Abstract
The Candida albicans ALS (agglutinin-like sequence) gene family encodes eight cell-surface glycoproteins, some of which function in adhesion to host surfaces. ALS genes have a central tandem-repeat-encoding domain comprised entirely of head-to-tail copies of a conserved 108-bp sequence. The number of copies of the tandemly repeated sequence varies between C. albicans strains and often between alleles within the same strain. Because ALS alleles can encode different-sized proteins that may have different functional characteristics, defining the range of allelic variability is important. Genomic DNA from C. albicans strains representing the major genetic clades was PCR-amplified to determine the number of tandemly repeated sequence copies within the ALS5 and ALS6 central domain. ALS5 alleles had 2 to 10 tandem repeat sequence copies (mean = 4.82 copies) while ALS6 alleles had 2 to 8 copies (mean = 4.00 copies). Despite this variability, tandem repeat copy number was stable in C. albicans strains passaged for 3000 generations. Prevalent alleles and allelic distributions varied among the clades for ALS5 and ALS6. Overall, ALS6 exhibited less variability than ALS5. ALS5 deletions can occur naturally in C. albicans via direct repeats flanking the ALS5 locus. Deletion of both ALS5 alleles was associated particularly with clades III and SA. ALS5 exhibited allelic polymorphisms in the coding region 5’ of the tandem repeats; some alleles resembled ALS1, suggesting recombination between these contiguous loci. Natural deletion of ALS5 and the sequence variation within its coding region suggest relaxed selective pressure on this locus, and that Als5p function may be dispensable in C. albicans or redundant within the Als family.
1. Introduction
The Candida albicans ALS gene family includes eight genes (ALS1 to ALS7, and ALS9) that encode large cell-surface glycoproteins (Hoyer et al., 2007). The ALS genes share a similar basic organization consisting of a relatively conserved 5’ domain, a central domain of tandemly repeated sequence units, and a 3’ domain of relatively variable length and sequence (Hoyer, 2001; Hoyer et al., 2007). Although the tandem repeat units in each ALS gene are 108 bp, the basic sequence of the repeat unit is variable and serves to group ALS genes into three subfamilies consisting of i). ALS1 to ALS4; ii). ALS5 to ALS7; and iii). ALS9 (Hoyer et al., 2007). Deletion of individual ALS genes and phenotypic testing of the resulting mutant C. albicans strains demonstrated that ALS1, ALS2, ALS3, ALS4 and ALS9 contribute to C. albicans adhesion (Fu et al., 2002; Zhao et al., 2004, 2005, 2007). Overexpression of ALS genes in S. cerevisiae suggested an adhesive role for ALS5 and ALS6 (Gaur and Klotz, 1997; Sheppard et al., 2004), but this role has not been demonstrated in C. albicans. Despite what is known about Als protein function, it is still unclear whether the family exists to provide C. albicans with different specificities of the same basic function (for example, adhesion to a variety of host surfaces) or to provide C. albicans with redundancy of critical functions. The high level of allelic variability found within the ALS family complicates studies that address these questions.
ALS allelic variability is most obvious within the central tandem repeat domain of each gene (Hoyer et al., 2007). For a few of the ALS genes, sequence variability exists within the 5’ domain, which is believed to encode the main adhesive domain of the Als protein (Hoyer and Hecht, 2000; Zhao et al., 2003), and in repeated regions within the 3’ domain, which encodes a serine/threonine-rich, heavily glycosylated portion of the mature protein (Zhang et al., 2003; Zhao et al., 2003). At the ALS3 locus, C. albicans strains tend to maintain heterozygous alleles with respect to the number of copies of the tandem repeat sequence present in the central domain (Oh et al., 2005). In a collection of clinical isolates, the mean difference in number of tandem repeat copies between two ALS3 alleles in the same strain was 2.6. Phenotypic testing of derivatives of C. albicans strain SC5314 showed that the ALS3 allele with 12 tandem repeat copies made the major contribution to C. albicans adhesion to endothelial and epithelial surfaces, while the ALS3 allele with 9 tandem repeat copies made a significant, but very minor adhesive contribution (Oh et al., 2005). This work suggested the possibility that C. albicans maintains two distinct ALS3 alleles, potentially for different functions. This theme was also illustrated by the study of ALS9, which displays the greatest number of types of allelic variability within the ALS family. Within the 5’ domain, ALS9 alleles are 11% different at the nucleotide level (16% different at the amino acid level; Zhao et al., 2003). Like all other ALS genes, ALS9 tandem repeat copy number varies within the central domain. Within the 3’ domain, certain ALS9 alleles have extra sequence blocks that are absent in other alleles (Zhao et al., 2003). In strain SC5314, the ALS9-2 allele, but not the ALS9-1 allele, restored adhesive function to an als9Δ/als9Δ strain (Zhao et al., 2007). Examination of the clinical isolate collection indicated extensive recombination at the ALS9 locus with an obvious preference for ALS9-2 allelic sequences (Zhao et al., 2003; Zhao et al., 2007).
These examples emphasize the allelic complexity within the ALS family and prompted analysis of other ALS loci to define their allelic variability. Knowledge of allelic variability provides the context required to draw accurate functional conclusions about Als proteins. The focus of this paper is ALS5 (Gaur and Klotz, 1997; Hoyer and Hecht, 2001) and ALS6 (Hoyer and Hecht, 2000). ALS5 and ALS6 share a cross-hybridizing tandem repeat motif and nearly 100% sequence identity within the 3’ domain (Hoyer and Hecht, 2000). The 5’ domain of ALS5 is nearly 80% identical to that of ALS1 and ALS3, while the 5’ domain of ALS6 is relatively unique within the ALS family (Hoyer etal., 2007). This study examines sequence variability within the central tandem repeat domain and also within other regions of the genes that may affect function of its encoded protein. The strain collection that was used for analysis of ALS3 alleles was also used in this study, which provides the opportunity for direct comparisons between the loci.
2. Materials and methods
2.1. C. albicans strains
The collection of clinical isolates used in this study was obtained from three populations previously analyzed by Ca3 fingerprinting (Blignaut et al., 2002; Pujol et al., 1997, 2002) and included 88 isolates from the United States and Canada, 71 from South Africa, 25 from Europe, 8 from South America, and 4 from Turkey and Israel. Clades in C. albicans were originally described by using the Ca3 fingerprinting method (Blignaut et al., 2002; Pujol et al., 1997, 2002; Soll and Pujol, 2003) and have since been confirmed by MultiLocus Sequence Typing (MLST; Robles et al., 2004; Tavanti et al., 2005; Odds et al., 2007; M. E. Bougnoux. C. Pujol, D. Diogo, C. Bouchier, D. R. Soll and C. d’Enfert, unpublished data). Clade status of most of the strains used here was confirmed by MLST (Odds et al., 2007 Tavanti et al., 2005). The collection included 51 clade I isolates, 47 clade II isolates, 40 clade III isolates, 22 clade E isolates and 36 clade SA isolates. clade E was subdivided into the two Ca3-defined subgroups (Pujol et al., 2002), Ea (12 strains in this study) and Eb (10 strains) that were recently shown to represent distinct clades by MLST analysis (Tavanti et al., 2005). An additional 1047 C. albicans isolates were assembled from those collected by the ARTEMIS Global Antifungal Surveillance Program (Pfaller et al., 2005). Clade assignments were not determined for this collection with the exception of ALS5-negative strains that were typed by the Ca3 method. Strains were screened for the presence of ALS5 using PCR- and Southern blot-based methods (see below). Additional verification that the ALS5-negative strains were C. albicans was provided by PCR with the LSUF and LSUR primers (Table 1; Boucher et al., 1996), by the PCR-RFLP method using primers ITS1 and ITS4 (Table 1; Mirhendi et al., 2005), and by Ca3 fingerprinting (Pujol et al., 2002).
Table 1.
