In most vertebrate cells, intermediate filaments (IF) form a continuous structural network extending from the nuclear surface to the cell periphery. Their unique viscoelastic properties render them more resistant than either microtubules or microfilaments to deformation and other external physical stresses. Through a growing family of associated proteins (IFAPs), IF are now known to be connected to other cytoskeletal systems such as microtubules and microfilaments, and to specialized cell surface structures such as hemidesmosomes and focal adhesions. IFAPs contain multiple binding domains that facilitate the cross talk between the different cytoskeletal systems, resulting in their interdependence (Fuchs and Cleveland 1998; Fuchs and Yang 1999; Herrmann and Aebi 2000). Based on their physical properties and their subcellular organization, IF are considered to be the major contributors to the mechanical integrity of cells and tissues. Their importance in this regard has been highlighted by the findings that numerous human skin and muscular diseases are caused by mutations in genes encoding IF and IFAPs (Fuchs and Cleveland 1998).
Although IF are considered to be the major structural backbone of the cytoplasm, they are by no means static protein polymers. Over the past decade, techniques such as microinjection of fluorescently tagged IF proteins and fluorescence recovery after photobleaching (FRAP) have been employed to determine the dynamic properties of IF during interphase. These studies have revealed that IF structure is regulated by a dynamic equilibrium between smaller subunits and polymerized IF. However, due to the relatively rapid photobleaching of fluorochrome-tagged (e.g., rhodamine) IF proteins, the direct observation of IF in living cells has been limited to short time intervals. In the last few years, this limitation has been alleviated by the use of green fluorescent protein (GFP) fusion proteins, which have made it possible to carry out detailed time-lapse observations of IF behavioral patterns in different cell types with increased temporal and spatial resolution. As a result, this new approach has yielded remarkable insights into the understanding of the dynamic properties of IF in living cells.
IF in the Fast Lane
The initial studies involving GFP-vimentin have shown that IF are constantly moving and changing shape within the cytoplasm of growing cultured cells (Ho et al. 1998; Yoon et al. 1998). One of the new insights on IF dynamics has come from observing the properties of GFP-vimentin in cultured fibroblasts that are actively engaged in spreading after trypsinization and replating (Prahlad et al. 1998). During this period of active cytoskeletal remodeling, a fraction of GFP-vimentin is found in non–membrane-bound and non-filamentous forms, termed vimentin “dots” or particles. They are most visible at the edge of cells during the early stages of the spreading process. As spreading progresses, these particles appear to be replaced by short fibrous structures, termed squiggles. Eventually, the number of vimentin particles and squiggles decrease, concomitant with the appearance of the extensive networks of long vimentin fibrils that typify IF patterns seen in fully spread fibroblasts. This phenomenon is not vimentin-specific, as similar keratin-containing structures have been observed at the edge of spreading epithelial cells (Windorffer and Leube 1999). From these observations, it has been hypothesized that at least part of the IF network is assembled sequentially in morphologically distinct steps: non-filamentous particles, short fibrous squiggles, and long fibrils (see video 1; this video contains information published in Prahlad et al. 1998, and is available at http://www.jcb.org/cgi/content/full/150/3/F101/DC1).
More interestingly, vimentin particles and squiggles appear to be translocated to the cell periphery at high rates of speed. Most vimentin particles move in a typical saltatory fashion: rapid movements along straight tracks, interrupted by pauses. Time-lapse measurements of motile particles yield an average speed of 0.6 μm/s with peak velocities ∼1 μm/s (see video 1 available at http://www.jcb.org/cgi/content/full/150/3/F101/DC1). Squiggle motility is somewhat slower with an average speed of ∼3 μm/m. The movements of these structures are primarily, but not exclusively, towards the cell periphery and they are sensitive to nocodazole treatment. This suggests that a plus end–directed microtubule-dependent motor is involved. Indeed, many of the vimentin particles colocalize with conventional kinesin, as determined by immunofluorescence (Prahlad et al. 1998). At the present time, there is no evidence for an association with a minus end–directed motor, such as dynein. However, it appears likely that this will help to explain particle and squiggle movements towards the nucleus (see Fig. 1).
