Abstract
We report a novel connection between nuclear pore complexes (NPCs) and spindle pole bodies (SPBs) revealed by our studies of the Saccharomyces cerevisiae NDC1 gene. Although both NPCs and SPBs are embedded in the nuclear envelope (NE) in yeast, their known functions are quite distinct. Previous work demonstrated that NDC1 function is required for proper SPB duplication (Winey, M., M.A. Hoyt, C. Chan, L. Goetsch, D. Botstein, and B. Byers. 1993. J. Cell Biol. 122:743–751). Here, we show that Ndc1p is a membrane protein of the NE that localizes to both NPCs and SPBs. Indirect immunofluorescence microscopy shows that Ndc1p displays punctate, nuclear peripheral localization that colocalizes with a known NPC component, Nup49p. Additionally, distinct spots of Ndc1p localization colocalize with a known SPB component, Spc42p. Immunoelectron microscopy shows that Ndc1p localizes to the regions of NPCs and SPBs that interact with the NE. The NPCs in ndc1-1 mutant cells appear to function normally at the nonpermissive temperature. Finally, we have found that a deletion of POM152, which encodes an abundant but nonessential nucleoporin, suppresses the SPB duplication defect associated with a mutation in the NDC1 gene. We show that Ndc1p is a shared component of NPCs and SPBs and propose a shared function in the assembly of these organelles into the NE.
Keywords: yeast, Ndc1p, spindle pole body, nuclear pore complex, Pom152p
The nuclear envelope (NE)1 remains intact throughout all stages of the cell cycle in the budding yeast, Saccharomyces cerevisiae. Two organelles, nuclear pore complexes (NPCs) and spindle pole bodies (SPBs), are embedded within the NE. Beyond their common localization to the NE, these organelles have not been thought of previously as related structures.
NPCs are macromolecular structures that function as the sole means by which molecules are exchanged between the nucleus and the cytoplasm. NPCs display eightfold symmetry, and are embedded within reflexed membrane pores in the NE. More than 30 NPC-associated proteins have been identified in S. cerevisiae using a combination of biochemical and genetic approaches (reviewed in Doye and Hurt, 1997; Wente et al., 1997). Mutations in NPC components can affect nuclear transport, NPC distribution, NE morphology, and even spindle morphology.
Pom152p was identified biochemically as a transmembrane glycoprotein that localizes to yeast NPCs (Wozniak et al., 1994). Although POM152 encodes one of the most abundant NPC components, this gene is not essential in yeast. However, POM152 becomes essential when the null allele is combined with mutations in a number of genes that encode NPC components (Aitchison et al., 1995b ; Nehrbass et al., 1996). Pom152p localizes to the yeast NPC membrane ring structure (Strambio-de-Castillia et al., 1995; Yang et al., 1998).
SPBs are estimated to be 20 times larger than NPCs in diploid yeast cells (Bullitt et al., 1997). The SPB functions as the centrosome equivalent organelle in yeast, serving as the sole microtubule organizing center of the cell (reviewed in Snyder, 1994; Winsor and Schiebel, 1997). The SPB is embedded in the NE where it nucleates both cytoplasmic and nuclear microtubules. Yeast cells contain a single SPB at the beginning of G1 that must be precisely duplicated once during each cell cycle to form the two poles of the mitotic spindle. Several genes have been identified in which mutations affect specific steps in SPB duplication (reviewed in Winey and Byers, 1993).
The S. cerevisiae NDC1 gene has been shown to function at a late step in SPB duplication (Winey et al., 1993). Although SPB duplication is initiated in ndc1-1 strains at the nonpermissive temperature, the newly synthesized SPB is not inserted into the NE. The defective SPB remains on the cytoplasmic face of the NE where it nucleates cytoplasmic microtubules, but it fails to nucleate nuclear microtubules. The preexisting SPB is functional in these cells, and all of the chromosomes remain associated with it. Thus, ndc1-1 cells arrest with large buds and a G2 DNA content in response to their monopolar spindles. Eventually, these cells undergo asymmetric cell division in which all of their chromosomal DNA segregates with the single, functional SPB; as a result, one cell doubles in ploidy and the other cell lacks chromosomal DNA (Thomas and Botstein, 1986). NDC1 encodes an essential 74-kD protein with six to seven potential transmembrane domains (Winey et al., 1993). Previous attempts to examine Ndc1p localization using overexpressed protein revealed perinuclear staining (Winey et al., 1993).
We show here that Ndc1p expressed at endogenous levels localizes to both NPCs and SPBs. Additionally, we have found that a deletion of POM152 suppresses the ndc1-1 SPB duplication defect. Our results uncover a previously unknown link between two NE-embedded organelles, NPCs and SPBs.
Materials and Methods
Yeast Strains and Media
Relevant yeast strains used in this study are listed in Table I and were constructed using standard techniques (Sherman et al., 1986). The chromosomal copy of NDC1 was epitope tagged at its 3′ end by creating an in-frame fusion to the IgG binding domains of protein A (ProA) as described by Aitchison et al. (1995a). NDC1-ProA strains grew at all temperatures ranging from 15 to 37°C and exhibited stable ploidy. The ndc1-1, pom152 null strain [HC5-31c(1166); Table I] was constructed by replacing the entire POM152 open reading frame, plus an additional 163 bp after the stop codon, with the HIS3 gene (Baudin et al., 1993). NDC1-ProA strains containing NUP49-GFP (HC26-15a/1a; Table I) were constructed by crossing a NDC1-ProA strain to yeast strain SWY809 (Bucci and Wente, 1997) that contains nup49-1::URA3 and nup49ΔGFLG::GFP-S65T-TRP1. Strains containing NDC1-ProA, NUP49-GFP, and the nup133 null allele (HC27-16d/16b; Table I) were constructed by crossing a NDC1-ProA strain to yeast strain SWY828 (Bucci and Wente, 1998) that contains nup133Δ::HIS3, nup49-1::URA3, and nup49ΔGFLG::GFP-S65T-TRP1. A yeast strain containing NIC96-ProA was constructed as described by Aitchison et al. (1995a). Yeast strains containing either NDC1-ProA or NIC96-ProA with SPC42-GFP (HC22-2b/1c and HC23-11d/16a, respectively) were constructed by crossing to yeast strain IAY18 (a kind gift from Ian Adams and John Kilmartin) that contains spc42Δ::LEU2 and TRP1::SPC42-GFP(3x) (Schutz and Winey, 1998). Yeast strain HC14-10c(1198)/HC29-6b is homozygous for two different null alleles of NDC1; the entire NDC1 open reading frame was removed in both cases. The ndc1Δ::HIS3 null allele was made by integrative transformation of a PCR product containing the HIS3 gene (Baudin et al., 1993). The ndc1Δ:: KANMX null allele was constructed using a two-step gene disruption technique (Rothstein, 1991); in this case, the NDC1 open reading frame was replaced with the KANMX gene (Wach et al., 1994).
Table I.
Yeast Strain List
| Strain* | Genotype | |
|---|---|---|
| ProA5-4d/4a | MAT a/MATα NDC1-ProA-HIS3-URA3/NDC1-ProA-HIS3-URA3 ura3-52/ura3-52 his3Δ200/his3Δ200 trp1-1/trp1-1 | |
| leu2-3,112/leu2-3,112 lys2-801/lys2-801 | ||
| ProA5-4d | MAT a NDC1-ProA-HIS3-URA3 ura3-52 his3Δ200 trp1-1 leu2-3,112 lys2-801 | |
| HC26-15a/1a | MAT a/MATα NDC1-ProA-HIS3-URA3/NDC1-ProA-HIS3-URA3 nup49-1::URA3/nup49-1::URA3 nup49ΔGFLG::GFP- | |
| S65T-TRP1/nup49ΔGFLG::GFP-S65T-TRP1 ura3/ura3 his3/his3 trp1-1/trp1-1 leu2-3,112/leu2-3,112 | ||
| HC27-16d/16b | MAT a/MATα nup133Δ::HIS3/nup133Δ::HIS3 NDC1-ProA-HIS3-URA3/NDC1 nup49-1::URA3/nup49-1::URA3 | |
| nup49ΔGFLG::GFP-S65T-TRP1/nup49ΔGFLG::GFP-S65T-TRP1 ura3/ura3 his3/his3 trp1-1/trp1-1 leu2-3,112/leu2-3,112 | ||
| HC22-2b/1c | MAT a/MATα NDC1-ProA-HIS3-URA3/NDC1-ProA-HIS3-URA3 spc42Δ::LEU2/spc42Δ::LEU2 | |
| TRP1::SPC42-GFP(3x)/TRP1::SPC42-GFP(3x) ura3/ura3 his3/his3 trp1-1/trp1-1 leu2-3,112/leu2-3,112 ade2-1/ade2-1 | ||
| HC23-11d/16a | MAT a/MATα NIC96-ProA-HIS3-URA3/NIC96-ProA-HIS3-URA3 spc42Δ::LEU2/spc42Δ::LEU2 TRP1::SPC42- | |
| GFP(3x)/TRP1::SPC42-GFP(3x) ura3/ura3 his3/his3 trp1-1/trp1-1 leu2-3,112/leu2-3,112 ade2-1/ade2-1 | ||
| HC14-10c(1198)/HC29-6b | MAT a/MATα ndc1Δ::KANMX/ndc1Δ::HIS3 ura3-52/ura3-52 his3Δ200/his3Δ200 trp1Δ63/TRP1 leu2Δ1/leu2Δ1 LYS2/ | |
| lys2-801 ade2Δ426/ade2Δ426 ade3Δ/ade3Δ (pRS315-NDC1-GFP) | ||
| HC10-42b/42c | MAT a/MATα ura3-52/ura3-52 ade2/ade2 his4-539/HIS4 | |
| HC10-42a/42d | MAT a/MATα ndc1-1/ndc1-1 ura3-52/ura3-52 ade2/ade2 his4-539/HIS4 | |
| HC13-1c | MATα NIC96-ProA-HIS3-URA3 ura3-52 his3Δ200 trp1-1 leu2-3,112 lys2-801 | |
| Wx340-3c | MATα ndc1-1 tub2-403 ura3-52 his4-539 lys2-801 | |
| Wx340-3d | MATα tub2-403 ura3-52 his4-539 | |
| HC14-10c | MAT a ura3-52 his3Δ200 trp1Δ63 leu2Δ1 ade2Δ426 ade3Δ | |
| HC5-31c | MAT a ndc1-1 ura3-52 his3Δ200 trp1Δ63 leu2Δ1 ade2Δ426 ade3Δ | |
| HC5-31c(1166) | MAT a ndc1-1 pom152Δ::HIS3 ura3-52 his3Δ200 trp1Δ63 leu2Δ1 ade2Δ426 ade3Δ |
All strains were constructed as a part of this study.
