Abstract
Regulation of vascular homeostasis depends upon collaboration between cells of the vessel wall and blood coagulation system. A direct interaction between integrin αVβ3 on endothelial cells and smooth muscle cells and prothrombin, the pivotal proenzyme of the blood coagulation system, is demonstrated and activation of the integrin is required for receptor engagement. Evidence that prothrombin is a ligand for αVβ3 on these cells include: (a) prothrombin binds to purified αVβ3 via a RGD recognition specificity; (b) prothrombin supports αVβ3-mediated adhesion of stimulated endothelial cells and smooth muscle cells; and (c) endothelial cells, either in suspension and in a monolayer, recognize soluble prothrombin via αVβ3. αVβ3-mediated cell adhesion to prothrombin, but not to fibrinogen, required activation of the receptor. Thus, the functionality of the αVβ3 receptor is ligand defined, and prothrombin and fibrinogen represent activation- dependent and activation-independent ligands. Activation of αVβ3 could be induced not only by model agonists, PMA and Mn2+, but also by a physiologically relevant agonist, ADP. Inhibition of protein kinase C and calpain prevented activation of αVβ3 on vascular cells, suggesting that these molecules are involved in the inside-out signaling events that activate the integrin. The capacity of αVβ3 to interact with prothrombin may play a significant role in the maintenance of hemostasis; and, at a general level, ligand selection by αVβ3 may be controlled by the activation state of this integrin.
Keywords: integrins, endothelial cells, smooth muscle cells, cell adhesion, ligands
The adhesive properties of vascular cells and the interaction of these cells with the blood coagulation system are intimately linked to the maintenance of vascular homeostasis. Whereas the endothelial cell lining of blood vessels is usually nonthrombogenic, vascular injury or the local generation of chemokines changes the surface properties such that the endothelial cells can initiate and efficiently propagate blood coagulation (Scarpati and Sadler, 1989; Stern et al., 1991; Bombeli et al., 1997). The culmination of these events is the activation of prothrombin to thrombin at the endothelial cell surface (Sueishi et al., 1995). In concert with changes in procoagulant activity, the adhesive properties of the endothelial cells are often altered. Expression and activation of a variety of adhesion receptors occur at the surface of stimulated endothelial cells (Pober and Cotran, 1990). Such changes are not restricted to endothelial cells; vascular smooth muscle cells also respond to injury and stimulation by changing their adhesive properties, such that they become migratory, and by expressing procoagulant activity on their cell surface (Taubman, 1993; Sueishi et al., 1995). Thus, the adhesive properties of vascular cells and their capacity to support prothrombin activation are intimately interwoven.
Recently, we have identified a previously unrecognized linkage between the major circulating cellular participant in thrombus formation, the platelet, its adhesive properties and thrombin generation by demonstrating that prothrombin serves as a ligand for the major integrin on the platelet surface, αIIbβ3 (Byzova and Plow, 1997). Prothrombin binds to αIIbβ3 on resting platelets in a specific, saturable, and divalent cation-dependent manner. This interaction accelerates prothrombin activation to thrombin, but thrombin itself does not bind to the receptor. Recognition of prothrombin by αIIbβ3 is mediated by an Arg-Gly-Asp (RGD)1 recognition specificity; RGD-containing peptides, which inhibit the binding of many ligands to αIIbβ3, also block prothrombin binding to the receptor. αVβ3 contains the same β subunit and an homologous α subunit as αIIbβ3 and plays a prominent role in vascular cell adhesion and migration. Cultured human umbilical vein endothelial cells (HUVEC) express αVβ3 as a major cell surface molecule (Cheresh, 1987) on both their luminal and basolateral surfaces (Conforti et al., 1992) and in endothelial cell–cell contacts (Glass and Kreisberg, 1993), and nonactivated SMC derived from large vessels also express αVβ3 (Brown et al., 1994). The binding of many ligands to αVβ3 is mediated by a RGD recognition specificity, and αVβ3 and αIIbβ3 share many common ligands, including von Willebrand factor, fibrinogen, fibronectin, and thrombospondin (Ruoslahti, 1996; Yamada, 1991).
In this study, we have sought to determine whether prothrombin can serve as a ligand for αVβ3 on vascular endothelial cells and smooth muscle cells. Our consideration of this possibility also was stimulated by the work of Bar-Shavit et al. (1991, 1993), who reported that cleavage products of thrombin or denatured thrombin, but not native thrombin, supported the adhesion of HUVEC in a RGD-dependent manner. In addition, recent studies showing prothrombin is deposited in the vessel wall at sites of injury (Hatton et al., 1995) and is synthesized by smooth muscle cells (McBane et al., 1997) adds further biological relevance to the role of prothrombin as a potential αVβ3 ligand. Here, we demonstrate that prothrombin can serve not only as an adhesive ligand for αVβ3 but also as a soluble ligand for the receptor. However, the interactions of prothrombin with αVβ3 and αIIbβ3 are fundamentally different: engagement of prothrombin by αVβ3 on vascular cells requires receptor activation, whereas its binding to αIIbβ3 on platelets does not (Byzova and Plow, 1997). Additionally, we also identify specific intracellular signaling molecules, which are involved in modulating αVβ3 to a prothrombin-competent binding state. Moreover, we show directly that αVβ3 discriminates between activation-dependent and activation-independent ligands, and prothrombin serves as the prototype of an activation-dependent ligand for αVβ3 on vascular cells.
Materials and Methods
Reagents
Human prothrombin purchased from Alexis Corp. (San Diego, CA) and Enzyme Research Corp. (South Bend, IN) was >99% pure as assessed by SDS-PAGE (Laemmli, 1970). The preparations used contained only one major Coomassie blue staining band, and this protein reacted with mAb to prothrombin (Biodesign International, Kennebunk, ME) in Western blots. Humanized mAb c7E3 was from Centocor (Malvern, PA); αVβ3-specific mAb LM609 (Cheresh, 1987) was from Chemicon (Temecula, CA). FITC-goat anti–mouse IgG was purchased from Zymed Laboratories (South San Francisco, CA); polyclonal affinity-purified antibodies against prothrombin were from Haematologic Technologies Inc. (Essex Junction, VT); BSA (fraction V, crystalline), calphostin C, bisindolylmaleimide I and V, and calpeptin were purchased from Calbiochem-Novabiochem Corp. (La Jolla, CA). Calpain inhibitors I and II were from Boehringer Mannheim (Mannheim, Germany). PMA, protease inhibitors (leupeptin, pepstatin, PMSF), adenosine 5′-diphosphate sodium salt (ADP) and cytochalasin B were from Sigma Chemical Co. (St. Louis, MO).
Purification of Proteins
Fibrinogen was purified from fresh human plasma by differential ethanol precipitation (Plow et al., 1984). αVβ3 was purified from detergent extracts of human placental tissues by affinity chromatography using a KGGRGDSP–Sepharose column followed by elution with 20 mM EDTA as described previously with minor modifications (Pytela et al., 1986; Smith et al., 1990b ). The preparations used exhibited only two major bands by SDS-PAGE and protein staining with Coomassie Brilliant Blue, which corresponded to the αV and β3 subunits, and was judged as being >95% pure. When immobilized in wells, αVβ3 preparation reacted with LM609, an mAb specific for αVβ3 (Cheresh, 1987), and did not react with CRC64, an mAb specific for αIIbβ3 (Mazurov et al., 1996), in an ELISA format.
Radioiodination
Na125I (specific activity = 15–17 mCi 125I/mg of iodine) from Nycomed Amersham Inc. (Princeton, NJ) was used for radioiodination. Prothrombin was radiolabeled using a modified chloramine-T method (Plow et al., 1984). The labeled prothrombin was indistinguishable from the unlabeled form upon SDS-PAGE under reducing and nonreducing conditions. When activated with Factor Xa + Va (5 mg/ml each; American Diagnostica Inc., Greenwich, CT). all of the radiolabeled prothrombin could be converted to thrombin within 30 min as assessed by gel analysis. Furthermore, the rate of activation of labeled and nonlabeled prothrombin by Factor Xa or Factor Xa/Va was the same as assessed with the Spectrozyme (American Diagnostics, Inc.) thrombin substrate (Byzova and Plow, 1997). Radioiodinated prothrombin was stored at 4°C and used within 3–4 d of labeling.
Solid-Phase Ligand Binding Assays
The binding of prothrombin to immobilized αVβ3 was performed as described (Charo et al., 1991; Byzova and Plow, 1997) with minor modifications. αVβ3 (280 μg/ml) was diluted 1:70 in a buffer containing 10 mM Tris, 150 mM NaCl, pH 7.4 (Buffer A), and immobilized onto 96-well microtiter plates (Costar Corp., Cambridge, MA) at 400 ng per well for overnight at 4°C. The plates were then washed and post-coated with 40 mg/ml BSA overnight at 4°C or 1 h at 37°C. The functional activity of the immobilized αVβ3 was assessed relative to 125I-fibrinogen binding to the same receptor preparations (Suehiro et al., 1996). 125I-prothrombin was added in Buffer A, containing 2 mg/ml BSA and the selected divalent cations. After incubation for selected times (75–120 min) at 37°C, wells were washed 4–5 times with Buffer A, and bound prothrombin was quantitated by counting the bound radioactivity in a γ-counter. In some experiments, αVβ3-coated wells were preincubated for 20 min with mAbs or peptides before addition of 125I-prothrombin. When fibrinogen was used as a competitor, H-D-Phe-Pro-Arg-chloromethylketone (Bachem, Torrance, CA) was included at a final concentration of 30 μg/ml. Nonspecific binding was measured in the presence of a 50-fold excess of unlabeled prothrombin. Data were determined as the means of triplicate or quadruplicate measurements at each experimental point.
