The L1 subgroup of the immunoglobulin G superfamily participates in neurite outgrowth, neurite fasciculation and inter-neuronal adhesion. Almost all functional studies of the L1 family have focused on their role in neurons. In this issue of the Journal of Cell Biology (Tait et al. 2000), the 155kD isoform of neurofascin, an ankyrin binding member of the L1 family is identified as the first glial constituent of the paranodal axo-glial junction. As the major anchor between myelin and axons, these septate-like junctions are also thought to contain the molecular orchestrators for the polarization of axonal and myelin membranes into nodes of Ranvier. The importance of this polarization and how it may occur is the focus of this commentary.
The sophisticated executive and motor functions we utilize to compete and survive depend upon rapid communication between billions of nerve cells. Rapid nerve communication can be achieved by increasing the diameter of the axons or cables that interconnect neurons to each other or to muscle and sensory endings. While this mechanism efficiently facilitates rapid nerve conduction in less sophisticated invertebrates, it would add unmanageable axonal bulk and brain mass to the mammalian CNS. Therefore, a mechanism for rapid nerve conduction evolved that concentrates the voltage-gated Na+ channels at discrete regions of the axon called nodes of Ranvier. The nodes are separated by a multilamellar, tightly compacted membrane called myelin that is synthesized by Schwann cells in the peripheral nervous system (PNS) and oligodendrocytes in the central nervous system (CNS). Myelin insulates axons and increases the resistance and decreases the capacitance of axonal membranes. The physiological end-product of myelination is saltatory conduction where the nerve impulse rapidly “jumps” from node to node. To obtain the same conductance without myelin, axonal diameters would have to increase 15,000-fold (Salzer 1997).
The clustering of Na+ channels at nodes requires the development of specialized domains of both axonal and myelin surface membranes. It is unknown, however, whether nodal distributions are dictated by intrinsic axonal properties or by ensheathing glial cells. Myelination is required for axonal Na+ channel clustering in vivo (Ching et al. 1999; Rasband et al. 1999). This does not necessarily mean, however, that myelination or a glial component dictates the distribution of nodes of Ranvier. Internodal distances are not the same on all axons. Nodes are spaced at approximately 100 times the diameter of the mature axon and thus range from 2.0 mm to 150 μm apart. Internodal distances are relatively constant along individual axons and established early in development when most axons have similar diameters. These observations support the concept that internodal distances are an inherent property of axons and thus dictated by an axolemmal molecule. The thickness of the myelin internode (number of spiral wraps) is also related to the mature diameter of the axon and thus to internodal distances. Mechanisms that determine nodal distributions may also regulate aspects of myelin gene expression.
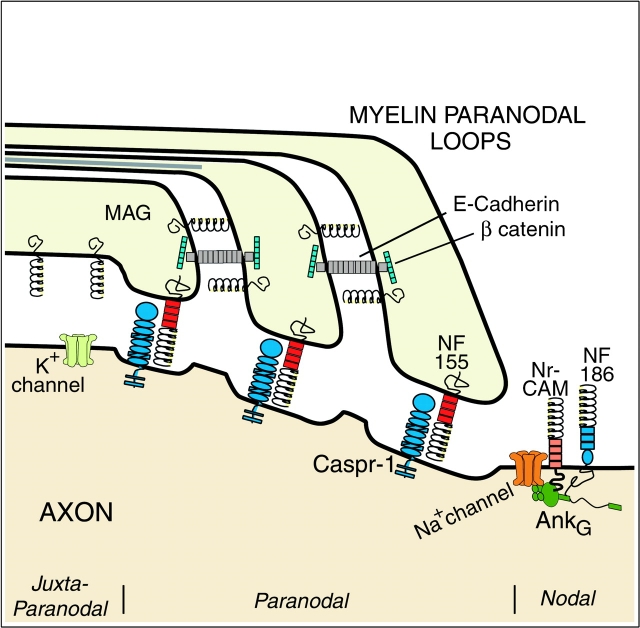
One key to unraveling the mechanisms responsible for node formation is elucidation of the molecular composition of the membranes that comprise and demarcate the node of Ranvier (Fig. 1). The myelinated axon can be divided into three domains: the internodal axon covered by compact myelin; the paranodal axon connected to the terminal ends or paranodal loops of the myelin internode by septate-like junctions; and the nodal axon which can be apposed by Schwann cell microvilli or astrocyte processes. The internodal axolemma contains high concentrations of K+ channels that are enriched in the juxtaparanodal region. The myelin-associated glycoprotein (MAG) is enriched in the internodal periaxonal or adaxonal membrane of the myelin sheath (Trapp et al. 1989), but is not essential for myelination or Na+ channel clustering.
