Abstract
Activation of the Wnt signaling pathway is important for induction of gene expression and cell morphogenesis throughout embryonic development. We examined the subcellular localization of dishevelled, the immediate downstream component from the Wnt receptor, in the embryonic mouse kidney. Using immunofluorescence staining, confocal microscopy, and coimmunoprecipitation experiments, we show that dishevelled associates with actin fibers and focal adhesion plaques in metanephric mesenchymal cells. Stimulation of Wnt signaling leads to profound changes in metanephric mesenchymal cell morphology, including disruption of the actin cytoskeleton, increased cell spreading, and increased karyokinesis. Upon activation of Wnt signaling, dishevelled also accumulates in and around the nucleus. Casein kinase Iε colocalizes with dishevelled along actin fibers and in the perinuclear region, whereas axin and GSK-3 are only present around the nucleus. These data indicate a branched Wnt signaling pathway comprising a canonical signal that targets the nucleus and gene expression, and another signal that targets the cytoskeleton and regulates cell morphogenesis.
Keywords: cytoskeleton, signaling, receptor, kidney, epithelia
Introduction
Wnt genes are expressed throughout embryogenesis, and play a role in tissue patterning and organogenesis (for reviews see Cadigan and Nusse 1997; Moon et al. 1997). In the embryonic kidney, metanephric mesenchyme is induced to differentiate into tubular epithelium by signals emanating from the ureteric bud, a branching epithelial tissue that invades the mesenchyme around embryonic day E11.5 of mouse development (for review see Saxen 1987). This inducing activity is mimicked by exposing naive mesenchyme to fibroblasts expressing Wnt1 in the absence of ureteric bud cells, demonstrating that Wnt signaling is sufficient to initiate epithelial differentiation in vitro (Herzlinger et al. 1994). Targeted disruption of Wnt4 results in no mesenchymal to epithelial transformation and kidney agenesis, although mesenchymal cells are induced by the ureteric bud to express the early induction markers Pax-2 and N-myc (Stark et al. 1994). These results demonstrate that Wnt signaling is necessary and sufficient for the mesenchymal to epithelial transformation of metanephric cells, a process that requires profound changes in cell shape and in gene expression (for review see Saxen 1987).
Wnt genes are known to mediate multiple cellular effects, and have been classified into two functional groups with separate downstream signaling pathways. The Wnt1 class stimulates the canonical Wnt/β-catenin signaling pathway, which leads to changes in cell fate and/or cell transformation. The Wnt5A class stimulates the Wnt/Ca2+ signaling pathway, which modulates cell adhesion and cell movement (for review see Miller et al. 1999a). Wnt4 possesses traits of both classes, as it is capable of altering gene expression and modulating cell adhesion and morphogenetic movements (Munsterberg et al. 1995; Ungar et al. 1995; Torres et al. 1996).
In the canonical Wnt/β-catenin signaling pathway, Wnts bind to frizzled receptors and signal to the intracellular protein dishevelled (dvl), which in turn inhibits the activity of glycogen synthase kinase-3 (GSK-3; for review see Miller et al. 1999a). In the absence of Wnt signals, GSK-3 exists in a complex with axin–conductin, the adenomatous polyposis coli protein (APC) and β-TrCP/Slimb, which together comprise the destruction complex for β-catenin degradation (for review see Miller et al. 1999a). Recently, casein kinase Iε also has been identified as a potential regulator of GSK-3 in conjunction with dvl (Peters et al. 1999; Sakanaka et al. 1999). In the presence of Wnt signals, inhibition of GSK-3 results in the stabilization of β-catenin, which translocates into the nucleus and stimulates transcription by associating with LEF/TCF transcription factors (for reviews see Miller et al. 1999a; Barker et al. 2000; Thorpe et al. 2000). The Wnt/Ca2+ signaling pathway also functions via a subclass of frizzled receptors, leading to release of intracellular calcium and activation of protein kinase C in a process that involves G protein activation (for review see Miller et al. 1999a). The mechanisms by which Wnt pathways mediate cellular effects other than changes in gene expression are very poorly understood.
Although the Wnt/β-catenin pathway has been characterized extensively using genetic and biochemical approaches in embryos and immortalized cell lines, it has been very difficult to determine the subcellular localization of endogenous Wnt signaling proteins in these model systems. Endogenous β-catenin is present at adherens junctions (for review see Aberle et al. 1996); in the nucleus (Yost et al. 1996), dvl is cytoplasmic (Fagotto et al. 1999) and may be associated with vesicle-like organelles (Miller et al. 1999b), and APC is present at the ends of microtubules (Nathke et al. 1996; Mimori-Koyusue et al. 2000). The detailed subcellular localization of other endogenous Wnt signaling proteins is unknown.
We chose to study the subcellular distribution of endogenous Wnt signaling proteins to gain insight into the possible mechanisms by which Wnt signaling might target the cytoskeleton and, hence, changes in cell morphogenesis. We examined embryonic mouse kidney development because Wnt signaling plays a role in the epithelial differentiation of metanephric mesenchymal cells in vivo (Stark et al. 1994) and because the epithelial phenotype of kidney tubule cells is very well understood. We focused on the subcellular localization of dvl because it is the most proximal intracellular branching site within the Wnt signaling pathway (Axelrod et al. 1998; Boutros et al. 1998; for review see Boutros and Mlodzik 1999). Our results indicate a role for Wnt signaling and dvl in actin cytoskeleton organization and cell morphogenesis.
Materials and Methods
Cell Culture
Kidneys from mouse CD1 E12 or E15 embryos were dissected and cultured for 3 d on collagen-coated coverslips. Embryonic E12 or E15 kidneys were dissociated by incubating 10 kidneys/ml in HDF (147 mM NaCl, 5.3 mM KCl, 5 mM dextrose, 4.2 mM NaHCO3, 0.5 mM EDTA) with 0.0625% trypsin solution for 20 min at 37°C, followed by repeated pipetting to resuspend cells. This cell suspension was diluted 1:4 with serum-containing culture medium to inactivate the trypsin. An average of 50,000 cells were plated on each collagen-coated coverslip in 1 ml total volume of culture medium per well and grown for a total of 3 d. This procedure yields a mix of ∼90% metanephric mesenchymal cells and 10% ureteric bud cells, based on the expression of the ureteric bud and mesenchymal markers cytokeratin 8 (K8) and vimentin, respectively. Control and Wnt1 expressing NIH 3T3 fibroblasts were provided by Dr. D. Herzlinger (Columbia University, New York, NY), and cultured as described previously (Herzlinger et al. 1994). Where indicated, 60,000–100,000 of these NIH 3T3 cells were added to each well of embryonic kidney cells plated 2 d earlier, and were cultured for an additional 24 h. In all cases, embryonic kidneys and dissociated kidney cells were cultured in DME/Ham's F-12 (1:1; GIBCO BRL) with 10% FCS and penicillin (0.5 U/ml), streptomycin (0.5 mg/ml) and kanamycin (1 mg/ml).
Immunofluorescence Staining
Whole cultured embryonic kidneys and dissociated embryonic kidney cells were fixed as indicated in 100% methanol at −20°C for 10 min, in 2% paraformaldehyde in 75 mM lysine, 37.5 mM NaPO4 and 0.1 M NaIO4 at room temperature for 10 min, or in 0.5% glutaraldehyde in 100 mM Pipes, pH 6.9, 4 mM MgCl2, 2 mM EGTA and 0.1% Triton X-100 (TX-100) at room temperature for 10 min, followed by quenching in PBS (pH 8.0, 2.7 mM KCl, 1.5 mM KH2PO4, 9.2 mM NaCl, 15.2 mM Na2HPO4), with 1 mg/ml NaBH4. After fixation, samples were washed in PBS, pH 7.5, and incubated overnight in blocking solution (PBS, pH 7.5 with 50 mM NH4Cl, 25 mM poly-l-lysine, 25 mM glycine, 20% normal goat serum, 0.2% BSA) at 4°C. When using mouse or rat mAbs, the blocking solution contained 18 μg/ml unlabeled goat anti–mouse or anti–rat IgG (Jackson ImmunoResearch Laboratories; Boehringer; Roche Molecular Biochemicals). Samples were incubated in blocking solution with the following primary antibodies: rabbit polyclonal antiuvomorulin (provided by R. Kemler, Max-Planck Institut fur Immunbiologie, Freiburg, Germany; Vestweber and Kemler 1984; Piepenhagen et al. 1995), rabbit polyclonal anti–dvl-1 and -2, rabbit polyclonal antiaxin (all provided by K. Willert in the laboratory of R. Nusse, Stanford, CA; Miller et al. 1999b, Willert et al. 1999), goat polyclonal anti–NH2 terminus or COOH terminus dvl antibodies (Santa Cruz Biotechnology), mouse monoclonal antiactin (Boehringer Mannheim; Roche Molecular Biochemicals), mouse monoclonal anti–E-cadherin, antipaxillin and anti–GSK-3 (all from Transduction Laboratories). After washing the samples with PBS and 0.2% BSA, they were incubated in blocking solution with rhodamine- or FITC-conjugated goat anti–rabbit, goat anti–mouse or donkey anti–goat secondary antibodies, as indicated. The samples were washed in PBS, incubated in PBS with 10 μg/ml Hoechst stain (Molecular Probes), washed in PBS, pH 7.5, and mounted in Vectashield (Vector Labs). Alternatively, nucleic acids were visualized by incubating samples in 50 μM SytoRed (Molecular Probes) in 50 mM Tris-HCl, pH 7.5, washed in 50 mM Tris-HCl, pH 7.5, and mounted as above. All samples were analyzed using a confocal scanning microscope (Molecular Dynamics).