Oligonucleotide primers used in this study
| Primer name | Primer sequence (5’ – 3’) |
|---|---|
| LSUF | ATC AAC TTA GAA CTG GTA CGG |
| LSUR | GAT AGT AGA TAG GGA CAG TGG |
| ITS1 | TCC GTA GGT GAA CCT GCG G |
| ITS4 | TCC TCC GCT TAT TGA TAT GC |
| ALS5RepF | TTT CTC CCT CAG ATA ATA ACC AGT ATC AAT |
| ALS5RepR | AAG ACA GTT CTT CCA ATG GAT CA |
| ALS5Geno2F | GAC GCT TAT ATT TCT CCC TCA GAT AA |
| ALS5Geno2R | ATA CTT GAT GAC TGC TCA ACC AGA |
| ALS6GenoF | GGA TGG CAA AAA GGG AAA TGA T |
| ALS6/7GenoR | AAC CCA ATT GAG CTT GAT GGA A |
| ALS6Geno2F | CCT CTT ATA TAC TTT TGG ACA TCA TAC ACA |
| ALS6Geno3R | GGT GGC AGA CGT ACT GGA CT |
| ALS3GenoF | ACC TTA CCA TTC GAT CCT AAC C |
| ALS3GenoR | GAT GGG GAT TGT GAA GTG G |
| ALS3GenoF2 | CCA CAA CAC ATA CTA ATC CAA CTG A |
| ALS3GenoR2 | TGT AGA CCA CAA AGT TGT ATG GTT G |
| ALS5dn2.5kF | CTG CAA TGA TAT TGA TCT CAT |
| ALS5dn2.5kR | ATG TAA CCT GTC TAG AGA TGA |
| ALS5up5.3kF | GTT GTC TCT GCA ATT GTT GTA |
| ALS5up5.3kR | ACA TTA TAG TGG CAG TGG TTG |
| ALS5up3kF | GGT ACA ACA TTT TAC TTG TGA CC |
| ALS5up3kR | GTA GCA CGT AAT CGA TAA TAA C |
| ALS519upF | TGT CTG AAT GCA GTA TTA GGA GGC |
| 5GapR1 | AAT TTT CGT GTA ATC GTG ATC |
| 5GapF2 | CAA AGA AAA TAC ACA GAG AAG |
| 5GapR2 | TCA TCT TCT ATC GAA CAG TAG |
| 5StartR | AGC ATT GGA CCA AGT TAA TGA GTC |
| ALS5endF | CCA CTA AAC ATC CTT CCT GGT TGC |
| ALS5dn1.8kR | CTT AGC AAA GAG CTG TAA AGG |
| ALS5Xho | CCC CTG GAG ATG ATT CAA CAA TTT ACA TTG TTA TTC C |
| RTALS5RALT | ATT GAT ACT GGT TAT TAT CTG AGG GAG AAA |
| ALS6XhoF | CCC CTC GAG ATG AAG ACA GTA ATA CTA TTA CAT C |
| RTALS6R | ATC ATT TCC CTT TTT GCC ATC C |
| ALS6GenoSeq3F | CTA TTG CAC GAT AGC AAT GGC |
| ALS6GenoSeq3R | CTG ATC CAG TCC ATA TAC TAG TG |
| 688B | GAA ACG ATA ACT ACA GGG C |
| ALS6R | CCC CTC GAG AAA AAC AGA ACA AAA AAA ACG ACA CC |
Tandem repeat copy number stability in ALS coding regions was assessed using four different C. albicans strains that were grown for 3000 generations (Pujol et al., 1999). To grow strains for 3000 generations, a single colony was inoculated into a flask of culture medium and incubated with shaking at 37°C. Every 200 generations, the culture was plated, a single colony isolated, and inoculated into fresh culture medium. Strains were stored on agar slants and eventually in glycerol at −80°C.
2.2. ALS tandem repeat genotyping reactions
Genomic DNA was extracted from each C. albicans isolate as described previously (Oh et al., 2005) and the size of the tandem repeat domain in each ALS allele determined by PCR using two independent primer pairs (Fig. 1, Table 1). Use of two primer pairs provided an additional control for the accuracy of the results. For ALS5, primer pairs were ALS5RepF/ALS5RepR and ALS5Geno2F/ALS5Geno2R. For ALS6, primer pairs were ALS6GenoF/ALS6/7GenoR and ALS6Geno2F/ALS6Geno3R. PCR reactions were described previously (Oh et al., 2005). Each used 1x Invitrogen Taq polymerase buffer with 1 mM MgCl2. PCR products were separated on 3.5% polyacrylamide/Tris Borate EDTA gels and stained with ethidium bromide. Amplicon sizes were determined by comparison to the 1 kb ladder (Invitrogen) and to ALS5 or ALS6 amplification products from strain SC5314 genomic DNA (Fig. 1). DNA sequencing of alleles from strain SC5314 showed two different ALS5 alleles with either 4 (GenBank accession number AY227439) or 5 (GenBank accession number AY227440) copies of the tandem repeat sequence. Within the central domain, both alleles of ALS6 in strain SC5314 have 4 copies of the tandemly repeated sequence (Fig. 1). The sequence of one allele is deposited in GenBank (accession number AY225310). Stability of the tandem repeat domain was assessed using C. albicans strains that were passaged serially in vitro for 3000 generations (Pujol et al., 1999). Genomic DNA from generation zero and generation 3000 was amplified by PCR to determine the number of copies of the tandem repeat sequence as described above for ALS5 and ALS6. ALS3 tandem repeat domain analysis used primer pairs ALS3GenoF/ALS3GenoR and ALS3GenoF2/ALS3GenoR2 (Table 1) as described by Oh et al. (2005).
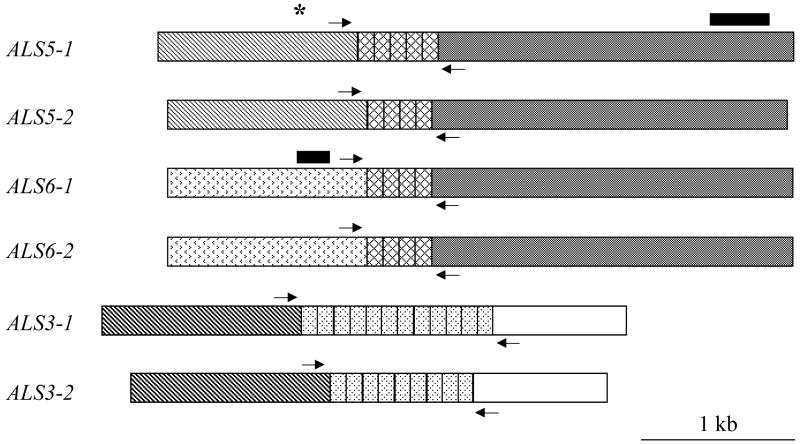
Fig. 1.
Schematic of ALS5, ALS6 and ALS3 from C. albicans strain SC5314 showing the 5’ domain, central tandem repeat domain, and 3’ domain. Genes are drawn to scale. Shading patterns indicate regions of sequence identity between the various genes. Individual tandem repeat units are indicated in the central domain of each gene, with five copies in ALS5-1, and 4 copies in ALS5-2 and in each of the ALS6 alleles. Although the tandem repeat units are similar across the ALS family, those in ALS3 (spotted) do not cross-hybridize at high stringency with the tandem repeats from ALS5 or ALS6 (cross-hatched; Hoyer et al., 1998). ALS genes can be grouped into subfamilies based on this difference in the sequence of the tandem repeat unit (Hoyer et al., 2007). Arrows (not drawn to scale) mark the approximate location of PCR primers used to determine the number of tandem repeat copies within each allele (see Materials and methods). Allelic sequences for ALS5 (AY227439, AY227440) and ALS3 (AY223551, AY223552) from strain SC5314 were deposited in GenBank, but only one ALS6 allele was reported (AY225310). Sequence AY225310 is identical to one reported by the genome sequencing project (AACQ01000074) except for two nucleotides that result in two amino acid sequence changes (755 S to R and 763 S to F) in the C-terminal domain of the protein. These differences could reflect allelic variation at the ALS6 locus. ALS5 is located in C. albicans chromosome 6, while ALS6 is on chromosome 3, and ALS3 is on chromosome R (Hoyer, 2001). Solid bars indicate Southern blot probes for ALS5 and ALS6 (see Materials and methods). The asterisk above the ALS5 schematic indicates the location of the oligonucleotide probe.