Cell Culture
Primary cultures of HUVEC, human aortic smooth muscle cells (HASMC), and human aortic endothelial cells (HAEC) were provided by Drs. Paul DiCorleto and Donald Jacobsen (Cleveland Clinic Foundation, OH). HUVEC were grown to preconfluence in 162-cm2 plastic flasks (Costar Corp.) in DME/F12 (BioWhittaker Inc., Walkersville, MD) supplemented with 15% FBS (BioWhittaker Inc.), 150 μg/ml endothelial growth factor (Clonetics Corporation, San Diego, CA), and 90 μg/ml heparin (Sigma Chemical Co., St. Louis, MO; D'Souza et al., 1996). The cells were used within the second to fourth passage. HAEC were grown to preconfluence in 162-cm2 plastic flasks (Corning Costar Corp.), coated with 0.1% gelatin (Sigma Chemical Co.), in DME/F12 (BioWhittaker Inc.) supplemented with 15% FBS (BioWhittaker Inc.), 150 μg/ml endothelial growth factor (Clonetics), and 90 μg/ml heparin (Sigma Chemical Co.) and used within the third to fifth passage. HASMC were grown in 162-cm2 plastic flasks (Costar Corp.) in DME Medium/F12 (GIBCO BRL, Gaithersburg, MD) supplemented with 10% FBS (GIBCO BRL), 75 μg/ml endothelial growth factor (Clonetics), and 45 μg/ml heparin (Sigma Chemical Co.), and used within the fourth to seventh passage. αVβ3 expression was verified by flow cytometry (as described below) and only αVβ3-positive cultures were used in adhesion assays. αVβ3-negative cultures did not demonstrate an agonist-induced increase in adhesion to prothrombin.
HUVEC, HAEC, and HASMC Adhesion Assays
HUVEC and HAEC were washed three times with PBS and harvested by gentle trypsinization (0.25 mg/ml trypsin, 0.01% EDTA solution; Clonetics). Cells were collected into a tube containing trypsin neutralizing solution (Clonetics) and immediately centrifuged at 500 g for 10 min. The cells were resuspended in 107 cells/ml in DME/F12, containing 1% BSA (adhesion buffer). Calcein AM (50 μg; Molecular Probes, Eugene, OR) was solubilized in 10 μl of DMSO (Sigma Chemical Co.), and then diluted with 500 μl PBS. This Calcein solution (200 μl) was added to 2 ml of cell suspension at 5 × 106 cells/ml. 24-well plates (Costar Corp.) were precoated with prothrombin for 1 h at 37°C (10 μg/well in 50 mM NaHCO3, 150 mM NaCl, pH 8.0) or fibrinogen (10 μg/well) and postcoated with 3% BSA for 1 h at 37°C. Cells were labeled by Calcein AM for 30 min as described above and diluted to 5 × 105 cells/ml in DME/F12, containing 1% BSA. For HASMC, all procedures were the same but the trypsin solution was from BioWhittaker Inc. Cells were preincubated with or without inhibitors, light-activated calphostin C (final concentration, 1 μM), bisindolylmaleimide I and V (20 nM each), calpeptin (50 μg/ml), the combination of calpain inhibitors I and II (100 μg/ml, each) in the presence of additional 1 mM CaCl2 or 1 mM MnCl2, and then stimulated with PMA (200 nM) or various concentrations of ADP. The cell suspension (0.3 ml) was added to the coated wells. In some experiments, PMA-stimulated cells were treated by cytochalasin B (final concentrations, 0.1, 1, or 10 μM). At selected times (50–70 min at 37°C), wells were gently washed three times with DME/F12. Adherent cells were quantitated in a Fluorescence Multi-Well Plate Reader (PerSeptive Biosystems, Framingham, MA) and examined microscopically.
Flow Cytometry
HUVEC, HAEC, or HASMC, harvested as described above, were suspended at 8 × 105/ml in adhesion buffer and incubated with LM609 (10 μg/ml) or with control mouse IgG for 60 min at 37°C. The cells were washed by centrifugation in DME/F12 + 1% BSA, incubated with FITC-goat anti–mouse IgG on ice for 20 min, and then analyzed by flow cytometry. Flow cytometry was performed using a FACScan® instrument; 10,000 events were recorded; and the data were analyzed using the CellQuest software program (version 1.2).
Binding of 125I-Prothrombin to HUVEC in Suspension
HUVEC were diluted to 7 × 105/ml in DME/F12, with or without 0.5 mM MnCl2. The cells were preincubated with mAb LM609 (20 μg/ml), nonimmune immunoglobulins (20 μg/ml), c7E3 (20 μg/ml), or fibrinogen (100 and 500 μg/ml) for 5 min, and 125I-prothrombin was then added at selected concentrations. Cells were activated with PMA at 200 nM as specified. After 75 min at 37°C, cell-bound ligand was separated by centrifugation through 20% sucrose for 2.5 min at 22°C in Beckman microfuge, and the cell-bound radioactivity was measured in a gamma-counter. Data were determined with quadruplicate measurements at each experimental point.
Binding of 125I-Prothrombin to the HUVEC Monolayer
HUVEC were suspended at 1 × 106/ml in DME/F12, containing 1% BSA and seeded into 24-well plates, precoated by 0.1% gelatin. After 4 h incubation, nonadherent cells were removed, and the media was changed to supplemented DME/F12 for overnight. 7 h before the experiment, the media was changed for DME/F12 containing 1% BSA and no serum. Cells were preincubated with c7E3 (30 μg/ml) or GRGDSP peptide (100 μM) or without inhibitors for 10 min and then treated by PMA at 200 nM or 0.5 mM MnCl2 as indicated. 125I-prothrombin was then added at concentration of 50 μg/ml. After 70 min at 37°C, wells were washed three times by PBS, and bound radioactivation solubilized in 0.3 ml 1 N NaOH, and measured in a γ-counter. Quadruplicate measurements were made at each experimental point.
Results
125I-Prothrombin Binding to Purified αVβ3
As an initial analysis, we sought to determine whether purified αVβ3 could bind prothrombin. The αVβ3 used was isolated from human placenta by affinity chromatography on a RGD column and contained no detected αIIbβ3, as assessed immunochemically. The functional activity of the isolated αVβ3 was evaluated with the receptor immobilized onto microtiter plates and using fibrinogen as a well-characterized αVβ3 ligand (e.g., Smith and Cheresh, 1990; Smith et al., 1990a ). The binding of fibrinogen was supported by Mn2+ and was inhibited by Ca2+, consistent with the data of Smith et al. (1994); and this interaction was completely inhibited by RGD-containing peptides and the αVβ3-specific mAb, LM609.
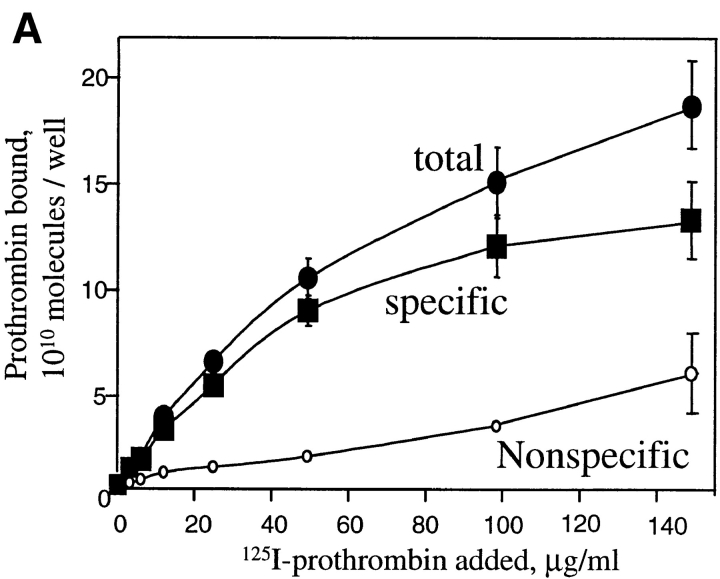
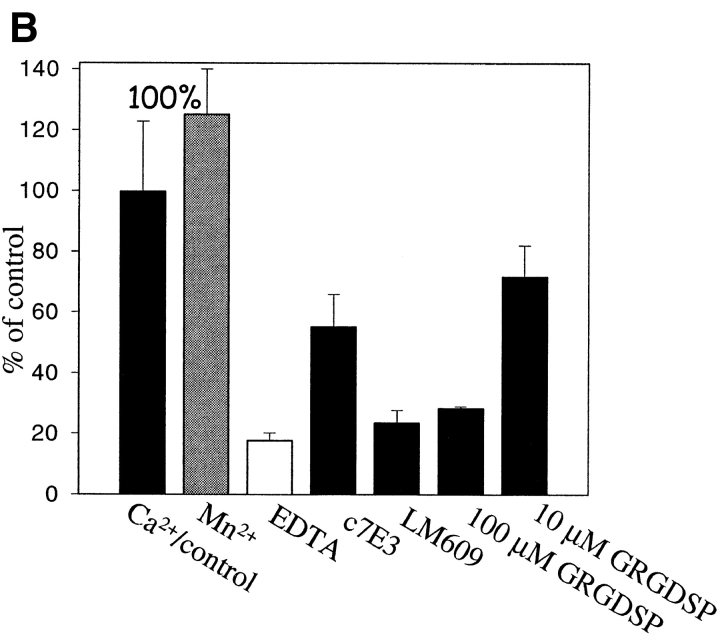
With evidence of receptor purity and function, the binding of prothrombin to αVβ3 was assessed. Increasing concentrations of 125I-prothrombin were added to wells coated with αVβ3. As shown in Fig. 1 A, prothrombin bound in concentration-dependent manner, and this interaction was inhibited by 50-fold excess of nonlabeled ligand. The concentration of 125I-prothrombin required for half-maximal binding was <25 μg/ml. At saturation, 13.6 × 1010 prothrombin molecules bound to the αVβ3-coated wells (see Fig. 1 A). In the presence of 1 mM MnCl2, ∼8.4 × 1010 fibrinogen molecules were maximally bound. Thus, the stoichiometry of binding of the two ligands to the receptor was similar. The specificity of prothrombin binding to αVβ3 is documented in Fig. 1 B. Typical of the binding of adhesive ligands to αVβ3, the interaction of prothrombin with the receptor was cation dependent: Ca2+ and Mn2+ supported binding, and EDTA inhibited the interaction. Two different mAbs reactive with αVβ3, LM609 and c7E3, also inhibited prothrombin binding to the receptor (Fig. 1 B) whereas nonimmune IgG had no effect. Although both mAbs were effective inhibitors of 125I-prothrombin binding to αVβ3, c7E3, even at higher concentrations, tended to be slightly less inhibitory than LM609, which may reflect the difference in the specificity of these mAbs (Cheresh, 1987; Jordan et al., 1997). The RGD-containing peptide, GRGDSP, produced dose dependent inhibition and, at a high concentration of 100 μM, was as effective as the mAbs and EDTA in inhibiting the interaction. Taken together, this inhibitory profile demonstrates that prothrombin can bind to αVβ3 via a RGD recognition specificity, which typifies the recognition of adhesive ligands by this integrin.
