Abstract
The lack of persistence of transferred autologous mature lymphocytes in humans has been a major limitation to the application of effective cell transfer therapies. The results of a pilot clinical trial in 13 patients with metastatic melanoma suggested that conditioning with nonmyeloablative chemotherapy before adoptive transfer of activated tumor-reactive T cells enhances tumor regression and increases the overall rates of objective clinical responses. The present report examines the relationship between T cell persistence and tumor regression through analysis of the TCR β-chain V region gene products expressed in samples obtained from 25 patients treated with this protocol. Sequence analysis demonstrated that there was a significant correlation between tumor regression and the degree of persistence in peripheral blood of adoptively transferred T cell clones, suggesting that inadequate T cell persistence may represent a major factor limiting responses to adoptive immunotherapy.
A major obstacle to the development of effective lymphocyte transfer therapies for patients with cancer and other diseases has been the inability to mediate the prolonged persistence of the transferred cells. In a prior study of five patients, the survival of tumor-reactive autologous tumor-infiltrating lymphocytes (TIL)2 that had been transduced with the neomycin phosphotransferase gene was analyzed using a sensitive PCR technique that was capable of detecting 1 in 105 cells (1). Using this technique, transduced cells were detected at levels of between 1 in 300 and 1 in 104 PBMC 2–3 days following adoptive transfer that decreased to less than 1 in 10,000 circulating cells by day 21 following cell transfer in all patients. In a second study using the adoptive transfer of highly avid melanoma-reactive CD8+ T cell clones in 13 patients, analysis was conducted using a clonotype-specific PCR capable of detecting between 1 and 103 and 1 in 105 T cells. Using this method, the transferred clones were detected in PBMC obtained 2–5 days following transfer in 40% of the patients that were analyzed, but could not be detected in peripheral blood samples collected 2–3 wk following transfer (2). Objective clinical responses were not observed either in this trial or in an additional trial using the adoptive transfer of melanoma-reactive T cell clones in which low levels of persistence were also observed (3).
In an attempt to enhance T cell engraftment and potentially improve clinical responses, 15 patients were treated with a non-myeloablative but lymphodepleting chemotherapy regimen before the adoptive transfer of avid tumor-reactive CD8+ T cell clones (4). Clinical responses were not observed in this trial and, in the majority of patients who were examined, T cells did not appear to persist in the peripheral blood of recipients at significant levels beyond 1–2 days following transfer (M. Dudley, unpublished data). This suggested that factors that were intrinsic to the T cells may have been responsible for the lack of clinical responses as well as the lack of T cell persistence, and lead to a modification of the clinical trial to allow the adoptive transfer of polyclonal populations of TIL following lymphodepleting chemotherapy. We initially reported that objective clinical responses were observed in 6 of the 13 patients who received TIL following nonmyeloablative conditioning (5), and 18 of the 35 patients that have been treated in this trial to date have now demonstrated objective responses. High levels of lymphocytosis that were associated with the engraftment of tumor-reactive T cells have been observed in two of the patients in this trial, and tumor-reactive clones derived from the transferred TIL persisted at high levels in the peripheral blood of these patients for a period greater than 4 mo following transfer.
Samples obtained from 25 of the patients who have been treated in this protocol including 7 of the patients who were examined in the prior report have now been analyzed in an attempt to identify factors that are critically involved with clinical responses. The results presented in this report demonstrate that substantial persistence of the transferred cells is observed in many of the treated patients and that the degree of persistence of adoptively transferred T cells is strongly associated with objective clinical responses.
Materials and Methods
Patient samples
The TIL used for this study were generated using high dose IL-2, followed by a rapid expansion using OKT-3 stimulation (5, 6). A single rapid expansion was conducted for all of the TIL with the exception of those from patient 7, which were expanded a second time. With the exception of patient 34, all of the patients that were treated expressed the HLA-A2 class I allele. The 25 patients selected for this study received chemotherapy conditioning with cyclophosphamide and fludarabine before adoptive transfer, as previously described (5). All of the patients analyzed in this study were selected on the basis of the availability of appropriate cryopreserved samples of PBMC obtained between 5 and 15 days following transfer to examine the short-term persistence of T cells, as well as ~1 mo following transfer, to examine relatively long-term persistence of the transferred T cells. For three of the responding patients, samples were not available 1 mo following transfer, and persistence was determined at the earliest time point available, which was ~2 mo following transfer. All of the samples were derived from the first nonmyeloablative treatment that was administered to patients, with the exception of three of the responders, patients 6, 7, and 30, each of whom had received a single prior nonmyeloablative treatment. Exclusion of these patients from the analysis of T cell persistence did not substantially alter the reported results.