2.3. DNA sequencing of the ALS5 deletion site
The ALS5 deletion site was defined by PCR amplification with primers upstream and downstream of the ALS5 coding region and genomic DNA from C. albicans strain 20535.027. Primers ALS5dn2.5kF and ALS5dn2.5kR (Table 1) yielded a product from genomic DNA from the ALS5-negative strains, suggesting that the region 2.5 kb 3’ of ALS5 was still present. The same result was obtained for primer pair ALS5up5.3kF and ALS5up5.3kR (Table 1) suggesting that sequences approximately 5.3 kb upstream of ALS5 were still present. A PCR product was not obtained with primers ALS5up3kF and ALS5up3kR (Table 1) suggesting that the region 3 kb upstream of ALS5 was deleted in the ALS5-negative strains. This information suggested that primers ALS519upF and ALS5dn2.5kR should produce a PCR product. A 2.2 kb product was obtained, suggesting that greater than 8.5 kb of sequence was deleted in the ALS5-negative strain. The DNA sequence of this PCR product was compared to information on the Candida Genome Database (www.candidagenome.org) to define the deleted region. To determine whether the deletion site was similar in all ALS5-negative strains in the collection, genomic DNA from the strains was PCR amplified with three different primer pairs: ALS519upF/5GapR1 which produces a product of 250 bp in strain 20535.027, 5GapF2/5GapR2 which amplifies a 196-bp product, and ALS519upF/5GapF2 which amplifies a 396-bp product (Table 1). PCR products were analyzed on acrylamide gels and their size compared to a standard fragment amplified from strain 20535.027. Strains that are hemizygous for ALS5 were identified by PCR screening of strains that appeared homozygous for tandem repeat copy number. Strains that yielded a PCR product with primers ALS519upF/ALS5dn2.5R had one chromosome from which ALS5 was deleted naturally and one chromosome with an intact ALS5 allele.
DNA sequencing of the region upstream of the ALS5 locus in clade I strains was complicated by the fact that the primer pairs used for strain 20535.027 (described above) produced multiple PCR products. The periodicity of the products suggested that the 5’ region flanking the ALS5 locus may contain tandem copies of a complex repeated sequence. Primer 5StartR (Table 1), which hybridizes to sequences within the ALS5 open reading frame, was paired with primer ALS519upF and a 3.8 kb PCR product cloned into pCRBlunt (Invitrogen). The sequence downstream of ALS5 was amplified using primers ALS5endF (which hybridizes to sequences at the 3’ end of the ALS5 open reading frame) and ALS5dn1.8kR (which hybridizes to sequences 1.8 kb downstream of the ALS5 stop codon; Table 1). The PCR product was cloned as described above. DNA sequencing was completed at the Roy J. Carver Biotechnology Center (Urbana, IL).
2.4. DNA sequencing of the ALS5 and ALS6 coding regions
ALS5 or ALS6 fragments were amplified by PCR for DNA sequence analysis to assess sequence divergence within the 5’ domain. All of these PCR reactions used Pfu proofreading polymerase (Stratagene). Primers for amplification of the ALS5 fragment were ALS5Xho and RTALS5RALT (Table 1), which amplified from nt 1 to 868 within the coding region (based on GenBank sequence AY227439). Primers for amplification of the ALS6 fragment were ALS6XhoF and RTALS6R (Table 1), which amplified from nt 1 to 927 within ALS6 (based on GenBank sequence AY225310). PCR reactions were described previously (Zhao et al., 2003). Initial genotyping analysis of the ALS6 tandem repeat domain in many clade SA strains did not yield a PCR product with primers ALS6GenoF and ALS6/7GenoR. To identify the altered DNA sequence in these strains, PCR products were generated within the 5’ domain (using primers ALS6GenoSeq3F and ALS6GenoSeq3R) and within the 3’ domain (using primers 688B and ALS6R). When detected, DNA sequence polymorphisms were verified by sequencing on both strands.
2.5. Southern blots
Southern blotting methods were described previously (Hoyer et al., 1995) and used the Genius non-radioactive labeling system (Roche). Probes included an oligonucleotide specific for ALS5 (Hoyer and Hecht, 2001), a PCR fragment from the 3’ domain of ALS5 that cross-hybridizes with ALS6 (Hoyer and Hecht, 2001) and a 215-bp PCR product specific for ALS6 (Hoyer and Hecht, 2000).
2.6. Statistical methods
The distributions of the number of tandem repeat copies in the different clades and the distributions of the difference in the number of tandem repeat copies per strain in the different clades were evaluated using the non-parametric Kolmogorov-Smirnov test. This approach was selected because the data were not normally distributed. The non-randomness of allelic combinations was calculated using Fisher’s Exact test. Allele frequencies were used to calculate the expected frequencies of the diploid genotypes under the null hypothesis that combinations of alleles were random. The observed and expected numbers were then compared using Fisher’s Exact test. The same strategy was used to obtain the difference in number of repeat copies per strain expected if combinations of alleles were random. Fst statistics were assessed using Multilocus 1.3 software package, available at http://www.agapow.net/software/multilocus (Agapow and Burt, 2001). The program calculates Weir’s (Weir, 1996) formulation of Wright’s Fst. The calculated statistic, θ, is an approximateion of the Fst. Fst statistics are used in population genetics studies as an estimate of the level of subdivision among populations to test their differentiation. An Fst of 0 means that there is no subdivision (the populations do not show any statistical difference), where an Fst of 1 means that the two populations are completely isolated genetically (they have nothing in common). In our study, Fst statistics were used to test if ALS5 and ALS6 gene frequencies reflected the genetic divergence shown by DNA fingerprinting among C. albicans clades.
3. Results and Discussion
3.1. ALS5 allelic diversity within the tandem repeat domain
The greatest amount of sequence divergence observed among ALS alleles occurs within the central tandem-repeat-encoding domain, mainly due to variability in the number of tandem repeat copies present (Hoyer et al., 2007). For this reason, much of the analysis of allelic variability presented here focused on that region. Within the central domain, ALS5 alleles in the strains examined encoded between 2 and 10 copies of the tandemly repeated 108-bp sequence (Table 2). The mean number of repeat copies per allele was 4.82 ± 1.61. Alleles containing 4 tandem repeat copies were the most common and also represented the median of the population. Overall, the clades differed with respect to prevalent ALS5 alleles and allele distribution (Table 2). Alleles with 4 tandem repeat copies were common in all clades except Eb where alleles with 6 repeat copies represented 88% of the total. Other less common alleles were relatively restricted to specific clades, such as 2 repeat copies in clade III, 5 in clade I, and 8 in clade II and Ea. The relative distribution of the number of repeat copies per allele was significantly different between all clades except III vs. Ea, III vs. SA, and Ea vs. SA (Table 2).
Table 2.
ALS5 alleles displayed by clade and tandem repeat copy number
| Percent alleles in each tandem repeat copy number group
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clade | No. alleles analyzed | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Mean no. of repeat copies per allelea |
| I | 96 | 0 | 0 | 65 | 33 | 0 | 1 | 1 | 0 | 0 | 4.43 ± 0.71 |
| II | 57 | 2 | 2 | 35 | 5 | 3 | 7 | 42 | 2 | 2 | 6.16 ± 2.03 |
| III | 44 | 25 | 0 | 61 | 0 | 0 | 2 | 5 | 0 | 7 | 4.16 ± 2.11 |
| Ea | 22 | 0 | 0 | 73 | 9 | 0 | 0 | 18 | 0 | 0 | 4.82 ± 1.56 |
| Eb | 17 | 6 | 0 | 6 | 0 | 88 | 0 | 0 | 0 | 0 | 5.65 ± 1.06 |
| SA | 41 | 2 | 5 | 78 | 10 | 0 | 0 | 0 | 2 | 2 | 4.27 ± 1.30 |
|
| |||||||||||
| Total | 277 | 5 | 1 | 57 | 15 | 6 | 3 | 11 | 1 | 2 | 4.82 ± 1.67 |
Mean number of repeat copies per allele ± standard deviation. Frequencies are for ALS5 alleles after strains were tested for hemizygous configuration. Using the Kolmogorov-Smirnov test, the P values between clades in the relative distribution of the number of tandem repeats per allele were the following: I vs. II, P < 0.001; I vs. III, P < 0.001; I vs. Ea, P < 0.001; I vs. Eb, P < 0.001; I vs. SA, P < 0.001; II vs. III, P < 0.001; II vs. Ea, P = 0.006; II vs. Eb, P = 0.001; II vs. SA, P < 0.001; III vs. Ea, not significant; III vs. Eb, P < 0.001; III vs. SA, not significant; Ea vs. Eb, P < 0.001; Ea vs. SA, not significant; Eb vs. SA, P < 0.001. Bold type is used for alleles that comprise more than 10% of the total alleles studied.
3.2. Spontaneous deletion of ALS5 in C. albicans clinical isolates
Some C. albicans strains did not produce PCR products with either ALS5 tandem repeat genotyping primer pair in this analysis suggesting large sequence polymorphisms at the priming sites or absence of the ALS5 locus. These strains were further studied by Southern blotting using ALS5-specific sequences as probes. Fourteen of the strains from the current collection (7.4%) did not have an ALS5-hybridizing band with either the oligonucleotide or PCR product probe (see Materials and methods), suggesting that these strains lacked the ALS5 locus. Previous work with a collection of 50 clinical isolates yielded 8% ALS5-negative strains (Hoyer and Hecht, 2001). Analysis of strains was expanded to a collection of 1047 C. albicans isolates (see Methods). In this collection, 17 ALS5-negative isolates (1.6%) were identified. Eight of the 17 strains were bloodstream isolates with the other strains isolated from the respiratory tract (3 isolates), abscess or tissue (3), pleural fluid (1), or ascitic fluid (1). These ALS5-negative strains were isolated from patients ranging in age from infancy (< 1 year of age) to elderly (> 80 years). Variations in the estimates of percent ALS5-negative isolates are likely the results of different clade representation within the various strain collections assayed (see below).
