Abstract
Abstract Capsule
Blood spot monitoring of FSH, LH, and progesterone appears equivalent to plasma levels for clinical research and care.
Objective
To compare LH, FSH, progesterone (P), estradiol (E2) levels obtained from bloodspot versus plasma (single visit study) and to determine if blood spots can document circulating hormone levels during ovulatory cycles (menstrual cycle study).
Design
Cross-sectional study.
Setting
Academic center.
Patients
Women age 18–35 years old with regular menstrual cycles and no recent use of hormonal contraception.
Intervention
Women contributed both a blood spot sample from a finger-stick and a plasma sample through venipuncture on a random day within their menstrual cycle (n = 100, single study visit). Five women were followed for an entire menstrual cycle with biweekly venipuncture and daily self-collected blood spot sampling. Samples were analyzed for FSH, LH, P, and E2.
Main Outcome Measure
Correlation between blood spot and plasma levels.
Results
Significant positive correlations were found between the blood spot and plasma samples in the single visit study (r2: FSH 0.91, LH 0.93, P 0.83, E2 0.70). Two out of the 5 menstrual cycle study women had ovulatory cycles based on P levels (>3 ng/mL) and an LH surge. Daily blood spot sampling was better able to document hormonal changes than biweekly venipuncture.
Conclusion
Blood spot monitoring of FSH, LH, progesterone, and to a lesser extent estradiol appears as valid as traditional plasma assays for clinical research and care.
Keywords: Blood spot, gonadotropins, FSH, LH, progesterone, estradiol, hormone monitoring, ovulation, enzyme immunoassay
Introduction
Plasma or serum samples obtained via repetitive venipuncture represent the accepted gold standard for monitoring circulating levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2) and progesterone (P) in published reproductive studies. Unfortunately for research subjects, the burden of frequent venipuncture is high (e.g. uncomfortable and time-consuming) and for researchers, venipuncture samples require immediate processing and storage facilities with freezers. Although less invasive techniques for measuring hormone levels currently exist, their reliability in reproductive research remains unproven.
One promising method involves self-collection of small blood samples through finger sticks (blood spot testing). The samples (collected on special paper and dried) do not require immediate processing, and can be stored at room temperature at home by a research subject for several weeks. Frequent office visits can therefore be avoided but frequent sampling can be performed. Blood spots have been successfully used to screen newborns for metabolic diseases and hypothalamic function and for anthropologic research, but neither of these situations requires the assay range and specificity that are crucial for reproductive research (1–4). Despite the apparent advantages, it remains unknown whether the results obtained from blood spot samples are reliable, specific, and sensitive enough to measure small changes in pituitary gonadotropins and ovarian steroid hormones important in studies of menstrual cyclicity and ovulation detection.
Further validation of blood spot assays is necessary before adopting this approach for reproductive research. This study was designed to compare LH, FSH, P, and E2 levels obtained from bloodspot versus plasma samples, to determine if blood spot assays can document peptide and steroid hormone changes seen in ovulatory cycles, and to determine the feasibility of relying on self-collected samples for reproductive studies.
Materials and Methods
The Institutional Review Board at Oregon Health & Sciences University (OHSU) and the General Clinical Research Center Scientific Advisory Committee approved the study protocol and all patients underwent informed written consent. Enrollment took place at OHSU from December 2004 to February 2005. Women age 18–35 years old with a history of regular menstrual cycles and no recent use of hormonal contraception were eligible for enrollment (n = 100). Women contributed both a blood spot sample from a finger-stick and a plasma sample through venipuncture during a single visit study during a random day within their menstrual cycles. Five additional women were followed for an entire menstrual cycle (menstrual cycle study) with bi-weekly venipuncture and daily self-collected blood spot sampling. Demographic data was collected on all patients at study entry.
Venipuncture samples (approximately 15 mL) were collected and centrifuged at 1500g for 10 minutes. The plasma samples were then stored at −80° C until analyzed. Plasma samples were analyzed separately by both the OHSU GCRC laboratory and a commercial laboratory specializing in blood spot testing (ZRT Laboratory, Beaverton, Oregon) using commercially available kits (OHSU: LH, FSH, and P with an automated chemiluminescent assay, Diagnostic Products Corporation, Los Angeles, CA, www.dpcweb.com and E2 with a radioimmunoassay, Diagnostic Systems Laboratories, Webster, TX, www.dslabs.com; ZRT: E2 and P with an enzyme immunoassay, DRG, Germany, www.drg-diagnostics.de and for FSH and LH with a fluoroimmunoassay, Perkin Elmer-Wallac, Wellesley, Massachusetts, las.perkinelmer.com ). Of note, one of the authors (Zava) is the President of ZRT laboratory, Beaverton, Oregon.