TCR β-chain V region (TRBV) analysis
Analysis of cell surface TRBV expression was conducted with a panel of Abs directed against either individual TRBV gene products or TRBV gene families, obtained from Beckman/Coulter (Fullerton, CA) and Pierce/Endogen (Rockford, IL) as well as using 5′ RACE analysis, as previously described (7). Briefly, a primer that was complementary to the TCR β-chain C region, 5′-CTCTTGACCATGGCCATC-3′, was used with the SMART RACE cDNA Amplification kit (BD Biosciences, Palo Alto, CA) to amplify the TRBV region sequences expressed by polyclonal TIL samples. The germline genes that encoded the expressed TRBV products were identified by aligning the cloned sequences with the known TRBV gene sequences using the VectorNTI AlignX protocol (Invitrogen Life Technologies, Carlsbad, CA) and the highly variable sequences that resulted from joining the TRBV genes to the Dβ and Jβ regions were then compared to identify T cell clonotypes.
Evaluation of T cell Ag reactivity
Ag reactivity of the infused TIL and T cell clones was evaluated by determining the production of IFN-γ in response to autologous and/or allogeneic melanoma cell lines as well as peptide-pulsed T2 cells. Two HLA-A2 tetramers (8) that were generated either with a modified MART-1:26–35 peptide (Beckman/Coulter), MART-1:26–35(2L), containing a substitution at the second amino acid from an alanine to leucine residue that enhances binding to HLA-A2 (9) or the native gp100:209–217 peptide (10) (Beckman/Coulter) were used to evaluate reactivity of the infused TIL and T cell clones from HLA-A2-positive patients. The Ag reactivity of TIL from patients 17 and 30 was determined by measuring the up-regulation of cell surface CD107a expression following stimulation with autologous tumor cells, as previously described (11).
Results and Discussion
General features of responding and nonresponding patients
The general characteristics of TIL that were administered to 25 patients who received prior nonmyeloablative chemotherapy, which included 7 patients who were analyzed in a prior study (5), were initially evaluated using multiple criteria. Thirteen of the 25 patients were objective clinical responders. The total number of T cells administered to patients that responded to therapy, 7.2 ± 1.2 ×1010 T cells (mean ± SD), and to patients that did not respond to therapy, 6.6 + 1.6 ×1010 T cells (mean ± SD), did not differ significantly. Neither the percentage of CD8+ T cells in the TIL that were administered to the patients, the ability of the administered TIL to recognize either known tumor Ags or autologous tumor cells, the length of time that TIL had been cultured in vitro, nor the rate of T cell proliferation before adoptive transfer were correlated with clinical response (data not shown). In addition, clinical response was not associated with general patient characteristics such as age and sex, or the sites of evaluable disease (data not shown).
Persistence of T cells following adoptive transfer
In the previous study of patients that had received TIL following nonmyeloablative chemotherapy (5), the persistence of populations of T cells was tracked through the use of Abs to individual TRBV gene products as well as through the use of tetramers of HLA class I molecules bound to an antigenic peptide. Approximately 50% of the TRBV gene products cannot be detected using the commercially available Abs, however, and many of the tumor-reactive T cell clones present in TIL appear to recognize unknown Ags and therefore cannot be tracked using MHC class I tetramers (data not shown).
