Abstract
CAP23 is a major cortical cytoskeleton–associated and calmodulin binding protein that is widely and abundantly expressed during development, maintained in selected brain structures in the adult, and reinduced during nerve regeneration. Overexpression of CAP23 in adult neurons of transgenic mice promotes nerve sprouting, but the role of this protein in process outgrowth was not clear. Here, we show that CAP23 is functionally related to GAP43, and plays a critical role to regulate nerve sprouting and the actin cytoskeleton. Knockout mice lacking CAP23 exhibited a pronounced and complex phenotype, including a defect to produce stimulus-induced nerve sprouting at the adult neuromuscular junction. This sprouting deficit was rescued by transgenic overexpression of either CAP23 or GAP43 in adult motoneurons. Knockin mice expressing GAP43 instead of CAP23 were essentially normal, indicating that, although these proteins do not share homologous sequences, GAP43 can functionally substitute for CAP23 in vivo. Cultured sensory neurons lacking CAP23 exhibited striking alterations in neurite outgrowth that were phenocopied by low doses of cytochalasin D. A detailed analysis of such cultures revealed common and unique functions of CAP23 and GAP43 on the actin cytoskeleton and neurite outgrowth. The results provide compelling experimental evidence for the notion that CAP23 and GAP43 are functionally related intrinsic determinants of anatomical plasticity, and suggest that these proteins function by locally promoting subplasmalemmal actin cytoskeleton accumulation.
Keywords: neurite outgrowth, neuromuscular junction, synaptic plasticity, actin dynamics, nerve sprouting
Introduction
The plasticity of the nervous system in the adult involves alterations in the functional properties of preexisting circuitry as well as physical rearrangements of the synaptic circuits. In the latter case, synapses are formed or dismantled, and nerves sprout or retract. Such anatomical changes are thought to provide a basis for persisting alterations in circuit function, which is important for learning, memory, and repair. The molecular mechanisms that regulate anatomical plasticity in the adult are poorly understood, but important constraints that shape the plasticity of neural systems derive from specific intrinsic properties of their neuronal components (Bisby and Tetzlaff 1992; Caroni 1997, Caroni 1999; Sengpiel et al. 1998; Antonini et al. 1999; Bravin et al. 1999). Consequently, identifying relevant intrinsic neuronal components and pathways that control growth in neurons should provide an entry point to dissect the mechanisms that control anatomical plasticity.
The neural growth–associated protein GAP43 has properties of a general intrinsic determinant of anatomical plasticity. First, its expression in neurons correlates closely with axonal elongation, synaptogenesis, and nerve sprouting during development and in the adult (Skene 1989; Bisby and Tetzlaff 1992). Second, overexpression of GAP43 promotes nerve sprouting in transgenic mice and process outgrowth in cultured cells (Yankner et al. 1990; Aigner et al., 1995). However, gene inactivation studies in mice did not reveal defects that would directly support the notion that GAP43 plays a general role in promoting axon elongation or nerve sprouting. Thus, although GAP43−/− mice exhibited high postnatal mortality, defects in the formation of axonal projections were restricted to growth cone guidance at specific choice points, and to the formation of specific topographic maps in the target region (Strittmatter et al. 1995; Sretawan and Kruger 1998; Maier et al. 1999; Zhu and Julien 1999). These results suggested that GAP43 is involved in translating signals important for growth cone guidance. However, mainly because of the fact that the molecular mechanisms through which GAP43 affects growth cone activity have remained unclear, the relation between this critical role of GAP43 and a hypothetical, more general function in promoting neurite outgrowth has been difficult to assess.
GAP43 may be related to the protein kinase C (PKC) substrates neurogranin, MARCKS, MacMARCKS, and CAP23 (Aderem 1995; Benowitz and Routtenberg 1997; Wiederkehr et al. 1997). GAP43 and neurogranin are expressed exclusively in the nervous system, whereas MARCKS and MacMARCKS are also expressed at high levels in many types of differentiating and motile nonneural cells. CAP23 is a neural protein, except for a widespread expression pattern during early embryonic development (Widmer and Caroni 1990). Although MARCKS, GAP43, and CAP23 have no sequence homologies, they share a number of characteristic properties (see Fig. 1 A). These include the following: (1) regulated, abundant expression related to contact-mediated differentiation, cell-surface activity, motility, and process outgrowth; (2) membrane association mediated by palmitoylation or myristoylation; (3) highly hydrophilic, with markedly acidic isoelectric points, rodlike structures, and a unique amino acid composition; (4) the presence of one unique stretch of 10–28 basic and hydrophobic residues, the effector domain (ED); this domain binds acidic phospholipids, calmodulin (CaM), and actin filaments in a mutually exclusive manner, and contains the PKC phosphorylation site(s); (5) colocalization at characteristic patches at the cell membrane; and (6) induction of dynamic actin structures at the surface of transfected cells (Aderem 1995; Benowitz and Routtenberg 1997; Wiederkehr et al. 1997). Like GAP43, expression of CAP23 is enhanced when axons elongate, and its overexpression in adult neurons of transgenic mice promotes nerve sprouting (Caroni et al. 1997). These observations have raised the possibility that CAP23 and GAP43 may be closely related functionally, possibly acting through a common mechanism to mediate morphogenic processes, including neurite outgrowth.
Figure 1.
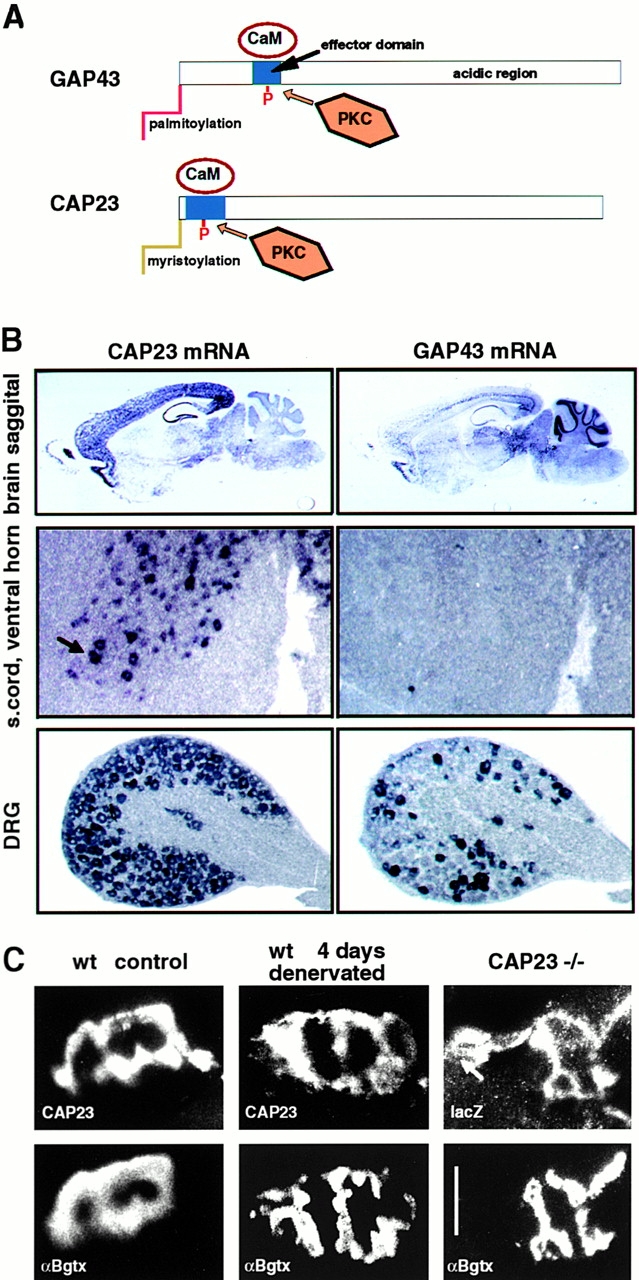
Expression patterns of mouse CAP23 and GAP43 in the adult. (A) Schematic representation of the structures of GAP43 and CAP23. The NH2-terminal with the acylation domains is to the left; (blue box) basic ED with PKC phosphorylation sites; and (white rectangles) predominantly acidic and highly hydrophilic regions with similar amino acid compositions. The relative dimensions of the proteins and the EDs are in scale. (B) In situ hybridization. Note the differential expression of CAP43 and GAP43 in adult brain regions, expression of CAP23 in large ventral horn neurons (arrow), and expression of GAP43 in DRG neuron subgroups. (C) CAP23 immunoreactivity at the adult nmj. Double labeling for postsynaptic acetylcholine receptors (bottom, αBgtx) and CAP23 or reporter gene lacZ (top). Note the persistence of CAP23 signal at denervated (i.e., nerve-free) nmj, and synaptic, plus extrasynaptic (arrow) accumulation of reporter gene signal in homozygous CAP23−/− mice. Bar, 50 μm.
