Abstract
Aims
The purpose of this study was to evaluate the effects of a polymerizable solubility enhancer, poly (ethylene glycol) dimethacrylate (PEGDMA) in BisGMA/HEMA model adhesives on adhesive phase separation, adhesive penetration and structural integrity of adhesive/dentin (a/d) interfaces.
Materials and methods
the occlusal one-third of the crown was removed from 10 unerupted human third molars, each tooth was separated in half by cutting perpendicular to the acid conditioned dentin surface and treated with BisGMA/HEMA model adhesives with and without PEGDMA. Five-micron-thick sections of adhesive/dentin interface specimens were cut and stained with Goldner’s trichrome for light microscopy. Companion slabs were analyzed with micro-Raman spectroscopy and scanning electron microscopy (SEM). The macrophase separation in the model adhesives with/without PEGDMA was also detected using cloud point measurements in the presence of water.
Results
The addition of PEGDMA enhanced the water solubility/compatibility of the BisGMA/HEMA model adhesives. Micro-Raman spectral analysis of the dentin/adhesive interface indicated that there was a gradual decrease in penetration of BisGMA component for model adhesive without PEGDMA, while homogeneous distribution of the hydrophobic BisGMA component was noted in the interface with adhesive containing PEGDMA. The addition of PEGDMA dramatically facilitated infiltration of the hydrophobic monomers into the wet demineralized dentin. The SEM and staining results showed that the addition of PEGDMA would also improve the integrity at the interface between pure adhesive and hybrid layers.
Significance
The addition of PEGDMA could reduce phase separation, enhance the infiltration of BisGMA-based adhesives into the wet, demineralized dentin substrates, and promote homogeneous distribution of the hydrophobic component throughout the interface.
Keywords: PEGDMA, dentin adhesives, solubility enhancer, micro-Raman spectroscopy, SEM
1. Introduction
The homogenous and thorough resin infiltration and entanglement of exposed collagen fibrils in wet demineralized dentin are the primary factors critical in determining an adequate adhesive/dentin (a/d) hybrid layer [1–3]. BisGMA (Bisphenol-A-glycerolate (1-glycerol/phenol) dimethacrylate), a widely used component in dentin adhesives has very good mechanical properties after curing, but this component is relatively hydrophobic and thus, does not adequately infiltrate the wet demineralized dentin collagen [4–8]. Complications such as phase separation have been associated with BisGMA based adhesives in the presence of wet, demineralized dentin. Collapse or aggregation of the demineralized dentin collagen as a result of drying has been frequently reported [4, 9–11]. The heterogeneous distribution of adhesives and/or the collapse of the collagen network compromise the integrity of the a/d hybrid layer (HL) as well as the marginal stability and strength of composite restorations. For example, recent studies have shown that collagen collapse means that adhesive does not infiltrate as much as one-half of the demineralized dentin [12–14]. Under clinical conditions, oral fluids may permeate the exposed collagen fibril network and bacterial enzymes may attack the collagen fibrils [15–18].
Much attention and effort has been devoted to the development of techniques that would lead to optimum adhesive infiltration of the hybrid layer. Many, if not all, of these efforts involve permutations of the so-called wet bonding technique [19–20]. Under wet bonding conditions the demineralized dentin is kept fully hydrated throughout the bonding procedure, i.e. the water supporting the collagen matrix is not removed [21]. However, the relatively hydrophobic BisGMA cannot adequately infiltrate the demineralized dentin layer under wet bonding conditions [4–8]. So developing adhesive formulations which can decrease the phase separation and promote enhanced penetration of the hydrophobic component into the wet demineralized dentin matrix is very important.
Poly (ethylene glycol) (PEG) based modifying reagents have been widely used as solubility enhancers and/or surfactants. Potentially, these reagents can facilitate the development of a homogenous adhesive formulation under wet bonding conditions. Investigations regarding the application of PEG based reagents in dentin adhesives have been conducted recently [22–25]. Tween 20, 40, 80, a series of PEG based non-ionic surfactants, were employed in the formulation of a dentin adhesive [22]. Stainless steel attachments were bonded to human enamel using adhesives with and without tweens. After bench curing, the bonded specimens were tested to failure in shear and the load at debond was recorded in each case. The conclusion was that the addition of tweens had little or no beneficial effect. There is no polymerizable moiety in tweens and this may be one reason that no beneficial effect was noted with the addition of these surfactants. PEG based polymerizable surfactant has been used in dentin adhesives [24, 25], such as Syntac (Ivoclar, Amherst, NY). An increase in shear bond strength has been noted in adhesives that include polymerizable PEGDMA [23]. However, effects of polymerizable PEG based reagents on the infiltration of adhesive into the demineralized wet dentin and the structure of hybrid layer have not been studied at the molecular level.
The micro-Raman technique has been shown to be a powerful tool for detecting and quantifying the molecular structure of HLs at a spatial resolution comparable to optical microscopy [4–8]. SEM observations of HLs exposed by acid-bleach methods and optical observation results of stained interfaces of dentin and model adhesives have been employed to evaluate the quality of HLs [26–32]. The objectives of the present study were to determine how PEGDMA, which can act as a polymerizable solubility enhancer, affects the infiltration of model BisGMA and HEMA based adhesives into the wet demineralized dentin layer via micro-Raman spectroscopy, and how it affects the integrity of HLs via SEM, staining and optical observations. This study tested thehypotheses that the addition of PEGDMA would increase the water-compatibility of thedentin adhesive formulation, provide decreased phase separation and thus, enhanced structural integrity of the hybrid layer.