In the current study, the representation of individual T cell clonotypes was evaluated by amplifying the TRBV genes expressed in samples of TIL and PBMC obtained following adoptive transfer using a primer that was complementary to the TCR β-chain region. The cDNA clones were assigned to a particular TRBV gene by alignment with germline sequences, and comparison of the unique sequences generated by joining of the TRBV, D, and J regions allowed the identification of individual T cell clonotypes. The relative representation of expressed TRBV gene products as determined by FACS analysis of samples of TIL and PBMC, which was conducted when Abs were available, was highly correlated with that obtained by sequence analysis of the same samples (p < 0.001) (Fig. 1). Distinct T cell clones that express rearranged products derived from the same TRBV germline gene cannot be distinguished using anti-TRBV Abs but can readily be identified by comparing the junctional regions of these transcripts. Therefore, patient samples were evaluated through the direct analysis of expressed TRBV gene products, which should provide the most reliable measurement of the relative frequency of individual clonotypes present in polyclonal populations of T cells.
FIGURE 1.
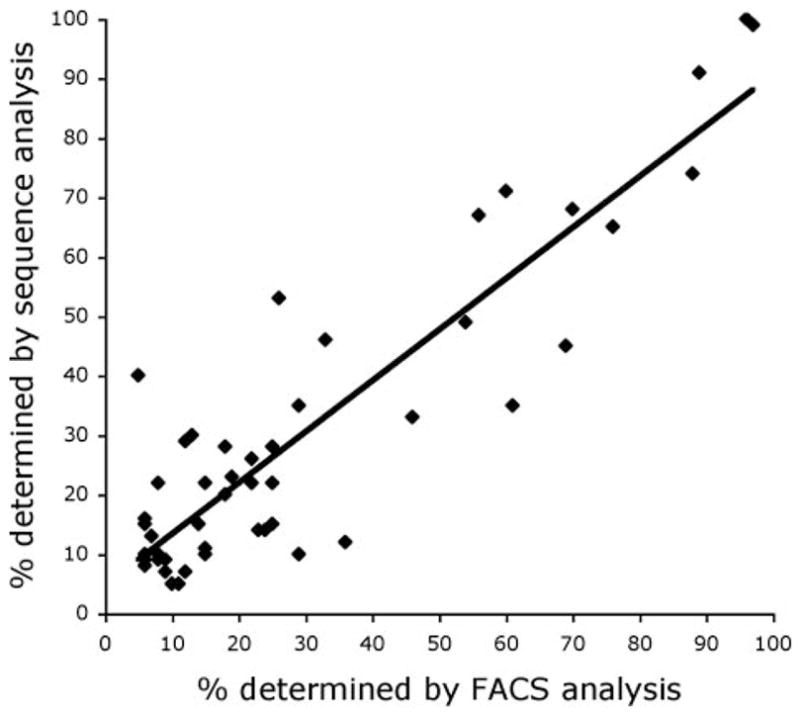
Comparison of TRBV sequencing results with FACS analysis. The percentages of individual rearranged TRBV sequences obtained using 5′ RACE analysis were compared with the results of FACS analysis conducted with specific TRBV Abs. Samples were only analyzed if the appropriate TRBV Ab was available, and TRBV products that were represented at levels below 5% as determined either by TRBV sequence analysis or by Ab staining were excluded from the analysis. The results obtained using these methods were highly correlated using linear regression analysis (R = 0.8975, p < 0.001).
Analysis of distribution of T cell clonotypes present in the 25 TIL that were administered to patients revealed that TIL from patients 11 and 14 were oligoclonal and contained only 4 and 3 distinct sequences, respectively (Table I). The majority of TIL were more heterogeneous, however, and from 8 to 78 distinct sequences were detected in samples from the remaining patients. A median of 22 distinct sequences was identified in the 25 TIL samples that were administered to the patients in this study (Table I). Dominant clonotypes represented at levels of ≥5% of the total sequences were identified in TIL from all but one of the nonresponding patients, patient 12, and TIL derived from responding and nonresponding patients demonstrated a similar distribution of clonotypes representing between 5 and 9%, 10 to 20%, and >20% of the total population of T cells that were administered. A total of 53 clones that were represented at levels of ≥5% were identified in TIL samples obtained from the 13 responding patients, and 37 over-represented clones were identified in TIL from the 12 nonresponding patients.
Table I.