Figure 1.
A schematic of nodal region in longitudinal orientation. Three regions can be distinguished based on ultrastructure and molecular composition. The juxtaparanodal axon membrane contains high concentration of K+ channels. The adaxonal myelin membrane and paranodal loops (PNS only) contain MAG. The paranodal loops are connected to each other by adherens junctions that contain E-cadherin and β catenin and to the axon by septate-like junctions that contain caspr-1 and neurofascin 155. Na+ channels cluster in the nodal axolemma and bind to the skeletal protein ankyrinG. Neurofascin 186 and NrCAM also bind ankyrinG and may help target Na+ channels to the node.
The paranodal loops tightly adhere to the axon through a continuous spiral of axo-glial junctions that resemble invertebrate septate junctions (SJs; Arroyo and Scherer 2000). The paranodal loops are a spiraled cytoplasmic channel that is contiguous with the perikaryon of the myelin-forming cell and thus can serve as a conduit for transmitting axonally induced signals that could regulate glial gene transcription. These junctions also form a physical barrier that prevents diffusion of nodal Na+ channels and juxtaparanodal K+ channels. Axo-glial paranodal junctions, therefore, share adhesion, diffusion barrier and putative intercellular communication functions with invertebrate SJs.
The paranodal axolemma contains the adhesion molecule caspr-1 (also called paranodin; Menegoz et al. 1997; Einheber et al. 1997). Caspr-1 has been included in the neurexin superfamily that also contains neurexin I, II, IIIα, and caspr-2 (Bellen et al. 1998). The caspr-1 homologue, neurexin IV, and gliotactin are localized to drosophila SJs along with a homologue of the cytoskeletal binding protein 4.1. Flies null for neurexin IV or gliotactin fail to form SJs. Gliotactin is related to neuroligins, heterophilic binding partners for the neurexins. The adhesion molecules, MAG and E-cadherin, are enriched in paranodal membranes, but not localized in axo-glial junctions. Tait et al. identified NF155 as the first glial molecule enriched in paranodal SJs using electron microscopic immunocytochemistry. In addition, NF155 and caspr-1 colocalized in confocal images of normal paranodal regions and at ectopic locations along dysmyelinated fibers in the Shiverer mouse. NF155 did not co-immunoprecipitate with caspr-1. However, this does not eliminate NF155 as a possible caspr-1 binding partner since adhesion could be regulated by cytoskeletal interactions and/or inside out signaling. As suggested (Tait et al. 2000), it is likely that caspr-1 and NF155 have unidentified binding partners.
An early event in node genesis is the formation of the initial paranodal loop with its characteristic septate junction. Longitudinal ensheathment may proceed until binding of axonal and glial molecules dictates the location of the first paranodal loop and septate junction. Tait et al. also demonstrate that NF155 mRNA levels peaked during initial stages of myelination in the optic nerve (postnatal day 8) supporting a possible role for NF155 in early paranodal formation. Formation of the first septate junction could also provide a stop signal for longitudinal ensheathment of the axon and a start signal for spiral wrapping of myelin. Adherens and tight junction proteins (β-catenin, Z0-1, ZONAB, and huASH1) can be part of signaling pathways that regulate cell growth and differentiation by acting as or binding to transcription factors (Kirkpatrick and Peifer 1995; Tsukita et al. 1999; Balda and Matter 2000; Nakamura et al. 2000). Transcription factors associated with the axo-glial junctions or adherens junctions that connect adjacent paranodal loops may help regulate myelin-forming cell gene expression and provide a mechanism for integrating internodal length and myelin sheath thickness.
In addition to Na+ channels, the nodal axolemma contains an isoform of Na+/K+ ATPase, the cell adhesion molecules, neurofascin 187 (NF187) and NrCAM, and the membrane skeletal proteins ankyrinG 480/270 kD (Davis et al. 1996; Lambert et al. 1997). Voltage-gated Na+ channels bind directly to ankyrinG as does NrCAM and NF187. AnkyrinG, with its ability to bind multiple transmembrane proteins, therefore, may be a key component in the assembly of Na+ channel clusters and functional nodes of Ranvier (Bennett and Lambert 1999). Na+ channel clusters are first detected adjacent to the ends of the developing myelin internodes as they longitudinally ensheath the axon (Ching et al. 1999; Rasband et al. 1999). They appear initially as doublets corresponding to heminodes and then as single clusters at the mature node. It is possible that Na+ channels are pushed and concentrated along the axons by the advancing myelin sheath. Since Na+ channel–cytoskeletal protein interactions differ in node and non-nodal locations (Bennett and Lambert 1999), selective recruitment and/or stabilization of nodal Na+ channels must also occur.