2. Materials and methods
2.1. Model adhesives
The monomer mixtures used in this investigation consisted of 2,2-bis[4-(2-hydroxy-3-methacryloxypropoxy) phenyl]-propane (BisGMA, Polysciences, Washington, PA) and hydroxyethylmethacrylate (HEMA, Acros Organics, NJ) with a mass ratio of 60/40. This composition is similar to that used in commercial dentin adhesive formulations such as Single Bond (3M ESPE, St. Paul, MN). The solvent used with the model resin composition was ethanol from Fisher (Fair Lawn, NJ). The following three-component visible light photoinitiators (all from Aldrich, Milwaukee, WI) were used in this study: camphorquinone (CQ, 0.5 wt%), 2-(dimethylamino) ethyl methacrylate (DMAEMA, 0.5 wt%) and diphenyliodonium hexafluorophosphate (DPIHP 1.0 wt %); the concentration of the photoinitiator is calculated with respect to the total amount of monomer. The mixtures were made with and without 10 wt% PEGDMA (average molecular weight 550). Ethanol at a concentration of 30 wt% was added to the above two mixtures to make model adhesives (30 wt% represents the approximate concentration of ethanol in the commercial BisGMA-based adhesives), which were applied to dentin surfaces. Shaking and sonication were required to yield well-mixed monomer/ethanol solutions. All the chemicals in this study were used as received.
2.2. Monomer/ethanol/water mixtures
Phase separation of two mixtures with/without PEGDMA was investigated at varying concentrations of ethanol (0, 15, 30 wt%) and different concentrations of water. The concentration of ethanol was based on the total final weight of the model mixture, then water was added to the mixtures at concentrations ranging from 0–40 wt%. The mixtures were sonicated for 30 s and then inspected for homogeneity. A loss in clarity was interpreted as evidence of phase separation. The water concentrations at which macrophase separation occurred were recorded. The monomer/ethanol/water mixtures were also cast as films on glass slides, covered with mylar, and polymerized for 40 s with visible light (Spectrum Light, Dentsply, Milford, DE); curing intensity was 550 mW/ cm2. Following polymerization, the mylar film was removed and specimens were imaged using a Nikon Eclipse E800 microscope with X60 objective.
2.3. Dentin/model adhesive specimen preparation
Ten extracted non-carious, unerupted human third molars stored at 4°C in phosphate buffered saline (PBS), containing 0.002% sodium azide, were used in this study. Teeth were collected after a patient’s informed consent was obtained under a protocol approved by the UMKC adult health sciences institutional review board. Dentin disks were prepared by first cutting the roots at the cementum–enamel junction with a water-cooled low-speed diamond saw (Buehler, Lake Bluff, IL), then the occlusal one-third of the crowns was removed by means of a second, parallel section. Dentin disks without any enamel remnants or exposure of the pulp chamber were prepared. Uniform standardized smear layers were created by wet-sanding the exposed dentin surfaces for 1 min with 600-grit silicon carbide sandpaper. The prepared dentin surfaces were conditioned with 35% phosphoric acid for 15 s, the dentin disks were sectioned equally perpendicular to the etched surfaces, the separated halves were randomly selected for treatment with model adhesives with/without PEGDMA. The control specimens were those treated with adhesive without PEGDMA and thus, the control specimen was recovered from the same tooth as the experimental sample. The wet bonding technique was used throughout the bonding procedures. The dentin adhesives were photo-cured for 20 s by exposure to a visible light source (Spectrum light, Dentsply, Milford, DE). The prepared specimens were stored for a minimum of 24 h in water before being sectioned. Cross-sectional specimens of the adhesive/dentin interface, approximately 2 mm thick, were cut from the teeth. Three dentin/adhesive rectangular slabs (~8 X ~2 X ~1.5 mm) were recovered from each half tooth; thus 30 interface specimens were analyzed for each model adhesive.
2.4. Micro-Raman spectroscopy
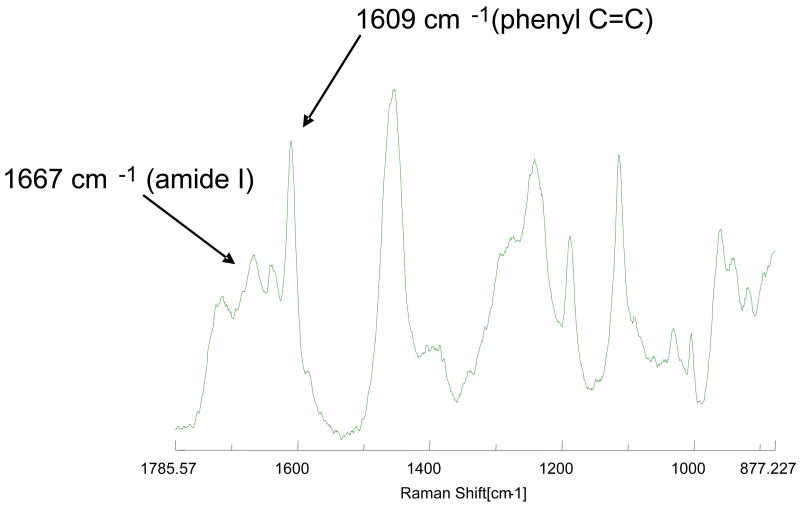
The specimens for micro-Raman analysis were stored in PBS buffer solution for 1 day before spectra collection. A Jasco NRS 2000 Raman spectrometer was employed to record the Raman spectra at the dentin/adhesive interfaces. The micro-Raman spectrometer consisted of an argon ion laser beam (514.5 nm) focused through an Olympus water-immersion lens (X 60) to a ~ 1.5 um beam diameter. Each interface slab was placed at the focus of the objective and covered with distilled water in preparation for micro-Raman analysis. Spectra were acquired at positions corresponding to 1-μm intervals across the adhesive/dentin interface with the use of the computer-controlled x-y-z stage with a minimum step width of 50 nm. Five sites across the interface of each specimen were examined. Spectra were obtained over the spectral region of 875–1785 cm−1 and with an integration time of 90 s. The laser power was approximately 8 mW. The degree of BisGMA monomer infiltration into the demineralized dentin matrices was determined based on the band ratio of 1609 cm−1(phenyl C=C, associated with BisGMA)/1667cm−1 (amide I, associated with collagen). The band ratio is calculated by the spectral subtraction technique [5]. Raman data on the degree of BisGMA infiltration into the demineralized dentin matrices were analyzed using 2-way ANOVA.