Clonal composition of TIL
| Number of Clonotypes Represented in TIL at:
|
||||||
|---|---|---|---|---|---|---|
| Total Number of TRBV Sequences Analyzed | Number of Distinct Clonal Sequencesa | <5% | 5–9% | 10–20% | >20% | |
| Responder | ||||||
| 6 | 118 | 41 | 37 | 1 | 3 | 0 |
| 9 | 128 | 10 | 4 | 2 | 2 | 2 |
| 10 | 92 | 20 | 17 | 0 | 0 | 3 |
| 16 | 64 | 20 | 16 | 2 | 0 | 2 |
| 17 | 81 | 24 | 21 | 0 | 2 | 1 |
| 19 | 78 | 38 | 32 | 4 | 2 | 0 |
| 21 | 91 | 37 | 32 | 2 | 3 | 0 |
| 25 | 81 | 39 | 35 | 3 | 1 | 0 |
| 26 | 84 | 37 | 32 | 3 | 1 | 1 |
| 28 | 78 | 15 | 12 | 1 | 0 | 2 |
| 30 | 81 | 37 | 33 | 0 | 3 | 1 |
| 31 | 75 | 18 | 13 | 4 | 0 | 1 |
| 34 | 80 | 21 | 19 | 1 | 0 | 1 |
| Nonresponder | ||||||
| 7 | 80 | 34 | 31 | 2 | 0 | 1 |
| 11 | 82 | 4 | 3 | 0 | 0 | 1 |
| 12 | 89 | 78 | 78 | 0 | 0 | 0 |
| 13 | 70 | 22 | 18 | 1 | 2 | 1 |
| 14 | 85 | 3 | 1 | 0 | 1 | 1 |
| 15 | 67 | 14 | 10 | 2 | 1 | 1 |
| 18 | 61 | 22 | 17 | 3 | 1 | 1 |
| 20 | 86 | 34 | 32 | 0 | 1 | 1 |
| 22 | 81 | 15 | 10 | 1 | 2 | 2 |
| 24 | 85 | 23 | 19 | 1 | 1 | 2 |
| 27 | 73 | 26 | 23 | 1 | 1 | 1 |
| 29 | 78 | 21 | 17 | 1 | 1 | 2 |
Clonotypes present within TIL samples were identified by comparison of the TRBV, D, and J region sequences of cDNA clones that were derived from individual TRBV genes.
The TRBV sequences that were amplified from the administered TIL were then aligned with sequences from PBMC samples collected at two time points following adoptive transfer in an attempt to determine the role of persistence of individual T cell clonotypes in clinical response to therapy. Total persistence, which represented the sum of the percentages of all of the clonotypes present in TIL that were also detected in PBMC following adoptive transfer, was evaluated for samples that were obtained at a time point that ranged between 5 and 15 days following adoptive transfer as well as at a later time point ~1–2 mo following transfer that was dependent on the availability of patient samples (Table II). The level of persistence measured in the peripheral blood of responders 5–15 days following transfer averaged 58 ± 5% (mean ± SEM), and ranged from 36 to 96%, while the level of persistence observed in nonresponders 5–15 days following adoptive transfer averaged 28 ± 8% (mean ± SEM), and ranged from <1 to 87%. The levels of persistence observed in the responding patients were significantly higher than those observed in nonresponding patient (p2 = 0.008) when nonparametric statistical analysis was used to compare the two patient groups.
Table II.