Figure 1.
125I-prothrombin binding to purified αVβ3. (A) Saturation isotherms of 125I-prothrombin binding (150 min at 37°C) to purified and immobilized αVβ3. Specific binding (▪) was derived by subtracting the nonspecific binding (○), the residual binding in the presence of a 50-fold excess of nonlabeled prothrombin, from the total binding (•), no inhibitor present. Data are derived in the presence of 1 mM Ca2+. (B) 125I-prothrombin binding to immobilized αVβ3 in the presence of 1 mM Ca2+ (black bars), 1 mM Mn2+ (gray bar), and 10 mM EDTA (open bar). Wells were preincubated with mAb c7E3 or LM609 (20 μg/ml each); or GRGDSP (100 μM and 100 μM) and 125I-prothrombin was added at a concentration of 30 μg/ml. The total binding (not corrected for nonspecific background) of prothrombin to αVβ3 in the presence of 1 mM Ca2+ without inhibitors was assigned a value of 100%. The data shown are means and SD of triplicates in one experiment and are representative of three separate experiments.
αVβ3-dependent Adhesion of Activated Vascular Cells to Prothrombin
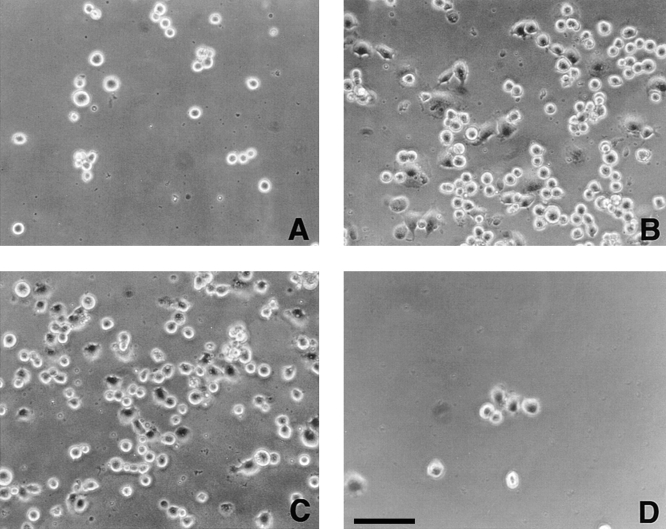
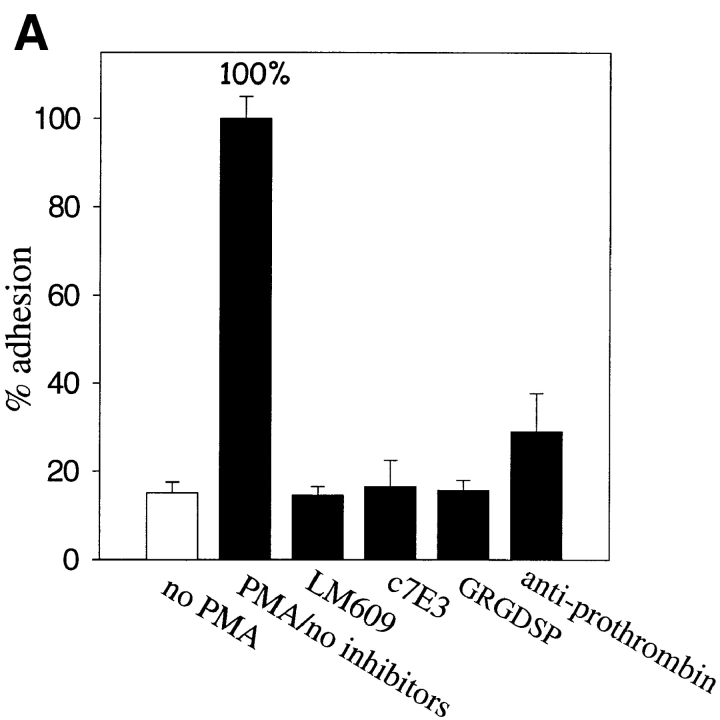
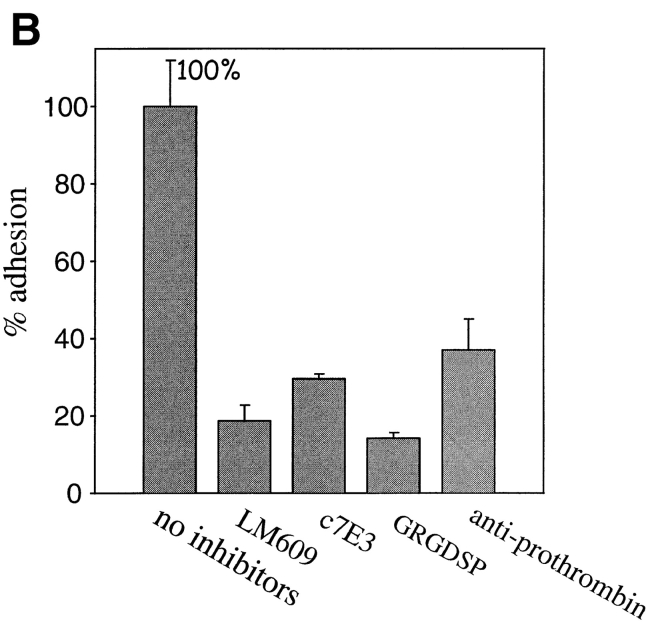
In view of recent evidence indicating that prothrombin is deposited in the vessel wall (McBane et al., 1997), we next sought to determine whether prothrombin could function as an adhesive ligand for αVβ3 in intact cells. The results of these analyses are shown microscopically in Fig. 2 and quantitatively in Fig. 3. Under conditions where prothrombin supported nonstimulated platelet adhesion, we found that nonstimulated HUVEC did not adhere to immobilized prothrombin (Figs. 2 A and 3 A). In contrast, adhesion of PMA-stimulated cells was evident within 30 min, and after 50–60 min, many of the adherent cells were spread on the prothrombin substratum (Fig. 2 B). With 2 × 105 HUVEC added to the prothrombin coated wells, the percentage of adherent cells ranged from 30 to 60% of the added cells; of the adherent cells, ∼30–40% were spread within 1 h (Fig. 2 B), and this percentage increased with longer incubation. Background cell adhesion to microtiter wells coated with BSA was not significantly affected by PMA stimulation, and the αVβ3 mAbs (LM609 and c7E3) and GRGDSP had no effect on the nonspecific adhesion of stimulated or nonstimulated cells to BSA (not shown). Verifying the role of αVβ3 in the adhesion of the PMA-stimulated HUVEC to prothrombin, mAbs LM609 and c7E3 completely blocked adhesion, as did GRGDSP (100 μM; Fig. 3 A). In contrast, neither nonimmune IgG nor a control peptide significantly affected HUVEC adhesion (Fig. 3 A). As an additional indication of specificity, antibodies to prothrombin also blocked adhesion to the immobilized substrate by >80% (Fig. 3 A, calculated by assigning the adhesion in the absence of PMA a value of 0%). This set of data demonstrates that PMA-activated HUVEC, but not resting cells, are capable of interacting with immobilized prothrombin via αVβ3 in a RGD-dependent manner.
Figure 2.
Photomicrographs of HUVEC adherent to immobilized prothrombin in the absence (A) and presence of 200 nM PMA (B) or in the presence of 1 mM MnCl2 (C). In D, cells were pretreated by mAb LM609 (20 μg/ml) to αVβ3 and then stimulated with PMA. Bar, 50 μm.
Figure 3.