All yeast strains were grown as described, in YPD (1% yeast extract, 2% bactopeptone, 2% glucose), YPR (1% yeast extract, 2% bactopeptone, 3% raffinose), synthetic media supplemented with the appropriate amino acids and 2% glucose, or synthetic media supplemented with the appropriate amino acids and 3% raffinose. We used YPR containing 2% galactose for galactose induction. Yeast strains were grown at the temperatures indicated. Techniques for yeast manipulation were carried out as described by Sherman et al. (1986). Cells were arrested in G1 using alpha-factor obtained from a custom peptide synthesis that was HPLC purified (Macromolecular Resources, Ft. Collins, CO), at a concentration of 11 μg/ml for 2 h.
Plasmids
Plasmids used in this study were constructed and introduced into Escherichia coli or yeast using standard techniques (Ausubel et al., 1994). pALR10-NDC1 contains an AgeI-SacI NDC1 fragment cloned into the XmaI/SalI and SacI sites of the pALR10 vector (a kind gift from Alain Camasses), a modified version of the centromeric plasmid pUN50 (Elledge and Davis, 1988) that contains the URA3 and ADE3 selectable markers. The pGAL-NIC96-ProA plasmid contains NIC96-ProA that by PCR amplification using genomic DNA from yeast strain HC13-1c (Table I) contained BglII sites on each end cloned into the BamHI site of the pBM272 vector, just after the GAL1 promoter (Johnston and Davis, 1984). The pRS315-NDC1-green fluorescent protein (GFP) plasmid was constructed by subcloning a PCR fragment containing GFP amplified from the pyEGFP3 plasmid (Cormack et al., 1997) with XbaI restriction sites into an AvrII linker introduced at the end of NDC1 cloned into the pRS315 vector (Sikorski and Hieter, 1989).
Protein Techniques
Fractionation of yeast strains was carried out as described previously (Rout and Kilmartin, 1990; Rout and Kilmartin, 1991; Rout and Blobel, 1993; Strambio-de-Castillia et al., 1995; Rout and Strambio-de-Castillia, 1998) using yeast strain ProA5-4d (Table I). Extractions of the isolated NEs were carried out using 0.1 M sodium carbonate, pH 11.5, as described previously (Wozniak et al., 1994). Peptide sequencing was carried out as described previously (Fernandez et al., 1994). Ndc1p-ProA and Pom152p were detected using purified rabbit IgG (1:1,000; ICN Pharmaceuticals, Inc., Costa Mesa, CA) and mAb 118C3 (1:5, culture supernatant) (Strambio-de-Castillia et al., 1995), respectively. Spc110p was detected using a combination of three monoclonal supernatants: 3D2, 36G6, and 45D10 (1:20, culture supernatants) (Rout and Kilmartin, 1990). Nup57p was detected using a mixture of mAb 414 and mAb 350 (1:5, culture supernatants) (Davis and Blobel, 1986; Davis and Blobel, 1987; Davis and Fink, 1990).
Cytological Techniques
Buffer solutions used for immunofluorescence (IF) microscopy include PBSA (10 mg/ml NaCl, 0.2 mg/ml KCl, 1.43 mg/ml KH2PO4), solution A (1.2 M sorbitol, 100 mM KPO4, pH 7.5), spheroplasting solution (solution A containing 4.83 μg/ml Zymolyase 100T, 0.1 M β-mercaptoethanol), and blocker (PBSA containing 10 mg/ml BSA, 0.1% Tween 20).
IF microscopy of proteins epitope-tagged with the IgG binding domains of ProA was carried out as follows. Log phase growing cells were fixed for 5 min using formaldehyde (3.7% in PBSA), followed by rinsing with PBSA. The cells were then resuspended in spheroplasting solution and incubated at 30°C for 45 min, followed by rinsing with PBSA and resuspension in solution A. Spheroplasted cells were adhered to poly-lysine–treated slides. The slides were submerged into chilled 100% methanol on ice, allowed to equilibrate to room temperature, and immersed into 100% acetone at room temperature for 30 s. Dried slides were treated with blocker, and primary antibody (rabbit IgG, 2 mg/ml in 0.15 M NaCl diluted 1:2,000; Sigma Chemical Co., St. Louis, MO) was applied overnight. After rinses with PBSA, the secondary antibody was applied; either FITC-conjugated donkey anti–rabbit IgGs (1:100; Amersham Life Science Inc., Arlington Heights, IL) or Texas red–conjugated goat anti–rabbit IgGs (1:200; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) were used. The slides were rinsed with PBSA and were treated with 4′,6-diamidino-2-phenylindole (Sigma Chemical Co.) to stain DNA. The slides were mounted in Citifluor (Ted Pella, Inc., Redding, CA).
Standard fluorescence microscopy was carried out using a Zeiss fluorescence microscope equipped with an Empix CCD camera. Images were captured using MetaMorph imaging software (Universal Imaging, West Chester, PA). Deconvolution microscopy was carried out using a Leica DMRXA/RF4/V automated universal microscope equipped with a Cooke SensiCam high performance digital camera. Images were acquired and deconvolved using the Slidebook software package (Intelligent Imaging Innovations, Denver, CO).
Immunoelectron microscopy was carried out as described previously (Ding et al., 1997). Approximately 5-ml aliquots of yeast cultures were harvested by vacuum filtration using 0.45-μm Milipore filters, and were high pressure frozen in a Balzers HPM-010 High Pressure Freezer (Winey et al., 1995). Samples were freeze substituted in 0.15% glutaraldehyde in acetone at −80°C for 3 d, gradually warmed to 4°C, and embedded in LR White resin. Thin sections were labeled with an anti-GFP rabbit polyclonal antiserum (a kind gift from Jason Kahana and Pamela Silver, Dana-Farber Cancer Institute). Sections were mounted on formvar-coated nickel grids and were incubated in 0.1 N HCl for 10 min, 50 mM ammonium acetate for 15 min, and in blocking buffer (0.8% BSA, 0.1% gelatin, 50 mM sodium phosphate, pH 7.2, 150 mM NaCl, and 0.1% Tween 20) for 1 h. The grids were then floated for 2 h on drops of primary antibody diluted 1:100 in blocking buffer, followed by rinses with PBST (50 mM sodium phosphate, pH 7.2, 150 mM NaCl, and 0.1% Tween 20). The grids were then reacted with goat anti–rabbit 15-nm colloidal gold conjugate (Ted Pella, Inc.) diluted 1:20 in blocking buffer for 1 h, followed by rinses and staining with uranyl acetate and lead citrate.
Flow cytometry was carried out as described (Hutter and Eipel, 1979) using propidium iodide to stain DNA (Sigma Chemical Co.). Stained cells were analyzed using a FACScan® flow cytometer and the Cell Quest software package for data analysis (Becton Dickinson, San Jose, CA).
Assays to Examine NPC Assembly
Assays to examine NPC assembly were carried out using yeast strains HC10-42b/42c and HC10-42a/42d (Table I) transformed with pGAL-NIC96-ProA. Asynchronous cultures grown in 30°C Ura− raffinose media were spun down and resuspended in prechilled YPR media. After 18 h, prechilled galactose was added to the cultures to a 2% final concentration. NIC96-ProA expression was induced for 12 h, followed by a glucose chase for 4 h. Throughout these experiments, samples were taken for IF microscopy, flow cytometry, and budding index analysis. In a separate assay, NPC numbers were determined directly using electron micrographs of serially sectioned cells with computer modeling techniques described by Winey et al. (1997) using yeast strains Wx340-3c and Wx340-3d (Table I).