Protein Extraction, Immunoprecipitation, and Western Blotting
Five E12, E15, or E16 embryonic mouse kidneys were homogenized on ice in 300 μl CSK buffer (10 mM Pipes, pH 6.8, 50 mM NaCl, 300 mM sucrose, 3 mM MgCl2, 0.5% TX-100, 1 mM pefablock, 10 μg/ml DNase, 10 μg/ml RNase) and centrifuged at 1,000 g for 10 min at 4°C. The TX-100–soluble fraction was separated from the pellet, which was solubilized on ice in 100 μl Tris-SDS buffer (50 mM Tris-HCl, pH 7.5, 1% SDS, 2 mM EDTA, 1 mM pefablock, 10 μg/ml DNase, 10 μg/ml RNase). The SDS was diluted to 0.1% by adding 900 μl CSK buffer. Primary antibodies, as indicated, and protein A–Sepharose beads (Sigma Chemical Co.) were incubated with the TX-100 and SDS-soluble protein fractions ranging from 2 h to overnight at 4°C, followed by washing three times in 1 ml CSK buffer (or PBS, pH 7.5, in the case of actin coimmunoprecipitations). Immunoprecipitated proteins were processed for SDS-PAGE (PAGE; 10% acrylamide gels) and transferred to 0.45-μm pore size nitrocellulose filters for Western blotting.
In all cases, antibodies used for Western blotting were the same as those used in at least a subset of the immunofluorescence experiments. Dishevelled and axin were detected using rabbit polyclonal primary anti–dvl-1 and antiaxin antibodies provided by K. Willert and R. Nusse (Miller et al. 1999b; Willert et al. 1999). Actin was detected using mouse monoclonal antiactin antibody (Boehringer; Roche Molecular Biochemicals). Paxillin and GSK-3 were detected using mouse monoclonal antipaxillin and mouse monoclonal anti–GSK-3 antibodies (Transduction Laboratories). All primary antibodies were visualized using HRP-conjugated anti-mouse or anti-rabbit secondary antibody (Amersham Pharmacia Biotech), except for GSK-3, which was detected using biotinylated anti-mouse antiserum, followed by HRP-conjugated streptavidin (Zymed) incubation. The HRP signal was visualized by ECL chemiluminescence reagent (Amersham Pharmacia Biotech).
Scoring Wnt1 Effects on Embryonic Kidney Cells
In all cases, embryonic kidney cells were distinguished from NIH 3T3 cells because of their large size, prominent stress fibers, and angular appearance. NIH 3T3 cells were easily identifiable by their small size and characteristic fibroblast morphology. The incidence of multinucleated cells, and increased cell spreading were scored by collecting a low magnification image from 10 random locations on a single coverslip per experiment. The frequency of morphological features listed above was determined at each site, and then an average and/or the total number of cells was calculated for each experiment. Multinucleated cells were identified by simultaneously visualizing the actin cytoskeleton and Hoechst-stained nucleic acids using a Zeiss Axioplan microscope. Nuclear dishevelled was visualized using a Molecular Dynamics confocal laser scanning microscope. SytoRed nucleic acid stain (Molecular Probes Inc.) was used as above to detect nuclei in intact cultured embryonic kidneys by confocal microscopy. Levels of nuclear dishevelled were compared from cell to cell using pseudocolor imaging to quantitate differences in fluorescent signals. Disassembly of the actin cytoskeleton was scored by acquiring high magnification, pseudocolorized images from five random locations within a single coverslip per experiment, and then determining the average number and average length of actin stress fibers present. Cell spreading was quantitated by measuring the average area of cells in controls versus Wnt1 treatments. All measurements were carried out using NIH Image software (http://rsb.info.nih.gov/nih-image/index.html).
Results
Dishevelled Is Present in Multiple Subcellular Compartments in the Developing Kidney
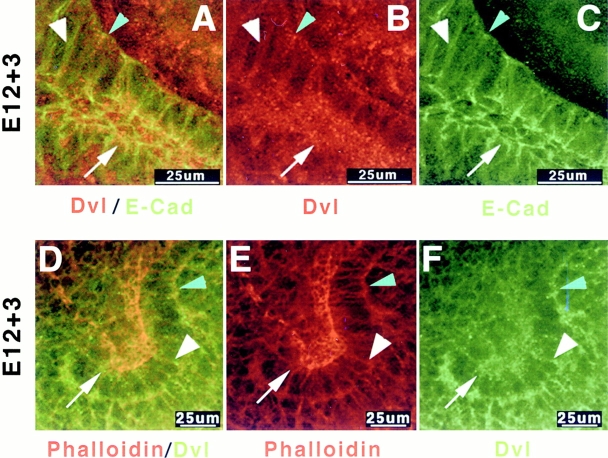
Wholemount immunofluorescence staining of E12 kidneys cultured ex vivo on collagen-coated coverslips for 3 d revealed diffuse staining of dvl along cell borders (Fig. 1, A–C, large white arrowhead) and in the cytoplasm of epithelial, (Fig. 1A and Fig. C; E-cad+) and mesenchymal cells (Fig. 1A and Fig. C, E-cad−). In addition, prominent punctate dvl staining was detected along the basal surface (Fig. 1A and Fig. B, small blue arrowhead) and at the apical pole of tubule epithelial cells (Fig. 1A and Fig. B, arrow). Dvl staining (Fig. 1D and Fig. F) overlapped with that of filamentous actin at cell–cell borders in both epithelial and mesenchymal cells (Fig. 1D and Fig. E). In epithelial cells, dvl also colocalized with F-actin at the apical and basal poles, although diffuse dvl staining was also present in areas lacking actin. These data raised the possibility that dvl might localize to the actin cytoskeleton during nephrogenesis.
Figure 1.
Endogenous dishevelled protein is present in multiple subcellular locations in embryonic kidney mesenchymal and epithelial cells. (A–C) Endogenous dishevelled and E-cadherin staining in E12 mouse kidneys cultured ex vivo for 3 d and fixed in 2% paraformaldehyde. Diffuse dvl is present throughout the cytoplasm. Dvl concentrates at lateral membranes (large arrowheads) and apical cell surfaces (arrows). Punctate dvl staining is present apically (arrows) and basally (small arrowheads). (D–F) Dvl staining partially overlaps with the rhodamine-phalloidin signal associated with filamentous actin along the basal (small arrowheads), lateral (large arrowheads), and apical (arrow) surfaces.