Figure 1.
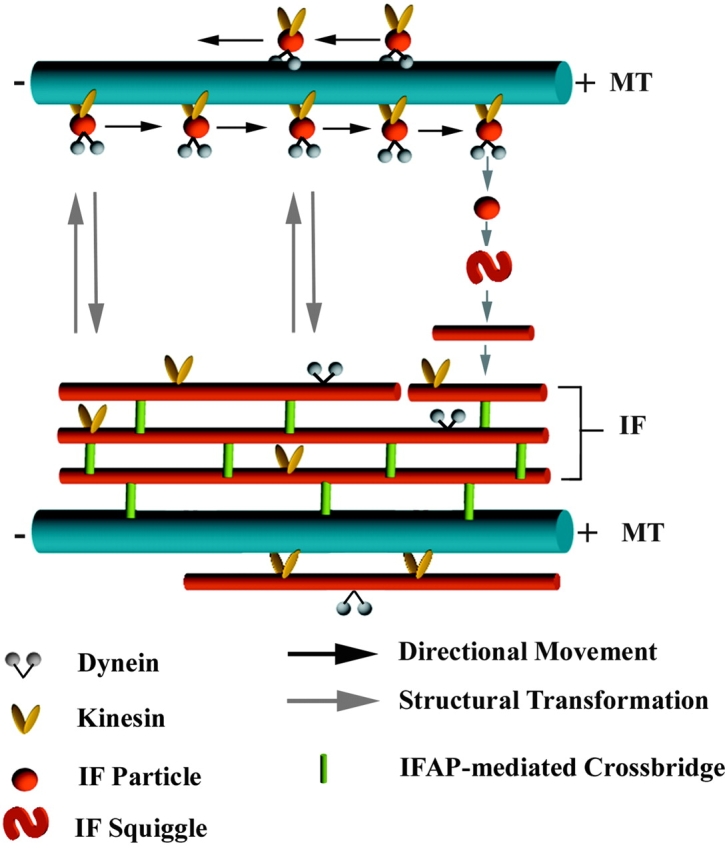
A model illustrating the possible mechanisms that underlie the dynamic properties of interphase IF networks. The model involves the interplay of three different regulatory processes: (a) reversible structural transformation between long filaments, short filaments (squiggles) and particles (non-filamentous oligomers), (b) transport of squiggles and filaments towards either the plus (cell periphery) or minus end (cell center) along microtubule tracks, (c) IFAP-mediated cross-links between neighboring IF and between IF and microtubules. The structural changes between different forms of IF are independent of microtubules and motors. The process is probably regulated at least in part by reversible phosphorylation of IF subunits. The higher the phosphorylation state of IF proteins, the more abundant the number of non-filamentous IF particles and squiggles. In spreading cells, the formation of new IF in the advancing cytoplasm is achieved by combined action of rapid microtubule-directed transport of IF particles from the cell center towards the periphery and of the local assembly of IF. The motility of individual filaments is counterbalanced by enhanced mechanical stability of IF. This may be accomplished by IFAP-mediated cross-links between neighboring IF and among different cytoskeletal systems, a process that is likely regulated by protein phosphorylation as well.
In contrast to the predominantly anterograde translocation of vimentin particles and squiggles in fibroblasts, GFP-keratin IF are seen to move from the cell periphery towards the nuclear region in epithelial cells. Short fibrils appear to merge with the bulk of the keratin IF network located in the perinuclear region (Windorffer and Leube 1999). Similar to vimentin IF movement, this inward-directed keratin migration is microtubule dependent, also suggesting the involvement of a minus end–directed microtubule-based motor molecule, such as dynein.