Results
Ndc1p Localizes to NPCs
We used a previously published biochemical fractionation technique to isolate highly enriched NPCs from Saccharomyces (Rout and Blobel, 1993). Individual protein components of the enriched NPCs were identified by direct peptide sequence analysis. One of the peptide sequences obtained corresponded exactly to the first 21 NH2-terminal amino acids of Ndc1p (MIQTPRELLNPRYTYHTIFSD) (Winey et al., 1993). Additionally, the protein band from which this peptide sequence was obtained corresponded in molecular weight to Ndc1p and was recognized by anti-Ndc1p antibodies (data not shown).
We tested whether Ndc1p from S. cerevisiae localizes to NPCs using a strain that contains NDC1 epitope-tagged at its COOH terminus with the IgG binding domains of ProA (NDC1-ProA). This epitope-tagged version of NDC1 is chromosomally integrated replacing the original copy of NDC1, is expressed using the endogenous NDC1 promoter, and is fully functional (data not shown). We used indirect IF deconvolution microscopy to examine the localization of Ndc1p-ProA in whole cells and found that it displayed punctate nuclear peripheral staining, suggestive of NPC localization (Fig. 1 A) (Rout and Wente, 1994). We saw similar localization using strains with a chromosomally integrated version of NDC1 that contains three repeats of the c-myc epitope at its COOH terminus (data not shown).
Figure 1.
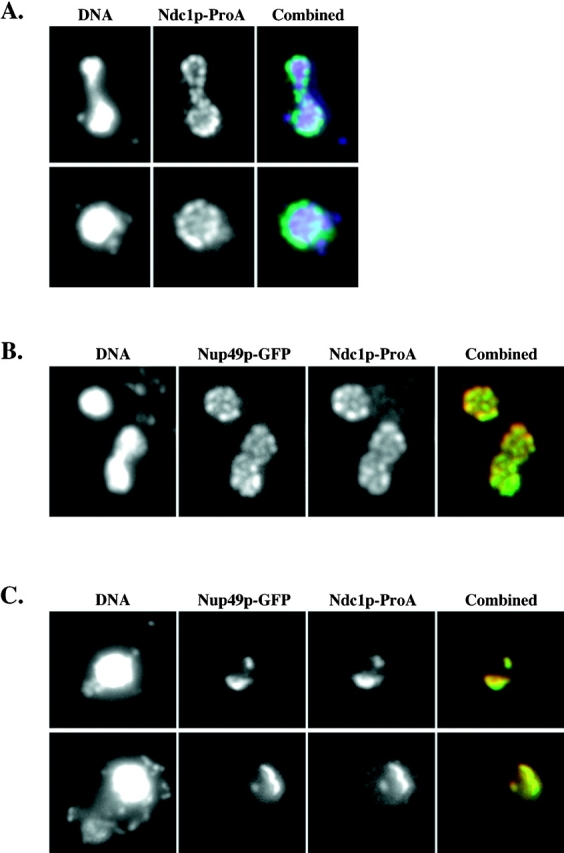
Ndc1p-ProA localizes to NPCs in whole cells. (A) Indirect IF deconvolution microscopy (see Materials and Methods) of yeast strain ProA5-4d/4a (Table I). Ndc1p-ProA was indirectly labeled using an FITC-conjugated secondary antibody, and DNA was visualized using 4′,6-diamidino-2-phenylindole. (B) Indirect IF deconvolution microscopy of yeast strain HC26-15a/1a (Table I) containing Ndc1p-ProA and Nup49p-GFP (Bucci and Wente, 1997). Ndc1p-ProA was indirectly labeled using a Texas red–conjugated secondary antibody, and Nup49p-GFP autofluorescence was detected using FITC filters. The combined image shows both Texas red (red) and GFP (green) signal (overlap = yellow). (C) Indirect IF deconvolution microscopy of Ndc1p-ProA localization and Nup49p-GFP localization in the presence of the nup133 null allele, which causes NPCs to cluster (HC27-16d/16b; Table I) (Doye et al., 1994; Pemberton et al., 1995). Ndc1p-ProA and Nup49p-GFP were detected as in B. The combined image shows both Texas red (red) and GFP (green) signal (overlap = yellow).
We then tested whether Ndc1p-ProA colocalizes with a known NPC component, Nup49p (Wente et al., 1992). We used strains that contain both NDC1-ProA and an allele of NUP49 that is epitope-tagged with the Aequorea victoria GFP (Bucci and Wente, 1997). When Ndc1p-ProA was indirectly labeled with Texas red, we observed colocalization with Nup49p-GFP signal (Fig. 1 B). It is important that this colocalization relied on the autofluorescence of Nup49p-GFP, because the ProA epitope on Ndc1p can cross-react with any other antibody used for double immunolabeling. We also examined this colocalization in a nup133 null strain in which NPCs become clustered (Doye et al., 1994; Pemberton et al., 1995). Ndc1p-ProA localization became clustered and colocalized with clusters of Nup49p-GFP signal in this strain (Fig. 1 C), suggesting that Ndc1p-ProA localizes to NPCs and not merely to the NE. Occasionally, we observed either one or two additional bright spots of Ndc1p-ProA staining that did not colocalize with Nup49p-GFP signal (see below).
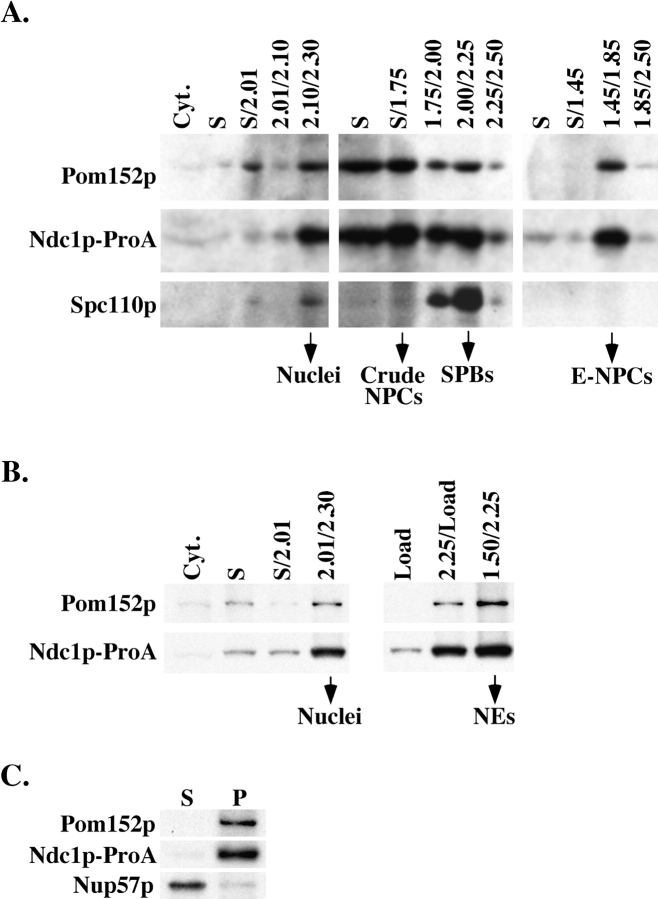
We also carried out biochemical fractionation experiments to isolate NPCs and NEs from strains that contain Ndc1p-ProA. In both cases, Ndc1p-ProA cofractionated with the known NPC marker, Pom152p (Fig. 2, A and B). We also examined these fractions using monoclonal antibodies recognizing numerous repeat motif-containing nucleoporins (Wente et al., 1997) and observed similar fractionation patterns (data not shown). Hydrophobicity plots of Ndc1p predict that it contains six to seven transmembrane domains (Winey et al., 1993). We extracted the NE fraction with carbonate to test whether Ndc1p-ProA behaves as an integral membrane protein. After centrifugation, soluble NPC components, such as Nup57p, were extracted by carbonate (Fig. 2 C). In contrast, Pom152p, a known integral membrane protein of the NE, remained in the pellet (Fig. 2 C). Ndc1p-ProA was not extracted from the NE by carbonate treatment (Fig. 2 C), suggesting that it is an integral membrane protein of the NE. Therefore, in combination with the whole cell IF localization data, these results indicate that Ndc1p-ProA is an integral membrane protein of NPCs.
Figure 2.
Biochemical analysis of Ndc1p-ProA. (A) Biochemical fractionation was carried out using a yeast strain that contains Ndc1p-ProA (ProA5-4d; Table I) (Rout and Kilmartin, 1990; Rout and Kilmartin, 1991; Rout and Blobel, 1993). Fractions containing nuclei, crude NPCs, SPBs, and enriched NPCs (E-NPCs) are labeled. Individual fractions were analyzed by SDS-PAGE and immunoblotted using antibodies to detect Pom152p, Ndc1p-ProA, or Spc110p. Pom152p is a NPC component (Wozniak et al., 1994), and Spc110p is a SPB component (Rout and Kilmartin, 1990; Kilmartin et al., 1993). Cell equivalents loaded were 1n for the left panels and 10n for the middle and right panels. (B) NEs were isolated from the Ndc1p-ProA yeast strain (ProA5-4d; Table I) using a previously described technique (Strambio-de-Castillia et al., 1995). Cell equivalents loaded were 1n for the left panels and 3n for the right panels. (C) Samples from the NE fraction were extracted with carbonate followed by centrifugation to determine whether Ndc1p-ProA was found in the supernatant (S) or in the membrane-containing pellet (P) (see Materials and Methods). Samples were analyzed by SDS-PAGE and immunoblotted using antibodies to detect Ndc1p-ProA, Pom152p, or Nup57p.