Endogenous Dishevelled Colocalizes and Coimmunoprecipitates with Actin in a Developmentally Regulated Manner
The subcellular distribution of endogenous dvl protein was examined in dissociated E12 embryonic kidney cells cultured for 3 d on collagen-coated coverslips (Fig. 2A, Fig. B, Fig. D, and Fig. E). In this, and all other experiments using dissociated E12 kidneys, 90% of cells in dissociated kidney cell cultures comprised mesenchymal cells and the remainder were epithelial cells derived from ureteric bud, based on the expression of vimentin, a mesenchymal marker, and cytokeratin 8 and E-cadherin, ureteric bud/epithelial markers (Torres, M.A., unpublished results). Note that dissociated metanephric mesenchymal cells retain the ability to differentiate into tubular epithelium, as nephric tubules form in reaggregated cultures grown for 3 d on collagen-coated coverslips (Torres, M.A., and W.J. Nelson, manuscript in preparation). Cells were stained with dvl antibody, and antiactin (Fig. 2A, Fig. C, Fig. D, and Fig. F, FITC) or anti–β-tubulin antibodies (Torres, M.A., unpublished results) to reveal that endogenous dvl colocalized with actin at leading edges and stress fibers, with an enrichment at the tips of stress fibers (Fig. 2, A–C). Dvl staining did not overlap with that of tubulin or APC; note that APC staining is also concentrated at the tips of membrane protrusions but in association with microtubules (Nathke et al. 1996; Torres, M.A., unpublished results). Immunofluorescence experiments carried out with nonimmune serum did not yield similar staining patterns, indicating that the signal detected using the anti-dvl antibodies is dvl-specific (M.A. Torres, unpublished results). In addition, we confirmed that the anti-dvl antibodies used detected a single ∼75-kD band, which is consistent with the size of endogenous mouse dvl (see below).
Figure 2.
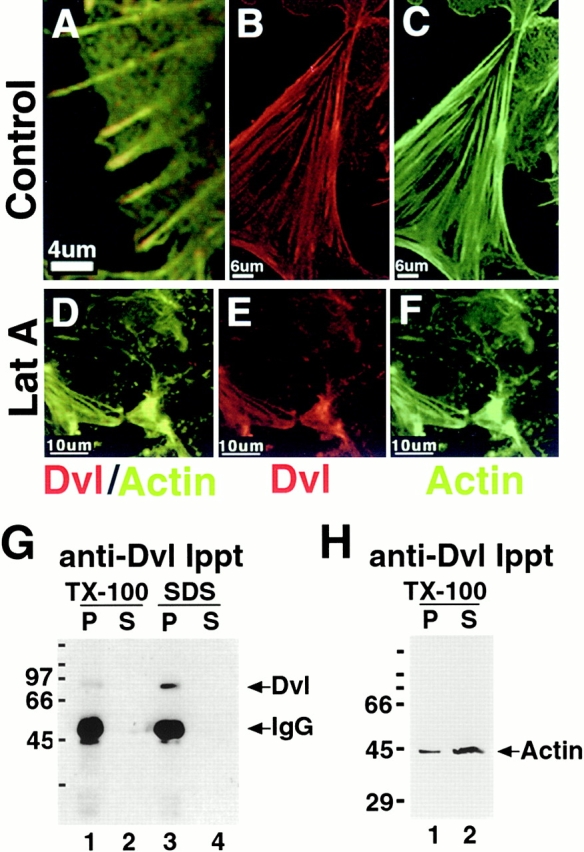
Dishevelled colocalizes and coimmunoprecipitates with actin in dissociated E12 embryonic kidney cells. E12 kidney cells were cultured for 3 d on collagen-coated coverslips and fixed with 100% cold methanol. (A–C) Dvl and actin signals overlap almost completely in dissociated E12 kidney cells cultured for 3 d on collagen-coated coverslips. Dvl appears to be enriched at the tips of actin stress fibers (A). (D–F) Dvl and actin signals continued to overlap after treatment with latrunculin A, which depolymerized the actin cytoskeleton, suggesting that dvl and actin are physically linked. (G) Immunoprecipitations were performed on TX-100–soluble and –insoluble (solubilized in SDS) protein fractions from five kidneys. 100% of the pellet (P) and 16% (lane 2) or 10% (lane 4) of the supernatant (S) fractions were analyzed by SDS-PAGE and Western blotting. A single 75-kD band was immunoprecipitated (lanes 1 and 3), which is consistent with the predicted size of dvl. (H) Dvl and actin are present in a complex in E12 kidney homogenates solubilized with TX-100. 100% of the pellet (lane 1) and 16% of the supernatant fractions (lane 2) were analyzed by SDS-PAGE and Western blotting. A single 44-kD actin band was detected in association with immunoprecipitated dvl (lane 1) and in the unbound supernatant fraction (lane 2), demonstrating that a fraction of TX-100–soluble actin is present in a complex with dishevelled in E12 mouse kidney cells.
Sibling cultures of embryonic kidney cells were treated with latrunculin A (Allison et al. 1998) to examine whether disruption of the actin cytoskeleton leads to alterations in dvl distribution. Latrunculin A disrupted both the actin cytoskeleton (Fig. 2D and Fig. F) and dvl distribution, although both proteins remained colocalized in cytoplasmic aggregates, indicating that dvl and actin are complexed. Treatment of embryonic kidney cells with nocodazole to disrupt microtubules did not alter the subcellular distribution of dvl (Torres, M.A., unpublished results).
Endogenous dvl expression in E12 embryonic kidneys was examined biochemically. Immunoprecipitation of endogenous dvl from E12 kidney homogenates sequentially extracted with TX-100 (Fig. 2 G; lane 1, pellet fraction [P], and lane 2, supernatant fraction [S]) and SDS (Fig. 2 G, lanes 3 [P] and 4 [S]), followed by Western blotting with dvl antibody revealed that endogenous dishevelled is present primarily in the TX-100–insoluble, SDS-extracted fraction (Fig. 2 G, compare lane 3 with lane 1). This is consistent with its association with the actin cytoskeleton. The apparent molecular mass of the immunoprecipitated protein is ∼75 kD, which is similar to the predicted size of mouse dvl-1 (Sussman et al. 1994) and dvl-2 (Klingensmith et al. 1996).
To test whether dvl and actin associate in a complex, dvl protein was immunoprecipitated from E12 kidney homogenates and the immunoprecipitate was probed by Western blotting with antiactin antibody. A fraction of the TX-100–soluble actin that was present in E12 kidneys coimmunoprecipitated with dvl (Fig. 2 H, lanes 1 [P] and 2 [S]), confirming that endogenous dvl and actin form a complex. We were unable to determine whether TX-100– insoluble actin also complexes with dvl because SDS disrupted protein–protein interactions (Torres, M.A., unpublished result). We did not detect APC or β-catenin in the complex; the axin antibody that we used does not work for Western blotting in our hands. We detected GSK-3 β in the complex, but the amount was very variable.
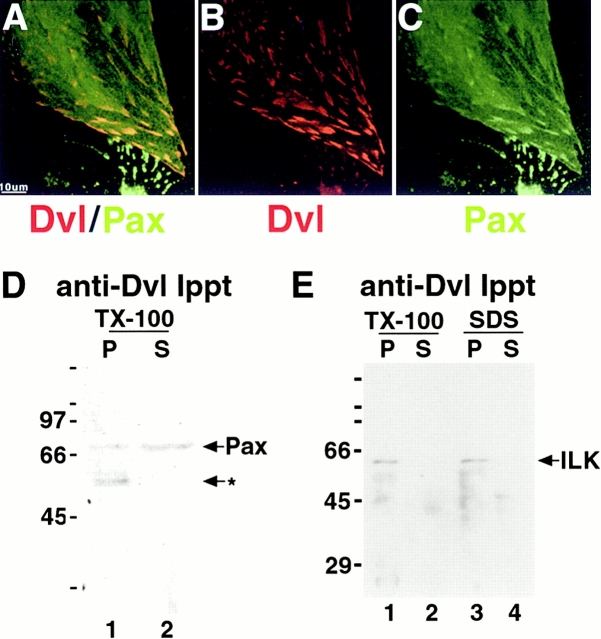
Dishevelled Localizes to Focal Adhesion Plaques in a Complex with Paxillin and Integrin-linked Kinase
The punctate distribution of endogenous dvl at the basal surface of embryonic kidney cells (Fig. 1) and at the end of actin stress fibers in dissociated cells (Fig. 2) indicated that dvl localizes to focal adhesion plaques. Significantly, when focusing on the basal surface of embryonic kidney cells below the actin cytoskeleton, the distribution of dvl (Fig. 3A and Fig. B, rhodamine) and the focal adhesion plaque protein paxillin (Fig. 3A and Fig. C, FITC) overlapped in distinctive patches (Fig. 3). To test whether endogenous dvl was present in a complex with paxillin, dvl-specific immunoprecipitates were probed by Western blotting with the antipaxillin antibody used above. A fraction of TX-100–soluble paxillin coimmunoprecipitated with dvl (Fig. 3 D, lane 1, P). A second TX-100–soluble protein of ∼58 kD detected by the antipaxillin antibody coimmunoprecipitated with dvl (Fig. 3 D, lane 1, arrow with asterisk); the identity of this protein is unknown. As expected, when the dvl–paxillin complex was completely denatured in SDS, we were unable to detect an interaction between dvl and paxillin (Torres, M.A., unpublished result). In addition, dvl coimmunoprecipitated with integrin-linked kinase (ILK; Fig. 3 F, lanes 1 and 3 [P] and lanes 2 and 4 [S]). From our combined biochemical and immunofluorescence data, we conclude that in metanephric mesenchymal cells, dvl is localized to focal adhesions in a complex with other components of the adhesion plaque.