Here, we tested the notion that GAP43 and CAP23 may be related functionally in vivo, and that they may play a critical role for nerve sprouting in the adult. CAP23-deficient mice exhibited high postnatal mortality, and pronounced abnormalities at the neuromuscular junction (nmj), including a nearly complete absence of stimulus-induced nerve sprouting. The nerve sprouting deficit in CAP23−/− mice was rescued by crossing into a transgenic background that overexpresses either CAP23 or GAP43 in adult motoneurons. In striking contrast, CAP23gap43/gap43 knockin mice expressing no CAP23, but instead GAP43, were essentially normal, indicating that GAP43 can functionally substitute for CAP23 in vivo. In a wild-type background, subtypes of dorsal root ganglion (DRG) neurons expressed different combinations of CAP23, GAP43, and MARCKS. A detailed analysis of DRG cultures derived from gene deletion and gene replacement mouse mutants revealed common and unique properties in the effects of CAP23 and GAP43 on the actin cytoskeleton and neurite outgrowth. The results establish CAP23 as a critical intrinsic component for stimulus-induced nerve sprouting at the adult nmj, and provide compelling experimental evidence for the notion that CAP23 and GAP43 are functionally related proteins that regulate subplasmalemmal actin dynamics and process outgrowth.
Materials and Methods
Targeting of Mouse CAP23 Gene
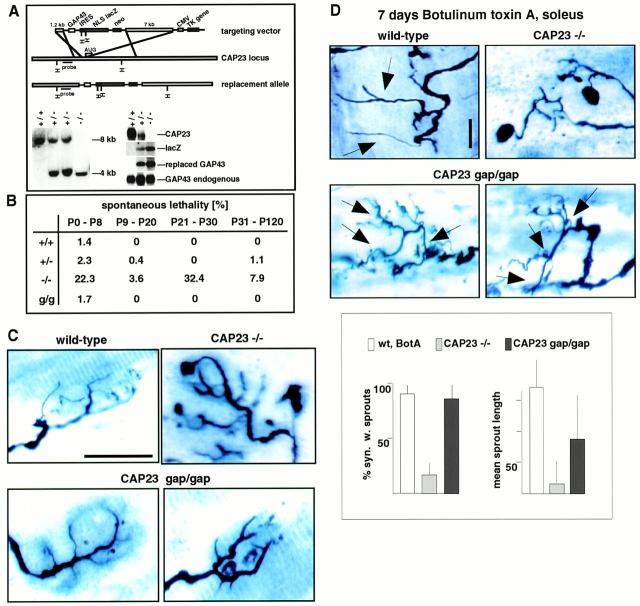
A genomic clone for the generation of a targeting construct was derived from a mouse 129/Sv BAC library (Genomsys), using mouse CAP23 cDNA as a probe. The targeting strategies are outlined in Fig. 2 A (knockout mice) and Fig. 4 A (knockin mice). NLSlacZ corresponds to lacZ cDNA with a nuclear targeting sequence. IRES corresponds to an internal ribosomal entry signal sequence for bicistronic mRNA production. Embryonic stem cells and mice were screened with genomic Southern blots, using probes corresponding to the regions indicated in Fig. 2 A and Fig. 4 A. Northern blots were carried out with total RNA samples from adult mouse brain according to standard procedures.
Figure 2.
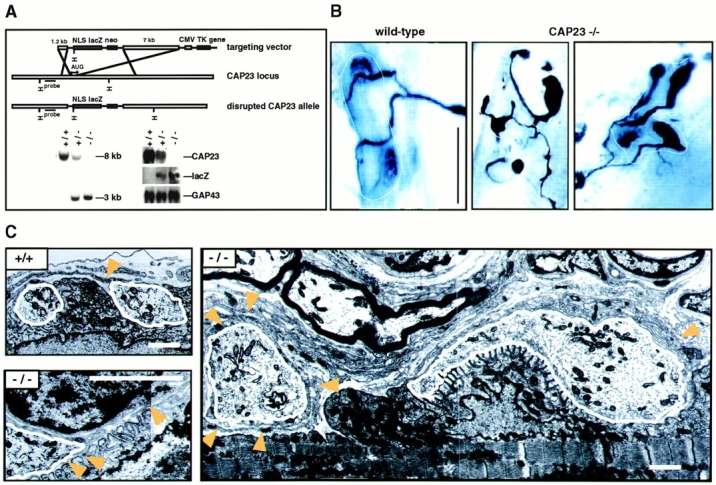
nmj phenotype of CAP23−/− mice. (A) Gene disruption strategy (top), and genomic Southern (left) and Northern (right; adult brain mRNA) blots of wild-type, CAP23+/−, and CAP23−/− mice. In the mutant mice, GAP43 expression was not noticeably affected (immunocytochemistry and in situ hybridization data not shown). (B) Altered arrangement of the presynaptic nerve at the nmj of CAP23-deficient mice. Combined silver esterase stains of fast-type medial gastrocnemius nmjs (2 mo); each photograph shows one nmj (black, nerve; blue, esterase reaction product). The dotted lines mark the synaptic regions. Note the highly irregular shapes of the nerve terminal branches in the mutants, with swellings and bloblike endings. More than 90% of the nmjs exhibited obvious structural abnormalities in CAP23−/− mice. (C) Ultrastructural appearance of adult medial gastrocnemius nmjs in wild-type and CAP23−/− mice. Muscle is down and nerve is up. The nerve terminal branches are outlined in white. Arrowheads point to tSC processes. Note the hypertrophy of tSC processes, and nerve blob (left end of the photograph, white outline) surrounded by tSC processes in the photograph on the right. The photograph on the lower left shows postsynaptic junctional folds unopposed by a nerve profile (local denervation), and tSC (nucleus on the upper part of the photograph) processes insinuating into the synaptic cleft on the left. Bars: (B) 50 μm; (C) 1.6 μm.
Figure 4.