These live cell studies clearly demonstrate that at least some aspects of the assembly of IF networks in vivo involve microtubules, microtubule-associated motors and an energy source. This complex mechanism of IF assembly is in contrast to that found for IF self-assembly in vitro, a process that requires neither energy nor cofactors (reviewed by Herrmann and Aebi 2000). The full significance of the rapid transport of complexes of IF proteins, such as vimentin particles, to the cell periphery and their subsequent conversion to squiggles and longer fibrils requires further exploration. However, it is tempting to hypothesize that this step-wise conversion of particles to squiggles and to higher order IF networks may represent a mechanism targeted for the regional construction of IF networks in areas of the cytoplasm engaged in rapid shape changes, such as those seen during spreading or locomotion. Such regional control of IF network assembly may be required for locally stabilizing cell shape and mechanical integrity in these areas of the cytoplasm (see Fig. 1).
Fast Moves in Slow Axonal Transport
The majority of neuronal IF are made of neurofilament triplet proteins, which are synthesized primarily in the cell body and then transported anterograde at rates of ∼1 mm/d. This mode of transport is known as slow axonal transport and is in contrast to the fast movement of membrane-bound vesicles, which typically move at an average rate of close to 1 μm/s. One of the unresolved issues in slow axonal transport is whether cytoskeletal proteins, such as neurofilaments, are transported as polymer or subunit (Baas and Brown 1997; Hirokawa et al. 1997). Recently, fast movements of longer IF fibrils have been reported in cultured sympathetic neurons transfected with the neurofilament triplet protein, GFP-NF-M (Wang et al. 2000). In these cells, fibrils up to ∼16 μm in length can be translocated rapidly in both anterograde and retrograde directions at average speeds of 0.38 and 0.49 μm/s, respectively. However, as in the case of the vimentin particles and squiggles, the movements of these fibrils are clearly biased (83%) in the anterograde direction, resulting in the net translocation (0.23 μm/s) of neurofilaments towards the distal tips of axons. Furthermore, most of these movements are discontinuous and are, more frequently than not, interrupted by long pauses that account for 73% of the observation time.
These observations provide direct evidence in support of the transport of polymerized neurofilaments yielding new insights into the mechanism underlying the phenomenon of slow axonal transport of cytoskeletal proteins. Based on these new results, the difference between slow and fast transport is not due to the speeds at which individual filaments travel; it is rather determined by the frequency and the duration of time individual filaments are engaged in motile activity (Wang et al. 2000). The mechanism underlying neurofilament transport remains unclear, however several possibilities can be envisioned. Neurofilaments may associate with microtubule motors as has been demonstrated for vimentin IF (see above), or alternatively, they may “piggyback” on microtubules that are propelled by motor molecules anchored in the cytoplasmic matrix (Baas and Brown 1997; Brady 2000).
However, the results of Wang et al. 2000 do not necessarily mean that all newly synthesized neurofilament proteins are transported exclusively in filamentous form. The transport of neurofilament proteins in non-filamentous oligomers is still a possibility. As mentioned above, a fraction of cellular vimentin in fibroblasts can be rapidly transported in the form of non-filamentous particles along microtubule tracks. It is likely that a proportion of neurofilament proteins may also be transported at these high speeds in similar oligomeric forms or even smaller complexes along axons. This would provide a mechanism by which neurofilament subunits synthesized in the cell body could be delivered to all regions of axons at high rates of speed, similar to the way membrane-bound molecules are conveyed. In support of this, there is evidence that phase-dense particles containing neurofilament components are capable of moving rapidly within axons (Hollenbeck and Bray 1987). Such rapid movements could provide subunits for the turnover of IF proteins at reasonable time intervals in the most distal regions of axons. It is also important to note that transport of non–membrane-bound protein cargo along microtubule tracks appears to represent a general mechanism present in many cell types. For example, a similar system of moving cytoskeletal precursors is observed in the assembly and maintenance of the flagellar axoneme in Chlamydomonas reinhardii. In this case, the building materials for the axoneme are synthesized in the cell body and then transported rapidly to the distal ends of flagella where assembly takes place. Mutational analysis has shown that axonemal precursors are transported bidirectionally in complexes driven by microtubule-dependent motors (Cole et al. 1998; Pazour et al. 1998).