Figure 3.
Dishevelled is present at focal adhesions and coimmunoprecipitates with paxillin and integrin-linked kinase (ILK). (A–C) E12 kidney cells were cultured for 3 d on collagen-coated coverslips and fixed in 100% cold methanol. Dvl and paxillin colocalize in patches at the basal cell surface. In addition, there is diffuse paxillin staining that does not overlap with dvl (A). (D and E) Endogenous dvl was immunoprecipitated from five E12 kidneys homogenized in the presence of TX-100, followed by SDS-PAGE and Western blotting to detect paxillin (D) or ILK (E). The supernatant represents 16% of the total TX-100–soluble fraction (D and E, lane 2) or 10% of the SDS-soluble fraction (E, lane 4). A small fraction of TX-100–soluble paxillin, a 68-kD protein (D, compare lanes 1 and 2), and all of an unidentified 58-kD protein (D, lane 1, arrow with asterisk) coimmunoprecipitated with dvl. We were unable to detect an interaction between paxillin and dvl in the TX-100–insoluble pellet after solubilization with SDS (data not shown). The majority of TX-100–soluble (E, lane 1) and insoluble (E, lane 3) ILK, a 59-kD protein (E), also coimmunoprecipitated with endogenous dishevelled.
Treatment of Metanephric Cells with Wnt1 Leads to the Shortening and Disappearance of Actin Stress Fibers and to Increased Cell Spreading
We tested whether stimulation of Wnt signaling alters the distribution of endogenous dvl along actin fibers or the structure of the actin cytoskeleton. Dissociated E12 kidney cells grown for 2 d on collagen-coated coverslips were cocultured for 24 h with Wnt1-expressing, or control NIH 3T3 fibroblasts. Wnt1-treated metanephric cells underwent marked morphological changes. Wnt1 stimulation resulted in a shortening of actin filaments, and a reduction in the number and length of actin fibers (compare control Fig. 4 A with Wnt1-treated Fig. 4, B–D, and Fig. 5A and Fig. B). Distribution analysis revealed that a greater proportion of embryonic kidney cells treated with Wnt1 possessed fewer actin fibers than control cells (Fig. 5 B). In addition, these fibers tended to be shorter relative to controls (Fig. 5 A): 13% of control versus 35% of Wnt1-treated cells possessed <5 actin stress fibers (Fig. 5 B); and in 57% of Wnt1-treated cells, the average actin fiber length was ≤15 μm, compared with 3% of control cells (Fig. 5 A).
Figure 4.
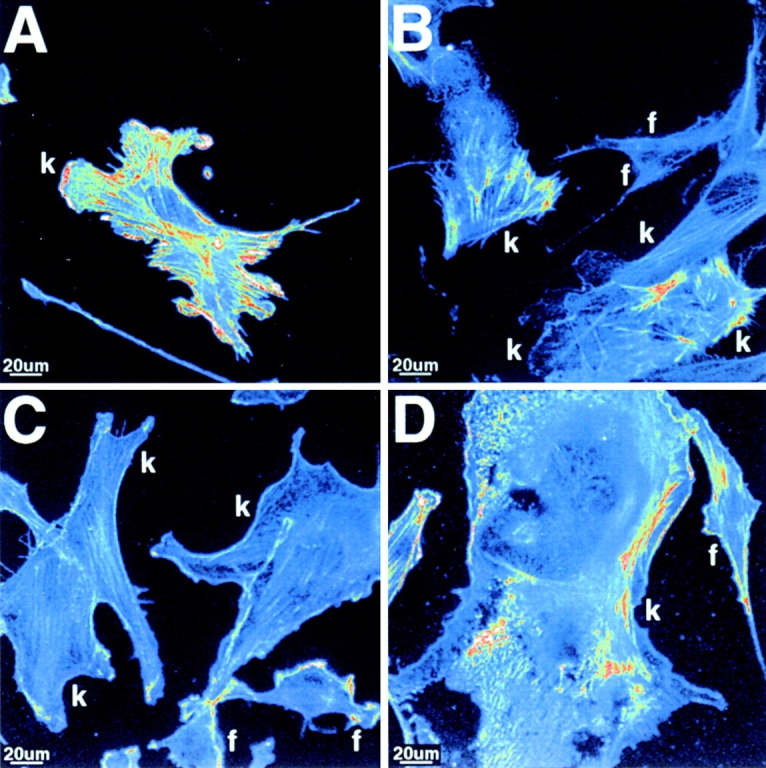
Wnt1 treatment of metanephric cells resulted in a shortening and reduction in the number of actin fibers and increased cell spreading. E12 kidney cells (k) were cultured for 2 d on collagen-coated coverslips, cocultured with control or Wnt1-expressing NIH 3T3 fibroblasts (f) for an additional 24 h, and fixed in 100% cold methanol. Actin stress fibers are shown in pseudocolor, to highlight differences in the antiactin signal between control and Wnt1 treatments. (A) Control-treated metanephric cells possessed long actin stress fibers throughout the cell body. (B–D) Wnt1 treatment resulted in a shortening of actin stress fibers for a subset of Wnt1-treated metanephric cells. Wnt1 treatment also led to an increased cell spreading in a subset of metanephric cells, compared with controls, with some cells exhibiting extreme cell spreading phenotypes (D).
Figure 5.
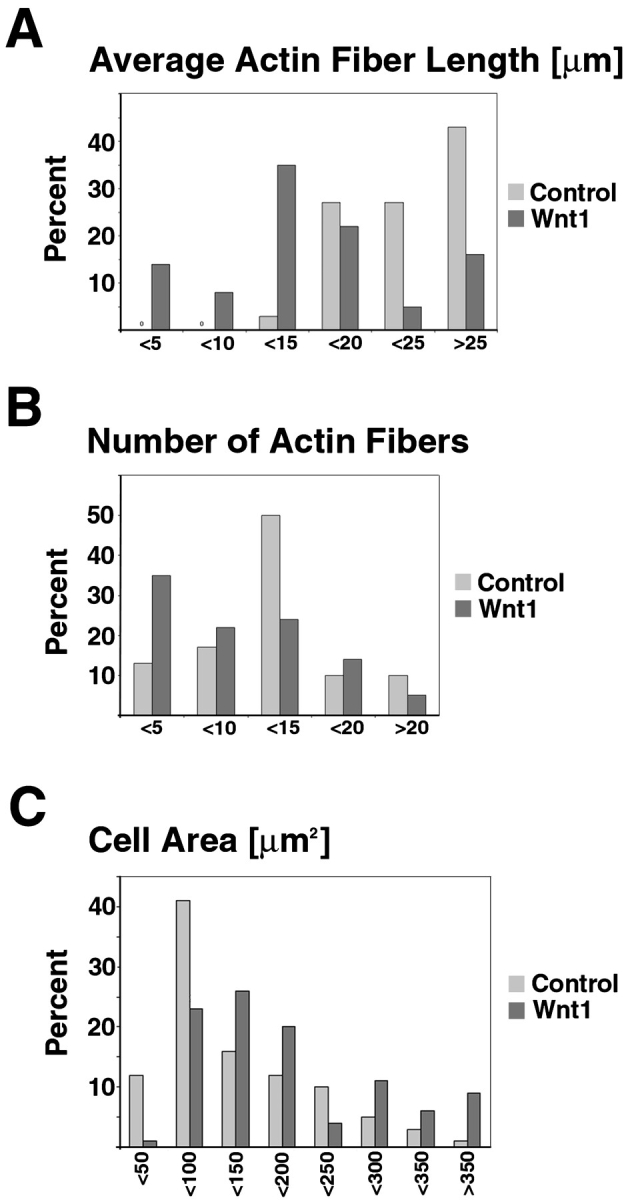
Quantitation of Wnt1 effects on actin fiber length, actin fiber number, and cell area in metanephric cells. Control- and Wnt1-treated metanephric cells were analyzed by measuring individual and average actin stress fiber lengths per cell, the total number of stress fibers per cell, and the total area per cell from confocal images detecting actin and dvl immunofluorescence signals. (A) A greater percentage of Wnt1-treated metanephric cells possess shorter actin stress fibers and, conversely, a smaller percentage of Wnt1-treated metanephric cells possess longer actin stress fibers, relative to controls. (B) A larger percentage of Wnt1-treated cells possess a small number of actin fibers, compared with controls. (C) Wnt1 treatment increases the percentage of cells with a larger cell area and, conversely, decreases the relative number of cells with a small cell area, compared with controls.