Blood spot samples (whole blood) were obtained via finger-sticks (lancets) and dropped onto specialized filter paper (Schleicher & Schuell #903, Bioscience, Keene, NH). Three to six ‘spots’ approximately 1 cm in diameter onto one card equaled one sample. Samples were dried for at least one hour and then stored at room temperature up to 1 month prior to being analyzed for FSH and LH. After processing the FSH and LH samples (see below), the blood spot specimens were desiccated, frozen at −70C and stored for approximately 10 months prior to being thawed for P and E2 analysis. Several random E2 blood spot samples were initially tested to try to improve the sensitivity of the assay, thereby leaving less samples for the final analysis.
Standard, control and participant disks (6.4 mm) were punched out using the Wallac Multipuncher 1296-081 Dried Bloodspot Puncher (Perkin Elmer-Wallac, Wellesley, Massachusetts) into 96 deep well (2 mL/well) plates and rehydrated in 200 μL/disk of assay buffer containing phosphate buffered saline (1000-3, Diamedix, Miami, FL), 0.025% Tween 20, and 0.01% ProClin 950 antimicrobial (Sigma-Aldrich, St Louis, MI). Blood spot assays were analyzed using modified immunoassays at ZRT laboratory (E2 and P with an enzyme immunoassay, DRG, Germany and for FSH and LH with a fluoroimmunoassay, Wellesley, Massachusetts).
Standards for blood spot assays were prepared by mixing E2 or P standards from DRG or LH and FSH standards from Perkin-Elmer 1:1 with washed human red blood cells prepared by the Red Cross (Pacific Northwest Regional Blood Services, Portland, OR). Control blood spots containing low (BioRad1), medium (BioRad2), and high (BioRad3) levels of E2, P, LH, and FSH are prepared by mixing the reconstituted BioRad samples 1:1 with washed red blood cells (Bio-Rad Laboratories, Anaheim, CA). Large lots of standards and controls were prepared by spotting multiple 50 μL (near equivalent to volume of a finger stick blood drop) aliquots onto large filter cards, desiccated, and then stored at −30 to −70 C until brought to room temperature for analysis.
For blood spot FSH and LH, intra- and inter-assay coefficients of variance (CV) were: FSH 8.6–16.0 % (BioRad1: 7.1 U/L), 8.0–15.8 % (BioRad2: 15.3 U/L), and 5.9–9.2 % (BioRad3: 41.7 U/L); for LH 16.317.7% (BioRad1: 1.6 U/L), 7.49.6% (BioRad2: 16.8 U/L), and 7.910.5% (BioRad3: 50.5 U/L). Blood spot intra-assay CVs for E2 and P were less than 10%. Inter-assay CVs for E2 were 22% (BioRad1: 113 pg/mL), 11.7% (BioRad2: 233 pg/mL), and 10.3% (BioRad3: 389 pg/ml). Inter-assay CVs for P were 19% (BioRad1: 0.94 ng/ml), 12.5% (BioRad2: 7.6 ng/ml) and 10%(BioRad3: 17 ng/ml). The limits of detection (sensitivity) based on blank average + 2SD for FSH, LH, E2, and P were, respectively, 0.078 U/L, 0.065 U/L, 17 pg/mL, and 0.18 ng/mL. Assay linearity for FSH, LH, E2, and P were, respectively, throughout the ranges 0.2–250 U/L, 0.2–256 U/L, 30–1000 pg/ml, and 0.3–40 ng/ml.
Blood spot and plasma samples were assigned unique identifying numbers such that the analysis was blind to the relationship between the samples. Only the principal investigator who was not involved in sample analysis had access to the key. Descriptive statistics were used to summarize demographic data (means, frequencies). Two replicates of each sample were performed and then averaged. ZRT and OHSU plasma results were compared using Pearson Correlation testing. ZRT and OHSU plasma results were separately compared to blood spot results for each paired sample from the single visit study using a paired t-test and Pearson Correlation testing. For the menstrual cycle study, the same statistics were used. All analysis was done based on intent to treat. Statistical analyses were performed using the Statistical Program for Social Sciences (SPSS version 10.0 for Windows; SPSS inc, Chicago, Ill).
