Abstract
Endometriosis (ENDO) is a painful disorder defined by extrauteral endometrial growths. It is created in rats by autotransplanting pieces of uterus (which form cysts), or, for shamENDO, fat (no cysts). ENDO induces vaginal hyperalgesia, likely via central sensitization. The severity of this hyperalgesia correlates with estradiol levels during the estrous cycle, suggesting the hyperalgesia is estradiol-modulated. If so, then hyperalgesic severity should track estradiol changes during reproductive senescence (estropause) when estradiol levels initially decrease, then increase. Using psychophysical methods to assess vaginal nociception, we found that the severity of ENDO-induced hyperalgesia paralleled estradiol changes during estropause: hyperalgesia first decreased, then returned. Furthermore, the return occurred regardless of the presence of the cysts (excised in some rats). This finding provides further support for ENDO’s likely centrally-mediated effects. Additionally, the results suggest that elimination of estradiol via ovariectomy (OVX) should alleviate ENDO-induced hyperalgesia and estradiol replacement should restore it. However, in healthy and shamENDO rats, OVX produces a vaginal hyperalgesia that is alleviated by estradiol, likely via estradiol’s peripheral influences on the vagina. Hence, we tested the hypothesis that OVX in ENDO rats would trigger a different type of vaginal hyperalgesia dependent on the loss of estradiol. We predicted that the opposing influences of estradiol on ENDO- and OVX-induced hyperalgesia would cancel each other. As predicted, OVX had no effect on ENDO-induced hyperalgesia and estradiol replacement alleviated it. These results suggest that, in intact rats, ENDO-induced vaginal hyperalgesia is exacerbated by estradiol, and that different mechanisms underlie ENDO-induced versus OVX-induced vaginal hyperalgesia.
Keywords: estrous cycle, pelvic pain, viscero-visceral effects, vulvodynia, aging
1. Introduction
Endometriosis (ENDO) is a poorly-understood condition defined by extrauteral endometrial growths. It is considered dependent on estrogens because it occurs in women of childbearing age and responds to hormonal treatments that produce a hypoestrogenic state (e.g., GnRH agonists). ENDO’s symptoms include subfertility, severe dysmenorrhea, dyspareunia (vaginal hyperalgesia), dyschezia, and chronic, mainly pelvic/abdominal and muscle pains (Giudice and Kao, 2004).
ENDO is surgically induced in rats by autotransplanting pieces of uterine horn, or, in controls, fat on abdominal arteries (shamENDO; Vernon and Wilson, 1985). The uterine but not fat transplants develop over weeks into cysts with characteristics similar to ectopic growths in women (Sharpe-Timms, 2002). In rats with ENDO, the cysts disappear after ovariectomy (OVX) and reappear after estradiol (E2) replacement (OVX+E2; Vernon and Wilson, 1985). Furthermore, like women with ENDO, rats with ENDO are subfertile (Sharpe-Timms, 2002) and exhibit vaginal and abdominal muscle hyperalgesia, whose severity varies with estrous in parallel with changes in circulating estradiol levels (Cason et al., 2003; Nagabukuro and Berkley, 2007). These findings suggest that the hyperalgesia in this model is estradiol-modulated and at least partially centrally-mediated, because cysts are located far from the vagina and the abdominal muscle hyperalgesia disappears after spinalization (Nagabukuro and Berkley, 2007).
If ENDO-induced hyperalgesia is E2-modulated, then hyperalgesic severity should track E2 changes during natural reproductive senescence (“estropause;” Chakraborty and Gore, 2004). At the beginning of estropause, rats, like women, cycle irregularly, but after cycling ceases, E2 levels in rats, unlike those in women and mice (vom Saal et al., 1994), fall, not to zero, but to steady moderate levels and then increase (Fig. 1; Lu et al., 1979; Nass et al., 1984).
Figure 1.
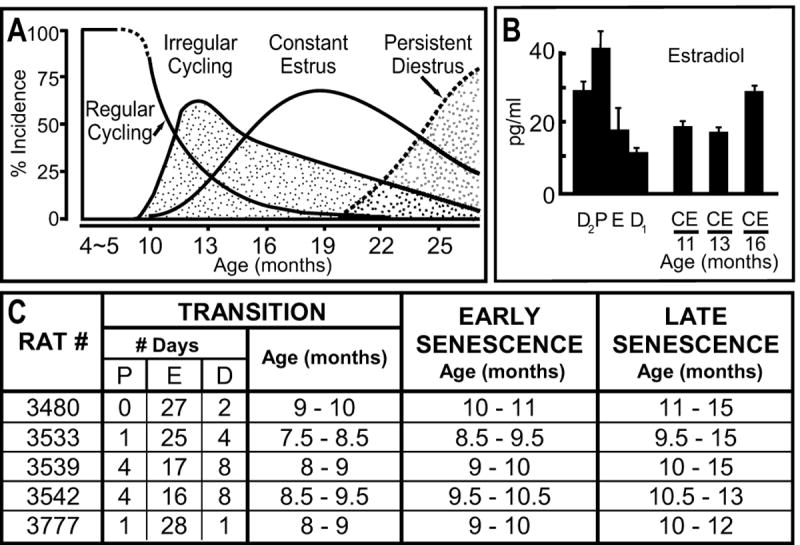
Changes in cycling patterns and plasma E2 levels as rats age through estropause. A and B are modified from Lu et al. (1979), with permission. A shows changes in cycling patterns of Long-Evans rats, whereas B shows how E2 levels change from regular four-day cycles of high and low E2 levels to moderate levels after the Long-Evans rats have stopped cycling and have entered into a constant estrus (CE) condition. Note that E2 levels then increase after several months of CE. Not shown here is the pattern of E2 changes during the transitional phase of irregular cycling. During irregular cycling, ovarian E2 levels are somewhat higher than they are in corresponding parts of the cycle in regularly cycling rats (Peluso et al., 1979). The E2 levels D1, D2, and E are, however, still lower than those in P in regularly cycling rats (Peluso et al., 1979). The table in C lists the pattern of vaginal cytological changes and ages of the rats in the present experiment as they progressed through estropause. Note two features of these rats. First, they progress through estropause at ages about 2 months younger than the rats in the Lu et al. (1979) study. Thus, estradiol levels in the rats in this study during their “late senescence” period are likely higher than their levels during “early senescence.” Second, during the transition period for the rats in this study, the predominant stage was E, indicating that overall the estradiol level in these rats during the transition period was less than it was when they were cycling. P, proestrus; E, estrus; D1, diestrus 1 (sometimes called metestrus); D2, diestrus 2 (sometimes called diestrus).