Morphometric analysis of the surface area of metanephric cells showed that Wnt1 treatment increased the average cell surface area from 124 μm2 for controls to 230 μm2 for Wnt1-treated cells. Using distribution analysis, we found that Wnt1 treatment resulted in a modest increase in cell area for most cells, along with a very pronounced increase in area for a subset of cells: 9% of Wnt1-treated cells had an area >350 μm2 versus only 1% of control-treated kidney cells (Fig. 5 A). Cultures of metanephric mesenchymal cells treated with Wnt1 also had a greater number of polynucleated cells (42%, n = 106) compared with controls (23%, n = 91; Fig. 6; Table ).
Figure 6.
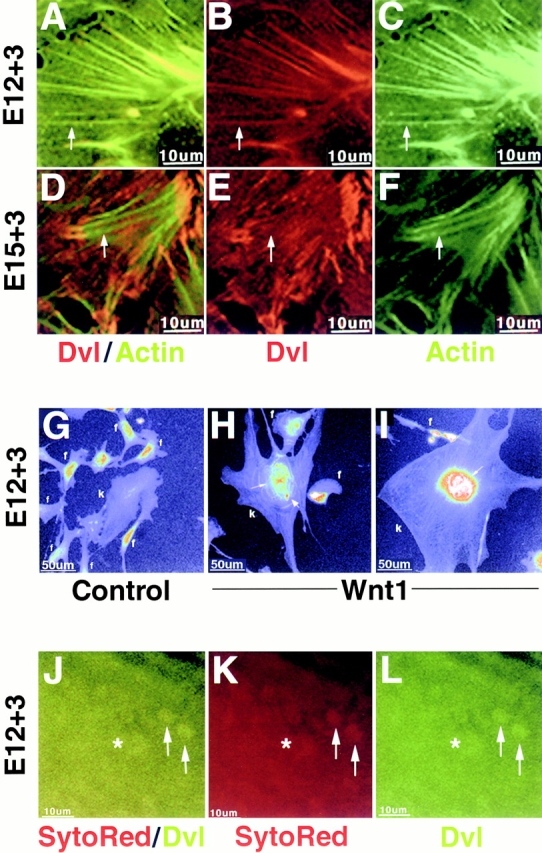
The subcellular localization of dishevelled is regulated during nephric tubule induction and by Wnt1 signaling. (A–F) Embryonic kidney cells were harvested at E12 (A–C) or at E15 (D–F) and cultivated for 3 d on collagen, followed by fixation in 100% cold methanol and immunofluorescence staining using anti-dvl and antiactin antibodies. (A–C) Cells from E12 kidneys exhibit a colocalization of endogenous dvl along actin stress fibers (arrows). (D–F) Cells from E15 kidneys exhibit decreased colocalization of dvl with actin stress fibers (arrows) and, instead display a punctate pattern of dvl staining on the basal cell surface and an accumulation of dvl at the end of actin stress fibers. (G–I) Wnt1 stimulation leads to the accumulation of dvl in and around the nucleus of metanephric cells. E12 kidney cells (k) were cultured for 2 d on collagen-coated coverslips and cocultured with control or Wnt1-expressing NIH 3T3 fibroblasts (f) for an additional 24 h. Cells were fixed in 0.5% glutaraldehyde and endogenous dvl was detected using anti-dvl antibodies. Pseudocolor was used to quantify the amount of nuclear dvl and to visualize cell boundaries. Control-treated metanephric cells possessed low levels of nuclear dishevelled (G), whereas Wnt1-treated metanephric cells exhibited high levels of nuclear and perinuclear dvl staining (H and I). In particular, dvl accumulated between nuclei (H and I, thin arrow) and in a punctate pattern adjacent to the nucleus (H, thick arrow). Wnt1 treatment also increased the incidence of polynucleated metanephric cells and promoted cell spreading (H and I). (J–L) Dvl is present in the nuclei of metanephric mesenchymal cells in E12 kidneys cultured ex vivo for 3 d on collagen-coated coverslips and fixed in 0.5% glutaraldehyde. The presence of dvl inside nuclei was determined by observing colocalization of dvl immunofluorescence and the nuclear stain SytoRed signal in confocal optical sections (arrows). Nuclear dvl (arrows) was observed in cells on the cortex of the developing kidney proximal to the tips of ureteric bud branches (asterisk), where mesenchyme is induced to transform into tubular epithelium.
Table 1.
Wnt1 Treatment Disrupts the Actin Cytoskeleton, Increases Cell Spreading and Promotes the Appearance of Multinucleated Embryonic Kidney Cells
| Treatment | Stress fiberlength | No. ofactin fibers | Cellarea | Percentmultinucleated |
|---|---|---|---|---|
| μm | μm2 | |||
| Control | 24.5 ± 7.1 (30) | 12.6 ± 6.6 (30) | 124 (102) | 23 (91) |
| Wnt1 | 15.6 ± 9.3 (37) | 9.3 ± 7.0 (37) | 230 (127) | 42 (106) |
E12 metanephric cells were cocultured with control or Wnt1-expressing cells as previously described. Actin fibers were identified using antiactin immunofluorescence and confocal imaging. Cell spreading was scored by determining the average area of cells in control versus Wnt1 treatments. Nuclei were identified using the nucleic acid Hoechst stain. The numbers in parentheses designate the total number of cells counted per treatment.
The Subcellular Distribution of Dishevelled Is Regulated during Nephric Tubule Induction and by Exogenous Wnt1
To test whether localization of dvl is developmentally regulated, dissociated cells from E15 kidneys, a developmental stage when most of the embryonic kidney mesenchyme has received the inducing signal emanating from the branching ureteric bud tips (for review see Saxen 1987), were examined by immunofluorescence microscopy. In dissociated kidney cell cultures derived from E15 kidneys, dvl was prominently present at the ends of actin fibers (Fig. 6, D–F), but was absent from the remainder of the actin fiber; note that in E12 cells, dvl is present along the length of the actin fibers (Fig. 6, A–C). We were unable to detect complexed dvl and actin in E16 kidney protein homogenates by immunoprecipitation (Torres, M.A., unpublished results). The timing of the transition from almost complete to partial colocalization of dvl and actin (Fig. 6, compare A–C with D–F) correlates with the induction of epithelial cell fate in the metanephric mesenchyme by the ureteric bud.
In addition to the more restricted colocalization of dvl with the ends of actin fibers in E15 cells, we observed other changes in dvl distribution when cells were fixed differently. Fixation with glutaraldehyde instead of methanol, and immunofluorescence staining using multiple dvl antibodies specific to either the NH2 and COOH terminus, revealed that in Wnt1-treated E12 mesenchymal cells, dvl accumulates in and around the nucleus (Fig. 6, compare G with H and I; Table ). The presence of dvl in the nuclei was confirmed using Hoechst stain to visualize nucleic acids. As expected, nuclear dvl was also observed in glutaraldehyde-fixed E12 kidneys cultured ex vivo on collagen-coated coverslips for 3 d (Fig. 6, J–L), since kidney mesenchyme is exposed to endogenous Wnt signaling during tubule induction. In intact E12 kidneys cultured ex vivo, nuclei were counterstained with the cell permeant nucleic acid stain SytoRed and examined by confocal microscopy (Fig. 6J and Fig. K, rhodamine).
Table 2.