2.5. Scanning electron microscopy
In order to evaluate the effect of PEGDMA on the structure of the dentin/adhesive interface, the specimens for SEM observation were separated into two groups (with/without PEGDMA) which were treated according to the following protocol [31]: HCl-bleach treatment: 5 N HCl for 30 s followed by 5% NaOCl for 30 min. After rinsing and air drying, the above specimens were mounted on 12 mm aluminum stubs and sputter coated with gold-palladium. Specimens were examined at a variety of magnifications using a Field Emission Philips XL30 ESEM-FEG 515 (Philips Electron Optics Inc., Hillsboro, OR) at 10 kV.
2.6. Differential staining technique
The rectangular, 8 x 2 x 1.5 mm, slabs of dentin interface specimens with two model adhesives (with/without PEGDMA) were mounted on a methacrylate support, and 5-θm-thick sections were cut from the face of the slab by means of a tungsten carbide knife mounted on a Polycut S "sledge" microtome. Differential staining of the microtomed sections was accomplished with Goldner's trichrome. Stained sections were dehydrated, cover-slipped, and examined under a Nikon E 800 light microscope.
3. Results
3.1. Effect of PEGDMA on water solubility of model adhesives
Macrophase separations in the model adhesives at the different amounts of ethanol and water were detected using cloud point measurements. The results listed in Table 1 were based on the visual observation of a loss of clarity in the monomer/ethanol/water mixtures. The BisGMA/HEMA formulation with 30% ethanol exhibits macrophase separation at 22–23% water. In comparison, with the addition of PEGDMA this formulation exhibits macrophase separation at 28–29% water. Representative light micrographs of the films cast from model mixtures with/without PEGDMA mixed with different amounts of ethanol and water are shown in Figure 1. The small particles in Figures 1a and 1c clearly demonstrate phase separation that occurs when BisGMA/HEMA monomer mixtures without PEGDMA are mixed with 12% water or 15% ethanol + 15% water. Distinct particles are not apparent at these water concentrations (1b and 1d) when PEGDMA is added to the BisGMA/HEMA monomer mixtures.
Table 1.
The water concentration at which macrophase separation occurs in BisGMA/HEMA mixtures without and with PEGDMA at different amounts of ethanol
| 0% Ethanol | 15% Ethanol | 30% Ethanol | |
|---|---|---|---|
| BisGMA/HEMA (60/40) | 8–9% | 15–16% | 22–23% |
| BisGMA/HEMA (60/40) With 10% of PEGDMA | 11–12% | 18–19% | 28–29% |
Figure 1.
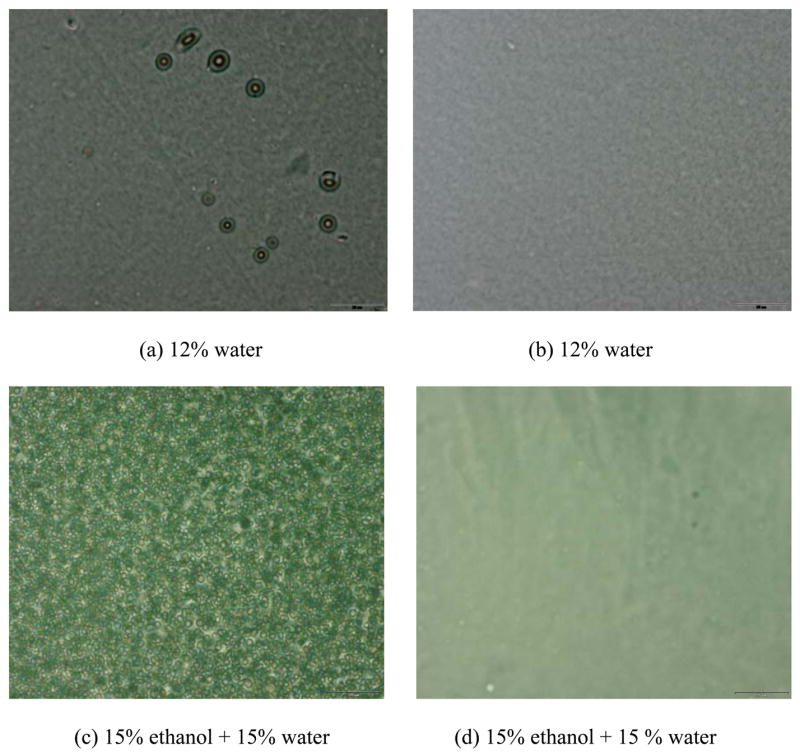
Representative light micrographs of the films cast from model mixtures without (a, c) and with PEGDMA (b, d) mixed with different amounts of ethanol and water. The scale bar is 20 microns.