NrCAM and neurofascin have been detected at developing nodal regions slightly before Na+ channels and ankyrinG 270/440, which appear in parallel. This has led to the hypothesis that NrCAM and neurofascin form a complex with Na+ channels through an interaction with ankyrinG. AnkyrinG is essential for Na+ channel cluster as well as NrCAM and NF187 distribution at the axon initial segment, a functional counterpart of nodes (Zhou et al. 1998). Two important questions are when does ankyrinG associate with Na+ channels and does it play a role in targeting Na+ channels to the node or stabilizing them once they are there? AnkyrinG binding is regulated by dephosphorylation of NrCAM and NF187 ankyrin binding domains (Bennett and Lambert 1999). Interestingly, myelination regulates the phosphorylation of internodal neurofilaments, which in turn increases axonal diameter. Nodal and paranodal neurofilaments are mostly nonphosphorylated. A central feature of the nodal axoplasm, therefore, includes regulation of kinase, phosphatase and possibly other enzymatic activities. A mechanism for local modification of nodal molecules and independent of transcription is essential as nodes can be located a meter or more from the neuronal nucleus.
While the molecular orchestrator for nodal distribution is likely to be part of the paranodal septate junction, proper nodal formation and distribution includes adhesion, signaling and cytoskeletal interactions of several multimolecular complexes that are located in paranodal, nodal and myelin membranes. Mice with point or null mutations in the myelin components myelin basic protein (MBP; Rasband et al. 1999; Tait et al. 2000), P0 protein (Martini et al. 1995), PMP.22 (Lambert et al. 1997), connexin 32 (Neuberg et al. 1999), and galactocerebroside (Dupree et al. 1999) can contain ectopic Na+ channel clusters and/or disrupted paranodes. The complexity and dependency of these interactions on nodal formation is highlighted by the fact that none of these molecules are located in septate junctions and P0 and MBP are enriched in compact myelin and absent from paranodal membranes.
The following can be considered as a working hypothesis for mechanisms involved in nodal formation. Adhesion molecules in the paranodal septate junctions dictate the sites of nodes. This adhesion induces binding between ankyrinG and multiple transmembrane proteins that in turn, target and stabilize Na+ channel clusters at the node. Dephosphorylation of the ankyrin binding domains of the axonal adhesion molecules is locally regulated and a requisite for ankyrinG binding and node formation. Further characterization of the molecular components of paranodal and nodal membranes is needed to test and expand these hypotheses and provide insights into the mechanisms that integrate node distribution and myelin sheath thickness.
Acknowledgments
The authors thank Wendy Macklin, Ph.D., for helpful comments and Vikki Pickett for editorial assistance.
Work in the authors' laboratory is supported by grants from the National Institutes of Health (NS29818, NS38186, NS3058, and NS38667) and the Multiple Sclerosis Society.