Clonal persistence following adoptive transfer
| PBMC Sample Daya | Lymphocyte Number Per mm3 (days 5–15) | Persistence Total (%)b | Lymphocyte Number Per mm3 (days 23–63) | Persistence Total (%)b | |
|---|---|---|---|---|---|
| Responder | |||||
| 6(2)c | 9, 55 | 2,085 | 49 | 1,646 | 24 |
| 9 | 9, 29 | 21,436 | 96 | 7,086 | 96 |
| 10 | 7, 23 | 12,150 | 94 | 3,410 | 84 |
| 16 | 7, 61 | 389 | 36 | 973 | 14 |
| 17(2)c | 8, 28 | 555 | 43 | 1,328 | 10 |
| 19 | 5, 33 | 1,956 | 57 | 2,740 | 12 |
| 21 | 6, 27 | 1,407 | 45 | 1,079 | 15 |
| 25 | 7, 31 | 168 | 56 | 455 | 8 |
| 26 | 8, 34 | 711 | 44 | 483 | 2 |
| 28 | 9, 63 | 576 | 74 | 1,382 | 7 |
| 30(2)c | 5, 26 | 111 | 61 | 283 | 11 |
| 31 | 7, 26 | 739 | 36 | 643 | 4 |
| 34 | 9, 37 | 82 | 62 | 639 | 51 |
| Mean | 3,258 | 58 | 1,704 | 26 | |
| SE | 1,756 | 5 | 515 | 9 | |
| Nonresponder | |||||
| 7 | 15, 37 | NDd | 9 | 1,893 | <1 |
| 11 | 8, 35 | 810 | 4 | 760 | <1 |
| 12 | 8, 35 | 791 | 5 | 864 | 9 |
| 13 | 8, 29 | 1,073 | 36 | 1,027 | <1 |
| 14 | 8, 29 | 532 | <1 | 1,792 | <1 |
| 15 | 7, 34 | 760 | 60 | 871 | 5 |
| 18 | 13, 29 | 287 | 2 | 385 | <1 |
| 20 | 8, 25 | 548 | 18 | 592 | 2 |
| 22 | 8, 34 | 305 | 41 | 307 | 1 |
| 24 | 7, 29 | 2,282 | 87 | 535 | 41 |
| 27 | 7, 24 | 2,496 | 47 | 200 | 1 |
| 29 | 8, 32 | 164 | 25 | 186 | <1 |
| Mean | 913 | 28 | 784 | 5 | |
| SE | 225 | 8 | 163 | 3 | |
| p2 = 0.663e | p2 = 0.008 | p2 = 0.115 | p2 = 0.001 | ||
The days given are relative to the date of TIL infusion.
The values indicate the sum of the percentages of individual clonotypes detected in PBMC samples that were also present in the administered TIL.
Patients 6, 17, and 30 had received a single prior ablation followed by an adoptive transfer of autologous TIL.
A PBL count was not carried out for patient no. 7 at this time point.
The lymphocyte counts and the percentage of persistent clonotypes present in responders and nonresponders were compared using the Wilcoxon rank sum test.
More striking differences were observed between responding and nonresponding patients when the levels of persistence between 1 and 2 mo following transfer were evaluated (Table II). The level of persistence measured in responding patients at this time was 26 ± 9% (mean ± SEM) and ranged from 2 to 96%, whereas the level of persistence observed in nonresponders averaged 5 ± 3% (mean ± SEM) and ranged from <1 to 9% with the exception of a single patient. Patient 24 demonstrated a total persistence level of 41% and experienced a 38% tumor regression that did not meet the criteria of an objective response and thus was classified as a nonresponder. The levels of persistence observed in responding patients were again significantly higher than those observed in nonresponding patients (p2 = 0.001) when nonparametric statistical analysis was used to compare the two patient groups. Neither the total lymphocyte count (Table II) nor the number of CD8+ or CD4+ T cells present in PBMC samples collected at either time point (data not shown) differed significantly between responders and nonresponders.
The frequencies of individual TIL clonotypes that were detected in peripheral blood between 1 and 2 mo following transfer were also evaluated in responders and nonresponders (Fig. 2). At this time, persistent clonotypes were identified in PBMC obtained from all of the responding patients following adoptive transfer, but were detected in PBMC from only 6 of the 12 non-responding patients. Two or more persistent clonotypes were detected in PBMC from 12 of the 13 responding patients, whereas multiple persistent clonotypes were detected in PBMC obtained from only 3 of the 12 nonresponding patients. There did not appear to be a significant degree of similarity between the sequences of dominant clonotypes identified from different patients.
FIGURE 2.