Ndc1p Also Localizes to SPBs
Because NDC1 function is required for proper SPB duplication, we tested whether Ndc1p also localizes to SPBs. This analysis was difficult because both NPCs and SPBs localize to the NE, and cells contain either one or two SPBs in the midst of 100–150 NPCs. We addressed this question using several experimental approaches. We used a biochemical fractionation technique to isolate SPBs from cells containing Ndc1p-ProA (Rout and Kilmartin, 1990). Immunoblotting of isolated fractions showed that Ndc1p-ProA partially cofractionated with SPBs (Fig. 2 A). However, one concern about these fractionation experiments was the heavy contamination of NPCs in the SPB fraction, making it difficult to distinguish the fractionation pattern of a protein that was only in the NPC from that observed for Ndc1p-ProA.
Therefore, we used whole cell indirect IF deconvolution microscopy to test whether any of the distinct spots of Ndc1p staining colocalize with signal from a known SPB component. This high-resolution fluorescence microscopy technique could potentially resolve SPB- and NPC-associated signals in the yeast NE. We used a strain that contains both NDC1-ProA and SPC42-GFP in these experiments. SPC42 encodes an essential SPB component that forms a crystalline layer between the central and outer plaque of the SPB (Rout and Kilmartin, 1991; Donaldson and Kilmartin, 1996; Bullitt et al., 1997). Additionally, we used a strain that contains both NIC96-ProA and SPC42-GFP as a negative control to ensure that overlapping signal was not merely due to a lack of resolution. Nic96p is known to localize to NPCs (Grandi et al., 1993), but it has been characterized extensively and is unlikely to localize to SPBs as well (Grandi et al., 1993; Aitchison et al., 1995b ; Grandi et al., 1995; Zabel et al., 1996; Schlaich et al., 1997; Ho et al., 1998).
We were able to distinguish between NPC and SPB signals using this technology. We observed a distinct spot of Ndc1p-ProA staining that colocalized with Spc42p-GFP signal in 91% of the SPBs examined (n = 63) (Fig. 3 A). In contrast, Nic96p-ProA staining did not exhibit colocalization with Spc42p-GFP signal in 82% of the SPBs examined (n = 51) (Fig. 3 B). Both Ndc1p and Nic96p contained the ProA epitope tag, allowing us to use the same antibodies to detect their localization. Additionally, the localization of Spc42p-GFP was visualized using GFP autofluorescence and therefore did not rely on potentially cross-reacting antibodies.
Figure 3.
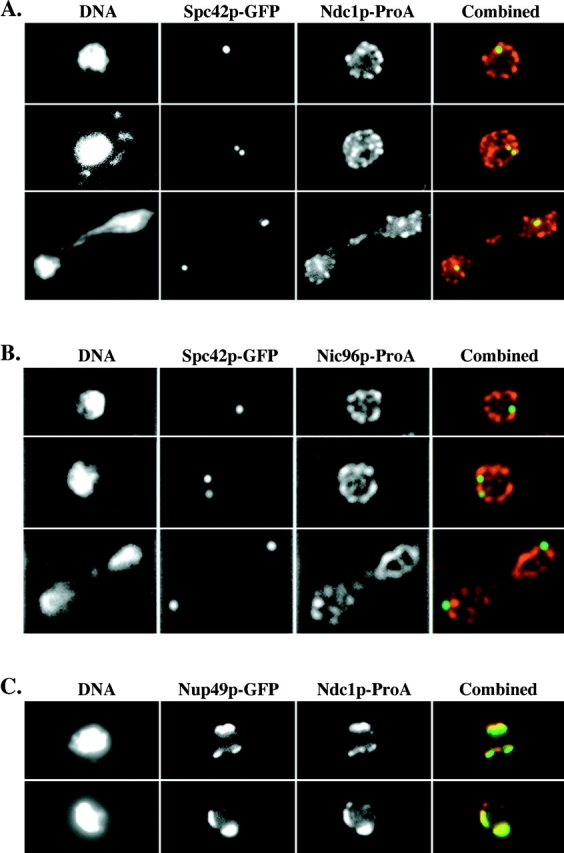
Ndc1p-ProA localizes to SPBs in whole cells. (A) Indirect IF deconvolution microscopy of cells (HC22-2b/1c; Table I) containing Ndc1p-ProA and Spc42p-GFP. Ndc1p-ProA was detected using a Texas red–conjugated secondary antibody, and Spc42p-GFP was detected by means of GFP autofluorescence using FITC filters (see Materials and Methods). The combined image shows both Texas red (red) and FITC (green) signal (overlap = yellow). (B) Indirect IF deconvolution microscopy of cells containing Nic96p-ProA and Spc42p-GFP (HC23-11d/16a; Table I) serves as a negative control for A. Samples were prepared as in A. The combined image shows both Texas red (red) and FITC signal (green). (C) Indirect IF deconvolution microscopy to examine the colocalization of Ndc1p-ProA with Nup49p-GFP in a nup133 null strain that exhibits NPC clustering (HC27-16d/16b; Table I). Ndc1p-ProA was detected as in A, and Nup49p-GFP was detected using FITC filters. The combined image shows both Texas red (red) and FITC (green) signals (overlap = yellow). Shown here are examples of cells that contain either one or two extra spots of Ndc1p-ProA staining that do not colocalize with Nup49p-GFP signal.
As described earlier, when examining the colocalization of Ndc1p-ProA with Nup49p-GFP in a NPC clustering mutant strain, we occasionally observed either one or two distinct spots of Ndc1p-ProA staining that did not overlap with Nup49p-GFP signal (Fig. 3 C). It is likely that these additional spots of Ndc1p-ProA staining correspond to SPB localization. Previous work demonstrated that the SPB often localizes within NPC clusters (Heath et al., 1995), explaining why we observed extra spots of Ndc1p-ProA staining only occasionally.
Finally, we examined the localization of Ndc1p-GFP in living cells. Although these cells displayed punctate nuclear peripheral localization of Ndc1p-GFP signal, they also contained either one or two intense spots of Ndc1p-GFP signal (Fig. 4 A). We propose that these bright spots correspond to SPB localization based on three criteria. First, SPBs are estimated to be 20 times larger than NPCs in diploid yeast cells (Bullitt et al., 1997), explaining why the signal might appear larger and brighter. Second, we observed either one or two bright spots of Ndc1p-GFP signal, corresponding to the number of SPBs normally found in cells. Finally, the bright spots were present at positions in the cell that are consistent with SPB localization. For example, the late mitotic cells displayed a bright spot at each end of the NE, where the SPBs are normally located. These bright spots were present in all of the cells we examined, suggesting that Ndc1p-GFP is associated with the SPB at all stages of the cell cycle.
Figure 4.
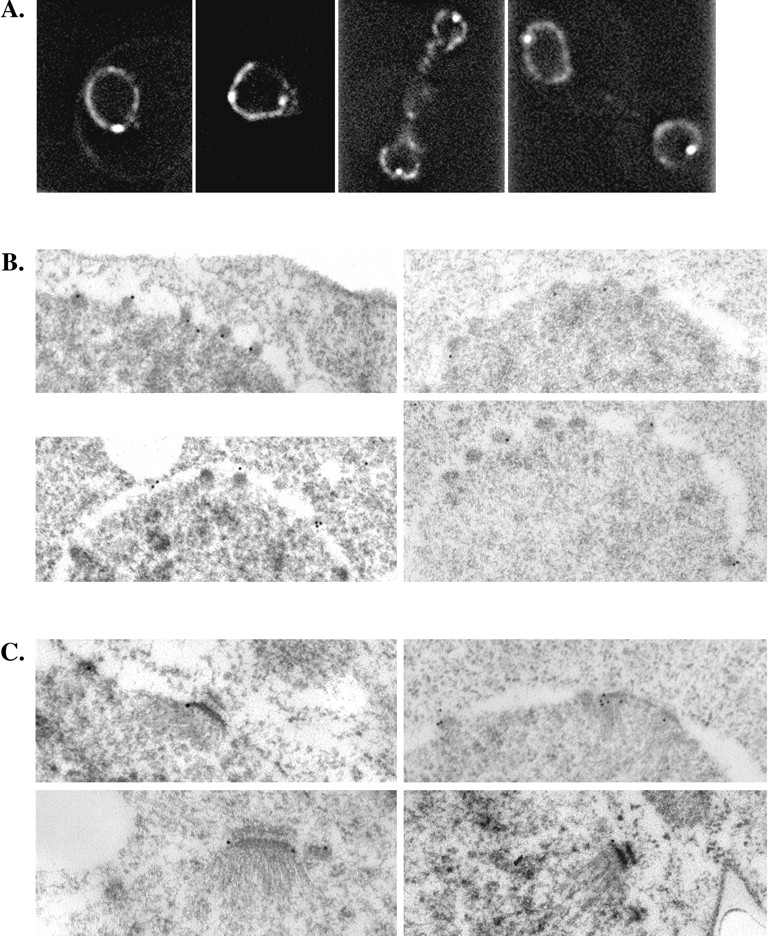
Ndc1p-GFP localizes to the membrane interacting regions of NPCs and SPBs. Ndc1p-GFP localization was examined using a diploid strain that is homozygous for the ndc1 null allele and is transformed with a centromeric plasmid containing NDC1-GFP [HC14-10c (1198)/HC29-6b; Table I]. Ndc1p-GFP localization was observed in living cells using deconvolution fluorescence microscopy (A). Additionally, this localization was examined using immunoelectron microscopy of whole cells (see Materials and Methods). Immunogold labeling, corresponding to Ndc1p-GFP, was detected at the periphery of NPCs (B) and at the edges of the central plaque region of SPBs (C); these are the regions of NPCs and SPBs that interact with the NE.