Wnt1 Treatment Promotes Nuclear and Perinuclear Accumulation of Endogenous dishevelled
| Treatment | Percent lownuclear Dvl | Percent highnuclear Dvl | Cellscounted |
|---|---|---|---|
| 3T3/anti–Dvl-1,-2 | 88 | 12 | 58 |
| Wnt1/anti–Dvl-1,-2 | 25 | 75 | 52 |
| 3T3/anti–Dvl-2 | 81 | 19 | 32 |
| Wnt1/anti–Dvl-2 | 88 | 71 | 69 |
E12 metanephric cells were cocultured with control or Wnt1-expressing cells as previously described. Overlap of dvl immunofluorescence with the signal from the nucleic acid Hoechst stain confirmed the localization of dvl to the nucleus after Wnt1 treatment. Nuclear dvl levels were defined as low for background staining (blue in pseudocolor) or high for signals above background staining (yellow, red, or white in pseudocolor). Wnt1 treatment increases the number of kidney cells with high levels of nuclear and perinuclear dishevelled between 370 and 625%. The two experiments shown above were randomly selected as samples for quantitation. These results were replicated in two additional experiments.
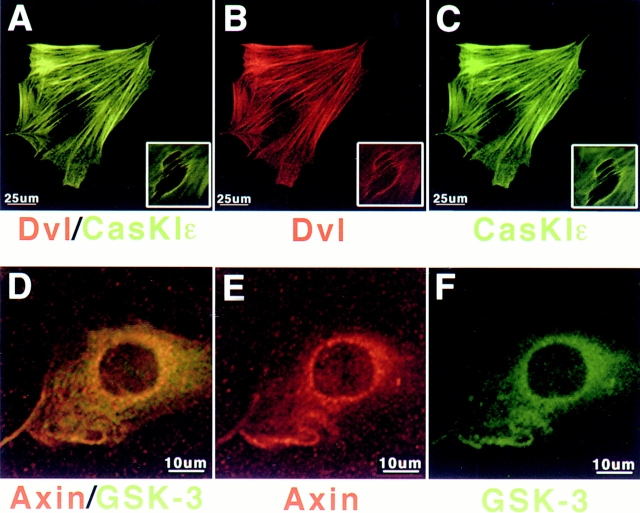
Casein Kinase Iε Colocalizes with dishevelled along Actin Stress Fibers
Additional downstream components of the Wnt/β-catenin pathway were localized in dissociated metanephric cells during embryonic kidney development to examine whether they colocalized with dvl. Endogenous β-catenin was detected primarily at sites of cell–cell contact (Torres, M.A., unpublished data), which is consistent with its function at adherens junctions (for review see Aberle et al. 1996). Axin (Fig. 7D and Fig. E, rhodamine) and GSK-3 (Fig. 7D and Fig. F, FITC) accumulated primarily at the nuclear envelope and in reticular structures surrounding the nucleus, with GSK-3 also being present diffusely in the cytoplasm. Endogenous dvl and casein kinase Iε colocalized along the length of actin fibers (Fig. 7, A–C) as well as in the perinuclear region (Fig. 7, A–C, inserts). These data show that a subset of the Wnt signal transduction machinery, dvl and casein kinase Iε, localize to the actin cytoskeleton in the absence of other downstream components, whereas the machinery to transduce canonical Wnt/β-catenin signals is located in the perinuclear region of the cell.
Figure 7.
Endogenous casein kinase Iε, dishevelled, GSK-3, and axin are present in the perinuclear region of embryonic kidney cells. E12 kidney cells were cultured for 3 d on collagen-coated coverslips and fixed in 100% cold methanol. (A–C) Endogenous casein kinase Iε colocalizes with dishevelled along actin stress fibers and around the nucleus (insets taken 4 μm above section detecting actin fibers). (D–F) Endogenous axin and GSK-3 are present perinuclearly and in reticular structures radiating from the perinuclear region. In addition, GSK-3 is present diffusely in the cytoplasm.
Discussion
A Putative Branch of Wnt Signaling Pathway Regulates the Actin Cytoskeleton
There is increasing evidence that there are multiple branch points within Wnt signaling pathways. For example, in addition to their roles in the canonical Wnt pathway, frizzled receptors and dvl are part of the planar cell polarity (PCP) pathway in developing epithelial tissues in Drosophila, whereas proteins further downstream of PCP signaling are not shared with the canonical Wnt pathway (Axelrod et al. 1998; Boutros et al. 1998; for review see Boutros and Mlodzik 1999). Within the Wnt/β-catenin signaling pathway, not all cellular effects are dependent on transcription. In particular, modulation of cell shape and cell adhesion appears to be mediated by a transcription-independent branch of the Wnt/β-catenin pathway. During endoderm specification in C. elegans, upstream components including GSK-3, but excluding β-catenin and LEF/TCF transcription factors, are required for correct mitotic spindle orientation in the EMS blastomere (Schlessinger et al. 1999), while the entire pathway is required to alter gene expression (for review see Thorpe et al. 2000). Similarly, in Drosophila mechanosensory bristle precursor cells, frizzled and dvl are required for correct mitotic spindle orientation (Gho and Schweisguth 1998). In Xenopus embryos, Wnt-1 and β-catenin increase gap junctional permeability independently of their roles in regulating gene expression and dorsal cell fate specification (Olson et al. 1991; Guger and Gumbiner 1995).
We propose that during nephrogenesis, a branch of the canonical Wnt/β-catenin signaling pathway alters cell morphology by targeting the actin cytoskeleton via dvl and casein kinase Iε, which strongly colocalize with actin fibers. Furthermore, we suggest that the other, canonical branch of the Wnt/β-catenin pathway is transduced in the perinuclear region of metanephric cells, because this is where the majority of Wnt/β-catenin signaling components overlap. In our experiments, Wnt1 signaling resulted in a reduction in actin fiber length and number in metanephric mesenchymal cells. We do not rule out the possibility that Wnt/Ca2+ or PCP signaling also targets the cytoskeleton via actin-associated dvl, although the downstream PCP component c-jun NH2-terminal kinase (Boutros et al. 1998) did not colocalize with dvl or actin (Torres, M.A., unpublished data). The Drosophila adenomatous polyposis coli 2 protein, which is related to the APC present in the destruction box, has been localized to the actin cytoskeleton, but it is unclear if this protein functions downstream of the Wnt signaling pathway (McCartney et al. 1999; Yu and Bienz 1999; Yu et al. 1999). At this time, we are unable to test whether the effects of Wnt signaling on the actin cytoskeleton are transcription-independent because of the lack of soluble Wnt1 to perform this experiment.
Wnt Signaling in Kidney Tubulogenesis
We found that Wnt1 stimulation of dissociated embryonic kidney cells results in the reorganization of the actin cytoskeleton, increased cell spreading, and the appearance of multiple nuclei within single metanephric cells (see below). These morphological changes are consistent with the program of epithelial differentiation observed in the embryonic kidney, which involves the formation of cell aggregates and cell proliferation (for review see Saxen 1987). The ability of these dissociated mesenchymal cells to respond with the appropriate differentiation program is supported by the fact that repelleted, dissociated mesenchymal cell cultures are capable of differentiating into tubular epithelium when cocultured with inducing ureteric bud cells (Torres, M.A., and W.J. Nelson, manuscript in preparation). It is possible that Wnt signaling mediates changes in cell shape during cell aggregation by modulating the actin cytoskeleton in a dvl- and/or casein kinase Iε–dependent manner. However, additional experiments are required to test whether dvl regulates the actin cytoskeleton directly.
In addition, we propose that Wnt signaling plays a role in increasing the apposition of aggregating cells by increasing cadherin-mediated cell adhesion (Bradley et al. 1993; Hinck et al. 1994), as well as by modulating integrin-mediated cell–substratum adhesion via dvl interacting with paxillin and/or ILK at focal adhesions. We have found that commercially available, dvl-specific polyclonal antibodies used to study the localization of dvl to the actin cytoskeleton do not detect dvl at focal adhesions in parallel experiments (Torres, M.A., unpublished results). Therefore, we conclude that the polyclonal antibody we used detects a specific epitope on dvl that is exposed at focal adhesions (Miller et al. 1999b; Willert et al. 1999). Presently, we are investigating the molecular nature of the association of dvl with actin, paxillin, and ILK to understand the mechanism by which dvl might transduce Wnt signals to modulate the actin cytoskeleton and cell adhesion.
Coculture of metanephric mesenchymal cells with Wnt1-expressing fibroblasts resulted in an increased incidence of multinucleated metanephric cells. Previous studies have reported that activation of Wnt signaling at multiple points in the pathway can promote cell proliferation (for review see Miller et al. 1999a). Therefore, we propose that in our experiments, the presence of multiple nuclei reflects a stimulation of cell division. The lack of cytokinesis that presumably is responsible for the multinucleated cells in cultured metanephric cells likely is due to the disruption of the actin cytoskeleton, combined with the extensive cell spreading that occurs in our cell plating protocol and in vitro culture conditions. In addition, the ability of dvl to form a complex with ILK, a putative oncogene (Novak et al. 1998; Wu et al. 1998), suggests that other signaling pathways might stimulate cell proliferation at least, in part, by activating Wnt signaling via dvl, or by synergizing with the Wnt signaling pathway.