By this logic, OVX, which immediately reduces E2 levels to near zero (Chakraborty and Gore, 2004), should eliminate the hyperalgesia, and E2 replacement (OVX+E2) should reinstate it. However, OVX in intact rats produces vaginal hyperalgesia similar in severity to that produced by ENDO (Bradshaw and Berkley, 2002). Similarly, women can develop vaginal hyperalgesia postmenopausally or after oophorectomy (Bachmann and Nevadunsky, 2000). In contrast to ENDO-induced hyperalgesia, OVX-induced hyperalgesia is thought to be due to peripheral effects of E2 depletion on the vagina (Pessina et al., 2006; Bachmann and Nevadunsky, 2000). As expected, E2 replacement reverses OVX-induced hyperalgesia in rats and women (Bradshaw and Berkley, 2002; Bachmann and Nevadunsky, 2000).
Thus, there are two hypotheses. (i) In an intact female rat, ENDO-induced vaginal hyperalgesia is centrally-mediated and exacerbated by E2. This hypothesis was tested by tracking the severity of ENDO-induced vaginal hyperalgesia through estropause. If correct, hyperalgesic severity should parallel E2 changes during estropause. (ii) A steady loss of E2, however, triggers a different type of vaginal hyperalgesia, due to peripheral effects of the loss of E2 on the vagina. This hypothesis was tested by tracking ENDO-induced hyperalgesic severity following OVX and OVX+E2. If correct, then, given the opposing influences of E2 on ENDO-induced and OVX-induced hyperalgesia, OVX should have either no effect on ENDO-induced hyperalgesia or exacerbate it. Effects of E2 replacement are, however, difficult to predict.
2. Methods
2.1. Subjects and vaginal cytology
Subjects were 13 adult virgin female Sprague-Dawley rats obtained from Charles River (Wilmington, MA; Raleigh NC facility). They weighed 175-250g at the start of the study and were housed individually in a temperature-controlled room (22.2 °C) in plastic cages lined with chip bedding, with ad libitum access to rat chow and water. They were maintained on a 12-h light/dark cycle, with lights on at 07:00.
Reproductive status was determined by daily vaginal lavage performed ~2h after lights on for all rats (Becker et al., 2005). Traditional nomenclature was used for the four estrous stages of proestrus, estrus, metestrus, and diestrus (Becker et al., 2005). The two groups of rats that received OVX maintained normal four-day estrous cycles throughout training and testing until OVX was performed. OVX was confirmed by daily vaginal lavages that consisted of leukocytes indicative of constant diestrus. Constant diestrus continued until E2 replacement was carried out ~6 weeks later. The continued effectiveness of the E2 replacement capsule (see below) in all eight rats was confirmed by daily vaginal lavages that consisted of cornified cells indicative of constant estrus (CE). CE continued in all rats until sacrifice. The remaining five rats that did not have OVX progressed naturally through reproductive senescence (estropause; Fig. 1). These “estropause” rats cycled normally for ~ 7.5 - 9 months, then progressed through a transition period of irregular cycling for ~1 month into a period of constant estrus (CE). The period of irregular cycling consisted predominantly of strings of estrus (Fig. 1C). Vaginal cytology during the period of constant estrus showed cornified cells every day, and the rats remained in CE until sacrifice.
All behavioral training and testing was done ~3-8 h after lights on. The study and all procedures were approved by Florida State University’s Animal Care and Use Committee as protocol # 9028.
2.2. Experimental groups and overview of experimental protocols
There were three experimental groups: (1) rats that underwent ENDO surgery followed by natural reproductive senescence (estropause; n = 5); (2) rats that underwent shamENDO surgery followed by OVX, followed by E2 replacement (n = 3); and (3) rats that underwent ENDO surgery followed by OVX, followed by E2 replacement (n = 5).
In all rats in all three experimental groups, baseline escape responses to vaginal distention were assessed in 1h-testing sessions 3-4 times/wk for ~2 months prior to the shamENDO or ENDO surgery. Post-ENDO and post-shamENDO responses continued to be assessed 3-4 times/wk for ~2.5 months after the surgery. We previously found that escape responses after ENDO surgery do not become significantly greater than baseline until one month post-surgery (i.e., the hyperalgesia develops in parallel with the growth of the cysts, which grow most rapidly during the first month, slowing up, then stabilizing thereafter; Cason et al, 2003). Accordingly, the post-ENDO (or post-shamENDO) data shown here in Figs. 2 and 3 are responses collected between one and two and one half months after the ENDO or shamENDO surgery was done (i.e., responses from the first post-operative month are not included). For both the baseline period and the post-ENDO or post-shamENDO period, the total number of testing sessions included approximately equal number of sessions obtained in each of the four estrous stages.
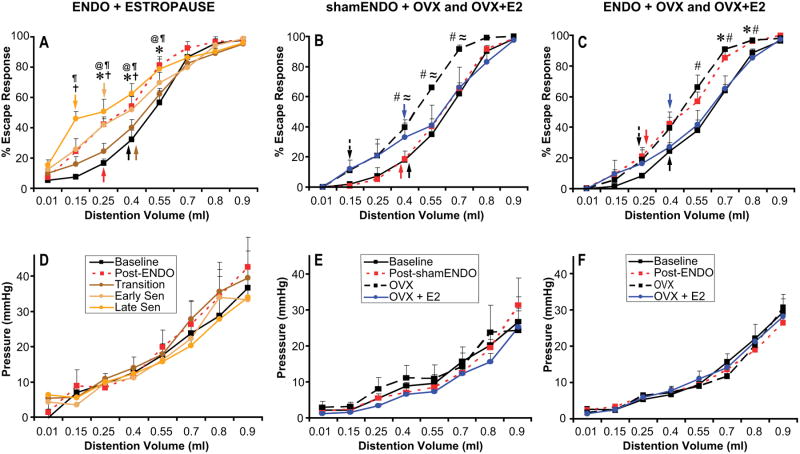
Figure 2.
Percent escape response (A,B,C) and vaginal pressure (D,E,F) to different volumes of distention of the vaginal canal. Results are shown from the three different experimental groups named at the top of the graphs and described in the text. Arrows in A-C indicate vaginal nociceptive thresholds for each condition. The other symbols indicate significant (P < 0.05) differences between conditions for each volume, as follows: *, baseline vs post-ENDO; #, baseline vs OVX; ≈, post-shamENDO vs OVX; †, baseline vs early senescence; @, baseline vs late senescence; ¶, transition vs late senescence.
Figure 3.