Immunoelectron Microscopy Demonstrates that Ndc1p-GFP Localizes to the Membrane-associated Regions of NPCs and SPBs
Although the experiments using whole cell IF microscopy provide strong evidence that Ndc1p localizes to both NPCs and SPBs, we also used immunoelectron microscopy to more closely examine the localization of Ndc1p-GFP within these two organelles. These experiments were carried out using a yeast strain that contains NDC1-GFP. Consistent with previous data, we observed immunogold labeling, corresponding to the localization of Ndc1p-GFP, at both NPCs and SPBs using this technique. Immunogold labeling was most often seen at the periphery of the NPCs (Fig. 4 B) and at the edges of the central plaque of the SPB (Fig. 4 C); these are the regions of NPCs and SPBs that interact with the NE. A similar localization was found for Ndc1p-ProA in biochemically isolated SPBs from the Ndc1p-ProA–containing strain using immunoelectron microscopy (data not shown).
ndc1-1 Strains Do Not Exhibit Defects in NPC Functions
A role for Ndc1p at SPBs has been established clearly (Winey et al., 1993); however, the localization of Ndc1p to NPCs was quite unexpected. We carried out assays to determine whether Ndc1p plays a role in NPC function. We tested whether ndc1-1 cells exhibit defects in NPC-related functions at the nonpermissive temperature. ndc1-1 strains arrest as large budded cells with a G2 DNA content after a shift to the nonpermissive temperature (Thomas and Botstein, 1986; Winey et al., 1993). This arrest indicates that NDC1 function, at least with respect to SPB duplication, has been lost (Winey et al., 1993). We examined NPC phenotypes at this arrest to ensure that ndc1-1 function was minimal.
We did not detect any defects in nuclear protein import in ndc1-1 strains after inducing the expression of histone H2B1 as a nuclear localization signal (NLS) fused to GFP (pJON280; Schlenstedt et al., 1995) at the ndc1-1 arrest (data not shown). Because the H2B1p-GFP fusion protein was detectable in ndc1-1 cells at the arrest, it seemed likely that its corresponding mRNA was properly synthesized and exported from the nucleus. We carried out a similar experiment using the same yeast strains transformed with a different plasmid containing a NLS derived from the yeast ribosomal protein L29 fused to β-galactosidase as a reporter expressed under the control of the GAL10 promoter (pNLS-E1; Underwood and Fried, 1990). Induced protein levels were detected by immunoblotting, and β-galactosidase expression was similar in both ndc1-1 and wild-type cells (data not shown), indicating that mRNA export was likely to be occurring properly at the ndc1-1 arrest. Finally, we examined NPC clustering, a phenotype observed when specific nucleoporins are mutated or deleted (Doye et al., 1994; Aitchison et al., 1995a ; Heath et al., 1995; Pemberton et al., 1995). We did not observe any clustering of NPCs in ndc1-1 strains using Nic96p localization as a marker for NPCs (Grandi et al., 1993) (data not shown).
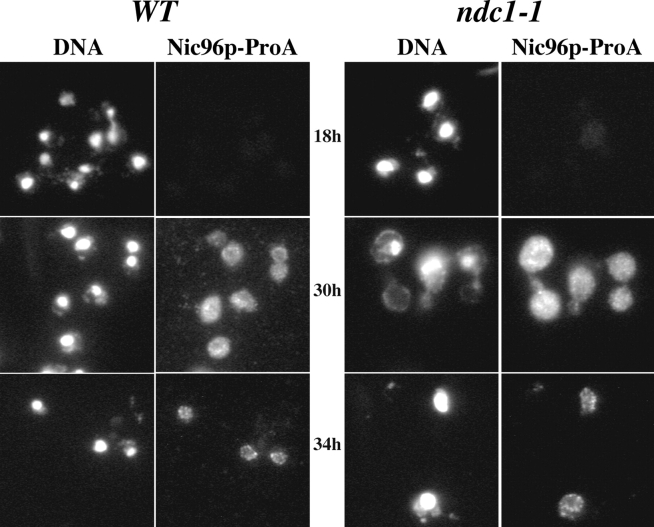
Because ndc1-1 cells are defective in the assembly of their new SPB into the NE, we examined whether ndc1-1 cells also exhibit defects in the assembly of newly synthesized NPCs into the NE. We tested whether ndc1-1 strains properly assemble an epitope-tagged version of Nic96p into their NE at the ndc1-1 arrest. Wild-type or ndc1-1 strains were transformed with a plasmid that contains NIC96-ProA expressed under the control of a galactose-inducible promoter. Thus, any ProA signal observed was due to induced Nic96p-ProA expression. These cells did contain their endogenous untagged chromosomal copy of NIC96, because this gene is essential (Grandi et al., 1993). Transformed yeast strains were shifted to the nonpermissive temperature of 15°C, and NIC96-ProA expression was induced at the ndc1-1 arrest (Fig. 5, 18h). The fusion protein was present throughout the cell as seen by indirect IF microscopy after 12 h of induction (Fig. 5, 30h). We then added glucose to the cultures to turn off further expression of Nic96p-ProA, allowing us to test whether the induced protein was assembled into NPCs. Nic96p-ProA was properly assembled into the NE in both wild-type and ndc1-1 cells (Fig. 5, 34h).
Figure 5.
ndc1-1 strains do not exhibit defects in NPC assembly. An assay to examine NPC assembly was carried out using either wild-type (WT) or ndc1-1 cells (HC10-42a/42d and HC10-42b/42c, respectively; Table I) transformed with a plasmid containing NIC96-ProA expressed under the control of a galactose-inducible promoter (pGAL-NIC96-ProA). Asynchronous cultures were shifted to 13.5°C for 18 h (18h); at this point, the ndc1-1 culture contained 80% large budded cells. NIC96-ProA expression was induced for 12 h (30h), followed by a glucose chase for 4 h to determine whether Nic96p-ProA was properly assembled into NPCs (34h). Nic96p-ProA localization was visualized using indirect IF microscopy with an FITC-conjugated secondary antibody (see Materials and Methods).
We further examined NPC assembly functions by using a recently published technique to directly count the number of NPCs in ndc1-1 cells at the nonpermissive temperature (Winey et al., 1997). We determined the number of NPCs in individual nuclei by generating computer-aided reconstructions of whole nuclei from electron micrographs of serially sectioned cells. The defective SPB in ndc1-1 cells is still capable of nucleating cytoplasmic MTs, allowing it to migrate and carry with it a thin finger of NE (Winey et al., 1993). This deformed NE is difficult to follow through serial sections. The tub2-403 allele is a cold-sensitive mutation in β-tubulin (Huffaker et al., 1988) that prevents the defective ndc1-1 SPB from migrating (Winey, M., unpublished observation). We used a ndc1-1, tub2-403 double mutant strain (Wx340-3c; Table I) for this analysis, and a strain containing the tub2-403 allele alone (Wx340-3d; Table I) as a control for mitotically arrested cells (Huffaker et al., 1988).
Samples were prepared as previously described (Winey et al., 1997) to directly count the number of NPCs in three nuclei from each strain after a shift to the nonpermissive temperature. Nuclei from the tub2-403 cells contained 162, 179, and 232 NPCs, and nuclei from the ndc1-1, tub2-403 double mutant cells contained 180, 193, and 209 NPCs. The maximum number of NPCs observed in wild-type cells occurs in early mitosis, with an average of 142 ± 16.4 NPCs (Winey et al., 1997). Our analysis demonstrated that both strains contained more NPCs than observed in wild-type cells at any point in the cell cycle, indicating that NPC assembly continues to occur when these cells are arrested.
A Deletion of POM152 Suppresses the ndc1-1 SPB Duplication Defect
To define a potential functional role for Ndc1p in NPCs, we examined genetic interactions between NDC1 and POM152. We were particularly interested in POM152 because it encodes an integral membrane protein that localizes to the pore membrane domain of NPCs (Wozniak et al., 1994). Although POM152 encodes an abundant nucleoporin, this gene is not essential. We initially examined potential synthetic lethal interactions between ndc1-1 and the pom152 null allele, but no synthetic lethality was observed when these two mutations were combined.