GAP43 can functionally substitute for CAP23. (A) Generation of CAP23gap43/gap43 knockin mice. (top) Targeting construct for gene replacement, (bottom) genomic Southern blot (left), and Northern blot of adult brain mRNA. The signs (+) and (−) refer to a wild-type or mutated CAP23 allele. (B) Enhanced mortality of CAP23+/− and CAP23−/− mice, and its rescue by GAP43. (g/g) CAP23gap43/gap43 background. (C) Correction by GAP43 of an nmj morphology phenotype because of the absence of CAP23. Combined silver esterase stains of fast-type medial gastrocnemius nmjs at 2 mo. Note the irregular diameter and blobs at nerve terminal branches of CAP23−/− mice. In the presence of GAP43, instead of CAP23, nerve terminal branches at the nmj were slightly broader than in a wild-type background, but regular in size, and without blobs. (D) Rescue of BotA-induced nerve sprouting at the soleus nmj of CAP23gap43/gap43 mice. Combined silver esterase stains and quantitative analysis of sprouting of soleus nmj's, 7 d after BotA injection; sprouts are indicated by arrows. Sprouts in the presence of GAP43, instead of CAP23, were shorter and more branched. Bar, 50 μm.
Histology, Immunocytochemistry, and Electron Microscopy
In situ hybridization for CAP23 and GAP43 mRNA was carried out with digoxigenin-labeled cRNA probes corresponding to amino acids 1–63 of mouse CAP23, and the entire coding sequence of mouse GAP43, according to Baumeister et al. 1997. Neuromuscular synapses and their innervation patterns were visualized with a combined silver esterase reaction, as described previously (Caroni et al. 1997). Where indicated, purified botulinum toxin A (Botox, clinical grade; Allergan AG) was applied at 0.01 U/g mouse (1–3-mo old). Immunocytochemistry of cultured DRG neurons was carried out according to the protocol described in Wiederkehr et al. 1997. The following antibodies were used: rabbit antisera to COOH-terminal peptide sequences from CAP23, GAP43, and MARCKS (Wiederkehr et al. 1997; Laux et al., 2000); rabbit antiserum to rat CGRP was from Genosys Biotechnologies Inc.; mAb 34HL to rat substance P was from Anawa Trading; rabbit antiserum to parvalbumin was from SWant AG; rabbit antiserum against calbindin was a gift from M. Celio (University of Fribourg, Switzerland); FITC-choleratoxin B subunit was from Sigma Chemical Co. For immunocytochemistry of muscle tissue, mice were perfused with 4% paraformaldehyde-containing PBS, and cryostat sections were incubated with antibodies as described in Caroni et al. 1997. RITC-α-bungarotoxin and Alexa-labeled secondary antibodies were from Molecular Probes Inc. Transmission electron microscopy of mouse medial gastrocnemius muscle was according to standard procedures.
Sensory Neuron Cultures
Sensory neurons were derived from dorsal root ganglia of P0-P6 mice. Briefly, ganglia freed of surrounding membranes were digested for 20 min at 37°C in the presence of 0.05% trypsin and 0.04% collagenase H. The digestion was stopped, and neurons were dissociated by four subsequent trituration steps. After washing, neurons were plated onto poly-l-lysine/laminin (40 μg/ml)–coated glass coverslips at a density of ∼2,000 cells/cm2, in the presence of L15, with antibiotics, 10% FCS, 30 mM NaHCO3, and 50 ng/ml of either NGF or NT3 (Sigma Chemical Co.). Cells were analyzed 15 h after plating. Where indicated, cytochalasin D (Sigma Chemical Co.) was added to the culture medium 2.5 h after plating, at a final concentration of 2–20 nM. For quantitative analysis of neurite outgrowth patterns, type-identified neurons (expression pattern and morphology) from three independent cultures (total of 15 neurons [n]; 2 neurites analyzed per neuron) were scored for the following parameters: neurite length (average length of two longest neurites); blebbing (average number of blebs [swellings] per neurite length); hairs (average number of filopodial side-branches per neurite length); and branching (average number of neuritic branch points per neurite length). To obtain a measure for neurite winding (winding factor), we subdivided neurites in 10-μm segments, and determined the number of times that the neurite crossed a straight line between the beginning and the end of the 10-μm segment. Total numbers of crossing points were normalized to neurite length, and the average values for 15 neurons were determined.
Results
Distinct Expression Patterns of CAP23 and GAP43 in the Adult Nervous System, and Expression of CAP23 at the Adult Neuromuscular Junction
During mouse nervous system development, CAP23, GAP43, and MARCKS mRNAs were coexpressed at high levels in most, if not all types of neurons (not shown). GAP43 and CAP23 were downregulated with comparable kinetics that correlated with the time, when axonal arborization and synaptogenesis in the target region decline (Caroni and Becker 1992; Caroni et al. 1997; Benowitz and Routtenberg 1997; Frey, D., and P. Caroni, unpublished results). In the adult, expression of these proteins was maintained in distinct, partially overlapping sets of neurons (Fig. 1 B; see McNamara and Lenox 1997, for a detailed analysis of MARCKS and GAP43 expression patterns). First, there were significant differences in the expression patterns of CAP23 and GAP43 throughout major brain structures in the adult (Fig. 1 B). CAP23 expression was particularly high throughout the adult neocortex, where GAP43 was mainly expressed in layer V projection neurons. In contrast, CAP23 was expressed at very low levels in thalamic and brain stem structures, and expression was not detectable in the cerebellar cortex. Strong CAP23 expression was detected in the hippocampal formation including dentate gyrus granule cells, which do not express GAP43 in the adult. Second, at a higher resolution, the analysis revealed differences in the expression patterns of subsets of neurons. Thus, for example, apparently all DRG neurons expressed high levels of CAP23 mRNA (Fig. 1 B) and protein (see Fig. 6), whereas the expression of GAP43 was restricted to a subset of DRG neurons in the adult (Fig. 1 B).
Figure 6.
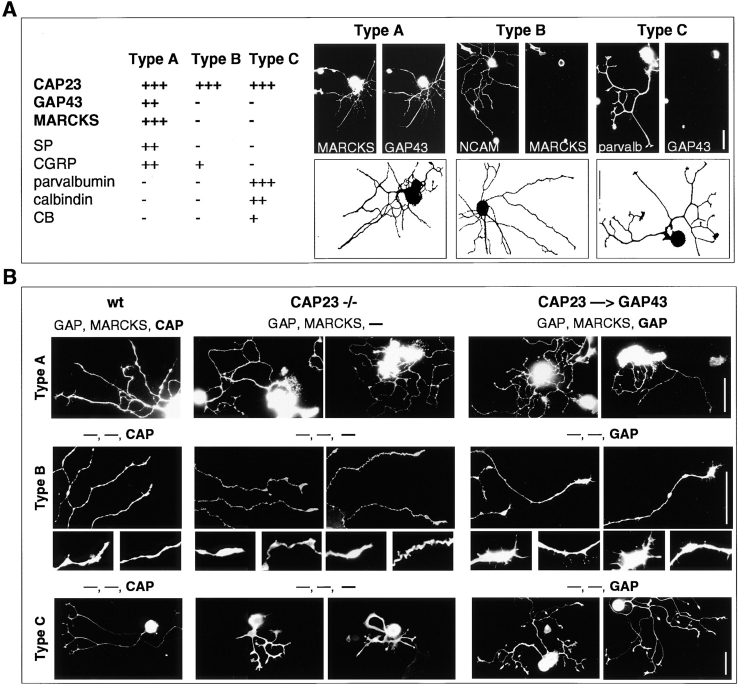
Common and unique roles of CAP23 and GAP43 in neurite outgrowth. (A) Operational definition of three subtypes of DRG neurons, based on the expression of marker antigens, and morphology. The camera lucida drawings show one representative neuron per subtype; the double labeling immunocytochemistry photographs show one further example per subtype. (B) Neurite outgrowth patterns of DRG neuron subtypes from wild-type (wt), CAP23−/−, and CAP23gap43/gap43 (CAP23→GAP43) mice (15-h cultures on 40 μg/ml laminin). The expression (or absence of expression, minus sign) of GAP43, MARCKS, and CAP23 is indicated on top of the photographs. Labeling antigens: MARCKS (type A), NCAM (type B), and parvalbumin (type C). Two details each of type B neuron photographs are also shown. Bars: 50 μm.
