the potential to interact with SH2 or PTB (phosphotyrosine-binding) domains through phosphorylated tyrosine residues (Craig et al. 1999). Fer is presumed to interact directly with p120ctn (Kim and Wong 1995), and p120ctn has a predicted coiled-coil domain at its NH2 terminus (Reynolds et al. 1994), which has been suggested to interact with a coiled-coil domain of Fer (Kim and Wong 1995; Craig et al. 1999). We find that FCC has no effect on the association of Fer with the cadherin complex or on cadherin function; thus, if coiled-coil interactions do mediate the interaction, it is not through CC1. CC1 does appear to be involved in targeting to the integrin complex. However, it is not clear whether FCC blocks targeting through an effect on oligomerization of Fer itself (Craig et al. 1999) or through blocking formation of heteromultimers. These results suggest that targeting of Fer to the two complexes is mediated by distinct domains.
Our results demonstrate for the first time that specific regulatory alterations in a cadherin complex of proteins affect integrin function through an epigenetic mechanism: translocation of regulatory proteins from one adhesion receptor to another, or reequilibration and redistribution of a pool of effectors. The protein tyrosine kinase Fer, and possibly other effectors, may thus coordinately regulate cadherin and integrin function. This provides a mechanism for rapid, specific, and coordinate regulation of cell–cell and cell–substrate adhesion during development.
Acknowledgments
We thank Nora Heisterkamp for providing anti-Fer antibody. We also thank Mark VanBerkum for his careful reading of the manuscript and helpful suggestions.
This study was supported by a grant from the National Institutes of Health (No. EY12132).
Footnotes
The present address of Carlos Arregui is INTECH, Camino Circunvalación Km 6, CC164, 7130 Chascomús, Argentina. The present address of J. Lilien and J. Balsamo is Department of Biological Sciences, University of Iowa, Iowa City, IA 52242-1324.
Abbreviations used in this paper: CBP, catenin binding peptide; COP, control antennapedia peptide; FCC, Fer coiled-coil domain peptide; FNT, Fer NH2-terminal peptide; JMP, juxtamembrane peptide; LN, L cells expressing N-cadherin; SBP, Shc binding region peptide.
References
- Aberle H., Schwartz H., Kemler R. Cadherin catenin complexprotein interactions and their implications for cadherin function. J. Cell Biochem. 1996;61:514–523. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C514::AID-JCB4%3E3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Aplin A.E., Howe A., Alahari S.K., Juliano R.L. Signal transduction and signal modulation by cell adhesion receptorsthe role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharm. Rev. 1998;50:199–263. [PubMed] [Google Scholar]
- Arregui C., Lilien J., Balsamo J. Impaired integrin-mediated adhesion and signaling in fibroblasts expressing a dominant negative mutant PTP1B. J. Cell Biol. 1998;143:861–873. doi: 10.1083/jcb.143.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsamo J., Ernst H., Zanin M.K., Hoffman S., Lilien J. The interaction of the retina cell surface N-acetylgalactosaminylphosphotransferase with an endogenous proteoglycan ligand results in inhibition of cadherin-mediated adhesion. J. Cell Biol. 1995;129:1391–1401. doi: 10.1083/jcb.129.5.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsamo J., Thiboldeaux R., Swaminathan N., Lilien J. Antibodies to the retina N-acetylgalactosaminylphosphotransferase modulate N-cadherin–mediated adhesion and uncouple the N-cadherin transferase complex from the actin-containing cytoskeleton. J. Cell Biol. 1991;113:429–436. doi: 10.1083/jcb.113.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsamo J., Hoffman S., Lilien J. Control of cadherin-mediated cell-cell adhesion through regulated association with the cytoskeleton. J. Braz. Assoc. Adv. Sci. 1996;48:341–346. [Google Scholar]