Unexpectedly, we found that the pom152 null allele suppressed the ndc1-1 cold-sensitive phenotype. ndc1-1 strains containing the pom152 null allele were able to grow on plates incubated at 15°C, the nonpermissive temperature for ndc1-1 (Fig. 6 A). This suppression was due specifically to a loss of POM152, because when a plasmid-borne copy of POM152 was reintroduced into the double mutant strain, the ndc1-1 cold-sensitive phenotype was restored (Fig. 6 A). Additionally, this suppression is specific for the ndc1-1 allele, because the pom152 null allele could not function as a bypass suppressor of the ndc1 null allele (data not shown).
Figure 6.
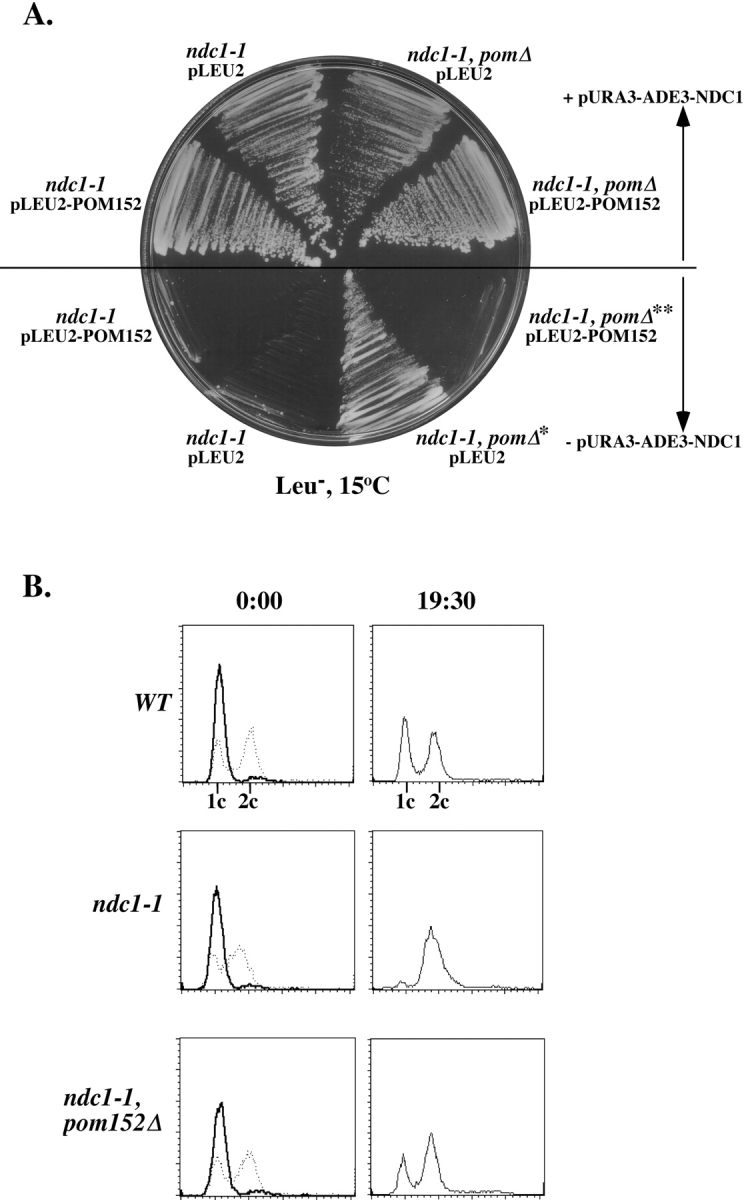
Deletion of POM152 suppresses the ndc1-1 SPB duplication defect in the first cell cycle. (A) ndc1-1 or ndc1-1, pom152 null double mutant strains [HC5-31c and HC5-31c(1166), respectively; Table I] containing pURA3-NDC1 (pALR10-NDC1, see Materials and Methods) or lacking pURA3-NDC1 were transformed with either pRS315 (pLEU2) or pPM1-HA (pLEU2-POM152) and were streaked to a 15°C Leu− plate. All strains containing pURA3-NDC1 were able to grow at the nonpermissive temperature. The ndc1-1 strain lacking pURA3-NDC1 failed to grow. However, the ndc1-1, pom152 null double mutant strain grew at 15°C when it contained the pLEU2 vector (*). When a plasmid-borne copy of POM152 (pLEU2-POM152) was reintroduced, the double mutant strain exhibited the ndc1-1 cold-sensitive phenotype (**). (B) DNA content of wild-type (WT), ndc1-1, and ndc1-1, pom152 null double mutant (ndc1-1, pom152Δ) strains [HC14-10c, HC5-31c, and HC5-31c(1166), respectively; Table I]. Asynchronous cultures (0:00, dashed lines) were arrested in G1 (1c DNA content; G2 and M cells have 2c DNA content) by treatment with alpha-factor at the permissive temperature (0:00, bold line) (see Materials and Methods). Cells were released from this arrest into media at the nonpermissive temperature for ndc1-1 for 19.5 h (19:30).
NDC1 function is required for SPB duplication in the first cell cycle after release from a G1 arrest induced by treatment with alpha-factor (Winey et al., 1993). We tested whether the deletion of POM152 suppresses the ndc1-1 SPB duplication defect in the first cell cycle after a shift to the nonpermissive temperature. Cells were arrested in G1 at the permissive temperature using alpha-factor, and released into prechilled media at the nonpermissive temperature for ndc1-1. Wild-type cells synchronously released from the G1 arrest, completed the cell cycle, and eventually became asynchronous (Fig. 6 B). In contrast, ndc1-1 cells synchronously released from the G1 arrest, but then arrested with a G2 DNA content and large buds due to failed SPB duplication in the first cell cycle (Fig. 6 B). The ndc1-1, pom152 null double mutant strains did not exhibit the ndc1-1 cell cycle arrest; they appeared similar to the wild-type cultures (Fig. 6 B). We conclude that the pom152 null allele suppresses the ndc1-1 SPB duplication defect in the first cell cycle.
Discussion
Two organelles, SPBs and NPCs, interrupt the NE and allow communication and coordination to occur between the nucleus and the cytoplasm. Although these organelles differ greatly in their characterized functions, our findings suggest that they may be more similar than previously thought; we show here that the integral membrane protein Ndc1p localizes to both SPBs and NPCs, the first such shared component identified. Endogenous Ndc1p was identified biochemically as a constituent of highly enriched yeast NPCs, and IF microscopy demonstrated that Ndc1p colocalized with a known nucleoporin.
We were interested in determining whether Ndc1p also localizes to SPBs because ndc1-1 cells exhibit defects in SPB duplication (Winey et al., 1993). SPBs are greatly outnumbered by NPCs in the NE, making it difficult to assess whether signal observed is specific to SPBs. We were able to distinguish between NPC and SPB signal using indirect IF deconvolution microscopy of whole cells. We identified distinct spots of Ndc1p signal that colocalized with a SPB marker. Control experiments using an abundant NPC component showed little colocalization with the SPB marker, indicating that NPCs and SPBs could be resolved. This suggests that Ndc1p is associated with the SPB, consistent with the SPB duplication defect observed in ndc1-1 mutant strains.
However, SPBs and NPCs are most clearly distinguished by using electron microscopy. Examination of Ndc1p- GFP localization using immunoelectron microscopy showed that immunogold labeling, corresponding to Ndc1p-GFP, was concentrated at both NPCs and SPBs. The distribution of immunogold particles at the periphery of NPCs and at the edges of the central plaque region of the SPB showed that Ndc1p-GFP often localized to the regions of the NPC and SPB that interact with the NE. Ultrastructural analysis of the yeast NPC shows that this organelle contains a unique membrane interacting ring (Strambio-de-Castillia et al., 1995; Yang et al., 1998). It is possible that the SPB contains a similar membrane interacting domain. Our results suggest that Ndc1p is a shared component of the membrane interacting regions of NPCs and SPBs.
Interestingly, the cut11 + gene of Schizosaccharomyces pombe, like NDC1, encodes a protein with seven potential membrane spanning regions, and mutations in cut11 + affect SPB function (West et al., 1998). Cut11p also localizes to NPCs and SPBs, and cut11 + and NDC1 also show limited functional homology (West et al., 1998). However, a function for Cut11p at NPCs has not been determined.
A function for Ndc1p at the SPB has been established clearly from previous work; ndc1-1 cells fail to insert their newly synthesized SPB into the NE at the nonpermissive temperature (Winey et al., 1993). We do not observe any defects in nuclear transport, in NPC distribution, or in NPC assembly at the nonpermissive temperature using the existing ndc1-1 allele (Thomas and Botstein, 1986). It is possible that new mutant alleles of NDC1 will uncover NPC related phenotypes. Studies of NDC1 gene dosage show that the ndc1-1 allele does not exhibit a complete loss of function at the nonpermissive temperature (Chial, H.J., unpublished observation). Several NPC components are not essential, suggesting that many nucleoporins carry out redundant functions. It is possible that other NPC components will have to be disrupted before a NPC-related function for NDC1 will be uncovered. NDC1 may perform a similar role for NPCs as has been established for SPBs, possibly being involved in their assembly into the NE.