Deletion of ALS5 is due to direct repeats that are present 5’ and 3’ of the coding region (Fig. 2). The boundaries of the deleted region were estimated by PCR and then pinpointed by DNA sequencing (see Materials and methods). Because of the size differences between ALS5 alleles, the length of the deleted region varies in each C. albicans isolate. However, PCR amplification using primers that span the deleted region (see Materials and methods) produced the same-sized products in each ALS5-negative strain suggesting that the deletion occurred in the same general location in all ALS5-negative isolates, regardless of their source. PCR amplification of genomic DNA from each of the ALS5-negative strains using a primer set specific for each of the other ALS genes (Green et al., 2004) suggested that the remainder of the ALS family is present in each strain.
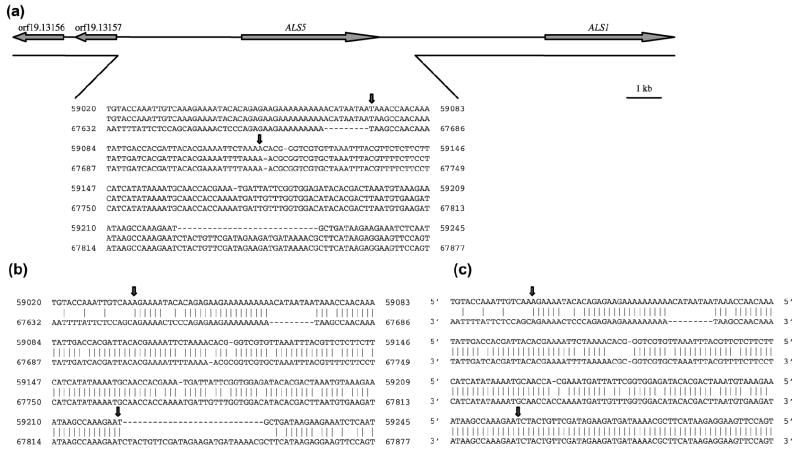
Fig. 2.
(a) Scale drawing of the chromosome 6 region that encodes ALS5 to indicate the sequences deleted in naturally occurring ALS5-negative strains. DNA sequence information was taken from the Candida Genome Database (http://www.candidagenome.org) Contig 19-20233; sequence coordinates are shown. Deletion of ALS5 is mediated by direct repeats that are 5’ and 3’ of ALS5. The gap in the line below the schematic chromosome indicates the deleted region, which extends from approximately 3.5 kb upstream of ALS5 to 1.0 kb 3’ of the ALS5 stop codon. The total size of the deleted fragment depends upon the size of the ALS5 allele encoded on that chromosome. An ALS5-negative strain has undergone recombination events to remove both alleles from the genome. Alignment of the direct repeat sequence from 5’ of ALS5 (top line; coordinates 59020 to 59425) and 3’ of ALS5 (bottom line; coordinates 67632 to 67877), and the sequence derived from an ALS5-negative clinical isolate (middle line), is shown. Vertical arrows above the sequence alignment delimit the region in which recombination occurs between the 5’ and 3’ direct repeats. (b) Same DNA sequences from upstream and downstream of ALS5 that were shown in (a), but with vertical lines to indicate sequence identity within the region of ALS5 deletion. Vertical arrows delimit the region used to calculate percentage identity (85.3%) between the sequences. Calculation of the length of the direct repeat sequences is complicated because regions of high identity are interspersed with sequence gaps. (c) DNA sequences from the same region in a clade I isolate (see Materials and methods). The similar percentage identity for these sequences (84.7%) to that for the sequences in (b) suggests that recombination to delete ALS5 should be able to occur in this isolate. The higher percentage of sequence identity 3’ of the region delimited by arrows suggests an even higher recombination potential in this strain. It is unclear why strains in clade I have not accumulated deleted ALS5 alleles similar to strains in other clades.
Identification of C. albicans clinical isolates in which both ALS5 alleles are deleted suggests that there is not a strong selective pressure to maintain this gene. Creation of a viable als5Δ/als5Δ mutant strain supports the conclusion that ALS5 is not essential (Zhao et al., in press). The ability of C. albicans to lose ALS5 spontaneously might also suggest that Als5p function is at least partially redundant. It is possible that some strains lose a single copy of ALS5 without consequence. Such hemizygous strains would be recorded as homozygous using the genotyping strategy presented above. Therefore, the strain collection was screened again using PCR to identify hemizygous C. albicans strains (Table 3). Allelic frequencies recorded in Table 1 were adjusted to reflect this new information. Overall, 38% of the strains tested were missing one ALS5 allele (Table 3). These strains were frequent in all clades except clade I (4% hemizygous). Fisher’s exact test showed that the proportion of clade I strains with at least one deleted ALS5 allele is significantly lower than in the remainder of the strain collection (P = 4 × 10-13). Similarly, the proportion of ALS5-deleted clade I alleles was significantly lower than in the remaining strains (P = 3 × 10-13). These results suggest the potential for selective pressure to maintain two functional ALS5 alleles in clade I strains. Alternatively, clade I may be more recently evolved and has not accumulated as many deleted ALS5 alleles. Strains with both ALS5 alleles deleted were found only in clades III and SA (Table 3). Despite the large number of hemizygous strains in clade II, no deleted strains were observed. Among the 43 clade II isolates analyzed, none were lacking both ALS5 alleles although 5 null strains were expected if allelic combinations were random (P = 0.019). This result suggests that selective pressure in clade II strains may maintain at least one functional ALS5 allele. Fst statistics were used to test if ALS5 allele composition reflected clade genetic divergence and showed significant genetic differentiation between most pairs of clades (Table 3). These results indicate that in addition to allelic divergence among clades, differences in frequency of deleted alleles were also present. Deleted alleles were found in all clades and appeared to be due to the same recombination mechanism. In contrast, the proportion of deleted alleles (clade I) or the frequency of ALS5 null strains (clade II) varied dramatically in function of clades. This observation demonstrates that ALS5 genetic diversity is strongly affected by genetic background and should be taken into consideration in future studies.
Table 3.
ALS5 allelic differences within clades
| Clade | Number of strains analyzed | Percent heterozygous strains | Percent heterozygous strains | Percent heterozygousa strains | Percent deleteda strains | Most common allelic combinationsb | |
|---|---|---|---|---|---|---|---|
| I | 49 | 43 | 53 | 4 | 0 | 4/4 (41%)
5/5 (12%) |
4/5 (41%) |
| II | 43 | 12 | 21 | 67 | 0 | 8/Δ (39%)
4/Δ (12%) |
4/4 (14%) |
| III | 39 | 10 | 18 | 56 | 15 | 4/Δ (49%) | Δ/Δ (15%) |
| Ea | 12 | 0 | 83 | 17 | 0 | 4/4 (58%)
4/Δ (17%) |
8/8 (17%) |
| Eb | 10 | 10 | 60 | 30 | 0 | 6/6 (60%) | 6/Δ (30%) |
| SA | 35 | 6 | 34 | 40 | 23 | 4/Δ (31%)
Δ/Δ (23%) |
4/4 (28%) |
|
| |||||||
| Total | 188 | 18 | 37 | 38 | 7 | 4/Δ (38%)
4/5 (12%) |
4/4 (25%) |
The following Fst statistics were computed between clades to test ALS5 genetic differentiation: I vs. II, θ = 0.247, P < 10-4; I vs. III, θ = 0.243, P < 10-4; I vs. Ea, θ = 0.058, not significant; I vs. Eb, θ = 0.506, P < 10-4; I vs. SA, θ = 0.183, P < 10-4; II vs. III, θ = 0.059, P = 0.0003; II vs. Ea, θ = 0.141, P = 0.0017; II vs. Eb, θ = 0.313, P < 10-4; II vs. SA, θ = 0.079, P = 0.0009; III vs. Ea, θ = 0.157, P = 0.0001; III vs. Eb, θ = 0.359, P < 10-4; III vs. SA, θ = 0.013, not significant; Ea vs. Eb, θ = 0.490, P = 0.0001; Ea vs. SA, θ = 0.111, P = 0.023; Eb vs. SA, θ = 0.397, P < 10-4.