Because of its unique anatomical arrangement, the neuromuscular junction provides a convenient experimental system to investigate stimulus-induced synaptic sprouting in the adult (Brown 1984; Caroni 1997). The expression of GAP43 in adult, unlesioned spinal motoneurons is undetectably low (Caroni and Becker 1992), and botulinum toxin A (BotA)–induced sprouting at the adult nmj of GAP43−/− mice was not impaired (data not shown). Likewise, we could not detect MARCKS immunoreactivity at the adult nmj (not shown). In contrast, although it was significantly lower than during development, CAP23 mRNA was clearly detectable at clustered, large ventral horn neurons, presumably motoneurons, in the adult, and the corresponding protein could be detected at the adult nmj (Fig. 1 C). In addition to spinal motoneurons, CAP23 was also expressed by terminal Schwann cells (tSCs), which are the nonmyelinating glial cells that cap the nmj, but not in muscle. Thus, 4 d after sciatic nerve crush, when there is complete degeneration and removal of the lesioned distal nerve, CAP23 immunoreactivity could still be detected in a characteristic cell-associated pattern at denervated nmjs (Fig. 1 C). In addition, an antibody to the reporter gene NLS-lacZ visualized tSC immunoreactivity at nonlesioned, adult nmjs of CAP23−/− mice (Fig. 1 C).
Critical Role of Motoneuron CAP23 for Stimulus-induced Nerve Sprouting at the Adult Neuromuscular Junction
To investigate the role of CAP23 in neurite outgrowth and anatomical plasticity, we generated mice with an inactivated CAP23 allele (Fig. 2 A). The targeting construct included a nuclear lacZ gene to monitor CAP23 gene activity in the mutant mice (Fig. 2 A). CAP23+/− and CAP23−/− were born with Mendelian frequencies, but in the absence of CAP23, only ∼10% of the mice survived to adulthood. Adult CAP23−/− mice weighed 50–70% of their wild-type littermates and were distinctly hyperactive, but they exhibited no elevated mortality. The brain of CAP23−/− mice exhibited enlarged ventricles, and pronounced axonal and synaptic ultrastructural abnormalities were detected in the hippocampus and neocortex (Frey, D., L. Xu, and P. Caroni, unpublished observations). In spinal motoneurons, GAP43 and CAP23 are expressed at high levels during axonal growth and target innervation, and are downregulated to undetectable (GAP43) or low (CAP23) levels between postnatal day 8 and P15, when neuromuscular synapses initiate their final maturation (Caroni and Becker 1992; Caroni et al. 1997). In the absence of CAP23, axon numbers in the peripheral nerves were normal, but both the nerve terminal and the tSC exhibited characteristic and unique abnormalities (Fig. 2B and Fig. C) that became apparent from P12-15. In the medial gastrocnemius muscle, nerve terminal subdomains were markedly heterogeneous in size, with frequent, grossly oversized terminal regions and occasional swollen endings that were completely wrapped by tSCs (Fig. 2B and Fig. C). The extent of these abnormalities had muscle region– and muscle type–specific features, and was least pronounced at slow-type neuromuscular synapses, e.g., in the soleus muscle (not shown). tSC processes were loosely packed and distinctly hypertrophic, with excess growth into the extracellular space (Fig. 2 C). These ultrastructural features and the corresponding overall appearance of these synapses are suggestive of instability with extensive ongoing remodeling (Bernstein and Lichtman 1999).
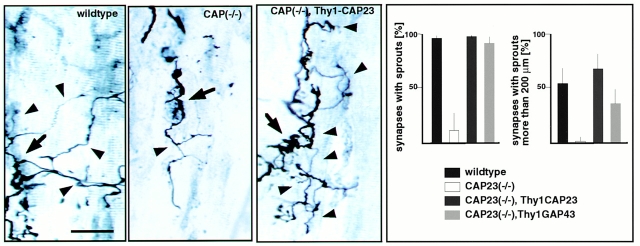
tSC process outgrowth at the adult neuromuscular junction is known to promote synaptic sprouting (Son and Thompson 1995). However, even when sprouting was induced by a local paralyzing injection of BotA, which induces strong sprouting in the soleus muscle of wild-type mice, CAP23−/− mice exhibited little or no nerve sprouting in this muscle (Fig. 3). To determine whether it was the absence of CAP23 in the motor nerve that impaired nerve sprouting, we crossed Thy1CAP23 mice that overexpress CAP23 from P7-9 on specifically and constitutively in neurons (Caroni et al. 1997) into the CAP23−/− background. This restored BotA-induced sprouting in the soleus (Fig. 3). Similar results were obtained when CAP23−/− mice were crossed into a Thy1GAP43 (Aigner et al., 1995) background (Fig. 3). Therefore, reexpression of CAP23 or GAP43 in adult motor nerves was sufficient to rescue nerve sprouting at the neuromuscular synapse.
Figure 3.
Critical role of motoneuron CAP23 for nerve sprouting at the adult nmj. Defective ultraterminal nerve sprouting at the soleus nmj of CAP23−/− mice, and rescue of nerve sprouting by reintroduction of CAP23 in motoneurons (CAP23−/− × Thy1CAP23 mice). (left) Combined silver esterase stains of soleus nmjs (arrowheads) 30 d after BotA injection. One nmj is shown in each photograph (arrows, broader nerve regions); some of the sprouts are indicated by arrowheads. (right) Quantitative analysis of nerve sprouting at the soleus, 30 d after BotA injection. The graphs refer to the fraction of synapses with sprouts (top), and to the fraction of synapses with a total length of sprouts >200 μm. The values are averages from three independent experiments (n = 50). Bar, 50 μm.
GAP43 Can Functionally Substitute for CAP23 In Vivo
To determine whether, and to what extent, GAP43 can substitute for CAP23, we generated CAP23gap43/gap43 knockin mice. The additional, bicistronic GAP43 mRNA species was larger than that of the endogenous GAP43 transcript, thus, allowing its independent detection on Northern blots. Heterozygous CAP23+/gap43 mice expressed reduced levels of CAP23, and two different GAP43 transcripts, whereas homozygous CAP23gap43/gap43 mice expressed no CAP23, and higher levels of the extra GAP43 transcript (Fig. 4 A). In situ hybridization analysis confirmed the additional expression of GAP43 in the characteristic CAP23 pattern, including strong signals throughout the neocortex and in dentate gyrus granule cells (not shown). Unlike CAP23−/− mice, CAP23gap43/gap43 mice exhibited no elevated mortality (Fig. 4 B), and were normal in size. Ultrastructural analysis revealed no gross abnormalities at the neuromuscular junction, in the neocortex, and in the hippocampus (not shown). At the light microscopic level, nmjs of CAP23gap43/gap43 mice were slightly broader than normal, but exhibited no signs of instability and no nerve terminal expansions (Fig. 4 C). Upon BotA–induced paralysis, nmjs in the soleus exhibited robust ultraterminal sprouting, although at close examination sprouts were more branched and shorter than in wild-type mice (Fig. 4 D). Therefore, GAP43 can effectively substitute for CAP23 in vivo, providing compelling experimental evidence for the notion that these proteins are related functionally, and exert partially redundant functions in vivo.