3.2. Effect of PEGDMA on adhesive penetration and interfacial structure of dentin/model adhesive bond
A representative Raman spectrum of the HL is presented in Figure 2. It reveals the molecular vibrations of penetrated adhesive and collagen. The spectral features associated with both the aliphatic and the aromatic components were identified, the most intense bands occur at 1720 cm−1 (carbonyl), 1457 cm−1 (CH2CH3), 1609 cm−1 (phenyl C=C), 1187 cm−1 (gemdimethyl) and 1113 cm−1 (C-O-C). The last three bands are strictly associated with BisGMA monomer in the model adhesives. The major bands associated with collagen appear at 1245 cm−1 (amide III) and 1667 cm−1 (amide I). The band ratio of 1609 cm−1(phenyl C=C)/1667cm−1 (amide I) can be calculated by the spectral subtraction technique [5], and this band ratio is used to determine the degree of BisGMA monomer infiltration into the demineralized dentin matrices
Figure 2.
Representative micro-Raman spectrum recorded at interface of dentin with the adhesive containing PEGDMA.
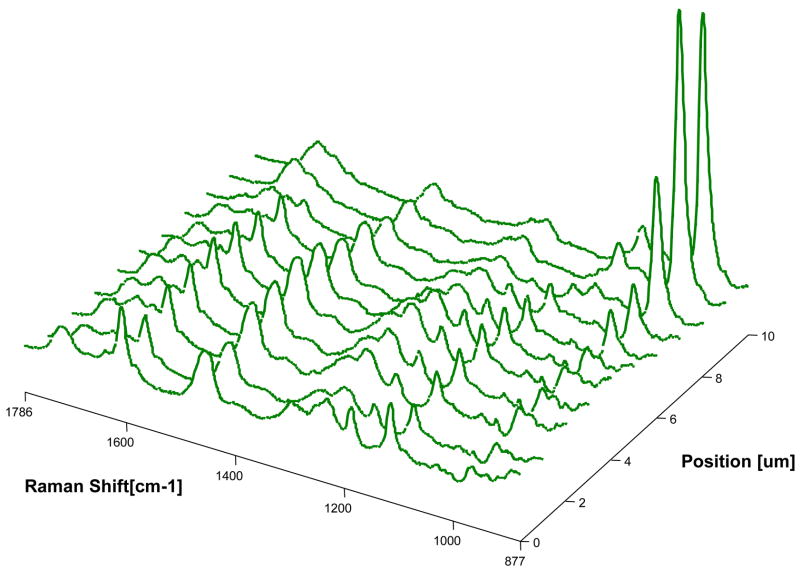
Micro-Raman mapping spectra of the dentin interfaces with model adhesives without and with PEGDMA are shown in Figures 3–4, respectively. Figure 3 represents a series of mapping spectra acquired at 1-um intervals across the dentin interface with adhesive without PEGDMA. The first two spectra were acquired from pure adhesive. Vibrational bands associated with the adhesive and collagen component of dentin were noted in the third spectrum, indicating the model adhesive monomers including BisGMA have penetrated into the HL. In the tenth spectrum, the relative intensity of the 960 cm−1 (PO4−3) band associated with the mineral component, as well as the weaker adhesive band 1609 cm−1 (phenyl C=C) suggested that this spectrum represented the bottom of the demineralized dentin. Thus the third through tenth spectra represent the HL zone of adhesive/dentin with a thickness of approximately 8 um. Figure 4 represents the series of mapping spectra acquired at 1-um intervals across the dentin interface with model adhesive with PEGDMA. The first spectrum was acquired from pure adhesive. The second through ninth spectra represent the HL zone of adhesive/dentin interface. The thickness of the HLs in the two model adhesives/dentin interfaces are approximately the same. This indicates that the addition of polymerizable solubility enhancer, PEGDMA, has no effect on the thickness of the HL. In comparing spectra from the interface in Figure 3 with those in Figure 4, it was noted that the bands associated with adhesive were dominant in Figure 4. The higher relative intensity of the adhesive bands indicated more diffusion of adhesive monomers into the zone of demineralized dentin at the interface with adhesive containing PEGDMA.
Figure 3.
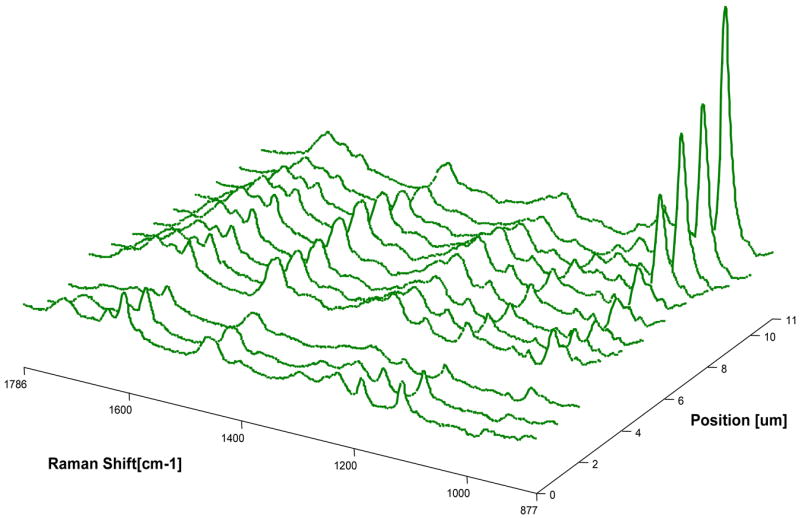
Micro-Raman mapping spectra acquired at 1-μm intervals across the dentin interface with adhesive without PEGDMA. The first two spectra were acquired from pure adhesive. The third spectrum includes collagen and adhesive, while the tenth spectrum represents underlying mineralized dentin identified by the high 960 cm−1 (PO4−3) band. Thus, the spectra from the third to the tenth represent the depth of the hybrid layer.