Although little is known about how yeast NPCs are assembled into the NE, studies of the NIC96 gene of S. cerevisiae have shown that it is involved in the formation of NPCs. Cells containing a conditional mutation in NIC96 exhibited decreased numbers of NPCs after exposure to the nonpermissive temperature (Zabel et al., 1996). Recently, a vertebrate homologue of NIC96, called NUP93, was identified (Grandi et al., 1997). After immunodepletion of Nup93p in a Xenopus nuclear reconstitution assay, the nuclei exhibited defects in NPC assembly (Grandi et al., 1997). Yeast Nic96p has been shown both in vivo and in vitro to be associated with a NPC subcomplex that contains Nsp1p, Nup49p, and Nup57p, which can be assembled into NPCs (Grandi et al., 1993; Grandi et al., 1995; Schlaich et al., 1997). Additionally, synthetic lethal interactions have been identified between mutant alleles of NIC96, NUP188, and POM152 (Aitchison et al., 1995b ; Nehrbass et al., 1996; Zabel et al., 1996).
Until now, only synthetic lethal interactions have been observed when the pom152 null allele is combined with mutations in a number of NPC components (Aitchison et al., 1995b ; Nehrbass et al., 1996). We report that the removal of Pom152p, one of the most abundant NPC components, strongly suppresses the ndc1-1 SPB duplication defect. In fact, this deletion suppresses the ndc1-1 SPB duplication defect within the first cell cycle after a shift to the nonpermissive temperature. Because a function for Ndc1p at NPCs has not been determined, we do not know if the suppression of ndc1-1 by the pom152 null allele has any functional consequence for NPCs. Nonetheless, this suppression lends support to the idea that SPBs and NPCs are somehow linked, perhaps in some mechanistic way beyond shared components.
The mechanism by which the pom152 null allele suppresses the ndc1-1 SPB duplication defect is not known, but it appears as if wild-type POM152 is antagonistic in ndc1-1 strains. To further understand this interaction, it will be necessary to learn whether Pom152p, in addition to its localization to NPCs, also localizes to SPBs. Although the POM152 gene is not essential, the overexpression of POM152 is toxic to yeast cells (Wozniak et al., 1994). We tested whether the toxicity associated with POM152 overexpression is due to a defect in SPB duplication, but we did not observe any SPB-related phenotypes in cells overexpressing POM152 (Chial, H.J., unpublished observation).
If Pom152p localizes only to NPCs, it is possible that a deletion of this nonessential NPC component releases Ndc1-1p from NPCs, allowing it to function at the SPB. This idea is supported by our observation that providing haploid ndc1-1 cells with an additional chromosomally integrated copy of the ndc1-1 allele relieves the ndc1-1 cold-sensitive phenotype, suggesting that Ndc1-1p can function in SPB duplication if present at slightly elevated levels in the cell (Chial, H.J., unpublished observation). It is possible that Ndc1p and Pom152p may directly interact at NPCs, because immunoelectron microscopy shows that these two proteins localize to similar regions of the NPC (Wozniak et al., 1994), and both are membrane proteins. However, if Pom152p localizes to both NPCs and SPBs, it is possible that Pom152p at the SPB somehow interferes with the ability of mutant Ndc1-1p to function in the proper insertion of the newly synthesized SPB into the NE. In this case, the removal of Pom152p might allow Ndc1-1p to function more efficiently at the SPB.
Experiments presented here reveal a link between two NE-embedded organelles that are functionally quite different. Little is known about how NPCs and SPBs are correctly assembled into the NE, but these processes are expected to be complex, requiring the organelles to be oriented correctly and possibly to be dependent on the function and/or assembly of each other. The localization of Ndc1p to both of these organelles suggests a common mechanism for the assembly of NPCs and SPBs into the NE.
Acknowledgments
We thank Elena Spichas for assistance with electron microscopy, Tari Suprapto for assistance with subcellular fractionations, and Colin Monks, Bill Betz, and Steve Fadul for assistance with IF deconvolution microscopy. We thank Ian Adams, Mirella Bucci, Alain Camasses, Jason Kahana, John Kilmartin, Jon Loeb, Pamela Silver, Susan Wente, and Rick Wozniak for providing reagents and strains. We also thank Andrea Castillo, Shawn Corey, Shelly Jones, Susan McBratney, Elisa Stone, and Bill Wood for critical reading of this manuscript, Jon Aitchison, Frank Luca, Estelle Steiner, Susan Wente, and Rick Wozniak for helpful discussions, and Betsy Siewert for technical assistance.
This work was supported by grants from the American Cancer Society (RPG-96-101-03-CSM to M. Winey), the Pew Scholars Program in the Biomedical Sciences (P0020SC to M. Winey), Günter Blobel (who we thank for his very generous support), the Howard Hughes Medical Institute (M.P. Rout), and by a National Institutes of Health training grant (GM-07135 to H.J. Chial). Deconvolution microscopy in MCD Biology was made possible, in part, by a gift from Virginia and Mel Clark.
Abbreviations used in this paper
- GFP
green fluorescent protein
- IF
immunofluorescence
- NE
nuclear envelope
- NLS
nuclear localization signal
- NPC
nuclear pore complex
- ProA
protein A
- SPB
spindle pole body
References
- Aitchison JD, Blobel G, Rout MP. Nup120p: a yeast nucleoporin required for NPC distribution and mRNA transport. J Cell Biol. 1995a;131:1659–1675. doi: 10.1083/jcb.131.6.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitchison JD, Rout MP, Marelli M, Blobel G, Wozniak RW. Two novel related yeast nucleoporins Nup170p and Nup157p: complementation with the vertebrate homologue Nup155p and functional interactions with the yeast nuclear pore-membrane protein Pom152p. J Cell Biol. 1995b;131:1133–1148. doi: 10.1083/jcb.131.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel, J.D., R. Brent, R.E. Kingston, D.D. Moore, J.G. Seidman, J.A. Smith, and K. Struhl. 1994. Current Protocols in Molecular Biology. John Wiley and Sons, New York.
- Baudin A, Ozier-Kalogeropoulos O, Denouel A, Lacroute F, Cullin C. A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. . Nucleic Acids Res. 1993;21:3329–3330. doi: 10.1093/nar/21.14.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci M, Wente SR. In vivo dynamics of nuclear pore complexes in yeast. J Cell Biol. 1997;136:1185–1199. doi: 10.1083/jcb.136.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci M, Wente SR. A novel fluorescence-based genetic strategy identifies mutants of Saccharomyces cerevisiaedefective for nuclear pore complex assembly. Mol Biol Cell. 1998;9:2439–2461. doi: 10.1091/mbc.9.9.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullitt E, Rout MP, Kilmartin JV, Akey CW. The yeast spindle pole body is assembled around a central crystal of Spc42p. Cell. 1997;89:1077–1086. doi: 10.1016/s0092-8674(00)80295-0. [DOI] [PubMed] [Google Scholar]
- Cormack BP, Bertram G, Egerton M, Gow NA, Falkow S, Brown AJ. Yeast-enhanced green fluorescent protein (yEGFP) a reporter of gene expression in Candida albicans. . Microbiology. 1997;143:303–311. doi: 10.1099/00221287-143-2-303. [DOI] [PubMed] [Google Scholar]
- Davis LI, Blobel G. Identification and characterization of a nuclear pore complex protein. Cell. 1986;45:699–709. doi: 10.1016/0092-8674(86)90784-1. [DOI] [PubMed] [Google Scholar]
- Davis LI, Blobel G. Nuclear pore complex contains a family of glycoproteins that includes p62: glycosylation through a previously unidentified cellular pathway. Proc Natl Acad Sci USA. 1987;84:7552–7556. doi: 10.1073/pnas.84.21.7552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LI, Fink GR. The NUP1gene encodes an essential component of the yeast nuclear pore complex. Cell. 1990;61:965–978. doi: 10.1016/0092-8674(90)90062-j. [DOI] [PubMed] [Google Scholar]
- Ding R, West RR, Morphew DM, Oakley BR, McIntosh JR. The spindle pole body of Schizosaccharomyces pombeenters and leaves the nuclear envelope as the cell cycle proceeds. Mol Biol Cell. 1997;8:1461–1479. doi: 10.1091/mbc.8.8.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson AD, Kilmartin JV. Spc42p: a phosphorylated component of the S. cerevisiaespindle pole body (SPB) with an essential function during SPB duplication. J Cell Biol. 1996;132:887–901. doi: 10.1083/jcb.132.5.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doye V, Hurt E. From nucleoporins to nuclear pore complexes. Curr Opin Cell Biol. 1997;9:401–411. doi: 10.1016/s0955-0674(97)80014-2. [DOI] [PubMed] [Google Scholar]
- Doye V, Wepf R, Hurt EC. A novel nuclear pore protein Nup133p with distinct roles in poly(A)+ RNA transport and nuclear pore distribution. EMBO (Eur Mol Biol Organ) J. 1994;13:6062–6075. doi: 10.1002/j.1460-2075.1994.tb06953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elledge SJ, Davis RW. A family of versatile centromeric vectors designed for use in the sectoring-shuffle mutagenesis assay in Saccharomyces cerevisiae. . Gene. 1988;70:303–312. doi: 10.1016/0378-1119(88)90202-8. [DOI] [PubMed] [Google Scholar]