Results
Of the 100 women enrolled in the single visit study, paired plasma and blood spot samples were available for analysis from ninety for LH and FSH (1 protocol violation with age >35, 1 unsuccessful venipuncture, and 8 insufficient plasma samples), sixty-six E2 samples (24 insufficient blood spot samples) and fifty-nine P samples (31 insufficient blood spot samples). A total of 5 women were enrolled in the menstrual cycle study. Overall for the entire study cohort, the average subject was a 28 year old (SD 3.9), Caucasian (82%), nulliparous (61%) woman with a body mass index of 25 kg/m2 (SD 5.8).
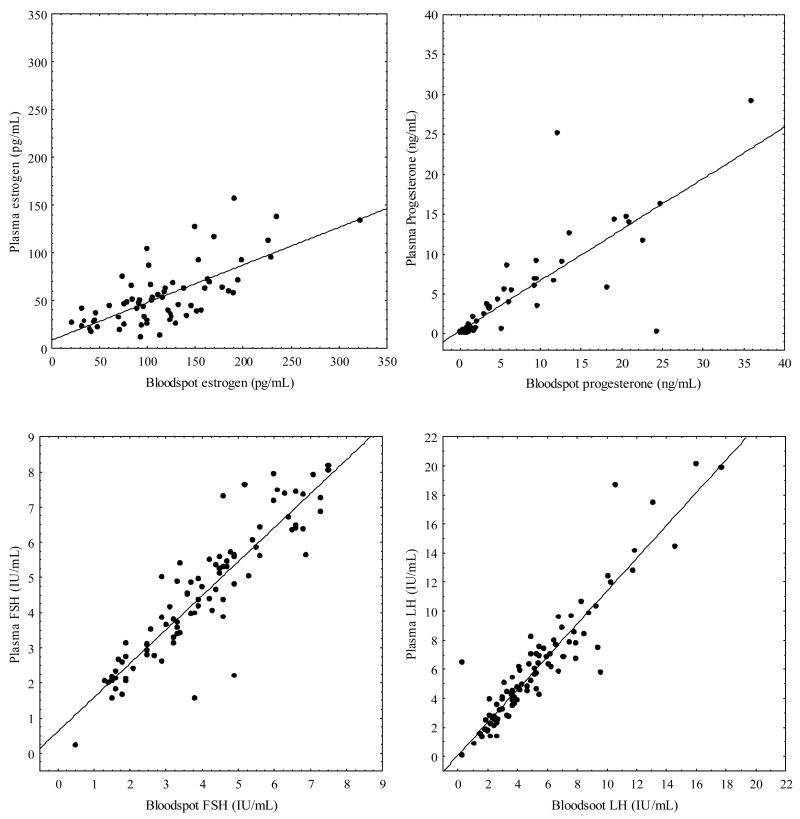
Plasma levels for gonadotropin and steroid levels were comparable when analyzed by the ZRT and OHSU GCRC laboratories (r2: FSH 0.97, LH 0.97, P 0.92, E2 0.86); therefore the plasma samples used for comparison to the blood spot samples are those from ZRT Laboratory. Significant positive correlations were found between the blood spot and plasma samples for the single visit study (Figure 1, r2: FSH 0.91, LH 0.93, P 0.83, E2 0.70). Excluding E2 data points below 50 or 100 pg/mL did not improve the correlation between plasma and bloodspot samples. The mean hormone values obtained from blood spots appeared modestly but significantly different (p< 0.001) than those derived from plasma samples (FSH 4.0 mIU/mL ± 1.8 vs. 4.5 mIU/mL ± 1.9; LH 5.3 mIU/mL ± 3.4 vs. 6.1 mIU/mL ± 4.1; P 6.1 ng/mL ± 8.0 vs. 4.2 ng/mL ± 6.2, E2 117.2 pg/mL ± 57.4 vs. 54.7 pg/mL ± 32.1)(Figure 1).
Figure 1.
Single visit study blood spot versus plasma values with regression line for E2 (top left panel, pg/mL, n = 66), P (top right panel, ng/mL, n = 59), FSH ( bottom left panel, IU/mL, n = 90), LH (bottom right panel, IU/mL, n = 90).