- Balsamo J., Leung T., Ernst H., Zanin M.K.B., Hoffman S., Lilien J. Regulated binding of a PTP1B-like phosphatase to N-cadherincontrol of cadherin-mediated adhesion by dephosphorylation of β-catenin. J. Cell Biol. 1996;134:801–813. doi: 10.1083/jcb.134.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsamo J., Arregui C., Leung T.-C., Lilien J. The nonreceptor protein tyrosine phosphatase PTP1B binds to the cytoplasmic domain of N-cadherin and regulates the cadherin–actin linkage. J. Cell Biol. 1998;143:523–532. doi: 10.1083/jcb.143.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauvais-Jouneau A., Thiery J.P. Multiple roles of integrins during development. Biol. Cell. 1997;89:5–11. doi: 10.1016/s0248-4900(99)80076-8. [DOI] [PubMed] [Google Scholar]
- Birge B., Fagardo J., Mayer B., Hanafusa H. Tyrosine phosphorylated epidermal growth factor receptor and p130 provide high affinity binding substrates to analyze Crk-phosphotyrosine-dependent interactions in vitro. J. Biol. Chem. 1992;267:10588–10595. [PubMed] [Google Scholar]
- Bixby J.L., Zhang R. Purified N-cadherin is a potent substrate for the rapid induction of neurite outgrowth. J. Cell Biol. 1990;110:1253–1260. doi: 10.1083/jcb.110.4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieher W.M., Yap A.S., Gumbiner B. Lateral dimerization is required for the homophilic binding activity of C-cadherin. J. Cell Biol. 1996;135:487–496. doi: 10.1083/jcb.135.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh D.A. Integrinsstructure, function, and biological properties. Adv. Mol. Cell. Biol. 1993;6:225–252. [Google Scholar]
- Craig A.W.B., Zirngibl R., Greer P. Disruption of coiled-coil domains in Fer protein tyrosine kinase abolishes trimerization but not kinase activity. J. Biol. Chem. 1999;274:19934–19942. doi: 10.1074/jbc.274.28.19934. [DOI] [PubMed] [Google Scholar]
- Critchley D.R., Holt M.R., Barry S.T., Priddle H., Hemmings L., Norman J. Integrin-mediated cell adhesionthe cytoskeletal connection. Biochem. Soc. Symp. 1999;65:79–99. [PubMed] [Google Scholar]
- Daniel J.M., Reynolds A.B. Tyrosine phosphorylation and cadherin/catenin function. Bioessays. 1997;19:883–891. doi: 10.1002/bies.950191008. [DOI] [PubMed] [Google Scholar]
- de Curtis I., Reichardt L.F. Function and spatial distribution in developing chick retina of the laminin receptor alpha6 beta1 and its isoforms. Development. 1993;18:377–388. doi: 10.1242/dev.118.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derossi D., Joliot A.H., Chassaing G., Prochiantz A. The third helix of the antennapedia homeodomain translocates through biological membranes. J. Biol. Chem. 1994;269:10444–10450. [PubMed] [Google Scholar]
- Finnemann S., Kühl M., Otto G., Wedlich D. Cadherin transfection of Xenopus XTC cells downregulates expression of substrate adhesion molecules. Mol. Cell. Biol. 1995;15:5082–5091. doi: 10.1128/mcb.15.9.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T., Kawano H., Ohyama K., Li H.P., Takeda Y., Oohira A., Kawamura K. Immunohistochemical localization of neurocan and L1 in the formation of thalamocortical pathway of developing rats. J. Comp. Neurol. 1997;382:141–152. [PubMed] [Google Scholar]