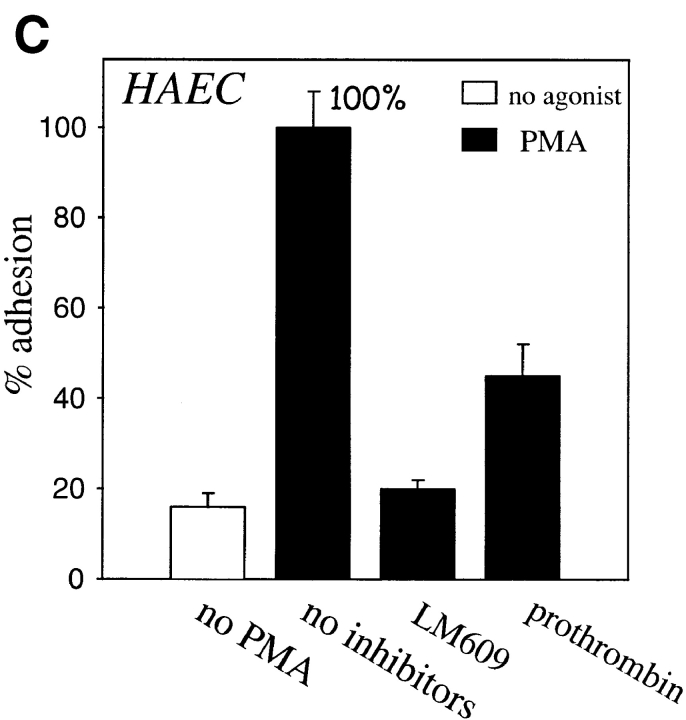
Endothelial cell adhesion to prothrombin requires stimulation. HUVEC (A and B) or HAEC were harvested, labeled by Calcein, and diluted to the 5 × 105 cells per ml in DME containing 0.2% BSA and 1 mM CaCl2. In A and B, HUVEC were stimulated with 200 nM PMA or 1 mM Mn2+, respectively. Inhibitors used were: mAb LM609 or mAb c7E3 (20 μg/ml each), GRGDSP peptide (100 μg), or polyclonal antibodies to prothrombin (anti-prothrombin). In C, HAEC were stimulated with PMA. In addition to the inhibitors used in A and B, soluble prothrombin was used at a concentration of 300 μg/ml. After 50 min, adhesion was measured. Adhesion in the presence of 1 mM CaCl2 and 1 PMA (A and C) or in the presence of 1 mM MnCl2 (B) and in the absence of inhibitors was assigned a value of 100%. The data shown are means and SD of quadruplicates in one experiment and are representative of seven separate experiments.
In the presence of Mn2+, a cation that stimulates the ligand binding function of αVβ3 (Smith et al., 1994), as well as many other integrins, (Mould et al., 1995; Suehiro et al., 1997), adhesion of the cells to prothrombin was observed without the requirement of an additional stimulus (Figs. 2 C and 3 B). This adhesion also was completely inhibited by GRGDSP, LM609, and c7E3, supporting the essential role for αVβ3 in the interaction (Figs. 2 D and 3 B). No additive effect on HUVEC adhesion was observed when PMA and Mn2+ were used together (not shown); however, we did find that after 6–7 passages, HUVEC required both PMA and Mn2+ to adhere to prothrombin. In Fig. 3 C, evidence is provided that adhesion to prothrombin also is observed with endothelial cells of a different origin. HAEC adhered to prothrombin in a similar manner as HUVEC, i.e., adhesion was stimulated by PMA and inhibited by LM609. Furthermore, as shown in Fig. 3 C, soluble prothrombin inhibited adhesion to the immobilized ligand. The later observation suggests that the soluble form of prothrombin is recognized by αVβ3, and surface denaturation is not required for prothrombin to become a ligand for the receptor. This interpretation is supported by subsequent binding studies using soluble prothrombin as a ligand for αVβ3 (see below).
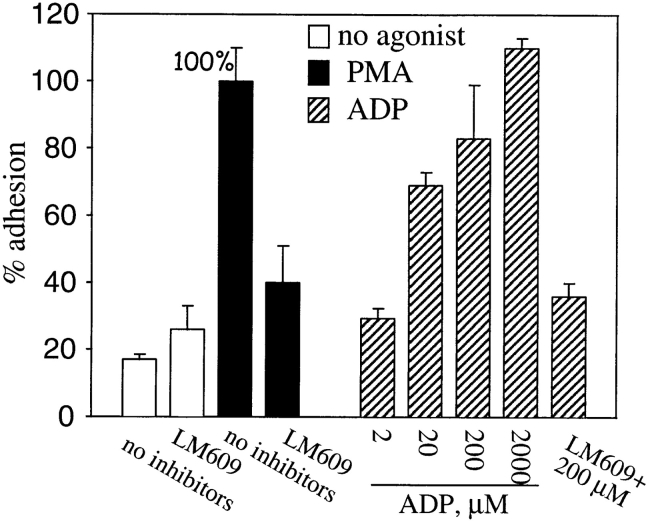
The capacity of prothrombin to support cell adhesion was not restricted to endothelial cells. Upon PMA stimulation, the adherence of a second type of vascular cell, HASMC, to prothrombin increased dramatically. The increased adhesion induced by PMA was 80% (calculated by assigning the adhesion in the absence of PMA a value of 0%) inhibited by mAb LM609 (Fig. 4) but not by nonimmune Ig. We also found that Mn2+ supported αVβ3-mediated adhesion of HASMC to prothrombin (not shown). Furthermore, with HASMC, a physiologically relevant agonist (Boarder and Hourani, 1998; Murthy and Makhlouf, 1998), ADP, was shown to induce adhesion to prothrombin. ADP increased adhesion in a dose-dependent manner; and, at higher concentrations (200–2,000 mM), adhesion was as extensive as that induced by PMA. The adhesion induced by ADP was αVβ3-mediated as LM609 was an effective inhibitor. We also observed that ADP induced adhesion of HUVEC to prothrombin (not shown) although higher concentrations of the agonist were required to obtain a comparable effect. Taken together, these data indicate that prothrombin serves as an adhesive ligand for αVβ3 on HUVEC, HAEC, and HASMC.
Figure 4.
PMA-stimulated adhesion of HASMC to prothrombin is αVβ3 dependent. HASMC were harvested, labeled by Calcein, diluted to the 5 × 105 cells per ml in DME containing 1% BSA and 1 mM CaCl2. HASMC were stimulated with either 200 nM PMA (black bars) or ADP (striped bars) at the indicated concentrations. mAb LM609 at a concentration of 20 μg/ml was included as indicated. After 40 min, adhesion was measured. Adhesion in the presence of PMA without inhibitors present was assigned a value of 100%. Nonspecific adhesion to BSA-coated wells was subtracted. The data shown are means and SD from three experiments.
Binding of Prothrombin to Endothelial Cells
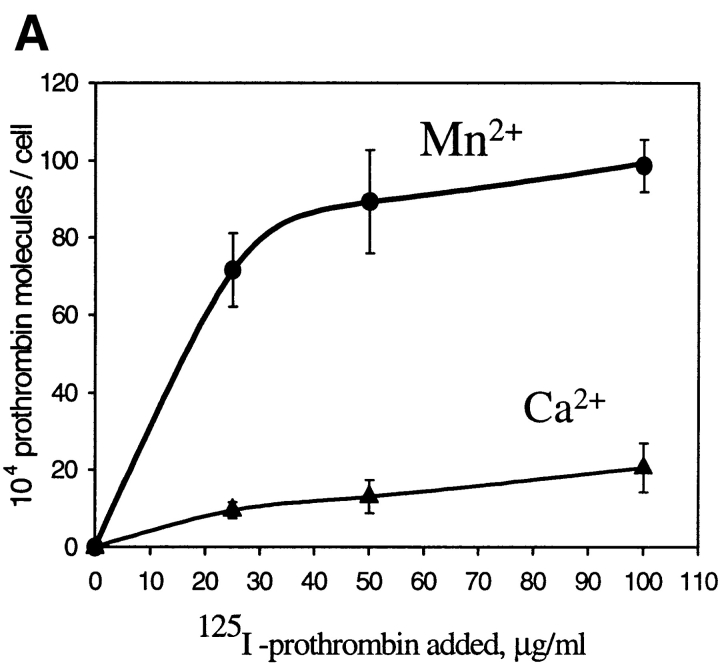
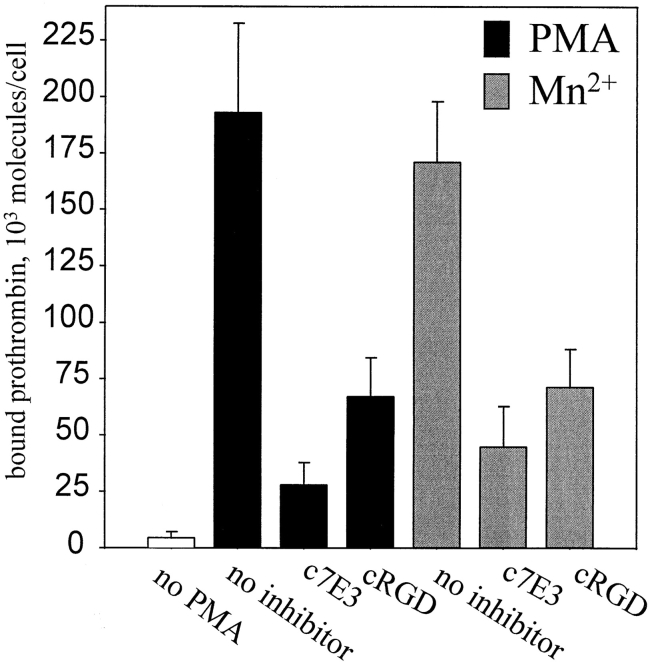
We next sought to assess whether αVβ3 on vascular cells is capable of recognizing not only immobilized but also soluble prothrombin and how cellular activation might influence this interaction. 125I-prothrombin was incubated with HUVEC in a single cell suspension in the presence of Ca2+ and Mg2+ (DME/F12 without additional cations) or in the presence of additional 1 mM Mn2+. Results of a typical experiment are shown in Fig. 5 A, which illustrates the influence of increasing concentrations of added 125I-prothrombin on specific prothrombin binding to HUVEC under the two divalent cation conditions. The nonspecific binding was determined in the presence of 50-fold molar excess of unlabeled prothrombin and corresponded to 10–15% of the total binding at the concentrations of 125I-prothrombin added. From the data presented in Fig. 5 A, it is evident that the addition of Mn2+ cause a dramatic increase of 125I-prothrombin binding to HUVEC. Saturation of binding was apparent in the presence of Mn2+ at prothrombin concentrations above 50 μg/ml, which corresponds to half the prothrombin concentration in plasma. At this saturating concentration, 958,000 ± 108,500 prothrombin molecules bound per cell in the presence of Mn2+, compared with 18,600 ± 2,050 molecules per cell in the presence of Ca2+. Thus, although Ca2+ did support limited specific binding of prothrombin to αVβ3 (inhibitable by nonlabeled prothrombin, c7E3 and LM609), Mn2+ enhanced this interaction by ∼50-fold.