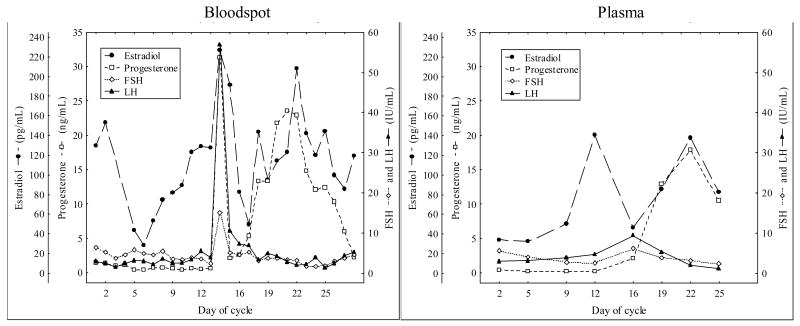
Two of the 5 women providing daily blood spots and biweekly plasma samples (menstrual cycle study) had ovulatory cycles based on P levels > 3 ng/mL with an LH surge by either blood spot or plasma sampling. Figure 2 demonstrates the FSH, LH, P and E2 variation during one subject’s ovulatory cycle with bi-weekly plasma sampling and daily, self-collected, bloodspot sampling. Although comparable in appearance, the LH peak was missed with bi-weekly venipuncture sampling.
Figure 2.
Daily blood spot (left panel) versus biweekly plasma (right panel) sampling for an entire menstrual cycle (FSH IU/mL, LH IU/mL, P ng/mL, E2 pg/mL) in one woman.
Discussion
Blood spot sampling appears to be an effective and accurate alternative to venipuncture for FSH, LH, and P monitoring. Although promising, E2 results are more varied. These results are not surprising since even radioimmunoassays for E2 on plasma samples experience this problem because of the low levels of E2 (pg/mL) in blood.
The mean hormone values for the single visit study were significantly different when comparing blood spot to venipuncture sampling. The magnitude of these differences is clinically insignificant given that these hormones normally have a much larger range over the course of a menstrual cycle and that a commercial assay’s coefficient of variation can be up to 10%. However, two paired P samples were extremely incongruous (Blood spot 24 ng/mL versus plasma 0.28 ng/mL; Blood spot 12 ng/mL versus plasma 25 ng/mL). The technique for both of these blood spot samples was not optimal (e.g. supersaturated or overlapping blood drops) and this may have affected the results, although exclusion of imperfect blood spot samples from the overall analysis did not affect correlation of blood spots and plasma samples.
The main advantage of blood spots over venipuncture is the opportunity to capture a greater number of data points without significantly increasing the burden to patients and/or research subjects. The menstrual cycle study participants successfully performed daily self-sampling and stored samples at home. As demonstrated by our results, biweekly venipuncture sampling missed the LH surge while daily blood spot sampling was able to document the peptide and steroid hormone changes seen in a typical ovulatory cycle (Figure 2).
Although diabetics perform serial self-sampling as a necessity, ours is the first study that has proven the feasibility of this method for research subjects in fertility studies. Blood spot testing should be given greater consideration for clinical and/or research scenarios when frequent testing of gonadotropins and ovarian hormones are needed in longitudinal studies.
Acknowledgments
The authors would like to thank Khanh Tran, BS and Sanjay Kapur, PhD for their technical assistance and ZRT Laboratory, Beaverton, Oregon for their generous support.
Financial support: HD 01243-03 Women’s Reproductive Health Research Fellow (NICHD K-12), PHS Grant 5 M01 RR000334 and Center For Women’s Health Grant 2004, Portland, Oregon.
Footnotes
Presented at American Society of Reproductive Medicine, Montreal, Quebec, Canada, October 2005.
Conflict of Interest: ZRT Laboratory donated supplies, time, and personnel for analysis of plasma and blood spot samples. Dr. Zava is the President of ZRT Laboratory, Beaverton, Oregon.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heinrichs C, Bourdoux P, Saussez C, Vis HL, Bourguignon JP. Blood spot follicle-stimulating hormone during early postnatal life in normal girls and Turner’s syndrome. J Clin endocrinol Metab. 1994;78:978–981. doi: 10.1210/jcem.78.4.8157730. [DOI] [PubMed] [Google Scholar]
- 2.Mei J, Alexander R, Adam BW, Hannon WH. Use of filter paper for the collection and analysis of human whole blood specimens. J Nutr. 1002;131:1631S–1636S. doi: 10.1093/jn/131.5.1631S. [DOI] [PubMed] [Google Scholar]
- 3.Worthman C, Stallings J. Hormone measures in finger-prick blood spot samples: new field methods for reproductive endocrinology. Am J Phys Anthropol. 1997;104:1–21. doi: 10.1002/(SICI)1096-8644(199709)104:1<1::AID-AJPA1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 4.Worthman C, Stallings J. Measurement of gonadotropins in dried blood spots. Clin Chem. 1994;40:448–453. [PubMed] [Google Scholar]