- Garton A.J., Burnbaum M.R., Bouton A.H., Tonks N.K. Association of PTP-PEST with the SH3 domain of p130cas; a novel mechanism of protein tyrosine phosphatase substrate recognition. Oncogene. 1997;15:877–885. doi: 10.1038/sj.onc.1201279. [DOI] [PubMed] [Google Scholar]
- Greve J.M., Gottlieb D.I. Monoclonal antibodies which alter the morphology of cultured chick myogenic cells. J. Cell Biochem. 1982;18:221–229. doi: 10.1002/jcb.1982.240180209. [DOI] [PubMed] [Google Scholar]
- Hall H., Williams E.J., Moore S.E., Walsh F.S., Prochiantz A., Doherty P. Inhibition of FGF-stimulated phosphatidylinositol hydrolysis and neurite outgrowth by a cell-membrane permeable phosphopeptide. Curr. Biol. 1996;6:580–587. doi: 10.1016/s0960-9822(02)00544-4. [DOI] [PubMed] [Google Scholar]
- Hatta K., Takeichi M. Expression of N-cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature. 1986;320:447–449. doi: 10.1038/320447a0. [DOI] [PubMed] [Google Scholar]
- Hemler M.E. Integrin associated proteins. Curr. Opin. Cell Biol. 1998;10:578–585. doi: 10.1016/s0955-0674(98)80032-x. [DOI] [PubMed] [Google Scholar]
- Hodivala K.J., Watt F.M. Evidence that cadherins play a role in the downregulation of integrin expression that occurs during keratinocyte terminal differentiation. J. Cell Biol. 1994;124:589–600. doi: 10.1083/jcb.124.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber A.H., Stewart D.B., Laurents D.V., Nelson W.J., Weis W.I. Physical characterization of the cadherin cytoplasmic domain and its interactions with β-catenin. Mol. Biol. Cell. 1999;10:70a. [Google Scholar]
- Huttenlocher A., Lakonishok M., Kinder M., Wu S., Truong T., Knudsen K.A., Horwitz A.F. Integrin and cadherin synergy regulates contact inhibition of migration and motile activity. J. Cell Biol. 1998;141:515–526. doi: 10.1083/jcb.141.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R.O. Integrinsversatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Jou T.-S., Stewart D.B., Stappert J., Nelson W.J., Marrs J.A. Genetic and biochemical dissection of protein linkages in the cadherin-catenin complex. Proc. Natl. Acad. Sci. USA. 1995;92:5067–5071. doi: 10.1073/pnas.92.11.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim L., Wong T.W. The cytoplasmic tyrosine kinase FER is associated with the catenin-like substrate p120 and is activated by growth factors. Mol. Cell. Biol. 1995;15:4553–4561. doi: 10.1128/mcb.15.8.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim L., Wong T.W. Growth factor-dependent phosphorylation of the actin-binding protein cortactin is mediated by the cytoplasmic tyrosine kinase Fer. J. Biol. Chem. 1998;273:23542–23548. doi: 10.1074/jbc.273.36.23542. [DOI] [PubMed] [Google Scholar]
- Kintner C. Regulation of embryonic cell adhesion by the cadherin cytoplasmic domain. Cell. 1992;69:225–236. doi: 10.1016/0092-8674(92)90404-z. [DOI] [PubMed] [Google Scholar]
- Klemke R.L., Leng J., Molander R., Brooks P.C., Vuori K., Cheresh D.A. CAS/Crk coupling serves a molecular switch for induction of cell migration. J. Cell Biol. 1998;140:961–972. doi: 10.1083/jcb.140.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Balsamo J., Hoffman S., Leung T.-C., Lilien J. Cloning and identification of the domain of chick neurocan essential for coordinate inhibition of cadherin and integrin function. J. Cell Biol. 2000;149:1275–1288. doi: 10.1083/jcb.149.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilien J., Hoffman S., Eisenberg C., Balsamo J. β-Catenin is a target for extracellular signals controlling cadherin functionthe neurocan GalNAcPTase connection. In: Pedersen R.A., Schatten G., editors. Current Topics in Developmental Biology. Vol. 35. Academic Press; San Diego: 1997. pp. 161–189. [DOI] [PubMed] [Google Scholar]