Figure 5.
HUVEC bind soluble 125I-prothrombin. (A) HUVEC in suspension were incubated with increasing concentrations of 125I-prothrombin in DME/F12 in the presence of 0.5 mM MnCl2 or 1 mM CaCl2 for 60 min, and cell-bound radioactivity was quantitated as described. Nonspecific binding was measured in the presence of 50-fold excess of nonlabeled prothrombin and subtracted to yield the specific binding data shown. Values represent the means and SD of four determinations. (B) Specificity of prothrombin binding to HUVEC. 125I-prothrombin (50 μg/ml) was incubated with HUVEC (8 × 105 cells/ml) in the presence of mAb LM609, mAb c7E3 (20 μg/ml each; a similar concentration of nonimmune IgG was without effect), or 100 μM GRGDSP. MnCl2 (1 mM) was added to the DME/F12 media, and cell-bound radioactivity was measured after 60 min incubation. Nonspecific binding was measured in the presence of 50-fold excess of nonlabeled prothrombin and subtracted. The data shown are means and SD of quadruplicates in one experiment and are representative of three separate experiments.
To investigate whether 125I-prothrombin binding in the presence of Mn2+ was attributable to αVβ3, binding studies were performed in the presence of mAbs LM609 and c7E3 and GRGDSP. As shown in Fig. 5 B, LM609 inhibited 125I-prothrombin binding to HUVEC by 75–80% and to c7E3 by ∼50%. At the same concentration, nonimmune IgG had no effect. The extent of inhibition by GRGDSP was similar to that produced by c7E3. The stimulatory effect of Mn2+ and the inhibition profile of 125I-prothrombin binding confirm the role of αVβ3 in interaction of HUVEC with soluble prothrombin.
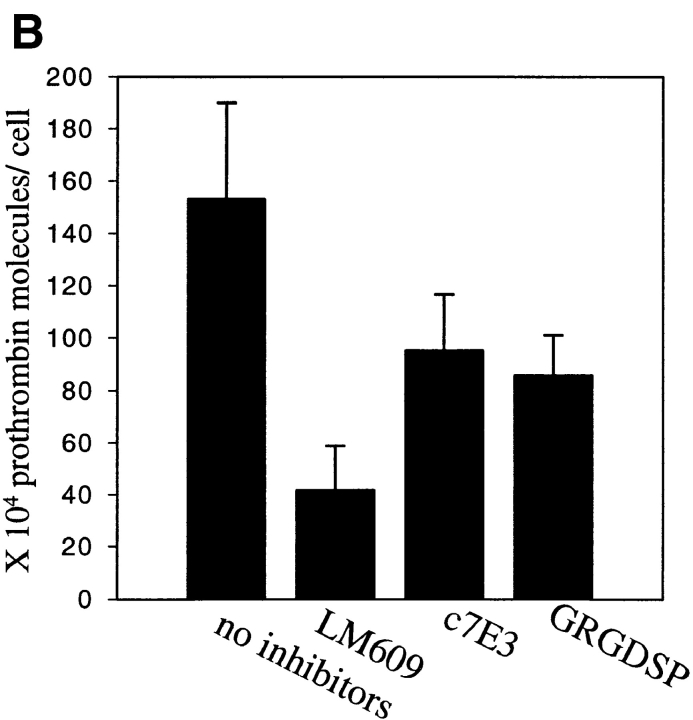
Next, we assessed whether 125I-prothrombin is capable of interacting with HUVEC in a monolayer. 125I-prothrombin at 50 μg/ml was added to a confluent and intact HUVEC monolayer in the presence of PMA, 0.5 mM Mn2+ or no addition. After 70 min, the cells were rapidly washed and HUVEC-associated radioactivity was extracted and counted. The αVβ3-mediated component of prothrombin binding accounted for 194,000 ± 5,780 molecules/cell. A contribution of αVβ3 to prothrombin binding also was demonstrable in the presence of 0.5 mM Mn2+ (Fig. 6). It should be noted that substantial specific binding of 125I-prothrombin (inhibited by excess nonlabeled prothrombin) to the untreated HUVEC monolayer was observed (not shown), but this interaction was unaffected by mAb LM609, i.e., the binding was not αVβ3-mediated. PMA-stimulation substantially increased the 125I-prothrombin binding, and this increment was abrogated by LM609 as well as c7E3 (Fig. 6). Thus, αVβ3 can recognize prothrombin, either as an adhesive substrate or as a soluble ligand, provided the HUVEC had been exposed to PMA or Mn2+. To determine whether 125I-prothrombin binding to the HUVEC monolayer resulted in internalization of ligand, we tested whether EDTA could elute bound prothrombin from the cells. 125I-prothrombin was bound to the cells for 70 min and then HUVEC were washed 4–5 times with phosphate buffer, containing 10 mM EDTA. This treatment did not disrupt the monolayer as assessed microscopically, but did elute 96% of the bound 125I-prothrombin, indicating that the reaction was reversible and that prothrombin was not internalized under the conditions used.
Figure 6.
125I-prothrombin binding to a HUVEC monolayer. Confluent cell monolayers were incubated with 50 μg/ml 125I-prothrombin in DME/F12-1% BSA in the presence or absence of 200 nM PMA (solid bars) or 0.5 mM MnCl2 (gray bars). Cells were preincubated with c7E3 (30 μg/ml) or a cyclic RGD peptide (10 μM) or without inhibitors for 10 min. After 70 min at 37°C, wells were washed three times with PBS, and the cells were solubilized in 1 N NaOH. Prothrombin binding to nonstimulated endothelial cells was subtracted from the total binding, and the difference is displayed. Values are means and SD of quadruplicates from one of five experiments with similar results.
Competition between Prothrombin and Fibrinogen for αVβ3
The capacity of a major αVβ3 ligand, fibrinogen, to compete with prothrombin for binding to the receptor was assessed. These analyses were conducted with both purified αVβ3 and with HUVEC in suspension, and the experiments were performed under different divalent cation conditions. The results are summarized in Table I. Previous studies have established that fibrinogen binds poorly to purified αVβ3 in the presence of Ca2+ (Smith et al., 1994; Suehiro et al., 1996), and fibrinogen, even in concentrations as high as 500 μg/ml, was a poor inhibitor of prothrombin binding, producing only 11% inhibition in 1 mM Ca2+. In the presence of Mn2+, fibrinogen was a more effective inhibitor, producing 40% inhibition at 500 μg/ml. Nevertheless, substantial binding of prothrombin was still observed. The data obtained for 125I-prothrombin binding to HUVEC were consistent with those obtained in the purified system. Specifically, in the absence of Mn2+, fibrinogen was a poor inhibitor of 125I-prothrombin binding. Inhibition was more extensive in the presence of 1 mM Mn2+, but substantial prothrombin binding was still observed, even at the higher fibrinogen concentration. Thus, under some, but not all conditions, fibrinogen competes but does not appear to be a particularly effective inhibitor of 125I-prothrombin binding to αVβ3.
Table I.
Effect of Fibrinogen on Prothrombin Binding to αVβ3 in a Purified System and on Endothelial Cells
| Cation conditions | 125I-Prothrombin binding to purified αVβ3 * | 125I-Prothrombin binding to HUVEC‡ | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 mM Ca2+ | 1 mM Mn2+ | 2 mM Ca2+ 2 mM Mg2+ | 1 mM Ca2+ 2 mM Mg2+ 1 mM Mn2+ | |||||
| No competitor | 100 ± 7.1% | 100 ± 9% | 100 ± 12% | 100 ± 18% | ||||
| Fibrinogen | 89 ± 9% | 60.7 ± 4% | 85.4 ± 11% | 38 ± 5% | ||||
| (500 μg/ml) | ||||||||
| Fibrinogen | 107 ± 14% | 93 ± 10.3% | 92.7 ± 11.9% | 76.9 ± 4% | ||||
| (100 μg/ml) | ||||||||
In all assays, H-D-Phe-Pro-Arg-chloromethylketone was present throughout at a final concentration of 30 μg/ml. Specific prothrombin binding, inhibited by excess nonlabeled prothrombin, was assigned the value of 100% under each cation condition. With purified and immobilized αVβ3, 100% was 7.7 × 1010 molecules/well in the presence of 1 mM Ca2+ and 9.8 × 1010 molecules/well in Mn2+. In the cell experiments, 100% was 110,000 molecules/cell in the presence of 2 mM Ca2+, 2 mM Mg2+, and 780,000 molecules/cell in the presence of 1 mM Ca2+, 2 mM Mg2+, and 1 mM Mn2+. Values are the means ± SD of quadruplicates in one experiment which is representative of at least five experiments.
Experimental conditions are the same as in Fig. 1 with purified αVβ3 immobilized onto microtiter wells.
Experimental conditions are the same as in Fig. 5 with HUVEC in suspension.