Hemizygous strains: strains where one ALS5 allele is missing. Deleted strains: strains that are lacking the ALS5 locus.
Genotypes found in more than 10% of the isolates of each sample. Δ, deleted allele.
3.3. ALS5 allelic diversity in the 5’ domain
Previous work showed the presence of allelic variability within the ALS5 5’ domain (Hoyer and Hecht, 2001). Subsequent analysis indicated that sequences from strains 1161 and CA1 represented the extremes of this DNA sequence variability (Zhao et al., 2003). Although ALS5 alleles from other strains tended to be more like the CA1 allele, strains that encoded 1161-like alleles were found in clades III, Ea and Eb (Table 4). However, not all strains in these clades encoded the sequence variations. The 1161-like ALS5 sequence variations resembled the sequence of ALS1 in this region suggesting the possibility of recombination between the highly similar 5’ domain sequences of the contiguous ALS5 and ALS1 coding regions (Fig. 2; Zhao et al., 2003).
Table 4.
Alignment of ALS5 5’ domain nucleotide sequences from various alleles
| Strain Name | Cladea | Nucleotide sequence (nt 324-nt 390)b | Number of Tandem Repeat Copiesc |
|---|---|---|---|
| CA1d | TCTTCATTAAAATGTACAGTGAACAATAATTTGAGATCATCTATTAAGGCTTTGGGTACGGTTACTTTA | 6 | |
| SC5314-1d | I | TCTTCATTAAAATGTACAGTGAACAATAATTTGAGATCATCTATTAAGGCTTTGGGTACGGTTACTTTA | 5 |
| SC5314-2d | I | TCTTCATTAAAATGTACAGTGAACAATAATTTGAGATCATCTATTAAGGCTTTGGGTACGGTTACTTTA | 4 |
| K221 | I | TCTTCATTAAAATGTACAGTGAACAATAATTTGAGATCATCTATTAAGGCTTTGGGTACGGTTACTTTA | 4,5 |
| P57047 | II | TCTTCATTAAAATGTACAGTGAACAATAATTTGAGATCATCTATTAAGGCTTTGGGTACGGTTACTTTA | 4,4 |
| P52013 | Eb | TCTTCATTAAAATGTACAGTGAACAATAATTTGAGATCATCTATTAAGGCTTTGGGTACGGTTACTTTA | 6,6 |
| OKP77 | SA | TCTTCATTAAAATGTACAGTGAACAATAATTTGAGATCATCTATTAAGGCTTTGGGTACGGTTACTTTA | 4,4 |
| OKP109 | SA | TCTTCATTAAAATGTACAGTGAACAATAATTTGAGATCATCTATTAAGGCTTTGGGTACGGTTACTTTA | 4,4 |
| P69 | SA | TCTTCATTAAAATGTACAGTGAACAATAATTTGAGATCATCTATTAAGGCTTTGGGTACGGTTACTTTA | 10,10 |
| P22095 | II | TCTTCATTAAAATGTACAATGAACAATAATTTGAGATCATCTATTAAGGCTTTGGGTACGGTTACTTTA | 8,Δ |
| P57045 | Eb | TCTTCATTAACATGTACAGTGAACAATACTTTGAGATCATCCATTAAGGCATTGGGTACGGTTACTTTA | 6,6 |
| P52073 | I | TCTACATTAACATGTACAGTGAACGACACTTTGAGATCATCCATTAAGGCTTTGGGTACGGTTACTTTA | 4,4 |
| P52098 | III | TCTACATTAACATGTACAGTGAACGACGATTTGAGATCATCCATTAAGGCATTGGGTACAGTTACTTTA | 4,4 |
| P22059 | Ea | TCTTCATTAACATGTACAGTGAACGATGCTTTGAGATCATCCATTAAGGCATTGGGTACGGTTACTTTA | 4,Δ |
| P22079 | III | TCTACATTAACATGTACTGTGAACGACGCTTTGAAATCATCCATTAAGGCATTTGGTACAGTTACTTTA | 2,Δ |
| 1161d | TCTACATTAACATGTACTGTGAACGACGCTTTGAAATCATCCATTAAGGCATTTGGTACAGTTACTTTA | 2 | |
| ALS1 | TCTACATTAACATGTACTGTGAACGACGCTTTGAAATCATCCATTAAGGCATTTGGTACAGTTACTTTA | N/A | |
|
| |||
| Consensus Sequence | TCT CATTAA ATGTAC_ TGAAC A TTTGA ATCATC_ATTAAGGC_TT GGTAC_GTTACTTTA | ||
Clade assignments, derived by Ca3 fingerprinting, are shown. Using this method, strains CA1 and 1161 do not cluster to any of the major clades. These strains are included because they are the source of ALS5 sequences in GenBank. Other strains chosen for this analysis represent different ALS5 tandem repeat allelic combinations within the various clades.
Nucleotide sequences from each strain in frame. Only the portion of the 5’ domain sequence that showed the greatest sequence variability is shown. Nucleotides that do not match the majority consensus sequence are underlined. Double underlining denotes nucleotides that alter the predicted amino acid sequence. The ALS1 sequence shown is from GenBank accession number L25902.
Δ, deleted allele. N/A, not applicable. The distribution of tandem repeat copy numbers is different for ALS1 and is not applicable to the current study.
Nucleotide sequences from these strains are in GenBank. CA1 = accession number AF025429; SC5314-1 = accession number AY227440; SC5314-2 = accession number AY227439; 1161 = accession number AF068866. For these alleles, the number of tandem repeat copies was determined by DNA sequencing of a cloned fragment, so the results for only one allele are shown. For all other entries, results were derived by PCR, so tandem repeat copy number for both alleles is shown.
3.4. ALS6 allelic diversity within the tandem repeat domain
ALS6 alleles encoded between 2 and 8 copies of the tandem repeat sequence within the central domain of the gene (Table 5). The mean number of tandem repeat copies per allele was 4.00 ± 0.98. Like ALS5, the median and most common alleles had 4 tandem repeat copies. Alleles with 3 or 4 tandem repeat copies were predominant in all clades except SA, where alleles with 6 repeat copies represented 65% of those tested. In clades I and Eb, nearly all alleles encoded 4 repeat copies. Despite this similarity in allelic composition, the relative distribution of the number of tandem repeat copies per allele differed significantly between all clades with the exception of II vs. III (Table 5).
Table 5.
ALS6 alleles displayed by clade and tandem repeat copy number
| Percent alleles in each tandem repeat copy number group
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Clade | No. alleles analyzed | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Mean no. of repeat copies per allelea |
| I | 102 | 0 | 2 | 97 | 1 | 0 | 0 | 0 | 3.99 ± 0.17 |
| II | 92 | 1 | 52 | 38 | 2 | 4 | 0 | 2 | 3.65 ± 1.00 |
| III | 74 | 0 | 54 | 38 | 0 | 5 | 3 | 0 | 3.64 ± 0.92 |
| Ea | 24 | 0 | 54 | 46 | 0 | 0 | 0 | 0 | 3.46 ± 0.51 |
| Eb | 20 | 0 | 10 | 90 | 0 | 0 | 0 | 0 | 3.90 ± 0.31 |
| SA | 60 | 0 | 8 | 22 | 5 | 65 | 0 | 0 | 5.27 ± 1.07 |
|
| |||||||||
| Total | 372 | 0.3 | 30 | 55 | 1.6 | 12 | 0.5 | 0.5 | 4.00 ± 0.98 |
Mean number of repeat copies per allele ± standard deviation. Using the Kolmogorov-Smirnov test, the P values between clades in the relative distribution of the number of tandem repeats per allele were the following: I vs. II, P < 0.001; I vs. III, P < 0.001; I vs. Ea, P < 0.001; I vs. Eb, P < 0.001; I vs. SA, P < 0.001; II vs. III, not significant; II vs. Ea, P = 0.004; II vs. Eb, P = 0.003; II vs. SA, P < 0.001; III vs. Ea, P = 0.008; III vs. Eb, P = 0.003; III vs. SA, P < 0.001; Ea vs. Eb, P = 0.019; Ea vs. SA, P < 0.001; Eb vs. SA, P < 0.001. Bold type is used for alleles that comprise more than 10% of the total alleles studied.