- Liu F., Hill D.E., Chernoff J. Direct binding of the proline-rich region of protein tyrosine phosphatase 1B to the Src homology 3 domain of p130cas . J. Biol. Chem. 1996;271:31290–31295. doi: 10.1074/jbc.271.49.31290. [DOI] [PubMed] [Google Scholar]
- Lu Q., Paredes M., Zhang J., Kosik K.S. Basal extracellular signal-regulated kinase activity modulates cell-cell and cell-matrix interactions. Mol. Cell. Biol. 1998;18:3257–3265. doi: 10.1128/mcb.18.6.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas A. Coiled coilsnew structures and new functions. Trends Biochem. Sci. 1996;21:375–382. [PubMed] [Google Scholar]
- Margolis R.K., Rauch U., Maurel P., Margolis R.U. Neurocan and phosphocantwo major nervous system specific chondroitin sulfate proteoglycans. Perspec. Dev. Neurobiol. 1996;3:273–290. [PubMed] [Google Scholar]
- Marrs J.A., Nelson W.J. Cadherin cell adhesion molecules in differentiation and embryogenesis. Int. Rev. Cytol. 1996;165:159–205. doi: 10.1016/s0074-7696(08)62222-6. [DOI] [PubMed] [Google Scholar]
- Matsuda M., Kurata T. Emerging components of the Crk oncogene productthe first identified adaptor protein. Cell Signal. 1996;8:335–340. doi: 10.1016/0898-6568(96)00067-8. [DOI] [PubMed] [Google Scholar]
- Matsui F., Nishizuka M., Yasuda Y., Aono S., Watanabe E., Oohira A. Occurrence of a N-terminal proteolytic fragment of neurocan, not a C-terminal half, in a perineuronal net in the adult rat cerebrum. Brain Res. 1998;790:45–51. doi: 10.1016/s0006-8993(98)00009-2. [DOI] [PubMed] [Google Scholar]
- Meyer-Puttlitz B., Milev P., Junker E., Zimmer I., Margolis R.U., Margolis R.K. Chondroitin sulfate and chondroitin/keratan sulfate proteoglycans of nervous tissuedevelopmental changes of neurocan and phosphocan. J. Neurochem. 1995;65:2327–2337. doi: 10.1046/j.1471-4159.1995.65052327.x. [DOI] [PubMed] [Google Scholar]
- Monier-Gavelle F., Duband J.-L. Cross talk between adhesion moleculescontrol of N-cadherin activity by intracellular signals elicited by β1 and β3 integrins in migrating neural crest cells. J. Cell Biol. 1997;137:1663–1681. doi: 10.1083/jcb.137.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A., Takeichi M. Transmembrane control of cadherin-mediated cell adhesiona 94kDa protein functionally associated with a specific region of the cytoplasmic domain of E-cadherin. Cell Regul. 1989;1:37–44. doi: 10.1091/mbc.1.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima Y., Morino N., Mimura T., Hamasaki K., Furuya H., Sakai R., Sato T., Tachibana K., Morimoto C., Yazaki Y., Hirai H. Integrin-mediated cell adhesion promotes tyrosine phosphorylation of p130cas, a Src-homology 3-containing molecule having multiple Src homology 2-binding motifs. J. Biol. Chem. 1995;270:15398–15402. doi: 10.1074/jbc.270.25.15398. [DOI] [PubMed] [Google Scholar]
- Ohkubo T., Ozawa M. p120ctn binds to the membrane-proximal region of the E-cadherin cytoplasmic domain and is involved in modulation of adhesion activity. J. Biol. Chem. 1999;274:21409–21415. doi: 10.1074/jbc.274.30.21409. [DOI] [PubMed] [Google Scholar]