A Neurite Outgrowth Phenotype in the Absence of CAP23 Is Phenocopied by Cytochalasin D, a Drug that Inhibits Actin Filament Polymerization
To investigate more directly cell autonomous roles of CAP23 in neurite outgrowth, we analyzed dissociated DRG neuron cultures, which in a wild-type background express substantial levels of CAP23. For these experiments, neurons were plated on a laminin substratum (40 μg/ml) and cultured in the presence of NGF or NT3. When compared to wild-type neurons, neonatal sensory neurons from CAP23−/− mice extended axons that were thin and strikingly twisted, with frequent varicosities and characteristic bulbous endings (Fig. 5). Time-lapse video microscopic analysis suggested that, in the absence of CAP23, growth cones are less stable, failing to maintain a spread configuration, frequently changing growth direction, and exhibiting a tendency to extend, forward or backward, along neighboring neurites in the culture (not shown). Some of these features are strikingly reminiscent of chick DRG neurons depleted of GAP43 by an antisense approach, where the phenotype included reduced accumulation of filamentous actin in growth cones (Aigner and Caroni 1995). Therefore, we determined whether the abnormal appearance of CAP23-deficient neurons was due to a defect in the actin cytoskeleton. As shown in Fig. 5, the neurite outgrowth phenotype could be reproduced to a large extent by growing wild-type neurons in the presence of low concentrations of cytochalasin D, which inhibits actin polymerization and induces loss of growth cone actin structures (Fig. 5). As discussed below, these findings are consistent with the notion that the absence of CAP23 leads to selective deficits in the stability of growth cone actin structures.
Figure 5.
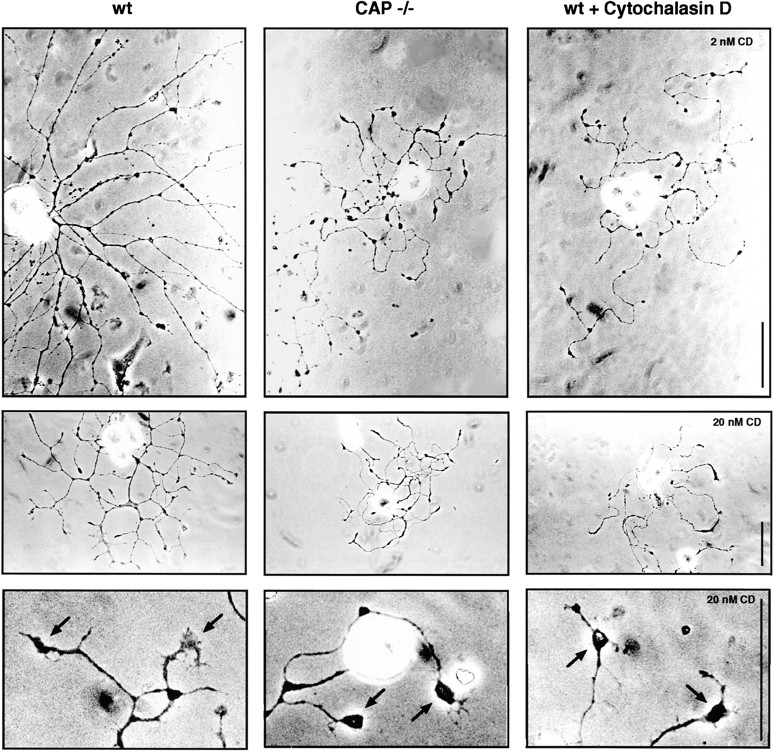
Alterations in neurite outgrowth because of the absence of CAP23 can be phenocopied by cytochalasin D, which interferes with actin filament polymerization. Representative phase-contrast photographs of 15-h DRG cultures on laminin, in the presence of NGF. Note the thin, meandering neurites, varicosities, and bulbous growth cones (arrows, bottom row) in the absence of CAP23 or in the presence of cytochalasin D. Bars: 50 μm.
Shared and Unique Roles of CAP23 and GAP43 in Neurite Outgrowth
To investigate the effects of CAP23 depletion and GAP43 replacement on defined subtypes of DRG neurons, we classified cultured neurons by their morphology, and by their expression patterns, using CAP23, GAP43, MARCKS, parvalbumin, substance P, CGRP, calbindin, and cholera toxin B as markers (Lawson 1992). In addition, we analyzed cultures in the presence of either NGF or the related neurotrophin NT3, which supports a different subpopulation of DRG neurons (Friedel et al. 1997). The expression of CAP23 and GAP43 was restricted to neurons, whereas MARCKS was also expressed at high levels in glial satellite cells (not shown). All neurofilament-positive neurons in wild-type cultures expressed substantial levels of CAP23, whereas GAP43 and MARCKS were only expressed in subsets of neurons. For the purpose of this analysis, we focused on three operationally defined DRG subtypes: type A expressed high levels of CAP23, GAP43, and MARCKS, whereas types-B and -C expressed high levels of CAP23, but no detectable GAP43 or MARCKS (Fig. 6 A). Types-A and -B neurons grew several thin processes, with small growth cones, whereas type C neurons extended comparatively broad processes, with large, well spread growth cones.
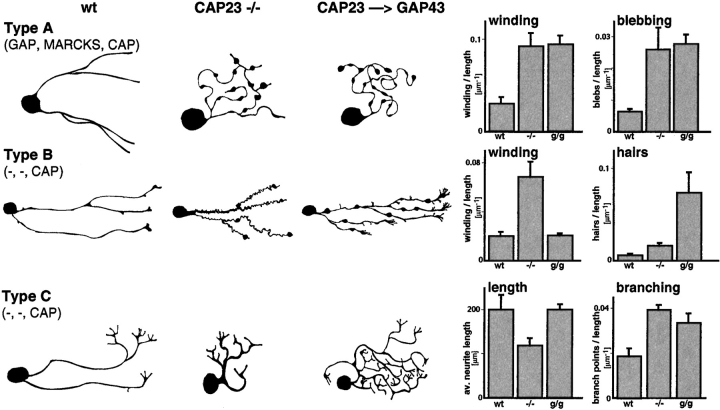
In the absence of CAP23, type A neurons grew twisted processes (winding), which failed to consistently extend away from the cell body (Fig. 6 B and 7). There also was an increase in varicosities and blobby growth cones (blebbing) (Fig. 7). These defects were not noticeably alleviated when these neurons expressed GAP43 instead of CAP23 (Fig. 6 B and 7). In the absence of CAP23, type B neurites (no GAP43, no MARCKS) grew in a strikingly thin and meandering pattern (winding), and growth cones were blobby. In these neurons, expressing GAP43 instead of CAP23 rescued the thin axon and the winding phenotype (Fig. 7). In addition, when compared to wild-type type B neurons, knockin neurites expressing GAP43 instead of CAP23 exhibited frequent filopodia side branches (hairs) and growth cones spread more (Fig. 6 B and 7). Finally, in the absence of CAP23 type C neurons grew shorter, broad processes, that lacked a well defined transition between growth cone and neurite shaft region, and branched frequently (Fig. 6 B and 7). In the presence of GAP43 instead of CAP23, neurite length was restored, but branching remained elevated (Fig. 6 B and 7).