- Fernandez J, Andrews L, Mische SM. An improved procedure for enzymatic digestion of polyvinyldiene difluoride-bound proteins for internal sequence analysis. Anal Biochem. 1994;218:112–117. doi: 10.1006/abio.1994.1148. [DOI] [PubMed] [Google Scholar]
- Grandi P, Doye V, Hurt EC. Purification of NSP1 reveals complex formation with “GLFG” nucleoporins and a novel nuclear pore protein NIC96. EMBO (Eur Mol Biol Organ) J. 1993;12:3061–3071. doi: 10.1002/j.1460-2075.1993.tb05975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandi P, Schlaich N, Tekotte H, Hurt EC. Functional interaction of Nic96p with a core nucleoporin complex consisting of Nsp1p, Nup49p and a novel protein Nup57p. EMBO (Eur Mol Biol Organ) J. 1995;14:76–87. doi: 10.1002/j.1460-2075.1995.tb06977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandi P, Dang T, Pane N, Shevchenko A, Mann M, Forbes D, Hurt E. Nup93, a vertebrate homologue of yeast Nic96p, forms a complex with a novel 205-kD protein and is required for correct nuclear pore assembly. Mol Biol Cell. 1997;8:2017–2038. doi: 10.1091/mbc.8.10.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath CV, Copeland CS, Amberg DC, Del Priore V, Snyder M, Cole CN. Nuclear pore complex clustering and nuclear accumulation of poly(A)+ RNA associated with mutation of the Saccharomyces cerevisiae RAT2/NUP120gene. J Cell Biol. 1995;131:1677–1697. doi: 10.1083/jcb.131.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho AK, Raczniak GA, Ives EB, Wente SR. The integral membrane protein Snl1p is genetically linked to yeast nuclear pore complex function. Mol Biol Cell. 1998;9:355–373. doi: 10.1091/mbc.9.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffaker TC, Thomas JH, Botstein D. Diverse effects of beta-tubulin mutations on microtubule formation and function. J Cell Biol. 1988;106:1997–2010. doi: 10.1083/jcb.106.6.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter KJ, Eipel HE. Microbial determinations by flow cytometry. J Gen Microbiol. 1979;113:369–375. doi: 10.1099/00221287-113-2-369. [DOI] [PubMed] [Google Scholar]
- Johnston M, Davis RW. Sequences that regulate the divergent GAL1-GAL10 promoter in Saccharomyces cerevisiae. . Mol Cell Biol. 1984;4:1440–1448. doi: 10.1128/mcb.4.8.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin JV, Dyos SL, Kershaw D, Finch JT. A spacer protein in the Saccharomyces cerevisiaespindle pole body whose transcript is cell cycle-regulated. J Cell Biol. 1993;123:1175–1184. doi: 10.1083/jcb.123.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehrbass U, Rout MP, Maguire S, Blobel G, Wozniak RW. The yeast nucleoporin Nup188p interacts genetically and physically with the core structures of the nuclear pore complex. J Cell Biol. 1996;133:1153–1162. doi: 10.1083/jcb.133.6.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton LF, Rout MP, Blobel G. Disruption of the nucleoporin gene NUP133results in clustering of nuclear pore complexes. Proc Natl Acad Sci USA. 1995;92:1187–1191. doi: 10.1073/pnas.92.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. Targeting, disruption, replacement, and allele rescue: integrative DNA transformation in yeast. Methods Enzymol. 1991;194:281–301. doi: 10.1016/0076-6879(91)94022-5. [DOI] [PubMed] [Google Scholar]
- Rout MP, Kilmartin JV. Components of the yeast spindle and spindle pole body. J Cell Biol. 1990;111:1913–1927. doi: 10.1083/jcb.111.5.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rout MP, Kilmartin JV. Yeast spindle pole body components. Cold Spring Harb Symp Quant Biol. 1991;56:687–692. doi: 10.1101/sqb.1991.056.01.077. [DOI] [PubMed] [Google Scholar]
- Rout MP, Blobel G. Isolation of the yeast nuclear pore complex. J Cell Biol. 1993;123:771–783. doi: 10.1083/jcb.123.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rout MP, Wente S. Pores for thought: nuclear pore complex proteins. Trends Cell Biol. 1994;4:357–365. doi: 10.1016/0962-8924(94)90085-x. [DOI] [PubMed] [Google Scholar]
- Rout, M.P., and C. Strambio-de-Castillia. 1998. Isolation of yeast nuclear pore complexes and nuclear envelopes. In Cell Biology: A Laboratory Handbook. 2nd edition, vol. 2. J.E. Celis, editor. Academic Press, New York. 143–151.
- Schlaich NL, Haner M, Lustig A, Aebi U, Hurt EC. In vitroreconstitution of a heterotrimeric nucleoporin complex consisting of recombinant Nsp1p, Nup49p, and Nup57p. Mol Biol Cell. 1997;8:33–46. doi: 10.1091/mbc.8.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenstedt G, Saavedra C, Loeb JD, Cole CN, Silver PA. The GTP-bound form of the yeast Ran/TC4 homologue blocks nuclear protein import and appearance of poly(A)+ RNA in the cytoplasm. Proc Natl Acad Sci USA. 1995;92:225–229. doi: 10.1073/pnas.92.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutz AR, Winey M. New alleles of the yeast MPS1gene reveal multiple requirements in spindle pole body duplication. Mol Biol Cell. 1998;9:759–774. doi: 10.1091/mbc.9.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman, F.G., G.R. Fink, and J.B. Hicks. 1986. Methods in Yeast Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. . Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M. The spindle pole body of yeast. Chromosoma. 1994;103:369–380. doi: 10.1007/BF00362281. [DOI] [PubMed] [Google Scholar]
- Strambio-de-Castillia C, Blobel G, Rout MP. Isolation and characterization of nuclear envelopes from the yeast Saccharomyces. . J Cell Biol. 1995;131:19–31. doi: 10.1083/jcb.131.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JH, Botstein D. A gene required for the separation of chromosomes on the spindle apparatus in yeast. Cell. 1986;44:65–76. doi: 10.1016/0092-8674(86)90485-x. [DOI] [PubMed] [Google Scholar]
- Underwood MR, Fried HM. Characterization of nuclear localizing sequences derived from yeast ribosomal protein L29. EMBO (Eur Mol Biol Organ) J. 1990;9:91–99. doi: 10.1002/j.1460-2075.1990.tb08084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wach A, Brachat A, Pohlmann R, Philippsen P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. . Yeast. 1994;10:1793–1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- Wente SR, Rout MP, Blobel G. A new family of yeast nuclear pore complex proteins. J Cell Biol. 1992;119:705–723. doi: 10.1083/jcb.119.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wente, S.R., S.M. Gasser, and A.J. Caplan. 1997. The nucleus and nucleocytoplasmic transport in Saccharomyces cerevisiae. In The Molecular and Cellular Biology of the Yeast Saccharomyces. Vol. 3. J.R. Broach, E. Jones, and J. Pringle, editors. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 471–546.
- West RR, Vaisberg EV, Ding R, Nurse P, McIntosh JR. cut11 +: a gene required for cell cycle-dependent spindle pole body anchoring in the nuclear envelope and bipolar spindle formation in Schizosaccharomyces pombe. . Mol Biol Cell. 1998;9:2839–2855. doi: 10.1091/mbc.9.10.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winey M, Byers B. Assembly and functions of the spindle pole body in budding yeast. Trends Genet. 1993;9:300–304. doi: 10.1016/0168-9525(93)90247-f. [DOI] [PubMed] [Google Scholar]
- Winey M, Hoyt MA, Chan C, Goetsch L, Botstein D, Byers B. NDC1: a nuclear periphery component required for yeast spindle pole body duplication. J Cell Biol. 1993;122:743–751. doi: 10.1083/jcb.122.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winey M, Mamay CL, O'Toole ET, Mastronarde DN, Giddings TH, Jr, McDonald KL, McIntosh JR. Three-dimensional ultrastructural analysis of the Saccharomyces cerevisiaemitotic spindle. J Cell Biol. 1995;129:1601–1615. doi: 10.1083/jcb.129.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winey M, Yarar D, Giddings TH, Jr, Mastronarde DN. Nuclear pore complex number and distribution throughout the Saccharomyces cerevisiaecell cycle by three-dimensional reconstruction from electron micrographs of nuclear envelopes. Mol Biol Cell. 1997;8:2119–2132. doi: 10.1091/mbc.8.11.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsor B, Schiebel E. Review: an overview of the Saccharomyces cerevisiaemicrotubule and microfilament cytoskeleton. Yeast. 1997;13:399–434. doi: 10.1002/(SICI)1097-0061(199704)13:5<399::AID-YEA126>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Wozniak RW, Blobel G, Rout MP. POM152is an integral protein of the pore membrane domain of the yeast nuclear envelope. J Cell Biol. 1994;125:31–42. doi: 10.1083/jcb.125.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Rout MP, Akey CW. Three-dimensional architecture of the isolated yeast nuclear pore complex: functional and evolutionary implications. Mol Cell. 1998;1:223–234. doi: 10.1016/s1097-2765(00)80023-4. [DOI] [PubMed] [Google Scholar]
- Zabel U, Doye V, Tekotte H, Wepf R, Grandi P, Hurt EC. Nic96p is required for nuclear pore formation and functionally interacts with a novel nucleoporin, Nup188p. J Cell Biol. 1996;133:1141–1152. doi: 10.1083/jcb.133.6.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]