Figure 4.
Micro-Raman mapping spectra acquired at 1-μm intervals across the dentin interface with adhesive containing PEGDMA. The first spectrum was acquired from pure adhesive. The second through ninth spectra represent the HL zone of adhesive/dentin interface.
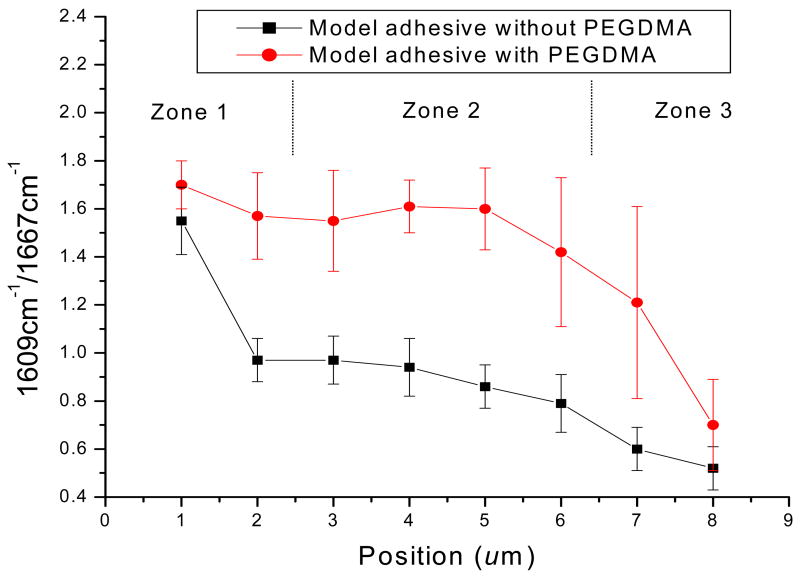
To determine the differences in adhesive penetration, the relative intensity ratios of 1609 cm−1 (phenyl C=C)/ 1667cm−1 (amide I) were calculated using the spectral subtraction technique. In this study, each model adhesive had been applied onto 10 teeth, 5 series of Raman mapping spectra were randomly recorded from each specimen. The statistically averaged band ratios of 1609 cm−1(phenyl C=C)/ 1667cm−1(amide I) as a function of spatial position across the adhesive/dentin interfaces are shown in Figure 5. The ratio of 1609/1667 shows a gradual decline for the model adhesive without PEGDMA, while the ratio for the adhesive with PEGDMA remains nearly unchanged across the breadth of the interface. The statistical comparisons between adhesive infiltration with and without PEGDMA as a function of position across the interface were collapsed into 3 zones. The first zone involved the first and second micrometers, the second zone involved the third through sixth micrometers and the third zone involved the seventh and eighth micrometers. There was a statistically significant difference (p < 0.02) in the band ratios (1609/1667) of the adhesive with and without PEGDMA at each of these zones. These results indicate a statistically significant increase in BisGMA monomer penetration into the wet demineralized dentin matrix with the inclusion of PEGDMA in the adhesive formulation.
Figure 5.
Micro-Raman intensity ratios of 1609 cm−1(phenyl C=C)/1667 cm−1(amide I) as a function of spatial position across the adhesive/dentin interfaces. The statistical comparisons between adhesive infiltration with and without PEGDMA as a function of position across the interface were collapsed into 3 zones. The first zone involved the first and second micrometers, the second zone involved the third through sixth micrometers and the third zone involved the seventh and eighth micrometers. There was a statistically significant difference (p < 0.02) in the band ratios (1609/1667) of the adhesive with and without PEGDMA at each of these zones.
Representative SEM micrographs of the dentin interfaces with model adhesives with and without PEGDMA are shown in Figures 6–7. The interface specimens were treated with 5 N HCl (30 s) followed by 5% NaOCl (30 min). This technique has been used routinely to determine the quality of hybrid layer [26]. An acid-resistant adhesive-infiltrated layer and adhesive tags were clearly observed in both model adhesives by this SEM evaluation. The thickness of the acid-resistant layer formed by model adhesive without PEGDMA in dentin was 2.25 ± 0.17 μm (n=6), while for adhesive with PEGDMA, the acid-resistant layer thickness was 3.96 ± 0.18 μm (n=6), which was much thicker than that without PEGDMA. There were gaps and/or cracks at the interface of pure adhesive and dentin when PEGDMA was not added in the formulation. In comparison, integrity between adhesive and dentin was improved when 10% of PEGDMA was added in the formulation. However, at higher magnification (Figs. 6B and 7B), the surface of HL in the interface with adhesive containing PEGDMA appears porous as compared to the HL in adhesive without PEGDMA.
Figure 6.
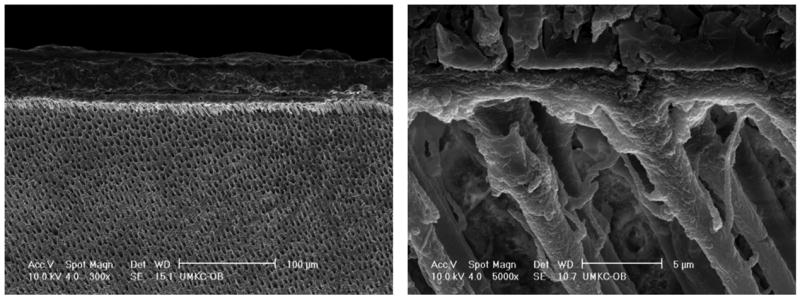
Representative SEM micrographs of acid-bleach treated interface specimens bonded to dentin using model adhesive without PEGDMA.
Figure 7.