References
- Arroyo E.J., Scherer S.S. On the molecular architecture of myelinated fibers. Histochem. Cell Biol. 2000;113:1–18. doi: 10.1007/s004180050001. [DOI] [PubMed] [Google Scholar]
- Balda M.S., Matter K. The tight junction protein ZO-1 and an interacting transcription factor regulate ErbB-2 expression. EMBO (Eur. Mol. Biol. Organ.) J. 2000;19:2024–2033. doi: 10.1093/emboj/19.9.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellen H.J., Lu Y., Beckstead R., Bhat M.A. Neurexin IV, caspr and paranodin--novel members of the neurexin familyencounters of axons and glia. Trends Neurosci. 1998;21:444–449. doi: 10.1016/s0166-2236(98)01267-3. [DOI] [PubMed] [Google Scholar]
- Bennett V., Lambert S. Physiological roles of axonal ankyrins in survival of premyelinated axons and localization of voltage-gated sodium channels. J. Neurocytol. 1999;28:303–318. doi: 10.1023/a:1007005528505. [DOI] [PubMed] [Google Scholar]
- Ching W., Zanazzi G., Levinson S.R., Salzer J.L. Clustering of neuronal sodium channels requires contact with myelinating Schwann cells. J. Neurocytol. 1999;28:295–301. doi: 10.1023/a:1007053411667. [DOI] [PubMed] [Google Scholar]
- Davis J.Q., Lambert S., Bennett V. Molecular composition of the node of Ranvieridentification of ankyrin-binding cell adhesion molecules neurofascin (mucin+/third FNIII domain-) and NrCAM at nodal axon segments. J. Cell Biol. 1996;135:1355–1367. doi: 10.1083/jcb.135.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupree J.L., Girault J.A., Popko B. Axo-glial interactions regulate the localization of axonal paranodal proteins. J. Cell Biol. 1999;147:1145–1152. doi: 10.1083/jcb.147.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einheber S., Zanazzi G., Ching W., Scherer S., Milner T.A., Peles E., Salzer J.L. The axonal membrane protein Caspr, a homologue of neurexin IV, is a component of the septate-like paranodal junctions that assemble during myelination. J. Cell Biol. 1997;139:1495–1506. doi: 10.1083/jcb.139.6.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick C., Peifer M. Not just gluecell-cell junctions as cellular signaling centers. Curr. Opin. Genet. Dev. 1995;5:56–65. doi: 10.1016/s0959-437x(95)90054-3. [DOI] [PubMed] [Google Scholar]
- Lambert S., Davis J.Q., Bennett V. Morphogenesis of the node of Ranvierco-clusters of ankyrin and ankyrin-binding integral proteins define early developmental intermediates. J. Neurosci. 1997;17:7025–7036. doi: 10.1523/JNEUROSCI.17-18-07025.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini R., Zielasek J., Toyka K.V., Giese P., Schachner M. Protein zero (P0)-deficient mice show myelin degeneration in peripheral nerves characteristic of inherited human neuropathies. Nat. Genet. 1995;11:281–286. doi: 10.1038/ng1195-281. [DOI] [PubMed] [Google Scholar]
- Menegoz M., Gaspar P., Le Bert M., Galvez T., Burgaya F., Palfrey C., Ezan P., Arnos F., Girault J.A. Paranodin, a glycoprotein of neuronal paranodal membranes. Neuron. 1997;19:319–331. doi: 10.1016/s0896-6273(00)80942-3. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Blechman J., Tada S., Rozovskaia T., Itoyama T., Bullrich F., Mazo A., Croce C.M., Geiger B., Canaani E. huASH1 protein, a putative transcription factor encoded by a human homologue of the drosophila ash1 gene, localizes to both nuclei and cell-cell tight junctions. Proc. Natl. Acad. Sci. USA. 2000;97:7284–7289. doi: 10.1073/pnas.97.13.7284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberg D.H., Sancho S., Suter U. Altered molecular architecture of peripheral nerves in mice lacking the peripheral myelin protein 22 or connexin32. J. Neurosci. Res. 1999;58:612–623. doi: 10.1002/(sici)1097-4547(19991201)58:5<612::aid-jnr2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Rasband M.N., Peles E., Trimmer J.S., Levinson S.R., Lux S.E., Shrager P. Dependence of nodal sodium channel clustering on paranodal axoglial contact in the developing CNS. J. Neurosci. 1999;19:7516–7528. doi: 10.1523/JNEUROSCI.19-17-07516.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer J.L. Clustering sodium channels at the node of Ranvierclose encounters of the axon-glia kind. Neuron. 1997;18:843–846. doi: 10.1016/s0896-6273(00)80323-2. [DOI] [PubMed] [Google Scholar]
- Tait S., Gunn-Moore F., Collinson J.M., Huang J., Lubetzki C., Pedraza L., Sherman D.L., Colman D.R., Brophy P.J. An oligodendroctye cell adhesion molecule at the site of assembly of the paranodal axo-glial junction. J. Cell Biol. 2000;150:657–666. doi: 10.1083/jcb.150.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp B.D., Andrews S.B., Cootauco C., Quarles R.H. The myelin-associated glycoprotein is enriched in multivesicular bodies and periaxonal membranes of actively myelinating oligodendrocytes. J. Cell Biol. 1989;109:2417–2426. doi: 10.1083/jcb.109.5.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S., Furuse M., Itoh M. Structural and signalling molecules come together at tight junctions. Curr. Opin. Cell Biol. 1999;11:628–633. doi: 10.1016/s0955-0674(99)00016-2. [DOI] [PubMed] [Google Scholar]
- Zhou D., Lambert S., Malen P.L., Carpenter S., Boland L.M., Bennett V. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J. Cell Biol. 1998;143:1295–1304. doi: 10.1083/jcb.143.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]