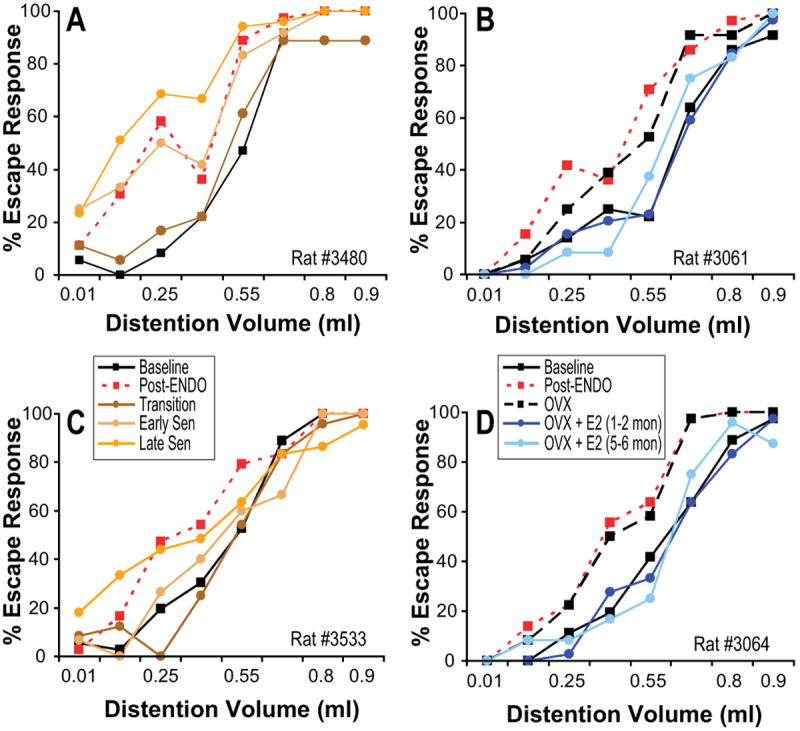
Percent escape responses for individual ENDO rats in the group that progressed through estropause (A,C) and in the group that had OVX, then OVX+E2 (B,D). A and C show results from two rats. One rat (B, #3480) received no treatment during estropause. The other rat (D, #3533) underwent cyst-removal surgery at the end of her transition period. Note that there are no differences between these two rats in the pattern of change in escape responses as the rats progress through estropause. In both, the hyperalgesia produced by ENDO is reduced during the transition phase, but then returns and increases during early and late senescence. B and D show results from two rats who had been treated with E2 for 6 months. Note that the responses of both rats (#3061 and #3064) increased from baseline after the ENDO surgery (i.e., they developed vaginal hyperalgesia) and that OVX had no influence on the responses. E2 replacement in the first two months reduced their responses to baseline levels (i.e., their hyperalgesia was completely reduced), and this reduction continued for another four months.
At the end of every rat’s entire testing period (which ranged from 7 to 11.5 months), the rat was anesthetized with urethane (1.2 g/kg, i.p.) for assessment of the cysts, and then sacrificed. To assess the cysts, the abdomen was opened and the sutures that were used to tie the uterine or fat autotransplants were located. When cysts were found, they were freed from surrounding fat and connective tissue, and their largest and smallest diameter measured (most cysts have an ovoid shape; Vernon and Wilson, 1985). A “cyst burden” was then calculated for each rat by multiplying the largest and smallest diameters of each implant that had formed a cyst and then adding those values together to obtain a total value (Nagabukuro and Berkley, 2007).
In group one (estropause), two of the five rats were tested continuously after ENDO surgery until sacrifice with no other intervening manipulation. In the other three rats, at the end of their transition period, they were anesthetized and their cysts removed (and measured) as described below. Testing resumed one week after the cyst-removal surgery. The ages at which the five rats progressed through the different phases of estropause are shown in Fig. 1.
In groups two and three, all eight rats were cycling regularly when the OVX was done. The OVX was done at comparable ages in the two groups ranging between 7-10 months old. E2 replacement was done ~6 wks after OVX in all eight rats. Testing under E2 replacement conditions continued for another ~two months. All of the shamENDO rats and three of the five ENDO rats were sacrificed at this time. The other two ENDO rats were not sacrificed; instead, testing resumed two months later, for a period of two months, when the rats were 17-19 months old.
2.3. ENDO, shamENDO, and cyst-removal surgeries
The ENDO and shamENDO surgeries were done following a protocol originally developed by Vernon and Wilson (1985). Rats in diestrus were anesthetized intraperitoneally with a mixture of ketamine hydrochloride (73 mg/kg) and xylazine (8.8 mg/kg), placed on heating pad to maintain body temperature ~37°C, and the surgery carried out using aseptic precautions. A midline abdominal incision was made to expose the uterus, and a ~1-cm segment of the left uterine horn and associated fat tissue were removed and placed in warm saline. Four pieces of uterine horn (~ 2mm × 2 mm) or, for shamENDO, four similarly-sized pieces of fat were cut from this segment. These pieces (uterine for ENDO, or fat for shamENDO) were sewn around alternate cascade mesenteric arteries that supply the caudal small intestine starting from the caecum using 4.0 nylon sutures. After making sure there was no bleeding in the abdominal cavity, the wound was closed in layers. Rats were closely observed during the postsurgical period for potential complications (none occurred). Postoperative recovery was uneventful, and regular estrous cyclicity resumed in all rats within one week.
For the cyst-removal surgery, performed in three of the rats in group one (#s 3533, 3539, and 3542, Fig. 1), at the end of their transition period they were anesthetized and treated during surgery the same way that rats had been treated for ENDO and shamENDO surgeries. The abdomen was opened to expose the area where the transplants had been sewn, and the cysts were located and carefully freed from surrounding fat and connective tissue and then measured. Next, using fine-tipped forceps, the sutures were carefully untied and removed, and the cysts were cut out using a combination of iris scissors and a small-tipped cautery. Care was taken, using absorbent sterile gauze, to make sure that none of the contents of the cysts leaked into the cavity and that bleeding was contained. In all three cases, bleeding was minimal. After assuring that all bleeding was stopped, the wound was closed in layers. Rats were closely observed during the postsurgical period for potential complications (none occurred), and behavioral testing resumed one week later. The surgery produced no changes in this groups’ vaginal lavage cytology (i.e., CE continued).
2.4. OVX and E2 replacement
For OVX, rats were anesthetized intraperitoneally with a mixture of ketamine hydrochloride (73 mg/kg) and xylazine (8.8 mg/kg), and the surgery carried out using aseptic precautions. A 2-cm incision was made to expose the abdominal cavity. The area where the autotransplants (either uterine or fat) had been placed was then gently examined, first locating the original sutures, then determining whether the transplants had formed cysts, and, if cystic, gently measuring their approximate small and large diameters. This procedure, however, allowed only an approximate estimate of the cyst burden during cycling.