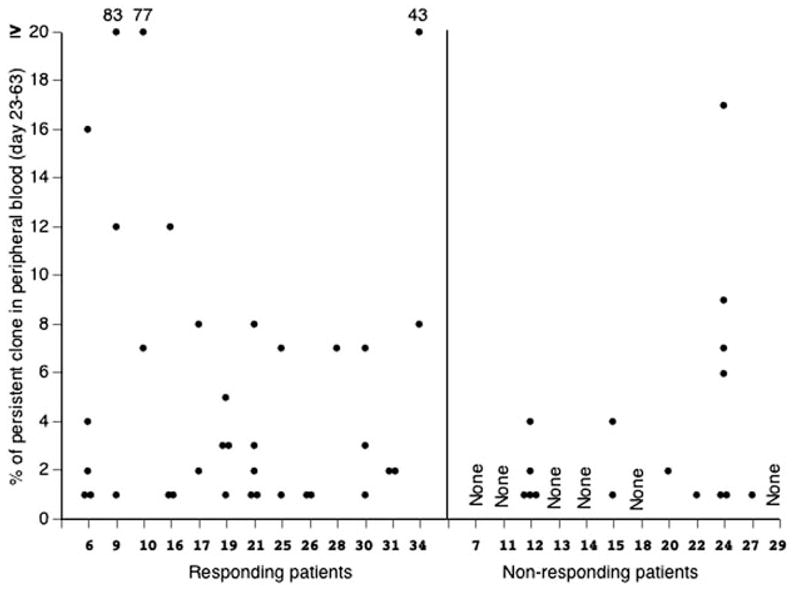
Comparisons of the persistence of individual T cell clonotypes in responders and nonresponders. The percentages of all persistent T cell clonotypes that were detected in samples of PBMC obtained between 23 and 63 days following adoptive transfer, as determined using TRBV sequence analysis, were plotted for the responding and nonresponding patients. Each point represents a distinct T cell clonotype that was identified in an individual patient sample.
Additional studies were then conducted to evaluate tumor Ag reactivity of the adoptively transferred T cells as well as the most prevalent of the persistent clones at the latest time point that was examined in patient PBMC. Only patients that had persistence clonotypes present at a level of ≥5% were studied because of the difficulty of determining the Ag reactivity of relatively small populations of cells. Eleven of the 13 responders had clonotypes that were persistent at a level ≥5%, and 8 of these were shown to be tumor reactive. The molecular identification of the Ags recognized was demonstrated in five of these patients (Table III). Only one of the nonresponders had a clone that persisted at a level ≥5%, and the reactivity of this clone is unknown.
Table III.
Ag reactivity of TIL and persistent T
| Patient No. | Ag Reactivity of TILa | Reactivity of Most Prevalent Persistent Clonotypeb | Most Prevalent Persistent Clonotype (%) |
|---|---|---|---|
| Responder | |||
| 6 | Autol. | Autol. | 16 |
| 9 | MART-1 | MART-1 | 83 |
| 10 | MART-1 | MART-1 | 77 |
| 16 | Autol. | Autol. | 12 |
| 17 | Autol. | Autol. | 8 |
| 19 | Autol. | N/Tc | 5 |
| 21 | Autol. | Autol. | 8 |
| 25 | Autol. | N/T | 7 |
| 26 | MART-1 | - | 1 |
| 28 | MART-1 | MART-1 | 7 |
| 30 | gp100:209–217 | Autol. | 7 |
| 31 | MART-1 | - | 2 |
| 34 | Autol. | N/T | 43 |
| Nonresponder | |||
| 7 | MART-1 | - | <1 |
| 11 | MART-1 | - | <1 |
| 12 | Autol. | - | 4 |
| 13 | MART-1 | - | 1 |
| 14 | Autol. | - | <1 |
| 15 | MART-1 | - | 4 |
| 18 | gp100:209–217 | - | <1 |
| 20 | MART-1 | - | 2 |
| 22 | Autol. | - | 3 |
| 24 | Autol. | N/T | 17 |
| 27 | MART-1 | - | 1 |
| 29 | MART-1 | - | <1 |
Ag reactivity was evaluated by determining the ability of TIL to release IFN-γ in response to autologous (Autol.) or HLA-matched allogeneic tumors, or to T2 cells that were pulsed with either the MART-1:26–35 or gp100:209–217 HLA-A2-restricted peptides.