Subcellular Localization of Wnt/β-Catenin Pathway Signaling Proteins
In metanephric mesenchyme, endogenous dvl, casein kinase Iε, GSK-3, and axin are all present in the perinuclear region. Casein kinase Iε functions in conjunction with dvl to transduce canonical Wnt/β-catenin signals by inhibiting GSK-3 activity (Peters et al. 1999; Sakanaka et al. 1999). GSK-3 and axin promote β-catenin degradation via the proteosome pathway in the absence of Wnt signaling (for review see Miller et al. 1999a). The colocalization of proteins involved in Wnt-mediated β-catenin stabilization in close proximity to nuclear pores may be significant, as β-catenin is able to traverse independently of the importin/karyopherin nuclear transport system (Fagotto et al. 1998). Interestingly, we find that perinuclear dvl levels also increase with Wnt1 treatment, suggesting that dvl itself might be stabilized by the canonical Wnt/β-catenin signaling pathway in the perinuclear region, followed by translocation of dvl into the nucleus, as observed in our studies. Translocation of dvl into the nucleus raises the possibility that some transduction of Wnt signals upstream of β-catenin might occur within the nucleus itself. Further studies are required to determine whether additional components of the Wnt signal transduction pathway are capable of accumulating in the nucleus as well.
Previously, ectopic green fluorescent protein–tagged dvl was observed to translocate from vesicular structures to the plasma membrane in response to planar cell polarity signals, whereas Wnt/β-catenin signaling did not alter its subcellular localization (Axelrod et al. 1998). Fagotta et al. (1999) also reported that over-expressed, ectopic HA-tagged dvl was present in cytoplasmic aggregates and at the plasma membrane when myc-tagged axin was overexpressed in the same cells. We found that accumulation of endogenous dvl in the nucleus and perinuclear region after Wnt1 treatment was detectable only after fixing cells in glutaraldehyde. As previous studies used ectopically expressed dvl-GFP and cells were fixed using different protocols, we suggest that perinuclear dvl was not observed in those cases because perinuclear dvl was not preserved, or because ectopic dvl does not accumulate in the same locations as the endogenous protein. Nuclear localization of endogenous cytoskeleton-associated proteins ZO-1 (Gottardi et al. 1999), plakophilin-2a and -2b (Merthens et al. 1996), and -3 (Bonne et al. 1999), and profilin (Mayboroda et al. 1997) also is dependent on extraction and fixation conditions. Some of the perinuclear dvl detected in our experiments appeared to be associated with the ER (Torres, M.A., unpublished results), which is consistent with localization of dvl to membrane-rich structures (for review see Miller et al. 1999a,Miller et al. 1999b).
In summary, we propose that during mouse embryonic kidney development, Wnt signals mediate changes in cell shape during mesenchymal to epithelial transformation by targeting the actin cytoskeleton via dvl and casein kinase Iε, but not the rest of the canonical Wnt/β-catenin signaling pathway. In addition, we hypothesize that Wnts mediate epithelial cell fate specification and cell proliferation by transducing signals in the perinuclear and nuclear regions of embryonic kidney cells. The novel component to this second model is that dvl accumulates in and around the nucleus, suggesting that even the upstream component most proximal to the frizzled receptor might transduce at least part of the Wnt signal within the nucleus. Future work is required to determine whether other Wnt signaling components are capable of entering the nucleus individually or as a complex, and what role perinuclear localization of Wnt signaling proteins plays in the transduction of the Wnt signal.
Acknowledgments
We thank members of the Nelson lab for helpful comments during the course of this work.
M.A. Torres was supported by a Minority Postdoctoral Fellowship from the National Science Foundation, and this study was supported by a National Institutes of Health grant to W.J. Nelson.
Footnotes
Abbreviations used in this paper: APC, adenomatous polyposis coli; dvl, dishevelled; GSK-3, glycogen synthase kinase-3; ILK, integrin-linked kinase; PCP, planar cell polarity; ZO-1, zonula occludens-1.
References
- Aberle H., Schwartz H., Kemler R. Cadherin-catenin complexprotein interactions and their implications for cadherin function. J. Cell. Biochem. 1996;61:514–523. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C514::AID-JCB4%3E3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Allison D.W., Gelfand V.I., Spector I., Craig A.M. Role of actin in anchoring postsynaptic receptors in cultured hippocampal neuronsdifferential attachment of NMDA versus AMPA receptors. J. Neurosci. 1998;18:2423–2436. doi: 10.1523/JNEUROSCI.18-07-02423.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod J.D., Miller J.R., Shulman J.M., Moon R.T., Perrimon N. Differential recruitment of dishevelled provides signaling specificity in the planar cell polarity and wingless signaling pathways. Genes Dev. 1998;12:2610–2622. doi: 10.1101/gad.12.16.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N., Morin P.J., Clevers H. The yin-yang of TCF-β-catenin signaling. Adv. Cancer Res. 2000;77:1–24. doi: 10.1016/s0065-230x(08)60783-6. [DOI] [PubMed] [Google Scholar]
- Bonne S., Van Hengel J., Nollet F., Kools P., Van Roy F. Plakophilin-3, a novel armadillo-like protein present in nuclei and desmosomes of epithelial cells. J. Cell Sci. 1999;112:2265–2276. doi: 10.1242/jcs.112.14.2265. [DOI] [PubMed] [Google Scholar]
- Boutros M., Mlodzik M. Dishevelledat the crossroads of divergent intracellular signaling pathways. Mech. Dev. 1999;83:27–37. doi: 10.1016/s0925-4773(99)00046-5. [DOI] [PubMed] [Google Scholar]
- Boutros M., Paricio N., Strutt D.I., Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94:109–118. doi: 10.1016/s0092-8674(00)81226-x. [DOI] [PubMed] [Google Scholar]
- Bradley R.S., Cowin P., Brown A.M. Expression of Wnt-1 in PC12 cells results in modulation of plakoglobin and E-cadherin and increased cellular adhesion. J. Cell Biol. 1993;123:1857–1865. doi: 10.1083/jcb.123.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadigan K.M., Nusse R. Wnt signalinga common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- Fagotto F., Gluck U., Gumbiner B.M. Nuclear localization signal independent- and importin/karyopherin-independent nuclear import of β-catenin. Curr. Biol. 1998;8:181–190. doi: 10.1016/s0960-9822(98)70082-x. [DOI] [PubMed] [Google Scholar]
- Fagotto F., Jho E.-H., Zeng L., Kurth T., Joos T., Kaufman C., Constantini F. Domains of axin involved in protein–protein interactions, Wnt pathway inhibition, and intracellular localization. J. Cell Biol. 1999;145:741–756. doi: 10.1083/jcb.145.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gho M., Schweisguth F. Frizzled signaling controls orientation of asymmetric sense organ precursor cell divisions in Drosophila . Nature. 1998;393:178–181. doi: 10.1038/30265. [DOI] [PubMed] [Google Scholar]
- Gottardi C.J., Arpin M., Fanning A.S., Louvard D. The junction-associated protein, zonula occludens-1, localizes to the nucleus before the maturation and during the remodeling of cell contacts. Proc. Natl. Acad. Sci. USA. 1999;93:10779–10784. doi: 10.1073/pnas.93.20.10779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guger K.A., Gumbiner B.M. Beta-catenin has Wnt-like activity and mimics the Nieuwkoop signaling center in Xenopus dorsal-ventral patterning. Dev. Biol. 1995;172:115–125. doi: 10.1006/dbio.1995.0009. [DOI] [PubMed] [Google Scholar]