The uterine horns were then visualized and followed rostrally to the fallopian tubes where a hemostat was clamped at each caudal end. The ovaries were removed by cutting through tissue on the rostral side of each hemostat. The hemostats were removed after making certain all bleeding, if any, had stopped. The wound was then closed. Postoperative recovery was uneventful. Blood levels of hormones were not assessed because of the stress that sampling procedures could have on the rats and their behavior. The loss of ovarian hormones was assured, however, by the fact that all rats maintained a continued daily vaginal cytology of leukocytes indicative of diestrus (i.e., constant diestrus) until E2 was replaced (vom Saal et al., 1994; Chakraborty and Gore, 2004).
Six to eight wks after OVX, the rats were briefly anesthetized with a gaseous mixture of 2/3 nitrous oxide—1/3 oxygen and 3% halothane. A silastic capsule (Dow Corning, Midland, MI) filled with crystalline 17β-estradiol (with the ends sealed with Silastic elastomer) was then inserted subcutaneously into the nape of the neck. The capsule was 5 mm in length with a 1.57-mm internal diameter. Capsules of this size have been shown to maintain a steady ~40–60 pg/ ml 17β -estradiol plasma level (Freeman and Sterman, 1978; Lerant and Freeman, 1998). As previously explained, blood levels of hormones could not be assessed due to potential stress effects. However, the continued presence of physiological levels of estradiol for two to six months after implantation of the capsule was assured by the rats’ continued daily vaginal cytology of cornified cells indicative of estrus; i.e., CE (Lu et al., 1979; vom Saal et al., 1994).
2.5. Behavioral testing procedures
Behavioral training and testing procedures were identical to those described in detail previously (Berkley et al., 1995; Bradshaw et al., 1999; Bradshaw and Berkley, 2002; Cason et al., 2003). Rats were trained to perform an escape response to terminate vaginal distention produced by an inflatable latex balloon. During each testing session, eight different distention volumes were delivered three times each in random order at intervals of ~ 60 sec. and percent escape response was assessed.
2.5.1. Apparatus and stimulator
The training and testing apparatus was a small rectangular plexiglass chamber of adjustable width designed to contain the rat just enough to prevent her from turning around. A hollow tube containing light-emitting diodes and a photosensor extended from the front of the chamber. If the rat extended her nose into this tube, she broke the light beam and terminated the stimulus (i.e., she made an escape response, see below). An opening in the rear of the chamber allowed the catheter (attached to the vaginal stimulator), to be connected to the computer-controlled, automated stimulus apparatus.
The vaginal stimulator was a small latex balloon (~ 10mm long × 1.5mm wide when uninflated) tied to a thin catheter with silk suture. Immediately prior to the training or testing session, the uninflated balloon was lubricated (K-Y jelly) and inserted into the mid-vaginal canal, located so that it would not touch the cervix even when inflated. Inflating the balloon with different volumes of water using a computer-controlled pump distended the vaginal canal. The pressure produced by each volume of distention (corrected for compliance characteristics of the balloon) was measured through a small-volume Cobe pressure transducer.
2.5.2. Training
After the rat was first adapted to the testing chamber by placing her in it for 10 min daily for 3-4 days (and feeding her small dabs of peanut butter on a small stick), training sessions began in which the trainer pinched the rat’s tail with a padded forceps, using its release to shape a required “escape” response, which involved the rat extending her head into the tube to interrupt the light beam. Training sessions of 10 pinches delivered at ~1min-intervals were run 3/week on non-consecutive days. Training was usually completed (>80% escape behavior) in 4-8 sessions.
The rat was next trained to make identical escape responses to deflate vaginal distention stimuli. These sessions were run 3/week on non-consecutive days for a total of 3-5 sessions. Ten large stimuli (0.80ml – 1.0ml, inflation rate 1ml/s) were delivered for a maximum of 15s at ~1-min intervals. All rats showed some behavioral response to the larger stimuli, which allowed the experimenter to use deflation of the vaginal balloon to shape the rat’s escape responses. All rats learned the escape response within 2-4 sessions. Once trained, testing sessions began.
2.5.3. Testing
Testing sessions were run 3 to 4 times/wk on non-consecutive days. Each testing session included a series of 24 computer-controlled escape trials that were run at ~1-min intervals (range 50 - 70s). Each trial consisted of rapid inflation of the balloon (1ml/s) to a fixed volume, where it remained until the rat made an escape response or 15 s elapsed, when the balloon rapidly deflated. Eight different distention volumes, including a control volume (0.01ml), were delivered three times each in random order. The computer recorded stimulus volume, stimulus pressure, and the response latency for each trial. The maximum latency of 15sec was considered to be no response. The experimenter was blind to the volumes being delivered to the rat. After the escape trials were run, the rat was given a dab of peanut butter on a small stick and removed from the testing chamber.
2.6 Data analysis
Percent escape responses and vaginal pressures as a function of distention volume were measured in each session. For each rat, the escape percentages and pressures for all of that rat’s sessions for each condition (at least 12 sessions/condition) were combined, and the mean values for each condition calculated. Conditions for group one included: baseline, post-ENDO, transition, early senescence, late senescence. Conditions for group two included: baseline, post-shamENDO, OVX, OVX+E2. Conditions for group three included: baseline, post-ENDO, OVX, OVX+E2.
The averages from each of the rats in the different conditions in each experimental group were combined by group and entered into spread sheets. Because the data did not violate assumptions of homogeneity of variance and normal distribution, statistical analyses were performed using parametric tests; i.e., repeated measure ANOVAs. If significant, these tests were followed by post-hoc comparisons. Because of the small number of rats in each group, as suggested by Saville (1990), the post-hoc comparison test we used was Fisher’s LSD procedure in an attempt to minimize both Type I and Type II errors. These analyses were followed, if conditions differed significantly, by one-way ANOVAs to determine the significance of differences between conditions for each distention volume.
A second set of one-way ANOVAs was carried out for every condition to determine the volume at which the percent escape response became significantly greater than the percent escape response to the control distention. This volume was considered the vaginal nociceptive threshold (Bradshaw et al., 1999; Cason et al., 2003).
Statistical analyses were done using Statistical Package for the Social Sciences software, version 15 (SPSS, Chicago, IL). Significance was set at P ≤ 0.05.
3. Results
A summary of the results that are reported below is provided in Table 1.
TABLE 1.