Figure 7.
Common and unique roles of CAP23 and GAP43 in neurite outgrowth. Schematic representation of characteristic features (drawings on the left), and quantitative analysis of 15-h DRG cultures as shown in Fig. 6 B. See Materials and Methods for details. n = 15.
Discussion
We have provided evidence that CAP23 in motoneurons is critical for stimulus-induced nerve sprouting at the nmj in the adult, and that GAP43 can, to a large extent, functionally substitute for CAP23 in mice. Abnormal neurite outgrowth in the absence of CAP23 was phenocopied by cytochalasin D, providing support for the notion that GAP43 (Aigner and Caroni 1995) and CAP23 act by promoting F-actin accumulation in neurites. These results establish GAP43 and CAP23 as functionally related proteins affecting actin cytoskeleton regulation and anatomical plasticity. In the companion article (Laux et al., 2000), we show that GAP43, CAP23, and MARCKS are closely related mechanistically: they accumulate at subplasmalemmal rafts, where they modulate PI(4,5)P2 and regulate actin dynamics. In the following sections, we discuss the roles of CAP23 at the nmj in the adult, and the implications of the finding that CAP23 and GAP43 have common as well as unique functions.
Roles of CAP23 at the Neuromuscular Junction
In the soleus muscle, blockade of neuromuscular transmission with BotA induces ultraterminal nerve sprouting, which can be detected at >90% of the neuromuscular synapses, starting 3–5 d after toxin application (Brown 1984; Frey et al. 2000). Sprouts grow in length and complexity for ∼4 wk, and form ectopic synapses that restore neuromuscular transmission (DePaiva et al. 1999). In CAP23−/− mice, <5% of the synapses exhibited any detectable sprouting 7 d after toxin application. This was not due to a reduced effectiveness of the toxin, as supported by the absence of muscle twitch upon nerve stimulation, pronounced muscle atrophy, and expansion of nerve branches at the nmj (see below). The absence of nerve sprouting was particularly striking, when considering the pronounced hypertrophy and process extension by synapse-associated tSCs in these mice, which can promote nerve sprouting in wild-type mice (Son and Thompson 1995). At 30 d after paralysis, when in wild-type mice sprouting and synaptogenesis peaked, sprouting in the absence of CAP23 was still restricted to less than half of the synapses in the soleus, where sprouts were very short, with no evidence of ectopic synapses. Reintroduction of either CAP23 or GAP43 in motoneurons after birth effectively rescued the sprouting defect, providing experimental evidence that intrinsic CAP23 or GAP43 is necessary for paralysis-induced ultraterminal sprouting at this synapse. At close examination, sprouts in the presence of GAP43 were shorter and more branched than in the presence of CAP23, a finding consistent with the effects of these proteins on nerve sprouting in the presence of wild-type CAP23 alleles (Caroni et al. 1997), and with the observation that CAP23 and GAP43 have shared, as well as unique functions in neurite outgrowth (see below). To our knowledge, these results provide the first experimental evidence for the notion that CAP23 (or GAP43) are intrinsic determinants promoting anatomical plasticity in the adult. These findings suggest that the expression levels of these proteins in a particular neuron may be one factor determining its tendency to exhibit anatomical plasticity in the target region (Caroni 1997). In analogy to the results with PC12 cells (Laux et al., 2000), this pathway is likely to involve stimulus-induced formation of dynamic peripheral actin structures that are required for sprout formation and growth.
In addition to motoneurons, CAP23 was also expressed in tSCs at the nmj. In the absence of CAP23, the nmj exhibited striking anatomical alterations, including highly irregular nerve profiles, large bloblike expansions at the end of terminal nerve branches, and tSC hypertrophy. These abnormal features became apparent during the third postnatal week, when nmjs expand in size, and when GAP43 cannot be detected anymore at this synapse. Although they recovered sprouting competence and nerve terminal branches were much more regular in shape, CAP23−/− × Thy1CAP23 mice still exhibited abnormally expanded synaptic regions, sprout endings (Fig. 3), and Schwann cell processes (not shown), suggesting that the absence of CAP23 in tSCs led to a defect in synaptic growth control. Analysis of nerve–muscle preparations from CAP23 mutant mice revealed that these neuromuscular synapses had average quantal contents about twice as high as controls, and that this phenotype was not affected by the reintroduction of CAP23 in motoneurons (Frey, D., L. Xu, P. Laroui, unpublished results). One possible interpretation of these findings is that tSCs control synaptic growth and synaptic strength at the adult neuromuscular junction through a mechanism that requires CAP23 in the tSC.
CAP23 and GAP43 Are Closely Related Functionally
Replacing the coding sequence of CAP23 with that of GAP43, thus, generating knockin mice that expressed no CAP23, but instead GAP43, largely rescued the phenotypes caused by the absence of CAP23. Rescued features included early mortality, body weight, nmj morphology, synaptic strength at the nmj (not shown), and stimulus-induced nerve sprouting at the nmj. Therefore, although they do not share homologous sequences, CAP23 and GAP43 are closely related functionally in vivo, suggesting that they may be components of the same regulatory pathway. In the companion paper, Laux et al. (2000), we show that GAP43, CAP23, and MARCKS are closely related mechanistically, as central components of a novel pathway, to modulate the accessibility of PI(4,5)P2 at plasmalemmal rafts, and regulate actin dynamics and process outgrowth.
Although characteristic defects specifically because of the absence of CAP23 could be detected in vitro (see below), nmj morphologies and sprouting patterns in knockin mice were slightly different than in wild-type mice, and homozygous knockin mice were sterile (not shown), the efficient functional replacement of CAP23 by GAP43 is striking, and was not anticipated to this extent. Thus, although the two proteins have similar effects in transfected cells (Wiederkehr et al. 1997), and both induce nerve sprouting at the adult nmj of transgenic mice (Caroni et al. 1997), they not only lack sequence homology, but their EDs have significantly different regulatory properties, and their association with the plasma membrane is also regulated differently (Fig. 8). Major differences include the following: (1) binding of calmodulin by the ED, which is calcium-dependent and of high affinity for CAP23, but calcium-independent and of low affinity for GAP43 (Skene 1989; Maekawa et al. 1994; Benowitz and Routtenberg 1997); (2) GAP43 is effectively phosphorylated by protein kinase C in situ, whereas under comparable experimental conditions, only a small fraction of CAP23 is phosphorylated (Widmer and Caroni 1990); and (3) GAP43 tightly associates with the plasma membrane via palmitoylation, whereas membrane association by CAP23 not only depends on myristoylation, but also on the presence of the ED (not shown). One interpretation of these findings is that certain cellular functions depend critically on the expression of a minimal amount of any of these related proteins. These minimal requirements may not depend critically on the specific regulatory properties of CAP23 or GAP43 (Fig. 8). This interpretation is consistent with the observation that CAP23+/− mice, which expressed about a third of wild-type CAP23 levels, exhibited a partial phenotype, with characteristic, but weaker, deformations and bloblike expansions at the nmj, average weights that were ∼80% of normal, slightly elevated mortality, but no alterations in nmj synaptic strength. This haplo-insufficiency of CAP23 supports the notion that CAP23 levels can be critically important, and that GAP43 may rescue these deficits via a quantitative mechanism. The expression of GAP43 and CAP23 (and MARCKS; Laux et al., 2000) is highly regulated with respect to cell type, stimulus responsiveness, and quantity, and there is a general tendency for markedly reduced levels of these proteins in adult neurons. We suggest that the quantitative decline in neuronal CAP23 and GAP43 levels may be one factor restricting anatomical plasticity in the adult nervous system.