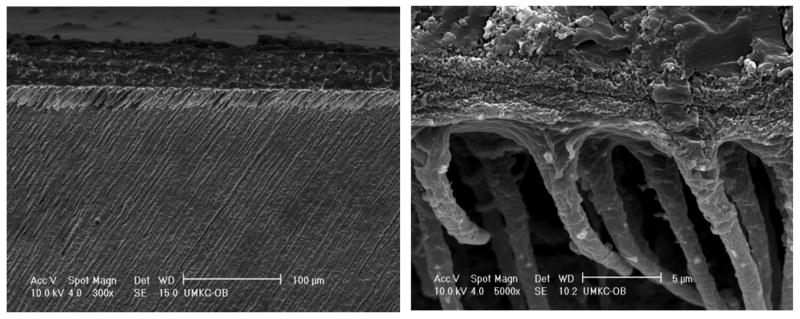
Representative SEM micrographs of acid-bleach treated interface specimens bonded to dentin using model adhesive with PEGDMA.
Representative optical micrographs of Goldner’s trichrome stained sections of the adhesive/dentin interface are presented in Figure 8. The mineralized dentin is green, while the pure adhesive layer is beige. The representative micrograph of dentin treated with model adhesive without PEGDMA is shown in Figure 8a. An orange/red layer distinct from either the adhesive or dentin is visible at the interface. The orange color indicates the HL is more encapsulated with adhesive [7]. A separation between the adhesive and HL was noticed along the length of the interface, indicating that the overall interface lacks structural integrity. Separation was not observed in the interface between dentin and adhesive with PEGDMA (Fig. 8b). However, the interfacial color turned into dark red, indicating the exposed collagen remains unprotected [7, 32]. The results were consistent with the SEM observations.
Figure 8.
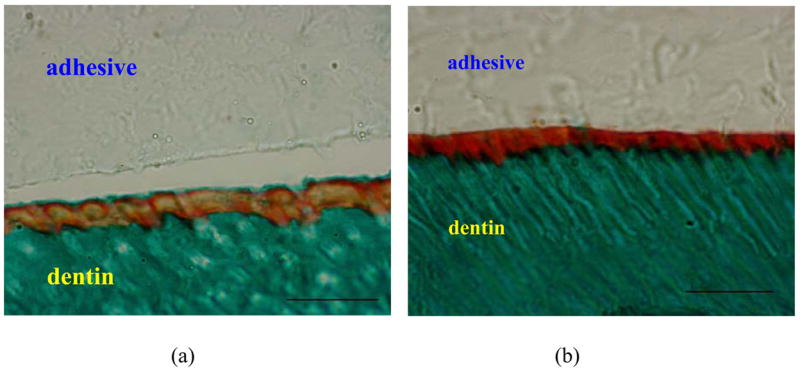
Representative optical micrographs of interface specimens of model adhesives bonded to dentin stained with Goldner’s trichrome. (a) adhesive without PEGDMA, (b) adhesive with PEGDMA. Green color zone is mineralized dentin; yellow (a) and red (b) zones are interfaces. The scale bar is 20 microns.
4. Discussion
It is generally accepted that the efficiency and quality of adhesive bonding to dentin are related to the infiltration and polymerization of adhesive monomers. The unpredictable water content on the etched dentin surface makes the bonding process very complex. In BisGMA/HEMA based adhesives, the presence of water may cause the phase separation between hydrophilic and hydrophobic monomers [4], and inhibit the infiltration of the relative hydrophobic BisGMA monomer. This will affect the integrity of the adhesive and dentin interface. Meanwhile, water can inhibit polymerization of dentin adhesives [33], especially, the hydrophilic component of adhesives [4, 34]. This is because the photoinitiators such as CQ and its co-initiators used in most dentin adhesives are relatively hydrophobic [34]. Thus, reducing phase separation of adhesives in the presence of water and keeping homogenous penetration of adhesives into wet demineralized dentin are very critical factors during the dentin bonding process.
The results from the cloud point measurements indicated that the addition of PEGDMA allowed the adhesive formulation to tolerate higher water concentrations before macrophase separation occurred. This is due to the solubility enhancing function of PEGDMA. PEGDMA can enhance the solubility of BisGMA in water and allow for a more homogeneous BisGMA/HEMA mixture in the presence of water. This basic function of PEGDMA will also enhance the infiltration of BisGMA into the wet demineralized dentin as revealed by Raman results, and lead to a more integrated interface (SEM and staining results).
The Raman results demonstrated that there was a distinct difference in the degree of adhesive monomer (especially BisGMA) penetration between formulations with and without PEGDMA. The diffusion of BisGMA monomer into the zone of wet demineralized dentin was much lower for the adhesive without PEGDMA. This was due to the relative hydrophobic nature of BisGMA. When applying BisGMA/HEMA based adhesives on the wet demineralized dentin, phase separation will occur, forming BisGMA-rich and HEMA-rich phases [4]. The water soluble monomer HEMA can easily dissolve in water on the dentin surface, and penetrate the collagen fibrils on the etched dentin surface. On the other hand, due to the hydrophobic nature of the BisGMA-rich phase, this phase will not readily penetrate the wet demineralized dentin matrices. Since the CQ based photoinitiator system is hydrophobic, most of the photoinitiator will remain associated with the BisGMA-rich phase [4, 34]. This will lead to poor polymerization of the HEMA which has penetrated the demineralized dentin zone. The crosslink density of the hybrid layer will be reduced because of the limited infiltration of the BisGMA into the wet, demineralized dentin matrices. The HEMA monomers and oligomers will leak into the aqueous environment, causing damage to HL integrity.