Summary of the effects of different manipulations in each experimental group.
| Group/Manipulation | (1) ESTROPAUSE | (2) shamENDO | (3) ENDO |
|---|---|---|---|
| ENDO surgery | hyperalgesia | hyperalgesia | |
| shamENDO surgery | no effect | ||
| transitional estropause | alleviation of hyperalgesia | ||
| early estropause | return of hyperalgesia | ||
| late estropause | maintained return of hyperalgesia | ||
| OVX | hyperalgesia | no effect | |
| OVX+E2 | alleviation of hyperalgesia | alleviation of hyperalgesia |
3.1. Effects of estropause on vaginal nociception and vaginal pressures in ENDO rats
Data from this group of rats, shown in Fig. 2A,D, were analyzed by repeated measures ANOVA as a function of five different conditions: baseline; post ENDO surgery during cycling; post ENDO surgery during the transition period; post ENDO surgery during early senescence; and post ENDO surgery during late senescence.
In the three rats who had their transplants removed at the end of their transition period, all transplants at this time were found to be cystic, but they were small, ranging from 1 × 1.5 mm to 3 × 4mm, with an average cyst burden of 15.8. By the end of the study, when these three rats were in late senescence, none of the removed cysts had returned (i.e., surgical removal had been complete). For the other two rats that had not undergone any surgery, the size of the transplants was measured only at the end of the study when the rats were in late senescence. In both of these rats, all four of their implants were cystic and very large, ranging from 6 mm × 5 mm to 14mm × 9mm, with a mean cyst burden of 310, which is ~2.5 times greater than the largest cyst burden found in cycling rats (~120, Nagabukuro and Berkley, 2007).
Despite these huge differences in cyst burden (zero for the cyst-removal group; 310 for the non-cyst removal group), there were no differences between the five rats in the pattern of their nociceptive changes across the five conditions. Thus, all five rats exhibited the general pattern described below, as can be seen by the two examples shown in Fig. 3A and C (rats #3480 and #3533). Therefore, the data from all five rats were combined for the graphs shown in Figs. 2A and 2D.
Percent escape responses to different volumes of vaginal distention in this group of rats differed significantly as a function of volume [F(7,140) = 366.12, P < 0.001], condition [F(4,20) = 5.07, P < 0.01], and there was a significant interaction between volume and condition [F(28,140) = 4.02, P < 0.001]. The pressures produced by different volumes of vaginal distention differed significantly as a function of volume [F(7,140) = 82.06, P < 0.001], but there were no significant effects of either condition [F(4,20) = 0.34, P = 0.845], nor was there a significant interaction between volume and surgical condition [F(28,140) = 0.55, P = 0.97].
Thus, in this ENDO/estropause group, percent escape responses (Fig. 2A) to some of the distention volumes, but not the pressures (Fig. 2D) produced by those volumes, were influenced by the condition of the rat. Post-hoc tests showed that escape responses increased significantly after the ENDO surgery during cycling (P = 0.01). During the transition period, responses decreased so that they were now not significantly different from baseline levels (P = 0.46). Next, during early senescence, responses increased (P = 0.025, compared with baseline), and increased somewhat further during late senescence (P = 0.001, compared with baseline), reaching levels insignificantly different from the post-ENDO-cycling period. As can be seen in Fig. 2A, these results indicate that escape responses increased after ENDO surgery, decreased during the transition period, then increased again during early and late senescence to reach levels seemingly even higher (but not significantly higher) than those in cycling rats after the ENDO surgery. In other words, rats became hyperalgesic after the ENDO surgery, but this hyperalgesia was alleviated during the transition period when the rats were irregularly cycling or had just begun CE. Regardless of the presence of cysts, the hyperalgesia returned as the rats continued through early and late senescence. Consistent with these effects, as shown by the arrows in Fig. 2A, nociceptive thresholds were lower in the ENDO-cycling and ENDO-late senescence groups than they were in the other groups.
3.2. Effects of OVX and OVX+E2 on vaginal nociception and vaginal pressures in shamENDO rats
Data from this group of rats, shown in Fig. 2B, E, were analyzed by repeated measures ANOVA as a function of four different conditions: baseline, post shamENDO surgery, post shamENDO surgery-OVX, and post shamENDO surgery-OVX+E2. Neither at the time of OVX, nor at the end of the study at sacrifice, were any cysts found in the abdomen of these rats.
Percent escape responses to different volumes of vaginal distention differed significantly as a function of distention volume [F(7,56) = 368.76, P < 0.001], and, although there was no significant effect of condition alone [F(3,8) = 2.80, P = 0.11], there was a significant interaction between volume and condition [F(21,56) = 2.65, P < 0.01]. The pressures produced by different volumes of vaginal distention differed significantly as a function of distention volume [F(7,56) = 58.12, P < 0.001], but there were no significant effects of condition alone [F(3,8) = 0.29, P = 0.83], nor was there a significant interaction between volume and surgical condition [F(21,56) = 0.56, P = 0.92].
Thus, percent escape responses (Fig. 2B) to some of the distention volumes, but not the pressures produced by those volumes (Fig. 2E), were influenced by the condition of the rat. Post-hoc tests showed no significant differences between baseline escape responses and shamENDO or OVX+E2, but there was a significant difference between baseline and OVX (P < 0.05), and between shamENDO and OVX (P < 0.05). As can be seen in Fig. 2B, what this result means is that whereas the shamENDO surgery had no influence on escape responses, OVX increased them, and then E2 replacement reduced the escape responses back to baseline. In other words, whereas the shamENDO surgery did not influence vaginal nociception, OVX induced hyperalgesia and estradiol replacement alleviated the hyperalgesia. Consistent with these effects, as shown by the arrows in Fig. 2B, nociceptive thresholds were lower in the OVX condition than in the three other conditions.
3.3. Effects of OVX and OVX+E2 on vaginal nociception and vaginal pressures in ENDO rats
Data from this group of rats, shown in Fig. 2C,F, were analyzed by repeated measures ANOVA as a function of four different conditions: baseline; post ENDO surgery, post ENDO surgery-OVX, and post ENDO surgery-OVX+E2.
Approximately 2.5 months post-ENDO surgery, when the rat’s abdomen was opened to carry out the OVX surgery, at least two of the implants in every rat were cystic, but as described in Methods, precise measurements were not made at this time to avoid disruption of the cysts. However, in previous studies, when rats with ENDO were sacrificed at this post-ENDO time period and their cysts carefully measured, we found that nearly all of the transplants in the rats form cysts; their sizes range from ~2 mm × 3 mm to ~7mm × 9 mm, and the cyst burden ranges from 9 – 120 (Nagabukuro and Berkley, 2007). Because the abdomen of the rats was not examined after OVX, it is not known whether OVX had any influence on the cysts, although others have reported that the cysts disappear following OVX (Vernon and Wilson, 1985; Rajkumar et al., 1990). At the time of sacrifice after E2 replacement, however, at least two of the transplants in each rat were cystic, ranging in size from 2 mm × 2mm to 3 mm × 4mm, with a mean cyst burden of 13.1. In other words, it is reasonable to assume that, as shown by others (Vernon and Wilson, 1985; Rajkumar et al., 1990), the cysts in the rats here disappeared after OVX, then reappeared after E2 replacement, but were generally smaller than cysts in regularly cycling rats.