The Ag reactivity of the most persistent clonotype detected in PBMC 23–63 days after transfer is indicated. Isolated T cell clones corresponding to the dominant persistent clonotypes from TIL from patients 9 and 10 released IFN-γ in response to T2 cells that were pulsed with the MART-1:27–35 peptide, and clones corresponding to the dominant persistent clonotypes within TIL from patients 6, 16, and 22, released IFN-γ in response to autologous tumor cells. The dominant persistent clonotype within the TIL from patient 28 was identified by staining with anti-TRBV Abs and a MART-1:26–35(2L) tetramer. Tumor reactivity of the dominant persistent clonotypes from patients 17 and 30 was determined by measuring the ability of these T cells, which were identified within the TIL using specific TRBV Abs, to specifically up-regulate expression of cell surface CD107a expression following stimulation with autologous tumor cells. A dash indicates either that no persistent clonotype was detected or that the most persistent clonotype was present at a frequency of between 1 and 5% and was not evaluated due to the limited number of cells available for analysis.
For samples designated N/T (not tested), the ability of the persistent clonotype to recognize autologous tumor has not been verified.
This clinical trial represents the first example in which high levels of persistence were observed following the adoptive transfer of autologous human effector T cells. Several factors may have been responsible for the enhanced engraftment of transferred T cells. The depletion of endogenous lymphocytes might lead to the enhanced engraftment of the transferred T cells, either by increasing the access of the transferred cells to homeostatic cytokines such as IL-15 and IL-7 or to APCs (12–14). Ablation may also lead to the depletion of regulatory T cells that under normal conditions suppress the proliferation of the adoptively transferred T cells either in response to direct tumor stimulation or by tumor Ags that are cross-presented by APC (15). Further investigation is needed, however, to determine the relative contribution of Ag-specific activation and homeostatic mechanisms to maintaining T cell persistence in patients receiving adoptive immunotherapy following nonmyeloablative chemotherapy.
Several additional questions remain concerning the mechanisms involved with T cell persistence, as well as the relationship between the degree of T cell persistence and patient response. Although the levels of persistence observed in this trial were high relative to those observed in any prior cell transfer of effector T cells, significant differences in these levels were observed, even among responding patients. Only a relatively small percentage of the administered T cell clonotypes analyzed in this report were capable of persisting in vivo at levels of 1% or greater in circulating PBMC. Another issue raised by these findings is whether or not the durability of clinical responses are correlated with the levels of T cell persistence; however, the number of responding patients in this study was too small to evaluate this relationship. The absolute lymphocyte counts measured following adoptive transfer varied widely within samples of PBMC obtained from both responding and nonresponding patients, irrespective of the number of infused T cells, and did not appear to be associated with clinical responses (Table II). Thus, tumor regression in this trial was associated with the presence of at least one T cell clonotype in the infused TIL that was capable of persisting at readily detectable levels in peripheral blood following adoptive transfer, rather than with the absolute number of persistent T cells.
Several additional factors may also have influenced responses to adoptive immunotherapy that were observed in this trial. Proliferative potential, which may be related to the stage of differentiation of individual T cell clonotypes, may have played an important role in the persistence of the transferred T cells. The ability of T cells to home to tumor sites may also have influenced the clinical responses that were observed; however, the visceral location of most tumor deposits makes it difficult to evaluate T cell clonotypes in these tumors. The in vivo persistence of T cells following adoptive transfer may also be influenced by the relative avidity of their respective TCRs, which was not directly addressed in this study. Variations in tumor characteristics such as the levels of Ag or HLA gene expression may also have influenced responses to therapy and represent factors that could have obscured the relationship between persistence and clinical response. The fact that such an association was still observed in the face of these potentially confounding factors provides further evidence that persistence may be causally related to the response to adoptive immunotherapy.
These results suggest that persistent T cells, derived from TIL and collected from the peripheral blood of patients following adoptive transfer, play an important role in mediating tumor regression. Differences in the levels of T cell persistence between responding and nonresponding patients was observed as early as 1 wk following therapy, suggesting that the failure of T cells to survive at significant levels for even a relatively short period of time following adoptive transfer may be responsible for the lack of clinical responses observed in ~50% of the patients treated in this protocol. A better understanding of the factors involved with maintaining the persistence of transferred tumor-reactive T cells may hopefully lead to the development of more effective adoptive immunotherapies.