Molecular Basis for Adhesion of Activated Vascular Cells to Prothrombin
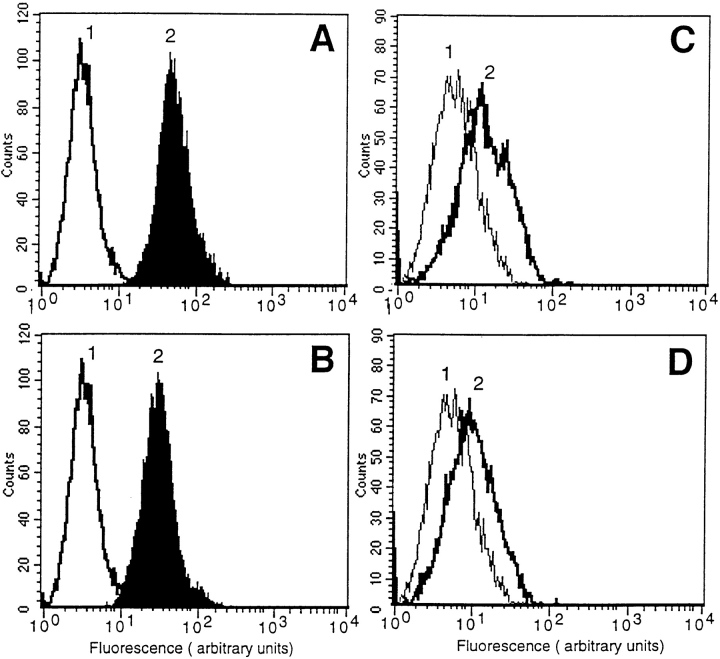
We sought to understand the mechanism by which αVβ3 became competent to bind prothrombin in the presence of agonist. FACS® analysis was used to determine whether the expression levels of αVβ3 on HUVEC and HASMC is altered by PMA stimulation. This analysis confirmed high expression of αVβ3 on HUVEC and lower expression on HASMC. PMA-stimulated cells were found to express αVβ3 at levels similar to that found on nontreated cells. These data indicate that short-term PMA treatment did not alter the expression level of αVβ3.
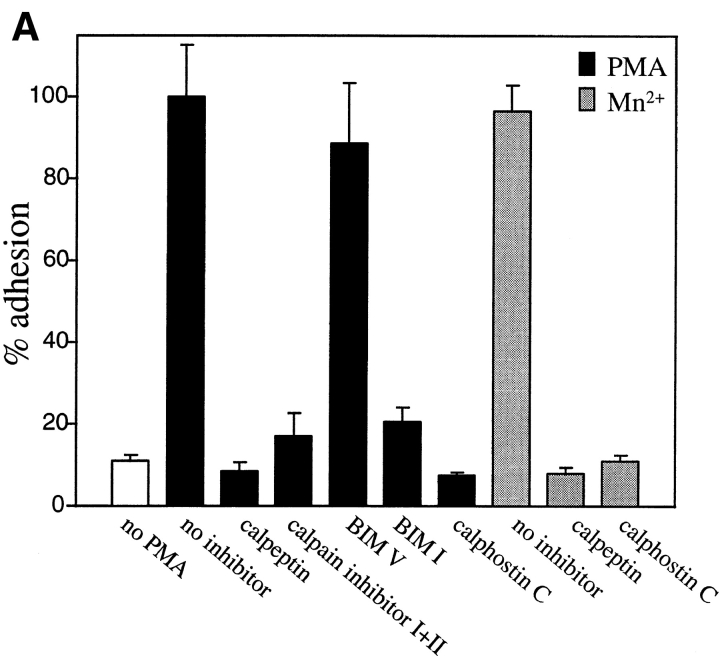
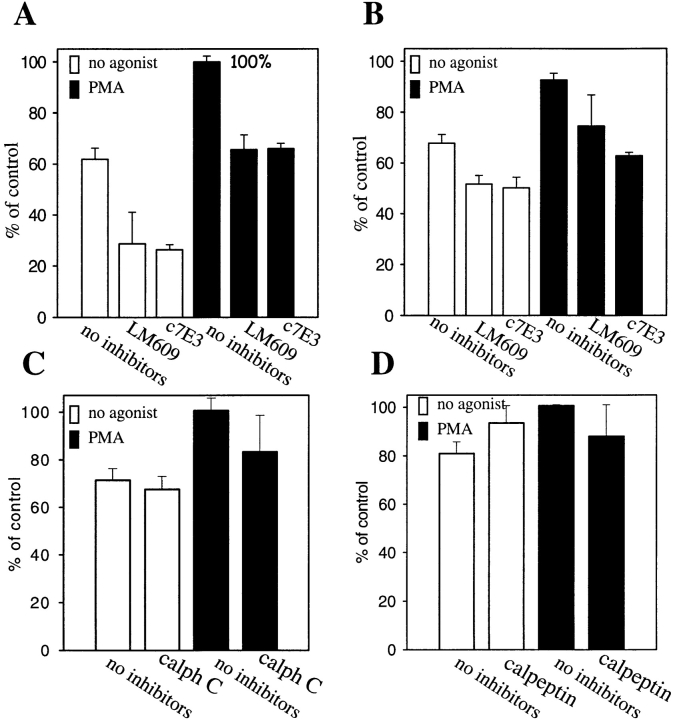
Since PMA is a potent activator of protein kinase C (PKC), the possible role of PKC in PMA-stimulated HUVEC adhesion on prothrombin was examined. Calphostin C, a specific and potent inhibitor of PKC (Kobayashi et al., 1989), completely blocked the effect of PMA on HUVEC adhesion to prothrombin (see Fig. 8 A). Similar results were obtained with a second PKC inhibitor, bisindolylmaleimide I, but not with the low affinity control inhibitor, bisindolylmaleimide V (Toullec et al., 1991). At the concentration used, calphostin C and bisindolylmaleimide I did not affect cell viability, as assessed by trypan blue staining, even with extended incubation times. These results indicate that the effect of PMA on increased adhesion depends upon PKC. Of note, treatment with calphostin C also abolished attachment and spreading of HUVEC on prothrombin in the presence of Mn2+ (see Fig. 8 A). This observation suggests that, like PMA, the induction of prothrombin binding to αVβ3 on HUVEC by Mn2+ requires inside-out signaling events.
Figure 8.
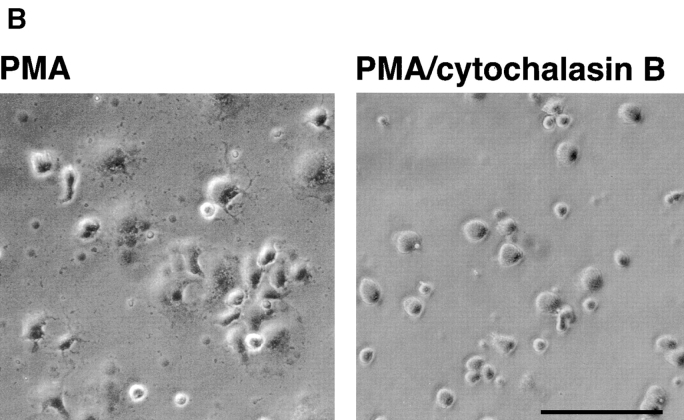
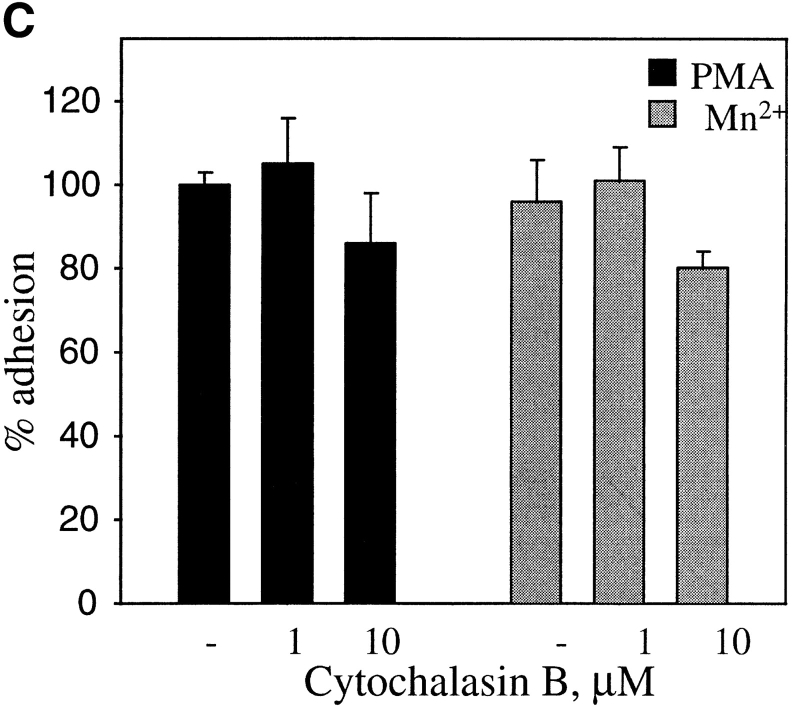
Effects of protein kinase C and calpain inhibitors (A) and cytochalasin B (B and C) on adhesion of PMA- and MnCl2-treated HUVEC to immobilized prothrombin. HUVEC adhesion was measured (see legend to Fig. 3 and Materials and Methods) in the absence (open bar) or presence of 200 nM PMA (black bars) or in the presence of 0.5 mM MnCl2 (gray bars). In A, cells were pretreated with calpeptin (50 μg/ml), calpain inhibitor I and II (100 μg/ml each), bisindolylmaleimide V (BIM V) and bisindolylmaleimide I (BIM I; 20 nM each) or calphostin C light-activated; 1 μM). In B, adherent cells in the absence or presence (0.1 μm) of cytochalasin B were photographed at 40×. Bar, 50 μm. In C, adhesion of stimulated cells was measured after 50 min in the absence or presence of cytochalasin B. Adhesion in the presence of 200 nM PMA without inhibitors was assigned a value of 100%. The data shown are means and SD from three experiments.