In comparison, the addition of PEGDMA decreased phase separation of the BisGMA/HEMA adhesive formulation in the presence of water. BisGMA, HEMA and PEGDMA can mix and remain as a homogenous formulation in the presence of the wet, demineralized dentin matrices. After polymerization, the pure adhesive resin and resin within the HL will retain this homogeneous structure. As compared to HEMA, PEGDMA serves as a better primer/enhancer for promoting penetration of BisGMA monomer into wet demineralized dentin. The cumulative effect of these factors will be an increase in the integrity of the penetrated adhesive as a result of the addition of PEGDMA in the adhesive formulation.
SEM observations of dentin interfaces with model adhesives are shown in Figures 7–8. Both of the formulations penetrate the open dentinal tubules as indicated by the adhesive tags. There is, however, a distinct difference in the integrity of the adhesive/dentin interfaces. There are cracks at the interface between dentin and the adhesive formulation that does not contain the polymerizable solubility enhancer, PEGDMA. These cracks reflect a loss of integrity and phase separation in the adhesive formulation without PEGDMA in the presence of the wet demineralized dentin matrices. As a result of phase separation, the low crosslink density and poorly polymerized adhesive cannot withstand the stresses generated during the polymerization and/or associated with specimen preparation. The addition of PEGDMA decreases adhesive phase separation in the presence of the wet demineralized dentin matrices. The integrity of the adhesive and hybrid layer are maintained and consequently, there are fewer cracks at the adhesive/dentin interface. Similar results are noted with the optical microscopic analyses.
Comparing the stained colors of HLs in Figures 8 (a) and (b), it can be seen that the color of the HL with the adhesive formulation containing PEGDMA was dark red. According to the authors’ previous study, this potentially means that the collagen fibrils in the HL were not adequately protected by the adhesive [7, 13]. In other words, there were collagen fibrils that were available for reaction with the Goldner’s trichrome stain. This may indicate one drawback with the addition of PEGDMA. From Figure 7, we can see that there were many micro-size pores in the hybrid layers when 10% of PEGDMA was added in the formulation. These pores may also indicate poor encapsulation of the collagen fibrils by the adhesive resin, so the collagen fibrils were accessible for degradation by the acid-bleach reagents. The reason for this weak entrapment of the collagen fibrils may be related to the increased penetration of the hydrophobic BisGMA into the HL as a result of the 10% PEGDMA. The hydrophobic BisGMA may not react appropriately with the water shell that surrounds the collagen fibrils. As a result, when the adhesive/dentin interface is stained, the exposed collagen fibrils will be available for reaction with the stain. The exposed collagen fibrils will also be degraded by the acid-bleach reagents used during the SEM sample preparation. The benefits of improved penetration of hydrophobic component deserve further exploration.
In summary, the results of the study support the hypotheses that addition of PEGDMA to BisGMA/HEMA model adhesives increased the water compatibility of the formulation and provided decreased phase separation of BisMGA, thereby enhancing infiltration. The study did not support the hypothesis that addition of BisGMA enhanced the structure integrity of the hybrid layer. In other words, the addition of PEGDMA can enhance the homogeneous infiltration of adhesives into the wet demineralized dentin substrates, decreasing the possibility of cracks at the adhesive/hybrid layer interface. However, additional penetration of the hydrophobic component, BisGMA, may lead to weaker interaction between the collagen fibrils and adhesive that infiltrates the channels between the demineralized dentin collagen fibrils. It is not known if the lack of this interaction will affect the long-term durability of the dentin bond. The effect of PEGDMA on the durability of the hybrid layers and the interaction between the collagen fibrils and the infiltrating adhesive are areas of ongoing investigation in the authors’ laboratory.
Acknowledgments
This work is a contribution from the UMKC Center for Research on Interfacial Structure & Properties (UMKC-CRISP). This investigation was supported by Research Grant: R01DE14392 (PI: Spencer), K25DE015281 (PI: Wang) from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD 20892.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eick JD, Gwinnet AJ, Pashley DH, Robinson SJ. Current concepts on adhesion to dentin. Crit Rev Oral Biol Med. 1997;8:306–335. doi: 10.1177/10454411970080030501. [DOI] [PubMed] [Google Scholar]
- 2.Eick JD, Cobb CM, Chappell RP, Spencer P, Robinson SJ. The dentinal surface: Its influence on dentinal adhesion. Part III Quintessence Int. 1993;24:571–582. [PubMed] [Google Scholar]
- 3.Erickson RL. Surface interactions of dentin adhesive materials. Oper Dent. 1992;(Suppl 5):81–94. [PubMed] [Google Scholar]