Acknowledgments
We thank Arnold Mixon and Shawn Farid for their assistance with FACS analysis and Donald White for his assistance with statistical analysis.
Footnotes
Abbreviations used in this paper: TIL, tumor-infiltrating lymphocyte; TRBV, TCR β-chain V region.
References
- 1.Rosenberg SA, Aebersold PM, Cornetta K, Kasid A, Morgan RA, Moen R, Karson EM, Lotze MT, Yang JC, Topalian SL, et al. Gene transfer into humans: immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med. 1990;323:570. doi: 10.1056/NEJM199008303230904. [DOI] [PubMed] [Google Scholar]
- 2.Dudley ME, Wunderlich J, Nishimura MI, Yu D, Yang JC, Topalian SL, Schwartzentruber DJ, Hwu P, Marincola FM, Sherry R, et al. Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J Immunother. 2001;24:363. doi: 10.1097/00002371-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Yee C, Thompson JA, Byrd D, Riddell SA, Roche P, Celis E, Green-berg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99:16168. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dudley ME, Wunderlich JR, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry RM, Marincola FM, Leitman SF, Seipp CA, et al. A phase I study of nonmyeloablative chemotherapy and adoptive transfer of autologous tumor antigen-specific T lymphocytes in patients with metastatic melanoma. J Immunother. 2002;25:243. doi: 10.1097/01.CJI.0000016820.36510.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riddell SR, Greenberg PD. The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand human antigen-specific T cells. J Immunol Methods. 1990;128:189. doi: 10.1016/0022-1759(90)90210-m. [DOI] [PubMed] [Google Scholar]
- 7.Huang J, El-Gamil M, Dudley ME, Li YF, Rosenberg SA, Robbins PF. T cells associated with tumor regression recognize frameshifted products of the CDKN2A tumor suppressor gene locus and a mutated HLA class I gene product. J Immunol. 2004;172:6057. doi: 10.4049/jimmunol.172.10.6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altman JD, Moss PAH, Goulder PJR, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- 9.Romero P, Gervois N, Schneider J, Escobar P, Valmori D, Pannetier C, Steinle A, Wolfel T, Lienard D, Brichard V, et al. Cytolytic T lymphocyte recognition of the immunodominant HLA-A*0201- restricted Melan-A/MART-1 antigenic peptide in melanoma. J Immunol. 1997;159:2366. [PubMed] [Google Scholar]
- 10.Kawakami Y, Eliyahu S, Jennings C, Sakaguchi K, Kang X, Southwood S, Robbins PF, Sette A, Appella E, Rosenberg SA. Recognition of multiple epitopes in the human melanoma antigen gp100 by tumor-infiltrating T lymphocytes associated with in vivo tumor regression. J Immunol. 1995;154:3961. [PubMed] [Google Scholar]
- 11.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 12.Mackall CL, Hakim FT, Gress RE. Restoration of T-cell homeostasis after T-cell depletion. Semin Immunol. 1997;9:339. doi: 10.1006/smim.1997.0091. [DOI] [PubMed] [Google Scholar]
- 13.Fry TJ, Connick E, Falloon J, Lederman MM, Liewehr DJ, Spritzler J, Steinberg SM, Wood LV, Yarchoan R, Zuckerman J, et al. A potential role for interleukin-7 in T-cell homeostasis. Blood. 2001;97:2983. doi: 10.1182/blood.v97.10.2983. [DOI] [PubMed] [Google Scholar]
- 14.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Inter-leukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu P, Spiotto MT, Lee Y, Schreiber H, Fu YX. Complementary role of CD4+ T cells and secondary lymphoid tissues for cross-presentation of tumor antigen to CD8+ T cells. J Exp Med. 2003;197:985. doi: 10.1084/jem.20021804. [DOI] [PMC free article] [PubMed] [Google Scholar]