Figure 8.
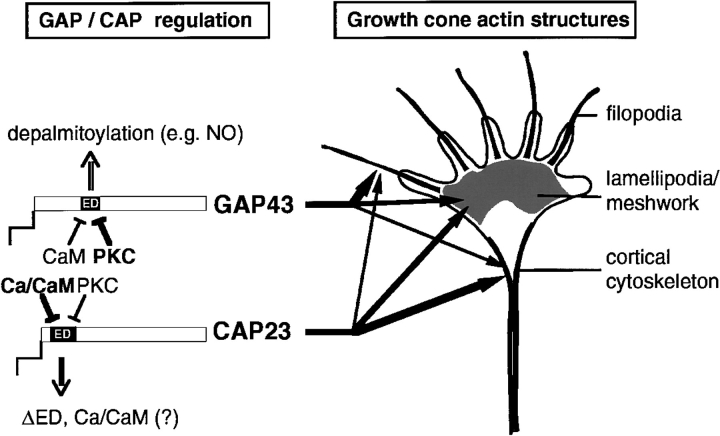
Proposed model of common and unique regulation and roles of CAP23 and GAP43 in growth cone actin dynamics. (left) CAP23 and GAP43 are regulated differently at the level of ED masking (relative roles of calmodulin [CaM] and PKC), and retention at the plasmalemma (ED masking versus depalmitoylation). These differences may result in differential impacts to the dynamics and accumulation of different types of actin structures at the growth cone (right).
Unique Properties of CAP23 and GAP43 in Neurite Outgrowth
The analysis of dissociated DRG neurons in culture revealed abnormalities in neurite outgrowth that were specifically due to the absence of CAP23, and were not compensated by GAP43. These were evident in the global effects of CAP23 depletion on DRG cultures (Fig. 5) as well as in the effects on type A neurons, which expressed CAP23, GAP43, and MARCKS in a wild-type background, and no CAP23, but substantially higher levels of GAP43 immunoreactivity in a knockin background (Fig. 6 and Fig. 7). Deficits specifically due to the absence of CAP23 were thinner neurites, a characteristic winding pattern of neurite outgrowth, and a prevalence of pronouncedly bulbous growth cones (Fig. 5 Fig. 6 Fig. 7). These defects could be phenocopied by low doses of cytochalasin D (Fig. 5), suggesting that they were specifically due to deficits in the actin cytoskeleton in the absence of CAP23. Interestingly, a striking excess of winding neurites, with large bloblike endings was also detected during the reinnervation of skeletal muscle by regenerating peripheral nerves of CAP23−/− mice (not shown). Time-lapse analysis revealed that in the absence of CAP23, growth cones frequently changed extension direction, indicating that the winding pattern was due to an alteration in growth cone behavior. At closer inspection, growth cones that did exhibit a spread configuration were abnormally phase-dense, and labeling for microtubules revealed unusually high densities and disordered arrangements of this cytoskeletal element in the growth cone (not shown). Combined with the similar effects of cytochalasin D on neurites and growth cones, these observations suggest that in the absence of CAP23, growth cone actin barriers that restrict microtubule invasion (Forscher and Smith 1988) are impaired. The frequent and abnormal occurrence of motile activity from the shaft region of the growth cone, which was again phenocopied by cytochalasin D (not shown), suggests that CAP23 may play a critical role to form an actin-based cortical cytoskeleton at the transition zone between growth cone and neurite. Such a role may also be required to prevent the unusual deformations and the striking bloblike expansions at the nmj of CAP23−/− mice.
The analysis of type B and type C DRG neurons that expressed high levels of CAP23, but no detectable GAP43 nor MARCKS provided further valuable information about common and shared properties of CAP23, GAP43, and MARCKS (Laux et al., 2000) in neurite outgrowth. First, although they exhibited anomalous growth patterns, neurites still extended in the absence of all three proteins (Fig. 6). This is in apparent contrast to the observation that the dominant-negative ΔED mutants suppressed neurite outgrowth in PC12 cells, and delayed peripheral nerve regeneration in transgenic mice (Laux et al., 2000). Possibly, DRG neurons express further functionally related proteins, such as MacMARCKS (Aderem 1995) or the more remotely related protein paralemmin (Kutzleb et al. 1998). Alternative explanations include that DRG neurons may extend neurites more vigorously than PC12 cells, thus not critically depending on the function of these proteins. Second, GAP43 partially restored neurite outgrowth properties in type B (neurite diameter and winding) and type C (neurite length) neurons, whereas neurites of type A neurons were comparable in knockout and knockin cultures. This observation further supports the notion that CAP23 and GAP43 are closely related functionally. Third, the expression of GAP43 instead of CAP23 in type B and type C neurons led to new neurite outgrowth features in these neurons, such as enhanced growth cone spreading and filopodia. These specific effects of GAP43 on neurite outgrowth in vitro are reminiscent of its effects on nerve sprouting at the nmj (Caroni et al. 1997), and suggest that GAP43 may predominantly affect different aspects of growth cone actin dynamics than CAP23 (Aigner and Caroni 1995).
What mechanisms could be responsible for the differential effects of CAP23 and GAP43 on neurite outgrowth? As summarized in Fig. 8, CAP23 and GAP43 are regulated differently, with respect to ED interactions and retention at the cell membrane. In addition, the ED is positioned differently relative to the NH2-terminal and the acylation site of these proteins. These differences could affect the retention of CAP23 and GAP43 at growth cone subregions, resulting in different contributions of the two proteins to growth cone actin dynamics. In addition, the different responsiveness of the EDs of CAP23 and GAP43 to calcium/calmodulin and PKC may lead to a predominant effectiveness of each protein at different points in the growth cone regulation cycle. A detailed elucidation of the mechanisms involved in differential regulation of actin dynamics and growth cone activity by CAP23 and GAP43 should shed light on their specific functions in the nervous system, during development and in the adult.
Acknowledgments
We thank S. Arber, J. Hagman, W. Krek, and U. Mueller (Friedrich Miescher Institute, Basel, Switzerland), and E. Stoeckli (University of Basel) for valuable comments on this manuscript.
Footnotes
Abbreviations used in this paper: BotA, botulinum toxin A; DRG, dorsal root ganglion; ED, effector domain; nmj, neuromuscular junction; PKC, protein kinase C; tSC, terminal Schwann cell.