- Oohira A., Matsui F., Watanabe E., Kushima Y., Maeda N. Developmentally regulated expression of a brain specific species of chondroitin sulfate proteoglycan, neurocan, identified with a monoclonal antibody Ig2 in the rat cerebrum. Neurosci. 1994;60:145–157. doi: 10.1016/0306-4522(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Ozawa M., Kemler R. The membrane-proximal region of the E-cadherin cytoplasmic domain prevents dimerization and negatively regulates adhesion activity. J. Cell Biol. 1998;142:1605–1613. doi: 10.1083/jcb.142.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petch L.A., Bockholt S.M., Bouton A., Parsons J.T., Burridge K. Adhesion induced tyrosine phosphorylation of the p130 src substrate. J. Cell Sci. 1995;108:1371–1379. doi: 10.1242/jcs.108.4.1371. [DOI] [PubMed] [Google Scholar]
- Polte T.R., Hanks S.K. Interaction between focal adhesion kinase and Crk-associated tyrosine kinase substrate p130cas . Proc. Natl. Acad. Sci. USA. 1995;92:10678–10682. doi: 10.1073/pnas.92.23.10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochiantz A. Getting hydrophilic compounds into cellslessons from homeopeptides. Curr. Opin. Neurobiol. 1996;6:629–634. doi: 10.1016/s0959-4388(96)80095-x. [DOI] [PubMed] [Google Scholar]
- Reynolds A.B., Daniel J.M., McCrea P.D., Wheelock M.J., Wu J., Zhang Z. Identification of a new cateninthe tyrosine kinase substrate p120cas associates with E-cadherin complexes. Mol. Cell. Biol. 1994;14:8333–8342. doi: 10.1128/mcb.14.12.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riehl R., Johnson K., Bradley R., Grunwald G.B., Cornel E., Lilienbaum A., Holt C.E. Cadherin function is required for axon outgrowth in retinal ganglion cells in vivo. Neuron. 1996;17:837–848. doi: 10.1016/s0896-6273(00)80216-0. [DOI] [PubMed] [Google Scholar]
- Rosato R., Veltmaat J.M., Groffen J., Heisterkamp N. Involvement of the tyrosine kinase Fer in cell adhesion. Mol. Cell. Biol. 1998;18:5762–5770. doi: 10.1128/mcb.18.10.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roura S., Miravet S., Piedra J., de Herreros A.G., Duñach M. Regulation of E-cadherin/catenin association by tyrosine phosphorylation. J. Biol. Chem. 1999;274:36734–36740. doi: 10.1074/jbc.274.51.36734. [DOI] [PubMed] [Google Scholar]
- Sakai R., Iwamatsu A., Hirano N., Ogawa S., Tanaka T., Mano H., Yazaki Y., Hirai H. A novel signaling molecule, p130 forms stable complexes in vivo with v-Crk and v-Src in a tyrosine phosphorylation dependent manner. EMBO (Eur. Mol. Biol. Organ.) J. 1994;15:3748–3756. doi: 10.1002/j.1460-2075.1994.tb06684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenwaelder S.M., Burridge K. Bidirectional signaling between the cytoskeleton and integrins. Curr. Opin. Cell Biol. 1999;11:274–286. doi: 10.1016/s0955-0674(99)80037-4. [DOI] [PubMed] [Google Scholar]
- Semb H., Christofori G. Insights from model systemsthe tumor suppressor function of E-cadherin. Am. J. Hum. Genet. 1998;63:1588–1593. doi: 10.1086/302173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithgall T.E., Rogers J.A., Peters K.L., Li J., Briggs S.D., Lionberger J.M., Cheng H., Shibata A., Scholtz B., Schreiner S., Dunham N. The c-Fes family of protein-tyrosine kinases. Crit. Rev. Oncog. 1998;9:43–62. doi: 10.1615/critrevoncog.v9.i1.40. [DOI] [PubMed] [Google Scholar]
- Stappert J., Kemler R. A short core region of E-cadherin is essential for catenin binding and is highly phosphorylated. Cell Adhes. Comm. 1994;2:319–327. doi: 10.3109/15419069409014207. [DOI] [PubMed] [Google Scholar]