Percent escape responses to different volumes of vaginal distention in this group of rats differed significantly as a function of volume [F(7,112) = 384.29, P < 0.001], and, although there was no significant effect of condition alone [F(3,16) = 2.80, P = 0.085], there was a significant interaction between volume and condition [F(21,112) = 2.18, P < 0.01]. The pressures produced by different volumes of vaginal distention differed significantly as a function of volume [F(7,112) = 206.12, P < 0.001], but there were no significant effects of either condition alone [F(3,16) = 0.06, P = 0.98], nor was there a significant interaction between volume and surgical condition [F(21,112) = 0.71, P = 0.82].
Thus, as in the other two groups, in the ENDO/OVX group, percent escape responses (Fig. 2C) to some of the distention volumes, but not the pressures (Fig. 2F) produced by those volumes, were influenced by the condition of the rat. The pattern of these effects, however, differed from the other two groups. Post-hoc tests in the ENDO group showed a significant difference between baseline escape responses and those after ENDO surgery (P < 0.05) or after OVX (P < 0.05), with no significant difference between the post-ENDO and OVX conditions. There were also no significant differences between baseline escape responses and those after OVX+E2. As can be seen in Fig. 2C, these results mean that the ENDO surgery increased escape responses, that OVX did not change this increased responding, but that E2 replacement reduced the escape responses back to baseline. In other words, rats became hyperalgesic after the ENDO surgery, and, whereas OVX surgery did not affect this hyperalgesia, E2 replacement alleviated it. Consistent with these effects, as shown by the arrows in Fig. 2C, nociceptive thresholds were lower in the ENDO and OVX conditions than they were in the baseline and OVX+E2 conditions.
In all five rats, vaginal nociception was studied for at least two months after the E2 capsule had been implanted. In two of these rats, however, the E2 capsule was left in place for an additional four months, and then testing resumed during last two of these four months. This procedure was followed in order to assess whether the increased length of exposure to E2 might reverse the alleviation of vaginal hyperalgesia, as occurs in rats as they proceed through ~ four months of estropause (in Fig. 2A, compare the dark green line with the lighter green lines). In these two rats with E2 capsules left in place for a longer period of time, however, there was no change in the alleviation of hyperalgesia, even after 4 months of E2, as shown by their individual graphs in Fig. 3B,D.
4. Discussion
The severity of ENDO-induced vaginal hyperalgesia in cycling rats parallels E2 levels during the estrous cycle (Cason et al., 2003). Consistent with this effect, ENDO-induced hyperalgesic severity paralleled E2 changes during estropause (compare Fig. 1 and Fig. 2A), providing further support for the hypothesis that E2 modulates (exacerbates) hyperalgesic severity. It may therefore seem surprising that the loss of E2 produced by OVX did not reduce the hyperalgesia that had originally been induced by ENDO. Also suprising is that E2 replacement following OVX in these rats alleviated the hyperalgesia that had originally been induced by ENDO. Of relevance to this situation are results from shamENDO rats, whose unchanged vaginal nociception was increased by OVX (i.e., OVX evoked vaginal hyperalgesia) and then decreased by E2 replacement. To explain these seemingly opposing findings, it is necessary to consider putative mechanisms underlying ENDO-induced vaginal hyperalgesia versus OVX-induced vaginal hyperalgesia.
4.1. Mechanisms of ENDO-induced versus OVX-induced vaginal hyperalgesia
ENDO-induced vaginal hyperalgesia is likely primarily centrally-mediated. The basis for this conclusion is that the abdominal cysts develop a nerve supply that sends afferents to mid-thoracic spinal segments and brainstem via the splanchnic and vagus nerves, respectively (Cason et al., 2003; Berkley et al., 2004; Berkley, 2005), and that spinalization eliminates ENDO-induced, vaginally-evoked muscle hyperalgesia (Nagabukuro and Berkley, 2007). Thus, ENDO likely affects vaginal nociception via descending connections from thoracic cord or brainstem to L6/S1 segments (which receive vaginal input; Berkley et al., 1993).
Regarding E2 modulation of ENDO-induced hyperalgesic severity, arguments have been made supporting E2’s influence on the cysts, spinal cord and brainstem, but not the vagina (Cason et al., 2003; Berkley et al., 2004, 2005). Although there are estrous changes in vaginal structure and physiology (Long and Evans, 1922), as well as in response properties of vaginal afferents (Robbins et al, 1992), the major vaginal changes occur between proestrus/estrus and metestrus/diestrus. Thus, vaginal changes across the estrous cycle do not parallel behavioral changes, which occur between proestrus and estrus (Cason et al., 2003). Therefore, changes in the vagina and vaginal afferent input are unlikely to contribute significantly to E2 effects on ENDO-induced hyperalgesia (Bradshaw et al, 1999).
OVX in unoperated rats induces vaginal hyperalgesia, and E2 replacement alleviates it (Bradshaw and Berkley, 2002). In contrast to the putative central mechanisms of ENDO-induced hyperalgesia, the effects of OVX and E2 replacement on vaginal nociception are likely due primarily to their effects on the vagina. Within a month after OVX, the rat’s vaginal canal becomes thinner, less muscular, and less vascularized. Furthermore, these changes are reversed within 2 wks of E2 replacement (Pessina et al., 2006; Kim et al., 2004). This same conclusion likely applies to results in shamENDO rats here.
4.2. Influence of estropause, OVX, OVX+E2, on ENDO-induced hyperalgesia
4.2.1. Estropause
During estropause, as rats transition from a regular 4-day cycle to constant estrus (CE; Fig. 1A), the pattern of E2 levels changes from regular 4-day cycles of large fluctuations to steady levels during CE that are close to those in estrus or diestrus, and lower than those in proestrus (Fig. 1B). During this transition period between cycling and CE, some rats have about a one-month period of irregular cycling when E2 levels are higher than they are in corresponding parts of the cycle in regularly cycling rats (Peluso et al., 1979), whereas others go directly into CE (Lu et al., 1979; vom Saal et al., 1994). Although hormone levels were not measured in the present study, the vaginal lavage data indicate it is reasonable to assume that similar changes occurred in the rats here.