IF Networks in the Slow Lane
IF motility is not confined to rapid linear movements of different structural forms. In cultured fibroblasts and epithelial cells, entire IF networks have been seen to constantly change their configuration without alterations in cell shape (Ho et al. 1998; Windorffer and Leube 1999; also see video 2, which contains information published in Yoon et al. 1998, and is available at http://www.jcb.org/cgi/content/full/150/3/F101/DC1). In the case of vimentin, long fibrils can bend, straighten, change their configuration, and translocate at speeds much slower than those seen for particles and squiggles. This translocation of long fibrils appears to depend on microtubule and microfilament related activities (Yoon et al. 1998). However, the shape changes exhibited by vimentin fibrils continue in the presence of both microtubule and microfilament inhibitors, but not in the presence of metabolic inhibitors (Yoon et al. 1998). This latter observation suggests that shape changes exhibited by IF involve unknown and potentially novel mechanisms.
Probably the least understood type of IF motility is the slow retraction or reorganization of complex IF networks from the cell periphery to the juxtanuclear region. This type of movement takes place under a variety of physiological conditions. In the case of vimentin IF, this movement requires energy and occurs in response to treatment with microtubule and microfilament inhibitors, heat shock, and various other agents or factors. These factors include those that affect the transport of IF proteins and those that modulate interactions between IF and between IF and other cytoskeletal elements. These are not mutually exclusive. For example, microinjection of kinesin antibodies (Gyoeva and Gelfand 1991; Prahlad et al. 1998) or expression of mutated kinesin cDNA (Navone et al. 1992) in cultured cells induces the aggregation of IF at the cell center. This aggregation is thought to be caused by the disruption of kinesin regulated IF-microtubule interactions required for maintaining an extended IF network. Since the transport of vimentin particles appears to be involved in the formation of IF networks in fibroblasts, the induction of juxtanuclear aggregates of IF after the microinjection of kinesin antibodies could reflect the dependence of an extended cytoskeletal network on the continuous microtubule-based anterograde transport of IF and their precursors.
Based upon these findings, it might be expected that modifications of microtubule tracks could affect IF organization. In support of this, it has been found that the retraction of fully extended IF networks can be induced by microinjection of antibodies directed against Glu-tubulins. In this latter case, antibodies appear to interrupt the interactions between IF and stable Glu-microtubules through a kinesin-dependent mechanism (Ho et al. 1998; Kreitzer et al. 1999). In addition, expression of the microtubule-associated protein (MAP) tau in cultured cells has been found to cause the accumulation of vimentin IF in the cell center, apparently due to a shift in the net movement of these IF from a predominantly anterograde to a retrograde direction (Trinczek et al. 1999). This may also be the basis for the retraction of IF networks seen in cells that have been microinjected with antibodies directed against other MAPs (reviewed in Prahlad et al. 1998; Trinczek et al. 1999). Therefore it appears that modulation of the biochemical properties of microtubules can be a contributing factor in regulating the distribution of IF networks in cells.
Other factors required for maintaining an extended IF network include those known to build connections between IFs, and between IF and the other major cytoskeletal elements. For example, the retraction of vimentin IF networks in cultured cells can be induced by the expression of mutated plectin (Nikolic et al. 1996). Plectin is an IFAP that can form cross bridges between IF and possesses binding domains for both microtubules and microfilaments. In sensory neurons, several isoforms of the IFAP bullous pemphigoid antigen 1 (BPAG1) are involved in the proper organization of all three cytoskeletal systems. Interestingly, in BPAG1-null mice (also known as dystonia musculorum mice), the major pathological features are axonal swellings which are filled with IF, due possibly to a defective axonal transport system (Guo et al. 1995; Yang et al. 1999).