As a next step, we investigated the role of another potential candidate in PMA-induced cell adhesion to prothrombin, calpain. Numerous activities have been ascribed to this neutral calcium-dependent protease, including the capacity to cleave the cytoplasmic tail of β3 subunit (Du et al., 1995) and to regulate cell migration (Huttenlocher et al., 1997). HUVEC, pretreated with membrane permeable calpain inhibitor, calpeptin (Tsujinaka et al., 1988), were unable to adhere to prothrombin either after PMA stimulation or in the presence of Mn2+ (Fig. 7 A). The combination of calpain inhibitors I and II also was effective in inhibiting HUVEC adhesion to prothrombin. These findings indicate that active calpain is required for modulation of αVβ3 affinity on endothelial cells induced by PMA and Mn2+. To determine if PMA stimulation exerted its effect on prothrombin binding by αVβ3 by influencing post-ligand binding events, such as integrin clustering and cell spreading, PMA-activated cells were treated with cytochalasin B, an effective inhibitor of actin cytoskeleton reorganization. Untreated HUVEC adhered and spread on prothrombin, whereas, in the presence of cytochalasin B, the cells remained adherent but entirely rounded (Fig. 8 B). This effect indicates that the cytochalasin treatment altered the cytoskeletal response of the cells. Quantitation of the number of adherent cells verified that the adhesion of PMA-stimulated HUVEC to prothrombin was not substantially changed by cytochalasin B at a concentration of 1 or 10 μM (Fig. 8 C). Neither of these concentrations were toxic for cells. HUVEC adhesion in the presence of Mn2+ or Mn2++ PMA also was unaffected by cytochalasin B. These results suggest that PMA stimulation modulates the affinity of αVβ3 for the prothrombin ligand and that cytoskeletal reorganization is not required for recognition of prothrombin by the receptor. Of note, we found that certain concentrations of cytochalasin B stimulated cell adhesion to prothrombin in the absence of PMA or Mn2+. Similar observations were reported by Qi et al. (1998), who found that cytochalasin D could activate αIIbβ3-mediated adhesion to fibrinogen.
Figure 7.
Short-term PMA treatment does not increase αVβ3 expression. HUVEC (A and B) or HASMC (C and D) were grown and harvested as described. For each experimental point, 106 cells were incubated in the presence of nonimmune IgG (1) or mAb LM609 (2) for 50 min. In B and D cells were treated by 200 nM PMA. After washing, the cells were incubated with anti-mouse IgG FITC-conjugated antibody and analyzed by flow cytometry.
To determine if the activation requirement for recognition of prothrombin by αVβ3 extends to other αVβ3 ligands, we assessed the effects of cell stimulation and of the inhibitors, calphostin C and calpeptin, on αVβ3-mediated HUVEC adhesion to fibrinogen. Consistent with previous reports (Cheresh, 1987; D'Souza et al., 1996; Suehiro et al., 1997), HUVEC adhere well to fibrinogen although only a portion of this adhesion was αVβ3 mediated. αVβ3-dependent adhesion was identified as that component of total cell adhesion that was sensitive to the anti-αVβ3 blocking mAbs, LM609 or c7E3 (Fig. 9 A). For nonstimulated cells, αVβ3-mediated adhesion was ∼37% (100% is defined as the total adhesion in the presence of PMA). Treatment with PMA caused an increase in total HUVEC adhesion, but the αVβ3-mediated portion of adhesion remained unchanged (35%). The same pattern was demonstrable in the presence of Mn2+. In the experiment shown in Fig. 9 B, αVβ3-mediated adhesion in the presence of Mn2+ was 17% of the total adhesion, and with Mn2+ + PMA present, 19% of the total adhesion was αVβ3 mediated (Fig. 9 B). In contrast to HUVEC adhesion to prothrombin, pretreatment of HUVEC with calphostin C did not significantly decreased the number of cells adherent to fibrinogen (Fig. 9 C). Furthermore, whereas pretreatment of HUVEC with calpeptin resulted in complete inhibition of cell adhesion to prothrombin, calpeptin had no effect on cell adhesion to fibrinogen (Fig. 9 D). Thus, the requirements for αVβ3-mediated adhesion to prothrombin and fibrinogen are quite distinct.
Figure 9.
HUVEC adhesion to immobilized fibrinogen. HUVEC were harvested, labeled by Calcein, diluted to the 5 × 105 cells per ml in DME containing 1% BSA and 1 mM CaCl2 (A) or 1 mM MnCl2 (B). Specific αVβ3-antagonists, mAb LM609, or mAb c7E3 (20 μg/ml each), were included as indicated. In C, the cells were pretreated with calphostin C at final concentration of 1 μM or calpeptin (50 μg/ml). In D, HUVEC were treated with 200 nM PMA (solid bars), and, after 50–60 min, adhesion was measured. Adhesion in the presence of 1 mM CaCl2 + 200 nM PMA was assigned a value of 100%. The data shown are means and SD of triplicates in one experiment and are representative of four separate experiments.
Discussion
In this study, we sought to assess whether prothrombin is a ligand for αVβ3 on vascular cells. A direct interaction between prothrombin and αVβ3 on human vascular cells, endothelial cells, derived from umbilical vein and from aorta, and smooth muscle cells was demonstrable, establishing a previously unrecognized interface between the adhesive and procoagulant properties of these cells. Moreover, in characterizing this interaction, we found that activation of αVβ3 by model agonists (PMA or Mn2+) or physiological agonists (ADP) is required for recognition of prothrombin, and this requirement is not necessary for fibrinogen to engage the receptor. Therefore, whereas recent studies have emphasized that αVβ3 can exist in different activation states (Pelletier et al., 1996; Bennett et al., 1997; Sadhu et al., 1998), it appears that the functionality of the receptor is defined by the ligand under analysis; and prothrombin and fibrinogen serve as prototypes of activation-dependent and activation-independent ligands for αVβ3 on vascular cells. Furthermore, our results implicate the PKC pathway and calpain activity in controlling the activation state of αVβ3 on endothelial and smooth muscle cells.
Three approaches were used to characterize the interaction of prothrombin with αVβ3. First, the binding of prothrombin to the purified receptor was analyzed. Specific and saturable binding of prothrombin to purified and immobilized αVβ3 was demonstratable. This interaction was cation-dependent and was mediated by a RGD recognition specificity as RGD-containing peptides blocked binding. These are characteristics of the interaction of αVβ3 with many of its adhesive ligands (e.g., Felding-Habermann and Cheresh, 1993). Second, the capacity of immobilized prothrombin to support αVβ3-dependent adhesion was measured. Prothrombin was found to support attachment and spreading of stimulated HUVEC, HAEC and HASMC. This adhesion was mediated by αVβ3 as evidenced by its blockade by mAbs to the receptor and by RGD-containing peptides. As an additional specificity control, antibodies to prothrombin also blocked this adhesion. Third, the ability of αVβ3 on cells to bind soluble prothrombin was evaluated. αVβ3 mediated the binding of prothrombin to stimulated HUVEC in suspension and in a monolayer, and this interaction required stimulation of the cells. Taken together, these analyses clearly document that prothrombin, presented either in a soluble or an immobilized form, is a ligand for αVβ3 on vascular cells.
Prothrombin contains a RGD sequence within its catalytic domain. Analysis of the crystal structure of thrombin revealed that the RGD is involved in the formation of the active site and lies at the bottom of the S1 specificity pocket (Stubbs and Bode, 1993). This positioning is likely to preclude access of αVβ3 and other integrins with a RGD recognition specificity to the sequence in native thrombin. The orientation of the RGD may be different in prothrombin and permit recognition by αVβ3. In the crystal structure of prethrombin 2 (Vijayalakshmi et al., 1994), a catalytically inactive intermediate generated during prothrombin activation, the RGD sequence resides in a surface-exposed configuration. As additional support for this possibility, Bar-Shavit et al. (1991, 1993) demonstrated that active thrombin was not adhesive but could be modified into a potent RGD-dependent adhesion molecule for endothelial cells. Our data showing that soluble prothrombin inhibits adhesion to the immobilized ligand suggest that the requisite sequence(s) for αVβ3 recognition are expressed on the surface of the native molecule. This interpretation by no means excludes the possibility that other sequences in prothrombin could mediate recognition of prothrombin by αVβ3; i.e., similar to the recognition of the γ chain, rather than the RGD sequence of fibrinogen by αIIbβ3 (Farrell et al., 1992), even though αIIbβ3 also has a RGD recognition specificity. Also to be resolved is whether factor X, which also contains a RGD sequence, can interact with αVβ3.