Fig. 1C shows that rats here began their transition at ~7.5 - 9 months (slightly younger than reported by Lu et al., 1979), and on most days were in estrus (E2 levels low). Because the rats had few days in proestrus (E2 levels high), the transition period likely represented an overall reduction in E2 levels from earlier overall levels when they cycled regularly. Therefore, during progression from cycling to the transition period, E2 likely acted in a manner similar to the change from proestrus to estrus. In other words, the initial overall reduction in E2 during the transition produced a reduction in severity of ENDO-induced vaginal hyperalgesia just like it did from proestrus to estrus during regular cycling. This reduction would be only temporary, because, as the estropause rats progressed into early and late senescence, E2 levels generally increased (Fig. 1B), which reinstated the hyperalgesia.
What mechanisms might underlie these effects? One possibility is that changes in cyst characteristics are responsible. For example, cysts undergo significant proestrus-to-estrus changes in sympathetic innervation, vascularization, and content of nerve growth factor and vascular endothelial growth factor (Zhang et al., 2006) that could contribute to the proestrus-to-estrus and possibly cycling-to-transitional estropause reductions in severity of ENDO-induced hyperalgesia. Such changes, however, cannot account for the return of hyperalgesia during the progression to later stages of estropause, because that return occurred even when the cysts had been removed (Fig. 3C). Therefore, the hyperalgesia during early and late estropause (Fig. 2A, lighter green lines), when E2 levels remain steady and increase (Fig. 1B), is more likely due to other effects. One possibility is that prolonged steady levels of E2 sensitize the vagina, but this effect seems unlikely because such E2 levels should protect the vagina (Pessina et al., 2006). A second possibility is that E2 modulates central processing of vaginal input that had previously been sensitized by ENDO. In other words, ENDO surgery might establish a central sensitization that remains, regardless of the presence of the cysts, and is modulated by E2. Thus, during transition into estropause, when E2 levels represent a decline, there is a reduction in hyperalgesic severity. Later, the increase in E2 results in a return of hyperalgesia (Figs. 2A and Fig. 3A,C).
4.2.2. OVX and OVX+E2
Given that OVX affects the vagina within weeks after surgery, and that ENDO rats were tested 2-8 wks post-OVX, peripheral effects on the vagina could have negated any potential ameliorative effects of E2 reduction on the putatively centrally-mediated ENDO-induced hyperalgesia. What is more difficult to explain, however, is how E2 replacement alleviated the now OVX+ENDO-induced hyperalgesia. Presumably, because E2 restored the vagina’s health, E2 should have alleviated the OVX-induced vaginal hyperalgesia. However, E2 should also have exacerbated the E2-dependent, ENDO-induced vaginal hyperalgesia. These two opposing influences should have resulted in E2’s having little or no influence on vaginal nociception. Instead, E2 reversed the vaginal hyperalgesia.
One possible explanation involves the effects of OVX and E2 on the cysts, but this possibility is unlikely, for two reasons: First, if the presence or size of the cysts is relevant, then the return of the cysts after E2 should have at least partially prevented the complete alleviation of the now OVX+ENDO-induced hyperalgesia. Second, we have previously shown that the amount of cystic material (cyst burden) does not correlate with ENDO-induced increases in visceromotor responses to vaginal distention (Nagabukuro and Berkley, 2007).
Another possibility is that other effects of the OVX surgery somehow override the central sensitizing effects of ENDO. One candidate is progesterone, which OVX reduces to near zero levels (Chakraborty and Gore, 2004). This possibility is unlikely, however, because progesterone levels also decrease to near zero levels during estropause (Lu et al., 1979) even when the hyperalgesia returns. Other candidates include OVX’s well-known influences on other reproductive hormones (e.g., GnRH, LH, FSH, prolactin; vom Saal et al., 1994), adrenal functioning (e.g., Figueiredo et al., 2007), central opioid peptides (e.g., Claiborne et al., 2006; Kalra et al., 1988), and the surgical effects of OVX surgery (Sanoja and Cervero, 2005).
4.3. Conclusions
In sum, the severity of ENDO-induced vaginal hyperalgesia appears to parallel E2 changes during estropause like it parallels E2 changes during the estrous cycle. A likely contributing mechanism in both cases is the modulating influence of E2 on ENDO-induced central sensitization. However, the effects of OVX and OVX+E2 on ENDO-induced hyperalgesia suggest that E2 modulation of the hyperalgesia applies only if the rat has not experienced a complete loss of E2.
Together, the findings support the hypothesis that the mechanisms that underlie ENDO-induced vaginal hyperalgesia differ from the mechanisms that underlie OVX-induced vaginal hyperalgesia. The most efficient explanation for the ENDO-induced hyperalgesia is central sensitization (Cason et al., 2003), whereas the most efficient explanation for the effects of OVX and E2 replacement on shamENDO or unoperated rats is E2’s peripheral effects on the vagina (Bradshaw and Berkley, 2002). Thus, explanations for the differing influences on ENDO-induced hyperalgesia of cycling and estropause versus OVX and OVX+E2 appear more complex, involving a mixture of various peripheral and central effects, the specifics of which require further study.
Regardless of which explanations eventually prove most plausible, the results here demonstrate that removal of ovarian hormones by OVX and replacement of E2 by implants are not sufficient to assess the influence of ovarian hormones on behavior. If we had not studied the effects of estrous cyclicity or estropause on ENDO-induced vaginal hyperalgesia, we would have concluded that ovarian hormones did not influence it.