- 4.Spencer P, Wang Y. Adhesive phase separation at dentin interface under wet bonding conditions. J Biomed Mater Res. 2002;62:447–456. doi: 10.1002/jbm.10364. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Spencer P. Quantifying adhesive penetration in dentin/adhesive interfaces using confocal Raman microscopy. J Biomed Mater Res. 2002;59:46–55. doi: 10.1002/jbm.1215. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Spencer P. Interfacial chemistry of class II composite restoration: Structure analysis. J Biomed Mater Res. 2005;75A:580–587. doi: 10.1002/jbm.a.30451. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Spencer P. Hybridization efficiency of the adhesive/dentin interface with wet bonding. J Dent Res. 2003;82:141–145. doi: 10.1177/154405910308200213. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Spencer P. Physicochemical interactions at the interfaces between self-etch adhesive systems and dentin. J Dent. 2004;32:567–579. doi: 10.1016/j.jdent.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Inokoshi S, Nakaoki Y, Shono T, Pereira PNR, Yamada T, Tagami J. Collagen layer collapse and inhibited resin penetration by dentin etching. J Dent Res. 1996;75:389. abstract #2976. [Google Scholar]
- 10.Tam LE, Pilliar RM. Fracture surface characterization of dentin-bonded interfacial fracture toughness specimens. J Dent Res. 1994;73:607–619. doi: 10.1177/00220345940730030601. [DOI] [PubMed] [Google Scholar]
- 11.Wieliczka DM, Kruger MB, Spencer P. Raman imaging of dental adhesive diffusion. Appl Spectrosc. 1997;51:1593–1596. [Google Scholar]
- 12.Lemor RM, Kruger MB, Wieliczka DM, Swafford JR, Spencer P. Spectroscopic and morphologic characterization of the dentin/adhesive interface. J Biomed Opt. 1999;4:22–27. doi: 10.1117/1.429917. [DOI] [PubMed] [Google Scholar]
- 13.Spencer P, Swafford JR. Unprotected protein at the dentin-adhesive interface. Quintessence Int. 1999;30:501–507. [PubMed] [Google Scholar]
- 14.Wieliczka DM, Spencer P, Kruger MB. Raman mapping of the dentin/adhesive interface. Appl Spectrosc. 1996;50:1500–1504. [Google Scholar]
- 15.Pashley DH, Ciucchi B, Sano H, Horner JA. Permeability of dentin to adhesive agents. Quintessence Int. 1993;24:618–631. [PubMed] [Google Scholar]
- 16.Roulet JF, Degrange M, editors. Adhesion: The silent revolution in dentistry. 1. Quintessence Publishing Co., Inc; 1999. p. 263. [Google Scholar]
- 17.Sano H, Takatsu T, Ciucchi BJAH, Matthews WG, Pashley DH. Nanoleakage: Leakage within the hybrid layer. Oper Dent. 1995;20:18–25. [PubMed] [Google Scholar]
- 18.Sano H, Yoshiyama M, Ebisu S, Burrow MF, Takatsu T, Ciucchi B. Comparative SEM and TEM observations of nanoleakage within the hybrid layer. Oper Dent. 1995;20:160–167. [PubMed] [Google Scholar]
- 19.Kanca J. Improved bond strength through acid etching of dentin and bonding to wet dentin surfaces. J Am Dent Assoc. 1992;123:235–243. doi: 10.14219/jada.archive.1992.0248. [DOI] [PubMed] [Google Scholar]
- 20.Gwinnett AJ. Dentin bond strength after air drying and rewetting. Am J Dent. 1994;7:144–148. [PubMed] [Google Scholar]
- 21.Kinney JH, Balooch M, Marshall SJ, Marshall GW. Atomic force microscope study of dimensional changes in dentine during drying. Arch Oral Biol. 1993;38:1003–1007. doi: 10.1016/0003-9969(93)90114-2. [DOI] [PubMed] [Google Scholar]
- 22.Ireland AJ, Ireland MJ, Sherriff M. Surfactants as part of a combined etchant and activator solution prior to the use of an anaerobic adhesive. Dent Mater. 2004;20:924–930. doi: 10.1016/j.dental.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Venz S, Dickens B. Modified surface-active monomers for adhesive bonding to dentin. J Dent Res. 1993;72:582–586. doi: 10.1177/00220345930720030501. [DOI] [PubMed] [Google Scholar]
- 24.6387982. Self etching adhesive primer composition and polymerizable surfactant. US patent. 2002
- 25.Watson TF, Wilmot DMD. A confocal microscopic evaluation of the interface between Syntac adhesive and tooth tissue. J Dent. 1992;20:302–310. doi: 10.1016/0300-5712(92)90055-h. [DOI] [PubMed] [Google Scholar]
- 26.Nakabayashi N, Takarada K. Effect on HEMA on bonding to dentin. Dent Mater. 1992;8:125–30. doi: 10.1016/0109-5641(92)90067-m. [DOI] [PubMed] [Google Scholar]
- 27.Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Res. 1982;16:265–73. doi: 10.1002/jbm.820160307. [DOI] [PubMed] [Google Scholar]
- 28.Perdigão J, Lambrechts P, Van Meerbeek B, Braem M, Yildiz E, Yucel T, et al. The interaction of adhesives systems with human dentin. Am J Dent. 1996;9:167–73. [PubMed] [Google Scholar]
- 29.Vargas MA, Cobb DS, Armstrong SR. Resin-dentin shear bond strength and interfacial ultrastructure with and without a hybrid layer. Oper Dent. 1997;22:159–66. [PubMed] [Google Scholar]
- 30.Yoshiyama M, Carvalho R, Sano H, Horner J, Brewer PD, Pashley DH. Interfacial morphology and strength of bonds made to superficial versus deep dentin. American J Dent. 1995;8:297–302. [PubMed] [Google Scholar]
- 31.Wang Y, Spencer P. Exploring the nature of acid-resistant hybrid layer with wet bonding. Oper Dent. 2004;29:650–5. [PubMed] [Google Scholar]
- 32.Wang Y, Spencer P, Christy Hager, Brenda Bohaty. Comparison of interfacial characteristics of adhesive bonding to superficial versus deep dentine using SEM and staining techniques. J Dent. 2006;34:26–34. doi: 10.1016/j.jdent.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Jacobsen T, Soderholm K-J. Some effects of water on dentin interface under wet bonding condition. Dent Mater. 1995;11:132–6. doi: 10.1016/0109-5641(95)80048-4. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Spencer P, Yao X, Ye Q. Effect of coinitiator and water on the photoreactivity and photopolymerization of HEMA/camphoquinone-based reactant mixtures. J Biomed Mater Res. 2006;78A:721–728. doi: 10.1002/jbm.a.30733. [DOI] [PMC free article] [PubMed] [Google Scholar]