Studies of IF motility to date reveal that different IF structures move over a wide range of speeds. Particles move the fastest, while networks move much more slowly. The differences in speed and direction of the different forms of IF that have been described so far, are probably determined by the different motors that power them, or by the different functional states of microtubule tracks that are in turn modulated by MAPs and/or IFAPs. In addition, the slower movements of long IF fibrils and networks may be a consequence of their large size and the cytoplasmic drag imposed by their connections to other cytoskeletal systems (see Fig. 1).
The Regulation and Potential Functions of IF Motility
The mechanism that regulates the morphological transformation of IF from non-filamentous particles to filaments and vice versa is currently unknown. When spreading cells are examined by electron microscopy, the vimentin particles are seen to consist of electron dense aggregates devoid of 10 nm IF and containing 3–5-nm short fibrils (Prahlad et al. 1998; unpublished observations). These are very similar to the disassembled IF aggregates seen in some cultured cells during cell division (Chou et al. 1996). Numerous studies have shown that the disassembly of IF networks during mitosis and cytokinesis is regulated by the phosphorylation of vimentin by protein kinases (Chou et al. 1996; Goto et al. 1998; Kosako et al. 1999). It therefore seems likely that phosphorylation also plays a role in the IF structural dynamics seen in spreading cells. In further support of this, it has been well established that IF proteins are substrates for a large number of protein kinases engaged in signal transduction pathways (reviewed by Inagaki et al. 1996).
Although phosphorylation by many of these kinases in vitro has been shown to induce IF disassembly, their complete disassembly is not commonly seen in interphase cells. More likely, cycles of protein phosphorylation and dephosphorylation are involved in facilitating the local exchange between IF polymers and subunits. This could provide a mechanism to explain the rapid recovery of fluorescence of GFP-vimentin IF in vivo following photobleaching (Yoon et al. 1998). The local control of phosphorylation also may selectively disassemble some IF into smaller structures (i.e., vimentin particles) in one region of the cytoplasm for rapid transport to other regions (see Fig. 1). This regional and temporal control of the phosphorylation of IF could provide a biochemical mechanism for enhancing the dynamics of IF networks in cytoplasmic domains where cytoskeletal elements need to rapidly reorganize to accommodate the shape changes that accompany cell spreading and locomotion. It is interesting to note that RhoA-kinase α, an enzyme involved in regulating actin-based structures such as focal adhesions and stress fibers, also phosphorylates and induces vimentin IF aggregation in vivo in response to treatment with growth factors (Sin et al. 1998).
The dynamic properties of IF may also play an important role in cell locomotion. This possibility is supported by the recent finding that fibroblasts derived from vimentin-free mice exhibit impairments in their mechanical and contractile properties, as well as their motile behavior. One manifestation of this loss of IF is seen in the slower motility of vimentin-free fibroblasts during wound healing (Eckes et al. 1998). There is also an apparent elevated expression of vimentin in motile human mammary epithelial cells at the edges of wounds (Gilles et al. 1999). Similarly, epidermal injury induces the elevated expression of keratins K6 and 16, which is accompanied by a dramatic reorganization of the endogenous IF networks and the enhanced motility of keratinocytes during the re-epithelialization process (Paladini et al. 1996). These observations are intriguing, as it has been thought that the combined functions of microtubules and microfilaments are mainly responsible for cell locomotion. IF, on the other hand, are thought to be responsible mainly for the mechanical properties of cytoplasm, and it is unclear at the present time how alterations in these properties are involved in the cell locomotion process. However, these divisions of functions of the three cytoskeletal systems are beginning to break down due to the ever growing evidence for interactions among them. For example, it has been shown that disassembly of vimentin IF by mimetic peptides results in dramatic changes in cell shape accompanied by the loss of the structural integrity of both microtubules and microfilaments (Goldman et al. 1996). Therefore, the three major cytoskeletal systems are functionally linked, and changes in these linkages can result in the less efficient operation of a cell's locomotory apparatus.