Although the overall analysis showed a minority of strains were heterozygous with respect to repeat copy number at the ALS6 locus (36%), great differences were observed among clades (Table 6). ALS6-homozygous strains were more prevalent in clades I, Eb and SA (Table 6). Similar to ALS5, the most common allelic combination was 4/4, but the 3/4 combination was observed almost as frequently (Table 6). The mean difference in number of repeat copies between the two alleles of the same strain was less than 1.0 for each clade and varied from 0 in clade Eb to 0.92 ± 0.29 in clade Ea. The mean difference for the entire strain collection was 0.38 ± 0.54. The mean difference in number of tandem repeats differed significantly between most clades except II, III and Ea (Table 6). Fst statistics were used to test if ALS6 allele composition reflected clade genetic divergence and showed significant ALS6 genetic differentiation between most pairs of clades (Table 6).
Table 6.
ALS6 allelic differences within clades
| Clade | Number of strains analyzed | Percent heterozygous strains | Percent homozygous strains | Mean difference in number of repeat copiesa | Most common allelic combinationsb | ||
|---|---|---|---|---|---|---|---|
| I | 51 | 4 | 96 | 0.06 ± 0.31 | 4/4 (96%) | ||
| II | 46 | 59 | 41 | 0.65 ± 0.64 | 3/4 (54%) | 3/3 (24%) | 4/4 (11%) |
| III | 37 | 57 | 43 | 0.55 ± 0.50 | 3/4 (57%) | 3/3 (27%) | 4/4 (11%) |
| Ea | 12 | 92 | 6 | 0.92 ± 0.29 | 3/4 (92%) | ||
| Eb | 10 | 0 | 100 | 0 | 4/4 (90%) | ||
| SA | 30 | 20 | 80 | 0.20 ± 0.41 | 6/6 (63%) | 3/4 (18%) | 4/4 (13%) |
|
| |||||||
| Total | 186 | 36 | 64 | 0.38 ± 0.54 | 4/4 (38%),
6/6 (12%) |
3/4 (34%) | 3/3 (12%) |
Mean ± standard deviation. The following P values were computed for the relative difference in distribution of the difference in number of repeat copies between the two alleles of a strain using the Kolmogorov-Smirnov test: I vs. II, P < 0.001; I vs. III, P < 0.001; I vs. Ea, P < 0.001; I vs. Eb, P < 0.001; I vs. SA, P = 0.025; II vs. III, not significant; II vs. Ea, not significant; II vs. Eb, P < 0.001; II vs. SA, P = 0.001; III vs. Ea, not significant; III vs. Eb, P < 0.001; III vs. SA, P = 0.007; Ea vs. Eb, P < 0.001; Ea vs. SA, P < 0.001; Eb vs. SA, P = 0.001. The following Fst statistics were computed between clades to test ALS6 genetic differentiation: I vs. II, θ = 0.491, P < 10-4; I vs. III, θ = 0.527, P < 10-4; I vs. Ea, θ = 0.648, P < 10-4; I vs. Eb, θ = 0.041, not significant; I vs. SA, θ = 0.681, P < 10-4; II vs. III, θ = −0.010, not significant; II vs. Ea, θ = −0.018, not significant; II vs. Eb, θ = 0.288, P < 10-4; II vs. SA, θ = 0.336, P < 10-4; III vs. Ea, θ = −0.019, not significant; III vs. Eb, = 0.306, P = 10-4; III vs. SA, θ = 0.344, P < 10-4; Ea vs. Eb, θ = 0.325, P = 1.3 × 10-3; Ea vs. SA, θ = 0.386, P < 10-4; Eb vs. SA, θ = 0.491, P = 10-4.
Genotypes found in more than 10% of the isolates of each sample.
3.5. ALS6 allelic diversity outside of the tandem repeat domain
The majority of ALS6 alleles with 6 tandem repeat copies were found in homozygous strains from clade SA (Table 6) and were rare in other clades (Table 5). The clade SA alleles with 6 tandem repeat copies also were notable in that many failed to produce a PCR product with primers ALS6GenoF and ALS6/7GenoR. However, Southern blotting showed the presence of a fragment that hybridized to an ALS6-specific probe, suggesting that ALS6 was present (data not shown). PCR products were amplified within the 5’ and 3’ domains near the sites of genotyping primer hybridization to detect sequence changes that may interfere with amplification of the tandem repeat domain (see Materials and methods). DNA sequence analysis of these PCR products indicated that the 5’ domain sequence was like other ALS6 alleles, but that there was an 18-bp deletion in the 3’ domain from nt 1885 to 1902 (numbered using GenBank sequence AY225310 as a reference). This deletion, which was unique to strains in clade SA, prevented annealing of primer ALS6/7GenoR and explained the negative PCR reactions. The 18-bp deletion maintains the ALS6 open reading frame and predicts a protein with a C-terminal domain almost as large as those encoded by other alleles. Because of the heavy glycosylation on this portion of the Als protein (Kapteyn et al., 2000), it is likely that loss of 6 amino acids does not have a great effect on Als6p function.
To determine whether ALS6 had 5’ domain sequence polymorphisms similar to those found in ALS5, strains were selected from each clade for DNA sequence analysis. Strains analyzed included P52073 (tandem repeat genotype 4/4) and K713 (3/4) from clade I, P22092 (3/3) and P22095 (3/4) from clade II, P22061 (3/4) and P52098 (3/3) from clade III, P22059 (3/4) from clade Ea and P52013 (4/4) from clade Eb, and G1 (4/4), P69 (3/4), P22073 (4/4), GC58 (6/6) and OKP109 (6/6) from clade SA. Of the clade SA strains, P22073, GC58 and OKP109 contained the 18-bp deletion within the 3’ domain of the ALS6 coding region. PCR primers were designed to amplify the first 927 nt of the ALS6 coding region (see Methods). Sequences from strains SC5314 (GenBank accession number AY223510) and 1161 (GenBank accession number AF075293) were included for comparison. Alignment of the resulting sequences showed only three positions where nucleotide changes occurred (nt 358, 414 and 474, numbered according to AY225310). In strains P52073 (clade I) and G1 (clade SA), one of the alleles had a T/G transversion that altered amino acid 120 from Ser to Ala. At position 414, one of the alleles of P52013 (clade Eb) and both alleles of P22073, GC58 and OKP109 (all clade SA with the 3’ deletion) had a C/T transition that did not affect the amino acid sequence. The T/C transition in these same strains at position 474 was also silent.
Data from analysis of ALS6 provides a contrast to those from ALS5. While strains with natural ALS5 deletions were detected among the isolates assayed, each of the isolates encoded ALS6. Also, the sequence of the ALS6 5’ domain was more stable than that of ALS5, possibly because the ALS6 5’ domain sequence is less conserved with other ALS 5’ domains. In comparison, the 5’ domain of ALS5 is 86% identical to the 5’ domain of ALS1, which is contiguous on chromosome 6 (Fig. 2). The proximity of ALS5 and ALS1 may provide greater opportunity for recombination between them.
3.6. Analysis of ALS allelic combinations
Comparisons of data from the current study of ALS5 and ALS6 tandem repeat allelic variation to that from analysis of ALS3 (Oh et al., 2005) shows that the range of alleles was narrower for ALS5 (2 to 10 tandem repeat copies) and ALS6 (2 to 8 tandem repeat copies) compared to ALS3 (6 to 19 tandem repeat copies). The mean number of tandem repeat copies in ALS3 (11.4 copies) was far larger than for ALS5 (4.82 copies) or ALS6 (4.00 copies). Within the same C. albicans strain, ALS3 alleles have a mean of 2.56 tandem repeat copies difference between the two alleles, while the ALS5 alleles (0.80 copies difference) and ALS6 alleles (0.38 copies difference) were much more closely matched in size (Table 7). Based on allele frequencies, we also compared the observed difference in number of repeat copies between the two alleles of a strain with that expected if combination of alleles was random (Table 7). For ALS3, the observed difference was significantly greater than the expected value. In contrast, ALS5 and ALS6 each had a significantly lower mean difference than expected. These results demonstrate that ALS6 alleles or ALS5 alleles present in a strain (with two ALS5 alleles) are globally more similar to each other with respect to tandem repeat copy number than expected by chance. This result is similar to the conclusion for analysis of ALS7 (Zhang et al., 2003), but opposite to observations for ALS3 (Table 7; Oh et al., 2005).
Table 7.