Acknowledgments
We thank Chad Samuelsen and Kristina McGinty for technical help, John Chalcraft for help with the figures, Chris Schatschneider, PhD for statistical advice, and Lisa Eckel, PhD for insightful discussions throughout the course of the study. This study was supported by NIH grant RO1-NS11892.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bachmann GA, Nevadunsky NS. Diagnosis and treatment of atrophic vaginitis. Am Fam Physician. 2000;61:3090–6. [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–73. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Berkley KJ. A life of pelvic pain. Physiol Behav. 2005;86:272–80. doi: 10.1016/j.physbeh.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Robbins A, Sato Y. Functional differences between afferent fibers in the hypogastric and pelvic nerves innervating female reproductive organs in the rat. J Neurophysiol. 1993;69:533–44. doi: 10.1152/jn.1993.69.2.533. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Wood E, Scofield SL, Little M. Behavioral responses to uterine or vaginal distention in the rat. Pain. 1995;61:121–31. doi: 10.1016/0304-3959(94)00150-D. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Dmitrieva N, Curtis KS, Papka RE. Innervation of ectopic endometrium in a rat model of endometriosis. Proc Natl Acad Sci U S A. 2004;101:11094–8. doi: 10.1073/pnas.0403663101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science. 2005;308:1587–9. doi: 10.1126/science.1111445. [DOI] [PubMed] [Google Scholar]
- Bradshaw HB, Berkley KJ. Estrogen replacement reverses ovariectomy-induced vaginal hyperalgesia in the rat. Maturitas. 2002;41:157–65. doi: 10.1016/s0378-5122(01)00261-4. [DOI] [PubMed] [Google Scholar]
- Bradshaw HB, Temple JL, Wood E, Berkley KJ. Estrous variations in behavioral responses to vaginal and uterine distention in the rat. Pain. 1999;82:187–97. doi: 10.1016/S0304-3959(99)00049-4. [DOI] [PubMed] [Google Scholar]
- Cason AM, Samuelsen CL, Berkley KJ. Estrous changes in vaginal nociception in a rat model of endometriosis. Horm Behav. 2003;44:123–31. doi: 10.1016/s0018-506x(03)00121-1. [DOI] [PubMed] [Google Scholar]
- Chakraborty TR, Gore AC. Aging-related changes in ovarian hormones, their receptors, and neuroendocrine function. Exp Biol Med. 2004;229:977–87. doi: 10.1177/153537020422901001. [DOI] [PubMed] [Google Scholar]
- Claiborne J, Nag S, Mokha SS. Activation of opioid receptor like-1 receptor in the spinal cord produces sex-specific antinociception in the rat: estrogen attenuates antinociception in the female, whereas testosterone is required for the expression of antinociception in the male. J Neurosci. 2006;26:13048–53. doi: 10.1523/JNEUROSCI.4783-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman ME, Sterman JR. Ovarian steroid modulation of prolactin surges in cervically stimulated ovariectomized rats. Endocrinology. 1978;102:1915–20. doi: 10.1210/endo-102-6-1915. [DOI] [PubMed] [Google Scholar]
- Figueiredo HF, Ulrich-Lai YM, Choi DC, Herman JP. Estrogen potentiates adrenocortical responses to stress in female rats. Am J Physiol Endocrinol Metab. 2007;292:E1173–82. doi: 10.1152/ajpendo.00102.2006. [DOI] [PubMed] [Google Scholar]
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–99. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- Kalra SP, Allen LG, Sahu A, Kalra PS, Crowley WR. Gonadal steroids and neuropeptide Y-opioid-LHRH axis: interactions and diversities. J Steroid Biochem. 1988;30:185–93. doi: 10.1016/0022-4731(88)90092-1. [DOI] [PubMed] [Google Scholar]
- Kim SW, Kim NN, Jeong SJ, Munarriz R, Goldstein I, Traish AM. Modulation of rat vaginal blood flow and estrogen receptor by estradiol. J Urol. 2004;172:1538–43. doi: 10.1097/01.ju.0000137744.12814.2e. [DOI] [PubMed] [Google Scholar]
- Lerant A, Freeman ME. Ovarian steroids differentially regulate the expression of PRL-R in neuroendocrine dopaminergic neuron populations: a double label confocal microscopic study. Brain Res. 1998;802:141–54. doi: 10.1016/s0006-8993(98)00583-6. [DOI] [PubMed] [Google Scholar]
- Long J, Evans H. The oestrous cycle in the rat and its associated phenomena. In: Leuschner A, editor. Memoirs of the University of California. Berkeley: University of California Press; 1922. pp. 1–149. [Google Scholar]
- Lu KH, Hopper BR, Vargo TM, Yen SS. Chronological changes in sex steroid, gonadotropin and prolactin secretions in aging female rats displaying different reproductive states. Biol Reprod. 1979;21:193–203. doi: 10.1095/biolreprod21.1.193. [DOI] [PubMed] [Google Scholar]
- Nagabukuro H, Berkley KJ. Influence of endometriosis on visceromotor and cardiovascular responses induced by vaginal distention in the rat. Pain. 2007 doi: 10.1016/j.pain.2007.04.039. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass TE, LaPolt PS, Judd HL, Lu JK. Alterations in ovarian steroid and gonadotrophin secretion preceding the cessation of regular oestrous cycles in ageing female rats. J Endocrinol. 1984;100:43–50. doi: 10.1677/joe.0.1000043. [DOI] [PubMed] [Google Scholar]
- Peluso JJ, Steger RW, Huang H, Meites J. Pattern of follicular growth and steroidogenesis in the ovary of aging cycling rats. Exp Aging Res. 1979;5:319–33. doi: 10.1080/03610737908257208. [DOI] [PubMed] [Google Scholar]
- Pessina MA, Hoyt RF, Jr, Goldstein I, Traish AM. Differential regulation of the expression of estrogen, progesterone, and androgen receptors by sex steroid hormones in the vagina: immunohistochemical studies. J Sex Med. 2006;3:804–14. doi: 10.1111/j.1743-6109.2006.00290.x. [DOI] [PubMed] [Google Scholar]
- Rajkumar K, Schott PW, Simpson CW. The rat as an animal model for endometriosis to examine recurrence of ectopic endometrial tissue after regression. Fertil Steril. 1990;53:921–925. doi: 10.1016/s0015-0282(16)53532-8. [DOI] [PubMed] [Google Scholar]
- Robbins A, Berkley KJ, Sato Y. Estrous cycle variation of afferent fibers supplying reproductive organs in the female rat. Brain Res. 1992;596:353–6. doi: 10.1016/0006-8993(92)91572-v. [DOI] [PubMed] [Google Scholar]
- Sanoja R, Cervero F. Estrogen-dependent abdominal hyperalgesia induced by ovariectomy in adult mice: A model of functional abdominal pain. Pain. 2005;118:243–253. doi: 10.1016/j.pain.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Saville DJ. Multiple comparison procedures: the practical solution. Am Stat. 1990;44:174–80. [Google Scholar]
- Sharpe-Timms KL. Using rats as a research model for the study of endometriosis. Ann NY Acad Sci. 2002;955:318–27. doi: 10.1111/j.1749-6632.2002.tb02792.x. [DOI] [PubMed] [Google Scholar]
- Vernon MW, Wilson EA. Studies on the surgical induction of endometriosis in the rat. Fertil Steril. 1985;44:684–94. [PubMed] [Google Scholar]
- vom Saal FS, Finch CE, Nelson JF. Natural history and mechanisms of reproductive aging in humans, laboratory rodents, and other selected vertebrates. In: Knobil E, Neill JD, editors. The physiology of reproduction. 2. New York: Raven Press; 1994. pp. 1213–314. [Google Scholar]
- Zhang GH, Liu Y, Dmitrieva N, Berkley KJ. Program No. 142.4. 2006 Neuroscience Meeting Planner. Atlanta (GA): Society for Neuroscience; 2006. Endometriosis in the rat: estrous changes in the content of cytokines, chemokines, and growth factors in the ectopic growths. Online. [Google Scholar]