Several IFAPs such as different isoforms of plectin and BPAG1 are known to regulate the structural interactions among the three cytoskeletal systems. These molecules are the likely candidates for coordinating activities or cross talk between IF and the cell's locomotory apparatus. In support of this, plectin-free fibroblasts (Andra et al. 1998) and BPAG1-free epidermal cells (Guo et al. 1995) have been shown to exhibit slower motility during wound healing. Fimbrin, an actin-binding protein, has been found to form complexes with vimentin and is another potential candidate for mediating cytoskeletal interactions (Correia et al. 1999). In the future a detailed analysis of the roles of these IFAPs in coordinating the activities of IF with the activities of the other cytoskeletal elements will most certainly reveal new insights into the mechanisms underlying cell motility.
In summary, recent insights into the motile properties of the various forms of IF and their constituent proteins have provided new opportunities to investigate the dynamics of IF assembly in vivo. The observations that IF proteins can be rapidly transported along microtubule tracks suggest that a fast and efficient system is involved in regulating the distribution of IF precursors for the localized assembly of IF networks. Such local control of IF assembly could provide regional mechanical stability in motile fibroblasts that undergo constant changes in their shape and adhesion properties. The finding that IF, one of the most abundant cellular proteins, can be transported in different structural forms along microtubule tracks also provides a unique opportunity to study how protein cargoes are packaged and targeted to microtubule motors. In addition, the realization that cell locomotion can be modulated by structural alterations in both IF and actin-networks suggests the interesting possibility that the dynamics of IF assembly may also be functionally linked to the actin-based cell locomotion machinery in migrating cells. Undoubtedly, a complete knowledge of how different regulatory molecules are involved in the assembly and function of IF should reveal important roles for IF in a variety of cellular functions ranging from cell shape maintenance to locomotion. Furthermore, an increased understanding of the regulation of IF transport should also shed new light on the molecular basis of the numerous human diseases, such as amyotrophic lateral sclerosis and Parkinson's disease, whose pathological hallmarks include abnormal aggregates of IF in different regions of the cytoplasm.
Acknowledgments
We wish to thank Drs. Bryan Clubb, Timothy Spann and Reynold Lopez for helping in various ways with this manuscript. We also thank the reviewers of this manuscript for constructive suggestions.
Work in this laboratory is supported by the National Institute for General Medical Sciences.
Footnotes
The online version of this article contains supplemental material.
Abbreviations used in this paper: BPAG1, bullous pemphigoid antigen 1; GFP, green fluorescent protein; IF, intermediate filaments, IFAPs, IF-associated proteins; FRAP, fluorescence recovery after photobleaching; MAP, microtubule-associated protein.