- Takeda H., Shimoyamo Y., Nagafuchi A., Hirohashi S. E-cadherin functions as a cis-dimer at the cell-cell adhesive interface in vivo. Nat. Struct. Biol. 1999;6:310–312. doi: 10.1038/7542. [DOI] [PubMed] [Google Scholar]
- Thoreson M.A., Anastasiadis P.Z., Daniel J.M., Ireton R.C., Wheelock M.J., Johnson K.R., Hummingbird D.K., Reynolds A.B. Selective uncoupling of p120ctn from E-cadherin disrupts strong adhesion. J. Cell Biol. 2000;148:189–201. doi: 10.1083/jcb.148.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaselli K.J., Reichardt L.F., Bixby J.L. Distinct molecular interactions mediate process outgrowth on nonneuronal cell surfaces and extracellular matrices. J. Cell Biol. 1986;106:2659–2672. doi: 10.1083/jcb.103.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaselli K.J., Hall D.E., Flier L.A., Gehlsen K.R., Turner D.C., Carbonetto S., Reichardt L.F. A neuronal cell line (PC12) expresses two β1-class integrinsα1β1 and α3β1, that recognize different neurite outgrowth-promoting domains in laminin. Neuron. 1990;5:651–662. doi: 10.1016/0896-6273(90)90219-6. [DOI] [PubMed] [Google Scholar]
- Varner J.A., Cheresh D.A. Integrins and cancer. Curr. Opin. Cell Biol. 1996;8:724–730. doi: 10.1016/s0955-0674(96)80115-3. [DOI] [PubMed] [Google Scholar]
- Vleminckx K., Kemler R. Cadherins and tissue formationintegrated adhesion and signaling. Bioessays. 1999;21:211–220. doi: 10.1002/(SICI)1521-1878(199903)21:3<211::AID-BIES5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Vuori K., Ruoslahti E. Tyrosine phosphorylation of p130cas and cortactin accompanies integrin-mediated cell adhesion to extracellular matrix. J. Biol. Chem. 1995;270:22257–22262. doi: 10.1074/jbc.270.38.22259. [DOI] [PubMed] [Google Scholar]
- Wheelock M.J., Knudsen K.A., Johnson K.R. Membrane-cytoskeleton interactions with cadherin cell adhesion proteinsroles of catenins as linker proteins. Curr. Top. Membr. 1996;43:169–185. [Google Scholar]
- Wu C., Keightley S.Y., Leung-Hagesteijn C., Radeva G., Coppolino M., Goicoechea S., McDonald J.A., Dedhar S. Integrin-linked protein kinase regulates fibronectin matrix assembly, E-cadherin expression, and tumorigenicity. J. Biol. Chem. 1998;273:528–536. doi: 10.1074/jbc.273.1.528. [DOI] [PubMed] [Google Scholar]
- Xu Y., Carpenter G. Identification of cadherin tyrosine residues that are phosphorylated and mediate Shc association. J. Cell Biochem. 1999;75:264–271. doi: 10.1002/(sici)1097-4644(19991101)75:2<264::aid-jcb9>3.3.co;2-2. [DOI] [PubMed] [Google Scholar]
- Xu Y., Guo D.-F., Davidson M., Inagami T., Carpenter G. Interaction of the adaptor protein Shc and the adhesion molecule cadherin. J. Biol. Chem. 1997;272:13463–13466. doi: 10.1074/jbc.272.21.13463. [DOI] [PubMed] [Google Scholar]
- Yap A.S., Brieher W.M., Pruschy M., Gumbiner B. Lateral clustering of the adhesive ectodomaina fundamental determinant of cadherin function Curr. Biol. 7 1997. 308 315a [DOI] [PubMed] [Google Scholar]
- Yap A.S., Brieher W.M., Gumbiner B. Molecular and functional analysis of cadherin-based adherens junctions Annu. Rev. Cell Dev. Biol. 13 1997. 119 146b [DOI] [PubMed] [Google Scholar]
- Yap A.S., Niessen C.M., Gumbiner B.M. The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120ctn . J. Cell Biol. 1998;141:779–789. doi: 10.1083/jcb.141.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu A.J., Watt F.M. Expression of a dominant negative cadherin mutant inhibits proliferation and stimulates terminal differentiation of human epidermal keratinocytes. J. Cell Sci. 1996;109:3013–3023. doi: 10.1242/jcs.109.13.3013. [DOI] [PubMed] [Google Scholar]