References
- Aderem A. The MARCKS family of protein kinase-C substrates. Biochem. Soc. Trans. 1995;23:587–591. doi: 10.1042/bst0230587. [DOI] [PubMed] [Google Scholar]
- Aigner A., Caroni P. Absence of persistent spreading, branching, and adhesion in GAP-43-depleted growth cones. J. Cell Biol. 1995;128:647–660. doi: 10.1083/jcb.128.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aigner, L., S. Arber, J.P. Kapfhammer, T. 0, C. Schneider, F. Botteri, H.-R. Brenner, and P. Caroni. 1995. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell. 83:269–278. [DOI] [PubMed]
- Antonini A., Fagiolini M., Stryker M.P. Anatomical correlates of functional plasticity in mouse visual cortex. J. Neurosci. 1999;19:4388–4406. doi: 10.1523/JNEUROSCI.19-11-04388.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister A., Arber S., Caroni P. Accumulation of muscle ankyrin repeat protein transcript reveals local activation of primary myotube end compartments during muscle morphogenesis. J. Cell Biol. 1997;139:1231–1242. doi: 10.1083/jcb.139.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz L.I., Routtenberg A. GAP-43an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997;20:84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- Bernstein M., Lichtman J.W. Axonal atrophythe retraction reaction. Curr. Opin. Neurobiol. 1999;9:364–370. doi: 10.1016/s0959-4388(99)80053-1. [DOI] [PubMed] [Google Scholar]
- Bisby M.A., Tetzlaff W. Changes in cytoskeletal protein synthesis following axon injury and during regeneration. Mol. Neurobiol. 1992;6:107–123. doi: 10.1007/BF02780547. [DOI] [PubMed] [Google Scholar]
- Bravin M., Morando L., Vercelli A., Rossi F., Strata P. Control of spine formation by electrical activity in the adult rat cerebellum. Proc. Natl. Acad. Sci. USA. 1999;96:704–709. doi: 10.1073/pnas.96.4.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M.C. Sprouting of motor nerves in adult musclea recapitulation of ontogeny. Trends Neurosci. 1984;7:10–14. [Google Scholar]
- Caroni P. Intrinsic neuronal determinants that promote axonal sprouting and elongation. Bioessays. 1997;19:767–775. doi: 10.1002/bies.950190906. [DOI] [PubMed] [Google Scholar]
- Caroni P. Neuro-regenerationplasticity for repair and adaptation. Essays Biochem. 1999;33:53–64. doi: 10.1042/bse0330053. [DOI] [PubMed] [Google Scholar]
- Caroni P., Becker M. The downregulation of growth-associated proteins in motoneurons at the onset of synapse elimination is controlled by muscle activity and IGF1. J. Neurosci. 1992;12:3849–3861. doi: 10.1523/JNEUROSCI.12-10-03849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroni P., Aigner L., Schneider C. Intrinsic neuronal determinants locally regulate extrasynaptic and synaptic growth at the adult neuromuscular junction. J. Cell Biol. 1997;136:679–692. doi: 10.1083/jcb.136.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePaiva A., Meunier F.A., Moldgo J., Aoki K.R., Dolly J.O. Functional repair of motor endplates after botulinum neurotoxin type A poisoningbiphasic switch of synaptic activity between nerve sprouts and their parent terminals. Proc. Natl. Acad. Sci. USA. 1999;96:3200–3205. doi: 10.1073/pnas.96.6.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forscher P., Smith S.J. Actions of cytochalasins on the organization of actin filaments and microtubules in a neuronal growth cone. J. Cell Biol. 1988;107:1505–1516. doi: 10.1083/jcb.107.4.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey D., Schneider C., Xu L., Borg J., Spooren W., Caroni P. Early and selective loss of neuromuscular synapse subtypes with low sprouting competence in motoneuron diseases. J. Neurosci. 2000;20:2534–2542. doi: 10.1523/JNEUROSCI.20-07-02534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedel R.H., Schnurch H., Stubbusch J., Barde Y.-A. Identification of genes differentially expressed by nerve growth factor- and neurotrophin-3-dependent sensory neurons. Proc. Natl. Acad. Sci. USA. 1997;94:12670–12675. doi: 10.1073/pnas.94.23.12670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutzleb C., Sanders G., Yamamoto R., Wang X., Lichte B., Petrasch-Parwez E., Kilimann M.W. Paralemmin, a prenyl-palmitoyl–anchored phosphoprotein abundant in neurons and implicated in plasma membrane dynamics and cell process formation. J. Cell Biol. 1998;143:795–813. doi: 10.1083/jcb.143.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson S.N. Morphological and biochemical cell types of sensory neurons. In: Scott S.A., editor. Sensory NeuronsDiversity, Development, and Plasticity. Oxford University Press; Oxford: 1992. pp. 27–59. [Google Scholar]
- Maekawa S., Murofushi H., Nakamura S. Inhibitory effect of calmodulin on phosphorylation of NAP-22 with protein kinase C. J. Biol. Chem. 1994;269:19462–19465. [PubMed] [Google Scholar]
- Maier D.L., Mani S., Donoval S.L., Soppet D., Tesarollo L., McCasland J.S., Meiri K.F. Disrupted cortical map and absence of cortical barrels in growth-associated protein (GAP)-43 knockout mice. Proc. Natl. Acad. Sci. USA. 1999;96:9397–9402. doi: 10.1073/pnas.96.16.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara R.K., Lenox R.H. Comparative distribution of myristoylated alanine-rich C kinase substrate (MARCKS) and F1/GAP-43 gene expression in the adult brain. J. Comp. Neurol. 1997;379:48–71. [PubMed] [Google Scholar]
- Sengpiel F., Godecke I., Stawinski P., Hubener M., Lowel S., Bonhoeffer T. Intrinsic and environmental factors in the development of functional maps in cat visual cortex. Neuropharmacology. 1998;37:607–621. doi: 10.1016/s0028-3908(98)00034-3. [DOI] [PubMed] [Google Scholar]
- Skene J.H.P. Axonal growth-associated proteins. Annu. Rev. Neurosci. 1989;12:127–156. doi: 10.1146/annurev.ne.12.030189.001015. [DOI] [PubMed] [Google Scholar]
- Son Y.J., Thompson W.J. Nerve sprouting in muscle is induced and guided by processes extended by Schwann cells. Neuron. 1995;14:133–141. doi: 10.1016/0896-6273(95)90247-3. [DOI] [PubMed] [Google Scholar]
- Sretawan D.W., Kruger K. Randomized retinal ganglion cell axon routing at the optic chiasm of GAP-43 deficient miceassociation with midline recrossing and lack of normal ipsilateral axon turning. J. Neurosci. 1998;18:10502–10513. doi: 10.1523/JNEUROSCI.18-24-10502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter S.M., Fankhauser C., Huang P.L., Mashimo H., Fishman M.C. Neuronal pathfinding is abnormal in mice lacking the neuronal growth cone protein GAP-43. Cell. 1995;80:445–452. doi: 10.1016/0092-8674(95)90495-6. [DOI] [PubMed] [Google Scholar]
- Wiederkehr A., Staple J., Caroni P. The motility-associated proteins GAP-43, MARCKS, and CAP-23 share unique targeting and surface activity-inducing properties. Exp. Cell Res. 1997;236:103–116. doi: 10.1006/excr.1997.3709. [DOI] [PubMed] [Google Scholar]
- Widmer F., Caroni P. Identification, localization, and primary structure of CAP-23, a particle-bound cytosolic protein of early development. J. Cell Biol. 1990;111:3035–3047. doi: 10.1083/jcb.111.6.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankner B.A., Benowitz L.I., Villa-Komaroff L., Neve R.L. Transfection of PC12 cells with the human GAP-43 geneeffects on neurite outgrowth and regeneration. Mol. Brain Res. 1990;7:39–44. doi: 10.1016/0169-328x(90)90071-k. [DOI] [PubMed] [Google Scholar]
- Zhu Q., Julien J.P. A key role for GAP-43 in the retinotectal topographic organization. Exp. Neurol. 1999;155:228–242. doi: 10.1006/exnr.1998.6989. [DOI] [PubMed] [Google Scholar]