References
- Andra K., Nikolic B., Stocher M., Drenckhaln D., Wiche G. Not just scaffoldingplectin regulates actin dynamics in cultured cells. Genes Dev. 1998;12:3442–3451. doi: 10.1101/gad.12.21.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas P.W., Brown A. Slow axonal transportthe polymer transport model. Trends Cell Biol. 1997;7:380–384. doi: 10.1016/S0962-8924(97)01148-3. [DOI] [PubMed] [Google Scholar]
- Brady S.T. Neurofilaments run sprints not marathons. Nature Cell Biol. 2000;2:E43–E45. doi: 10.1038/35004071. [DOI] [PubMed] [Google Scholar]
- Chou Y.-H., Opal P., Quinlan R.A., Goldman R.D. The relative roles of specific N- and C-terminal phosphorylation sites in the disassembly of intermediate filaments in mitotic BHK-21 cells. J. Cell Sci. 1996;109:817–826. doi: 10.1242/jcs.109.4.817. [DOI] [PubMed] [Google Scholar]
- Cole D.G., Diener D.R., Himelbleu A.L., Beech P.L., Fuster J.C., Rosenbaum J.J. Chlamydomonas kinesin II-dependent intraflagellar transport (IFT)IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J. Cell Biol. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia I., Chu D., Chou Y.-H., Goldman R.D., Matsudaira P. Integrating the actin and vimentin cytoskeletonsadhesion-dependent formation of fimbrin-vimentin complexes in macrophages. J. Cell Biol. 1999;146:831–842. doi: 10.1083/jcb.146.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckes B., Dogic D., Colucci-Guyon E., Wang N., Maniotis A., Ingber D., Merckling A., Langa F., Aumailley M., Delouvee A., Koteliansky V., Babinet C., Krieg T. Impaired mechanical stability, migration and contractile capacity in vimentin-deficient fibroblasts. J. Cell Sci. 1998;111:1897–1907. doi: 10.1242/jcs.111.13.1897. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Cleveland D.W. A structural scaffolding of intermediate filaments in health and disease. Science. 1998;279:514–519. doi: 10.1126/science.279.5350.514. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Yang Y. Crossroads on cytoskeletal highways. Cell. 1999;98:547–550. doi: 10.1016/s0092-8674(00)80041-0. [DOI] [PubMed] [Google Scholar]
- Gilles C., Polette M., Zahm J.-M., Tournier J.-M., Volders L., Foidart J.-M., Birembaut P. Vimentin contributes to human mammary epithelial cell migration. J. Cell Sci. 1999;112:4615–4625. doi: 10.1242/jcs.112.24.4615. [DOI] [PubMed] [Google Scholar]
- Goldman R.D., Khuon S., Chou Y.-H., Opal P., Steinert S.M. The function of intermediate filaments in cell shape and cytoskeletal integrity. J. Cell Biol. 1996;134:971–983. doi: 10.1083/jcb.134.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto H., Kosako H., Tanabe K., Yanageda M., Sakurai M., Amano M., Kaibuchi K., Inagaki M. Phosphorylation of vimentin by Rho-associated kinase at a unique N-terminal site that is specifically phosphorylated during cytokinesis. J. Biol. Chem. 1998;273:11728–11736. doi: 10.1074/jbc.273.19.11728. [DOI] [PubMed] [Google Scholar]
- Guo L., Degenstein L., Dowing J., Yu G.-C., Wollman R., Penman R., Fuchs E. Gene targeting of BPAG1abnormalities in mechanical strength and cell migration in stratified epithelia and neurologic degeneration. Cell. 1995;81:233–243. doi: 10.1016/0092-8674(95)90333-x. [DOI] [PubMed] [Google Scholar]
- Gyoeva F.K., Gelfand V.I. Coalignment of vimentin intermediate filaments with microtubules depends on kinesin. Nature. 1991;353:445–448. doi: 10.1038/353445a0. [DOI] [PubMed] [Google Scholar]
- Herrmann H., Aebi U. Intermediate filaments and their associated multi-talented structural elements specifying cytoarchitecture and cytodynamics. Curr. Opin. Cell Biol. 2000;12:79–90. doi: 10.1016/s0955-0674(99)00060-5. [DOI] [PubMed] [Google Scholar]
- Hirokawa N., Terada S., Funakoshi T., Tadeda S. Slow axonal transportthe subunit transport model. Trends Cell Biol. 1997;7:384–388. doi: 10.1016/S0962-8924(97)01133-1. [DOI] [PubMed] [Google Scholar]