Distinct patterns of non-random ALS allele combinations indicated by difference in the number of tandem repeat copies per strain
| ALS gene (number of strains) | Mean difference in number of repeat copies per strain ± SD
|
Number of homozygous strains
|
||||
|---|---|---|---|---|---|---|
| Observed | Expectedb | Significancec | Observed | Expectedb | Significanced | |
| ALS3a (196) | 2.56 ± 1.70 | 1.97 ± 1.61 | P < 10-3 | 31 | 41.6 | Not significant |
| ALS5 (188) | 0.80 ± 1.85 e | 1.59 ± 1.68 e | P < 10-3 | 70f | 37.4f | P < 10-3 |
| ALS6 (186) | 0.38 ± 0.54 | 0.95 ± 1.01 | P < 10-3 | 119 | 75.2 | P < 10-3 |
Data from Oh et al. (2005).
Expected under the hypothesis that combination of alleles were random.
The significance was calculated by using the non-parametric Kolmogorov-Smirnov test to compare the distribution of the difference in number of repeat copies between the two alleles of a strain for observed and expected data.
The significance was calculated by using the χ2 statistic.
Observed and expected values were for 101 isolates with two ALS5 alleles. The remaining 87 isolates had lost one or two ALS5 alleles and were not used to compute averages.
Strains that were missing one or two ALS5 alleles were not included among ALS5-homozygous genotypes.
In previous work, gene frequency analysis of the tandem repeat domain from ALS3 showed significant deficits of some ALS3-homozygous genotypes and more globally, the preferential pairing of alleles with less that 12 repeat copies with alleles containing more than 12 repeat copies (Oh et al., 2005). In that study, 84% of strains tested were heterozygous for ALS3 repeat copy number. By comparison, the ALS5 and ALS6 loci showed a majority of strains homozygous at these loci (Tables 3 and 6), with only 18% (ALS5) and 36% (ALS6) of strains having a heterozygous genotype. Overall ALS5 and ALS6 presented significant excesses of homozygotes and ALS3 displayed a deficit of homozygotes that was, nevertheless, non-significant (Table 7). While these global comparisons indicate that allelic distributions in ALS5 and ALS6 were distinct from ALS3, they can hardly summarize the diversity encountered between clades for these genes. In contrast to ALS3 where heterozygosity was rather homogeneous among clades (Oh et al., 2005), the percentage of heterozygous strains for both ALS5 and ALS6 differed widely in function of the clades, as did the frequency of deleted ALS5 loci (Tables 3 and 6). Intraclade allelic pairing of tandem repeat copy number in ALS5 did not significantly differ from the numbers expected if allelic combinations were random. For ALS6, clade SA strains presented a highly significant deficit in 6/x heterozygotes (where x represents any ALS6 allele with a repeat copy number other than 6; P = 2.1 × 10-4). Altogether, our data indicate that alleles of the various ALS genes have unique distributions within the major genetic clades of C. albicans, and that ALS3 allelic combinations are distinct.
3.7. Stability of the tandem repeat domain over serial passage
Analysis of ALS3 (Oh et al., 2005), ALS5 and ALS6 demonstrates the variability present within the tandem repeat domain. Tandem repeat instability has been examined in detail for Saccharomyces cerevisiae FLO1, which is part of a gene family encoding cell surface proteins involved in yeast flocculation (Verstrepen et al., 2004, 2005; Verstrepen and Klis, 2006). This work showed that frequent recombination events between intragenic tandem repeat sequences results in readily detected expansion and contraction of gene size, creating quantitative alterations in adhesion phenotypes (Verstrepen et al. 2005). The work in S. cerevisiae was suggested to serve as a model for how tandemly repeated sequences contribute to plasticity of adhesin-encoding genes in pathogenic fungi. Previous Southern-blot-based attempts to detect changes in ALS gene tandem repeat copy number over 530 generations of serial passage of C. albicans strains in vitro did not detect any changes (Hoyer et al., 1995). These results suggested that the ALS tandem repeats are relatively stable compared to other types of repeated DNA in the C. albicans genome (Hoyer et al., 1995). This analysis was revisited using PCR genotyping in order to detect smaller changes that might not be apparent in the context of larger restriction fragments, and to extend analysis over a greater number of generations of passage. Tandem repeat genotyping primers for ALS3, ALS5 and ALS6 (Table 1) were used to amplify genomic DNA from four C. albicans strains that had been grown for 3000 generations in vitro (Fig. 3; Pujol et al., 1999). The size of the tandem repeat region for each gene in each strain did not change, consistent with the conclusion that, despite the diversity of ALS alleles present among C. albicans clinical isolates, the number of tandem repeat copies present in a given allele is relatively stable. Additional investigation is required to determine if copy number changes would be more obvious for C. albicans strains passaged under different environmental conditions, such as in vivo.
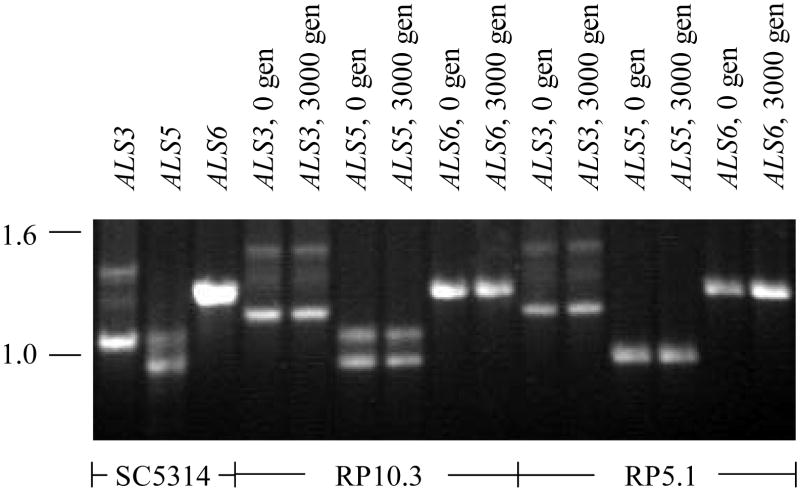
Fig. 3.
Ethidium bromide-stained agarose gel of PCR products from amplification of the tandem repeat region of various ALS genes. Products from amplification of strain SC5314 genomic DNA are shown to demonstrate the PCR strategy illustrated in Fig. 1. Primers ALS3GenoF2 and ALS3GenoR2 were used to amplify the tandem repeat domain of ALS3, primers ALS5Geno2F and ALS5Geno2R for ALS5, and ALS6Geno2F with ALS6Geno3R for ALS6 (Table 1). The tandem repeat domain from each of these genes was also amplified from genomic DNA extracted from strains RP10.3 and RP5.1 grown for either zero or 3000 generations (labeled “0 gen” and “3000 gen”, respectively in the figure). Co-migration of the PCR products from the strains at generation 0 and 3000 suggests stability of the tandem repeat copy number in each gene over serial passage in vitro. A similar result was obtained from analysis of two other C. albicans strains grown for 3000 generations (RP39.1 and RP39.2) and with the second set of PCR genotyping primers for each ALS gene (data not shown). Molecular sizes (in kb) are shown to the left of the figure.