- Herzlinger D., Qiao J., Cohen D., Ramakrishna N., Brown A.M.C. Induction of kidney epithelial morphogenesis by cells expressing Wnt-1. Dev. Biol. 1994;166:815–818. doi: 10.1006/dbio.1994.1360. [DOI] [PubMed] [Google Scholar]
- Hinck L., Nelson W.J., Papkoff J. Wnt-1 modulates cell–cell adhesion in mammalian cells by stabilizing β-catenin binding to the cell adhesion protein cadherin. J. Cell Biol. 1994;124:729–741. doi: 10.1083/jcb.124.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingensmith J., Yang Y., Axelrod J.D., Beier D.R., Perrimon N., Sussman D.J. Conservation of dishevelled structure and function between flies and miceisolation and characterization of Dvl-2 . Mech. Dev. 1996;58:15–26. doi: 10.1016/s0925-4773(96)00549-7. [DOI] [PubMed] [Google Scholar]
- Lee J.S., Ishimoto A., Yanagawa S. Characterization of mouse dishevelled (Dvl) proteins in the Wnt/Wingless signaling pathway. J. Biol. Chem. 1999;274:21464–21470. doi: 10.1074/jbc.274.30.21464. [DOI] [PubMed] [Google Scholar]
- Mayboroda O., Schluter K., Jockusch B.M. Differential colocalization of profiliin with microfilaments in PtK2 cells. Cell. Motil. Cytoskel. 1997;37:166–177. doi: 10.1002/(SICI)1097-0169(1997)37:2<166::AID-CM9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- McCartney B., Dierick H.A., Kirkpatrick C., Moline M.M., Baas A., Peifer M., Bejsovec A. Drosophila APC2 is a cytoskeletally associated protein that regulates Wingless signaling in the embryonic epidermis. J. Cell Biol. 1999;146:1303–1318. doi: 10.1083/jcb.146.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merthens C., Kuhn C., Franke W.W. Plakophilins 2a and 2bconstituitive proteins of dual location in the karyoplasm and the desmosomal plaque. J. Cell Biol. 1996;135:1009–1025. doi: 10.1083/jcb.135.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.R., Hocking A.M., Brown J.D., Moon R.T. Mechanism and function of signal transduction by the Wnt/β-catenin and Wnt/Ca2+ pathways Oncogene. 18 1999. 7860 7872a [DOI] [PubMed] [Google Scholar]
- Miller J.R., Rowning B.A., Larabell C.A., Yang-Snyder J.A., Bates R.L., Moon R.T. Establishment of the dorsal–ventral axis in Xenopus embryos coincides with the dorsal enrichment of dishevelled that is dependent on cortical rotation J. Cell Biol 146 1999. 427 437b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori-Koyusue Y., Nobuyuki S., Tsukita S. Adenomatous polyposis coli (APC) protein moves along microtubules and concentrates at their growing ends in epithelial cells. J. Cell Biol. 2000;148:505–518. doi: 10.1083/jcb.148.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon R.T., Brown J.D., Torres M.A. Wnts modulate cell fate and behavior during vertebrate development. Trends Genet. 1997;13:157–162. doi: 10.1016/s0168-9525(97)01093-7. [DOI] [PubMed] [Google Scholar]
- Munsterberg A.E., Kitajewski J., Bumcrot D.A., McMahon A.P., Lassar A.B. Combinatorial signaling by Sonic hedgehog and Wnt family members induces myogenic bHLH gene expression in the somite. Genes Dev. 1995;9:2911–2922. doi: 10.1101/gad.9.23.2911. [DOI] [PubMed] [Google Scholar]
- Nathke I.S., Adams C.L., Polakis P., Sellin J.H., Nelson W.J. The adenomatous polyposis coli tumor suppressor protein localizes to plasma membrane sites involved in active cell migration. J. Cell Biol. 1996;134:165–179. doi: 10.1083/jcb.134.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak A., Hsu S.C., Leung-Hagesteijn C., Radeva G., Papkoff J., Montesano R., Roskelley C., Grosschedl R., Dedhar S. Cell adhesion and the integrin-linked kinase regulate the LEF-1 and beta-catenin signaling pathways. Proc. Natl. Acad. Sci. USA. 1998;95:4374–4379. doi: 10.1073/pnas.95.8.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson D.J., Christian J.L., Moon R.T. Effect of Wnt-1 and related proteins on gap junctional communication in Xenopus embryos. Science. 1991;252:1173–1176. doi: 10.1126/science.252.5009.1173. [DOI] [PubMed] [Google Scholar]
- Peters J.M., McKay R.M., McKay J.P., Graff J.M. Casein kinase I transduces Wnt signals. Nature. 1999;401:345–350. doi: 10.1038/43830. [DOI] [PubMed] [Google Scholar]
- Piepenhagen P.A., Peters L.L., Lux S.E., Nelson W.J. Differential expression of Na+-K+-ATPase, ankyrin, fodrin, and E-cadherin along the kidney nephron. Am. J. Physiol. 1995;269:C1417–C1432. doi: 10.1152/ajpcell.1995.269.6.C1417. [DOI] [PubMed] [Google Scholar]
- Sakanaka C., Leong P., Xu L., Harrison S.D., Williams L.T. Casein kinase I epsilon in the Wnt pathwayregulation of beta-catenin function. Proc. Natl. Acad. Sci. USA. 1999;96:12548–12552. doi: 10.1073/pnas.96.22.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxen, L. 1987. Organogenesis of the kidney. In Dev. Cell Biol. Series. P.W. Barlow, P.B. Green, and C.C. Wylie, editors. Cambridge University Press.
- Schlessinger A., Shelton C.A., Moloof J.N., Meneghini M., Bowerman B. Wnt pathway components orient a mitotic spindle in the early C. elegans embryo without requiring gene transcription in the responding cell. Genes Dev. 1999;13:2028–2038. doi: 10.1101/gad.13.15.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark K., Vainio S., Vassileva G., McMahon A.P. Epithelial transformation of metanephric mesenchyme in the developing kidney is regulated by Wnt-4 . Nature. 1994;372:679–683. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- Sussman D.J., Klingensmith J., Salinas P., Adams P.S., Nusse R., Perrimon N. Isolation and characterization of a mouse homolog of the Drosophila segment polarity gene dishevelled . Dev. Biol. 1994;166:73–86. doi: 10.1006/dbio.1994.1297. [DOI] [PubMed] [Google Scholar]
- Thorpe C.J., Schlesinger A., Bowerman B. Wnt signalling in Caenorhabditis elegansregulating repressors and polarizing the cytoskeleton. Trends Cell Biol. 2000;10:10–17. doi: 10.1016/s0962-8924(99)01672-4. [DOI] [PubMed] [Google Scholar]
- Torres M.A., Yang-Snyder J.A., Purcell S.M., DeMarais A.A., McGrew L.L., Moon R.T. Activities of the Wnt-1 class of secreted signaling factors are antagonized by the Wnt-5A class and by a dominant negative cadherin in early Xenopus development. J. Cell Biol. 1996;133:1123–1137. doi: 10.1083/jcb.133.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungar A.R., Kelly G.M., Moon R.T. Wnt4 affects morphogenesis when misexpressed in the zebrafish embryo. Mech. Dev. 1995;52:153–164. doi: 10.1016/0925-4773(95)00386-f. [DOI] [PubMed] [Google Scholar]
- Vestweber D., Kemler R. Rabbit antiserum against a purified surface glycoprotein decompacts mouse preimplantation embryos and reacts with specific adult tissues. Exp. Cell Res. 1984;152:169–178. doi: 10.1016/0014-4827(84)90241-6. [DOI] [PubMed] [Google Scholar]
- Willert K., Shibamoto S., Nusse R. Wnt-induced dephosphorylation of axin releases β-catenin from the axin complex. Genes Dev. 1999;13:1768–1773. doi: 10.1101/gad.13.14.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Keightley S.Y., Leung-Hagesteijn C., Radeva G., Coppolino M., Goicoechea S., McDonald J.A., Dedhar S. Integrin-linked protein kinase regulates fibronectin matrix assembly, E-cadherin expression, and tumorigenicity. J. Biol. Chem. 1998;273:528–536. doi: 10.1074/jbc.273.1.528. [DOI] [PubMed] [Google Scholar]
- Yost C., Torres M., Miller J.M., Huang E., Kimelman D., Moon R.T. The axis-inducing activity, stability, and subcellular distribution of β-catenin is regulated in Xenopus embryos by glycogen synthase kinase-3. Genes Dev. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- Yu X., Bienz M. Ubiquitous expression of a Drosophila adenomatous polyposis coli homolog and its localization in cortical actin caps. Mech. Dev. 1999;84:69–73. doi: 10.1016/s0925-4773(99)00072-6. [DOI] [PubMed] [Google Scholar]
- Yu X., Waltzer L., Bienz M. APC homologue associated with adhesive zones of epithelial cells. Nat. Cell Biol. 1999;1:144–151. doi: 10.1038/11064. [DOI] [PubMed] [Google Scholar]