- Ho C.L., Martyr J.L., Mikhailov A., Gundersen G.G., Liem R.K. Novel features of intermediate filament dynamics revealed by green fluorescent protein chimeras. J. Cell Sci. 1998;111:1767–1778. doi: 10.1242/jcs.111.13.1767. [DOI] [PubMed] [Google Scholar]
- Hollenbeck P.J., Bray D. Rapidly transported organelles containing membrane and cytoskeletal componentstheir relation to axonal growth. J. Cell Biol. 1987;105:2827–2835. doi: 10.1083/jcb.105.6.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki M., Matsuoka Y., Tsujimura K., Ando S., Tokui T., Takahashi T., Inagaki N. Dynamic property of intermediate filamentsregulation by phosphorylation. Bioessays. 1996;18:481–487. [Google Scholar]
- Kosako H., Go H., Yanagida M., Matsuzawa K., Fujita M., Tomono Y., Okigaki T., Odai H., Kaibuchi K., Inagaki M. Specific accumulation of Rho-associated kinase at the cleavage furrow during cytokinesiscleavage-specific phosphorylation of intermediate filaments. Oncogene. 1999;18:2783–2788. doi: 10.1038/sj.onc.1202633. [DOI] [PubMed] [Google Scholar]
- Kreitzer G., Liao G., Gundersen G.G. Detyrosination of tubulin regulates the interaction of intermediate filaments with microtubule in vivo via a kinesin-dependent mechanism. Mol. Biol. Cell. 1999;10:1105–1118. doi: 10.1091/mbc.10.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolic B., Mac Nulty E., Mir B., Wiche G. Basic amino acid residue cluster within nuclear targeting sequence motif is essential for cytoplasmic plectin-vimentin network junctions. J. Cell Biol. 1996;134:1455–1467. doi: 10.1083/jcb.134.6.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladini R.D., Takahashi K., Bravo N.S., Coulombe P.A. Onset of re-epithelialization after skin injury correlates with a reorganization of keratin filaments in wound edge keratinocytesdefining a potential role for keratin 16. J. Cell Biol. 1996;132:381–397. doi: 10.1083/jcb.132.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour G.J., Wilkerson C.G., Witman G.B. A dynein light chain is essential for the retrograde particle movement of intraflagellar transport (IFT) . J. Cell Biol. 1998;141:979–992. doi: 10.1083/jcb.141.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prahlad V., Yoon M., Moir R.D., Vale R.D., Goldman R.D. Rapid movement of vimentin on microtubule trackskinesin-dependent assembly of intermediate filament networks. J. Cell Biol. 1998;143:159–170. doi: 10.1083/jcb.143.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin W.-C., Chen X.-Q., Leung T., Lim L. RhoA-binding kinase alpha translocation is facilitated by the collapse of the vimentin intermediate filament network. Mol. Cell. Biol. 1998;18:6325–6339. doi: 10.1128/mcb.18.11.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinczek B., Ebneth A., Mandelkow E.-M., Mandelkow E. Tau regulates the attachment/detachment but not the speed of motors in microtubule-dependent transport of single vesicles and organelles. J. Cell Sci. 1999;112:2355–2367. doi: 10.1242/jcs.112.14.2355. [DOI] [PubMed] [Google Scholar]
- Wang L., Ho C.-L., Sun D.-M., Liem R.K.H., Brown A. Rapid movement of axonal neurofilaments interrupted by prolonged pauses. Nature Cell Biol. 2000;2:137–141. doi: 10.1038/35004008. [DOI] [PubMed] [Google Scholar]
- Windorffer R., Leube R.E. Detection of cytokeratin dynamics by time lapse fluorescence microscopy in living cells. J. Cell Sci. 1999;112:4521–4534. doi: 10.1242/jcs.112.24.4521. [DOI] [PubMed] [Google Scholar]
- Yang Y., Bauer C., Strasser G., Wollman R., Julien J.-P., Fuchs E. Integrators of the cytoskeleton that stabilize microtubules. Cell. 1999;98:229–238. doi: 10.1016/s0092-8674(00)81017-x. [DOI] [PubMed] [Google Scholar]
- Yoon M., Moir R.D., Prahlad V., Goldman R.D. Motile properties of vimentin intermediate filament networks in living cells. J. Cell Biol. 1998;143:147–157. doi: 10.1083/jcb.143.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]