Acknowledgments
We thank Richard Hollis and Lauren Wrobel for their work with the collection of C. albicans clinical isolates. This research was funded by NIH grants DE14158 to L.L.H. from the National Institute of Dental and Craniofacial Research and AI2392 to D.R.S. from the National Institute of Allergy and Infectious Disease, National Institutes of Health. The ARTEMIS Global Antifungal Surveillance Program is supported by a research grant from Pfizer Inc. to M.A.P. and D.J.D. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR16515-01 from the National Center for Research Resources, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agapow P-M, Burt A. Indices of multilocus linkage disequilibrium. Mol Ecol Notes. 2001;1:101–102. [Google Scholar]
- Blignaut E, Pujol C, Lockhart S, Joly S, Soll DR. Ca3 fingerprinting of Candida albicans isolates from human immunodeficiency virus-positive and healthy individuals reveals a new clade in South Africa. J Clin Microbiol. 2002;40:826–836. doi: 10.1128/JCM.40.3.826-836.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher H, Mercure S, Montplaisir S, Lemay G. A novel group I intron in Candida dubliniensis is homologous to a Candida albicans intron. Gene. 1996;180:189–196. doi: 10.1016/s0378-1119(96)00453-2. [DOI] [PubMed] [Google Scholar]
- Fu Y, Ibrahim AS, Sheppard DC, Chen YC, French SW, Cutler JE, Filler SG, Edwards JE., Jr Candida albicans Als1p: an adhesin that is a downstream effector of the EFG1 filamentation pathway. Mol Microbiol. 2002;44:61–72. doi: 10.1046/j.1365-2958.2002.02873.x. [DOI] [PubMed] [Google Scholar]
- Gaur NK, Klotz SA. Expression, cloning, and characterization of a Candida albicans gene, ALA1, that confers adherence properties upon Saccharomyces cerevisiae for extracellular matrix proteins. Infect Immun. 1997;65:5289–5294. doi: 10.1128/iai.65.12.5289-5294.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CB, Cheng G, Chandra J, Mukherjee P, Ghannoum MA, Hoyer LL. RT-PCR detection of Candida albicans ALS gene expression in the reconstituted human epithelium (RHE) model of oral candidiasis and in model biofilms. Microbiology. 2004;150:267–275. doi: 10.1099/mic.0.26699-0. [DOI] [PubMed] [Google Scholar]
- Hoyer LL. The ALS gene family of Candida albicans. Trends Microbiol. 2001;9:176–180. doi: 10.1016/s0966-842x(01)01984-9. [DOI] [PubMed] [Google Scholar]
- Hoyer LL, Scherer S, Shatzman AR, Livi GP. Candida albicans ALS1: domains related to a Saccharomyces cerevisiae sexual agglutinin separated by a repeating motif. Mol Microbiol. 1995;15:39–54. doi: 10.1111/j.1365-2958.1995.tb02219.x. [DOI] [PubMed] [Google Scholar]
- Hoyer LL, Payne TL, Hecht JE. Identification of Candida albicans ALS2 and ALS4 and localization of Als proteins to the fungal cell surface. J Bacteriol. 1998;180:5334–5343. doi: 10.1128/jb.180.20.5334-5343.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer LL, Hecht JE. The ALS6 and ALS7 genes of Candida albicans. Yeast. 2000;16:847–855. doi: 10.1002/1097-0061(20000630)16:9<847::AID-YEA562>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Hoyer LL, Hecht JE. The ALS5 gene of Candida albicans and analysis of the Als5p N-terminal domain. Yeast. 2001;18:49–60. doi: 10.1002/1097-0061(200101)18:1<49::AID-YEA646>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Hoyer LL, Green CB, Oh S-H, Zhao X. Discovering the secrets of the Candida albicans agglutinin-like sequence (ALS) gene family—a sticky pursuit. Med Mycol. 2007 doi: 10.1080/13693780701435317. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapteyn JC, Hoyer LL, Hecht JE, Muller WH, Andel A, Verkleij AJ, Makarow M, Van Den Ende H, Klis FM. The cell wall architecture of Candida albicans wild-type cells and cell wall-defective mutants. Mol Microbiol. 2000;35:601–611. doi: 10.1046/j.1365-2958.2000.01729.x. [DOI] [PubMed] [Google Scholar]
- Mirhendi H, Makimura K, Zomorodian K, Maeda N, Ohshima T, Yamaguchi H. Differentiation of Candida albicans and Candida dubliniensis using a single-enzyme PCR-RFLP method. Jpn J Infect Dis. 2005;58:235–237. [PubMed] [Google Scholar]
- Odds FC, Bougnoux ME, Shaw DJ, Bain JM, Davidson AD, Diogo D, Jacobsen MD, Lecomte M, Li SY, Tavanti A, Maiden MC, Gow NA, d’Enfert C. Molecular phylogenetics of Candida albicans. Eukaryot Cell. 2007 doi: 10.1128/EC.00041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S-H, Cheng G, Nuessen JA, Jajko R, Yeater KM, Zhao X, Pujol C, Soll DR, Hoyer LL. Functional specificity of Candida albicans Als3p protein and clade specificity of ALS3 alleles discriminated by the number of copies of the tandem repeat sequence in the central domain. Microbiology. 2005;151:673–681. doi: 10.1099/mic.0.27680-0. [DOI] [PubMed] [Google Scholar]
- Pfaller MA, Boyken L, Messer SA, Tendolkar S, Hollis RJ, Diekema DJ. Comparison of results of voriconazole disk diffusion testing for Candida species with results from a central reference laboratory in the ARTEMIS globl antifungal surveillance program. J Clin Microbiol. 2005;43:5208–5213. doi: 10.1128/JCM.43.10.5208-5213.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol C, Joly S, Lockhart SR, Noel S, Tibayrenc M, Soll DR. Parity among the randomly amplified polymorphic DNA method, multilocus enzyme electrophoresis, and Southern blot hybridization with the moderately repetitive DNA probe Ca3 for fingerprinting Candida albicans. J Clin Microbiol. 1997;35:2348–2358. doi: 10.1128/jcm.35.9.2348-2358.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol C, Joly S, Nolan B, Srikantha T, Soll DR. Microevolutionary changes in Candida albicans identified by them complex Ca3 fingerprinting probe involve insertions and deletions of the full-length repetitive sequence RPS at specific genomic sites. Microbiology. 1999;145:2635–2646. doi: 10.1099/00221287-145-10-2635. [DOI] [PubMed] [Google Scholar]
- Pujol C, Pfaller MA, Soll DR. Ca3 fingerprinting of Candida albicans bloodstream isolates from the United States, Canada, South America, and Europe reveals a European clade. J Clin Microbiol. 2002;40:2729–2740. doi: 10.1128/JCM.40.8.2729-2740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles JC, Koreen L, Park S, Perlin DS. Multilocus sequence typing is a reliable alternative method to DNA fingerprinting for discriminating among strains of Candida albicans. J Clin Microbiol. 2004;42:2480–2488. doi: 10.1128/JCM.42.6.2480-2488.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard DC, Yeaman MR, Welch WH, Phan QT, Fu Y, Ibrahim AS, Filler SG, Zhang M, Waring AJ, Edwards JE., Jr Functional and structural diversity in the Als protein family of Candida albicans. J Biol Chem. 2004;279:30480–30489. doi: 10.1074/jbc.M401929200. [DOI] [PubMed] [Google Scholar]
- Soll DR, Pujol C. Candida albicans clades. FEMS Immunol Med Microbiol. 2003;39:1–7. doi: 10.1016/S0928-8244(03)00242-6. [DOI] [PubMed] [Google Scholar]
- Tavanti A, Davidson AD, Fordyce MJ, Gow NA, Maiden MC, Odds FC. Population structure and properties of Candida albicans, as determined by multilocus sequence typing. J Clin Microbiol. 2005;43:5601–5613. doi: 10.1128/JCM.43.11.5601-5613.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstrepen KJ, Klis FM. Flocculation, adhesion and biofilm formation in yeasts. Mol Microbiol. 2006;60:5–15. doi: 10.1111/j.1365-2958.2006.05072.x. [DOI] [PubMed] [Google Scholar]
- Verstrepen KJ, Reynolds TB, Fink GR. Origins of variation in the fungal cell surface. Nat Rev. 2004;2:533–540. doi: 10.1038/nrmicro927. [DOI] [PubMed] [Google Scholar]
- Verstrepen KJ, Jansen A, Lewitter F, Fink GR. Intragenic tandem repeats generate functional variability. Nat Genet. 2005;37:986–990. doi: 10.1038/ng1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir BS. Genetic data analysis II. Sinauer Associates, Inc; Sunderland, MA, USA: 1996. [Google Scholar]
- Zhang N, Harrex AL, Holland BR, Fenton LE, Cannon RD, Schmid J. Sixty alleles of the ALS7 open reading frame in Candida albicans: ALS7 is a hypermutable contingency locus. Genome Res. 2003;13:2005–2017. doi: 10.1101/gr.1024903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Pujol C, Soll DR, Hoyer LL. Allelic variation in the contiguous loci encoding Candida albicans ALS5, ALS1 and ALS9. Microbiology. 2003;149:2947–2960. doi: 10.1099/mic.0.26495-0. [DOI] [PubMed] [Google Scholar]
- Zhao X, Oh S-H, Cheng G, Green CB, Nuessen JA, Yeater KM, Leng RP, Brown AJP, Hoyer LL. ALS3 and ALS8 represent a single locus that encodes a Candida albicans adhesin; functional comparisons between Als3p and Als1p. Microbiology. 2004;150:2415–2428. doi: 10.1099/mic.0.26943-0. [DOI] [PubMed] [Google Scholar]
- Zhao X, Oh S-H, Yeater KM, Hoyer LL. Analysis of the Candida albicans Als2p and Als4p adhesins suggests the potential for compensatory function within the Als family. Microbiology. 2005;151:1619–1630. doi: 10.1099/mic.0.27763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Oh S-H, Hoyer LL. Unequal contribution of ALS9 alleles to adhesion between Candida albicans and vascular endothelial cells. 2007 doi: 10.1099/mic.0.2006/005017-0. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Oh S-H, Hoyer LL. Deletion of ALS5, ALS6 or ALS7 increases adhesion of Candida albicans to human vascular endothelial and buccal epithelial cells. 2007 doi: 10.1080/13693780701377162. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]