The αVβ3 integrin is widely expressed on vascular cells. It is present on luminal and basolateral surfaces of endothelial cells, on smooth muscle cells, and is also expressed on certain circulating blood cells (Cheresh, 1987; Savill et al., 1990; Moulder et al., 1991; Conforti et al., 1992; Brown et al., 1994). This expression profile suggests that αVβ3 is directly exposed to plasma proteins, including prothrombin. From our analyses of the interaction of prothrombin with αVβ3, either in purified form or on cells, half-maximal binding occurred at input concentrations of ∼50 μg/ml and almost 106 prothrombin molecules were bound per endothelial cell. Thus, the plasma concentration of prothrombin at ∼100 μg/ml would potentially place substantial quantities of prothrombin on cell surfaces that must be nonthrombogenic to maintain hemostasis. In addition to its presentation as a soluble ligand from plasma, prothrombin also may be a relevant substrate for vascular cell adhesion. Recent studies have demonstrated that prothrombin is synthesized by smooth muscle cells (McBane et al., 1997). Furthermore, prothrombin accumulates within the vascular matrix, particularly at sites of lesion formation. High levels of prothrombin have been identified in the aortic intima after deendothelializing injury (Hatton et al., 1995) and in early atherosclerotic lesion (Smith and Staples, 1981). Based on our studies of prothrombin binding to αIIbβ3, a potential functional consequence of prothrombin binding to αVβ3 would be its more efficient activation to thrombin (Byzova and Plow, 1997). Also, with the deposition of prothrombin in the vessel wall under pathophysiological conditions (Hatton et al., 1995; Smith and Staples, 1981), adhesion itself may be a biologically relevant endpoint of prothrombin–αVβ3 interactions. With these potential biological ramifications, the interaction of prothrombin with this receptor requires tight regulation. Such regulation appears to be established by the activation state of αVβ3. Whether competition with other αVβ3 ligands provides an additional level of control remains to be established. In this regard, based upon their plasma levels, the two primary competitors for plasma prothrombin binding to αVβ3 are predicted to be vitronectin and fibrinogen. Denaturation is required for vitronectin to become a soluble ligand for αVβ3 (Seiffert and Smith, 1997); with fibrinogen, the role of αVβ3 in mediating its binding to HUVEC has been variable (Languino et al., 1993). In our analyses, we found that fibrinogen did inhibit 125I-prothrombin binding to HUVEC, but only under specific cation conditions, i.e., when Mn2+ was present. This result is consistent with the suppression of fibrinogen binding to purified αVβ3 that has been previously reported (Smith et al., 1994; Suehiro et al., 1996). Thus, competition between prothrombin and fibrinogen will be determined by specific microenvironmental cation conditions and the relative affinity of the two ligands for the receptor. Detailed studies are in progress to assess this latter parameter. Also, concentration is not the sole determinant of the competition between these ligands, e.g., although von Willebrand factor is present at much lower concentrations in plasma than fibrinogen, it is still a preferred substrate at high shear conditions (Savage et al., 1996). In the matrix, still other conditions will determine the importance of prothrombin, fibrinogen, vitronectin, and other αVβ3 ligands as adhesive substrates. Ultimately, the relative capacity of these various ligands to support cell migration, as well as adhesion, will be functionally important. Thus, it is uncertain whether ligand competition will play a significant role in regulating prothrombin binding to αVβ3.
Interaction of prothrombin with αVβ3 on intact cells was not observed unless the cells were stimulated. Such activation was induced by a well-characterized, model integrin agonist, PMA. In addition, a physiological agonist, ADP (Boarder and Hourani, 1998), also activated smooth muscle cells and endothelial cells to adhere to prothrombin. ADP is a physiologically relevant agonist (Nurden et al., 1995) for activation of αIIbβ3 on platelets, and the second β3 integrin, αVβ3, also responds to this stimulus (Boarder and Hourani, 1998). Higher concentrations of ADP were required to activate αVβ3 on HUVEC than on HASMC. This may reflect the higher levels of CD39, an ecto-ADPase, on HUVEC (Marcus et al., 1997). Three potential explanations for the effects of these agonists on αVβ3 function may be considered. First, PMA stimulation could increase the number of αVβ3 receptors. However, FACS® analysis of treated and untreated HUVEC and SMC showed that expression levels of αVβ3 were not changed upon stimulation. In addition, the induction of adhesion was observed after short-term treatment by the agonists (1 h or less), a time insufficient for extensive de novo synthesis. Second, receptor clustering may enhance ligand binding to integrins (Miyamoto et al., 1995; Detmers et al., 1987; Hato et al., 1998). When PMA-stimulated cells were treated with cytochalasin B at concentrations that inhibited actin cytoskeleton rearrangements as evidenced by the abolition of cell spreading on prothrombin, cell adhesion to prothrombin was not diminished. This observation does not exclude a role of integrin clustering in αVβ3 activation. Indeed, we observed that cytochalasin B in the absence of PMA could induce cell adhesion to prothrombin. Integrin activation by cytochalasins has been observed by others (Kucik et al., 1996; Qi et al., 1998) and may arise from the increased mobility of receptors in the plane of the membrane. Therefore, αVβ3 multimerization may regulate vascular cell adhesion to prothrombin. Third, PMA stimulation may change the affinity state of αVβ3 for prothrombin. It is well established that integrins can exist in multiple conformational states (Schwartz et al., 1995; Shattil and Ginsberg, 1997), which exhibit distinct functions. Such affinity modulation is a consequence of inside-out signaling and is central to the function of αIIbβ3 on platelets (Schwartz et al., 1995). Indeed, PMA is one of the agonists that activates αIIbβ3 (Shattil and Brass, 1987). Affinity modulation also has been ascribed to αmβ2 (Altieri et al., 1988), α4β1 (Masumoto and Hemler, 1993), α5β1 (Faull et al., 1993), and α6Aβ1 (Delwel et al., 1996). The capacity of αVβ3 to exist in different functional states also has been previously demonstrated (Bennett et al., 1997) although the mechanisms underlying these functional differences were not fully resolved.
PMA induced the conversion of αVβ3 from a low- to a high-affinity/avidity state for prothrombin. The activity of this agonist suggests that PKC activation may be important in the activation of αVβ3 (Danilov and Juliano, 1989; Vuori and Ruoslahti, 1993). This conclusion was supported by the observation that known inhibitors of PKC, calphostin C (Kobayashi et al., 1989) and bisindolylmaleimide I (Toullec et al., 1991), abolished the effect of PMA on cell adhesion to prothrombin. Calphostin C also blocked adhesion of HUVEC to prothrombin induced by Mn2+, indicating a role of this cation in activation of PKC. Thus, the influence of Mn2+ on integrin function is not restricted to its effects on the extracellular ligand binding domains of integrins (Smith et al., 1994). In addition, a second intracellular signaling molecule, the neutral protease calpain, was implicated in the activation pathway of αVβ3 based upon the effects of the membrane permeable and highly potent calpain inhibitor, calpeptin. Calpain can influence multiple intracellular signaling pathways by cleaving any of a variety of substrates including PKC, phospholipase C, pp60 Src, as well as cytoskeletal proteins including talin and paxillin (Kishimoto et al., 1989; Suzuki et al., 1992; Al and Cohen, 1993). Indeed, it has been reported that calpain can directly cleave the cytoplasmic tail of β3-subunit (Du et al., 1995). Thus, the proteolysis of any one of many potential substrates by calpain could lead to activation of αVβ3, and careful dissection will be required to identify the requisite event(s). Whereas calpain activity and integrin function have been previously linked, to date, the effects of calpain have been assigned to post-ligand binding events, outside-in signaling (Suzuki et al., 1992; Cooray et al., 1996). Our results suggest a potential role of calpain in agonist-induced activation of integrins, inside-out signaling.
In contrast to prothrombin, HUVEC adhesion to fibrinogen occurred in the absence of added agonists, and PMA treatment of the cells did not effect αVβ3-dependent adhesion to this protein. This observation provides the first direct evidence that different activation states of αVβ3 can discriminate between different ligands. While we cannot presume that cultured HUVEC necessarily present αVβ3 in a resting or basal state, it is clear that these cells adhere to fibrinogen, with or without additional stimulation, whereas interaction with prothrombin requires additional activation of the receptor. This distinction suggest that αVβ3 ligands may be classified as being activation-dependent or as activation-independent. Fibrinogen is an activation-independent ligand, and prothrombin represents the activation-dependent ligands. Of note, platelet adhesion to both prothrombin (Byzova and Plow, 1997) and fibrinogen (Savage et al., 1995) does not require activation of αIIbβ3, emphasizing the fine differences in the recognition specificity of these two β3 integrins.
Acknowledgments
This work was supported in part by National Institutes of Health grant HL54924. T.V. Byzova is the recipient of a fellowship from the American Heart Association, Northeast Ohio Affiliate.
Abbreviations used in this paper
- HAEC
human aortic endothelial cells
- HASMC
human aortic smooth muscle cells
- HUVEC
human umbilical vein endothelial cells
- PKC
protein kinase C
- RGD
Arg-Gly-Asp
References
- Al Z, Cohen CM. Phorbol 12-myristate 13-acetate-stimulated phosphorylation of erythrocyte membrane skeletal proteins is blocked by calpain inhibitors: possible role of protein kinase M. Biochem J. 1993;296:675–683. doi: 10.1042/bj2960675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altieri DC, Bader R, Mannucci PM, Edgington TS. Oligospecificity of the cellular adhesion receptor MAC-1 encompasses an inducible recognition specificity for fibrinogen. J Cell Biol. 1988;107:1893–1900. doi: 10.1083/jcb.107.5.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shavit R, Sabbah V, Lampugnani MG, Marchisio PC, Fenton JW, II, Vlodavsky I, Dejana E. An arg-gly-asp sequence within thrombin promotes endothelial cell adhesion. J Cell Biol. 1991;112:335–344. doi: 10.1083/jcb.112.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shavit R, Eskohjido Y, Fenton JW, II, Esko JD, Vlodavsky I. Thrombin adhesive properties: induction by plasmin and heparan sulfate. J Cell Biol. 1993;123:1279–1287. doi: 10.1083/jcb.123.5.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JS, Chan C, Vilaire G, Mousa SA, DeGrado WF. Agonist-activated αVβ3on platelets and lymphocytes binds to the matrix protein osteopontin. J Biol Chem. 1997;272:8137–8140. doi: 10.1074/jbc.272.13.8137. [DOI] [PubMed] [Google Scholar]
- Boarder MR, Hourani SM. The regulation of vascular function by P2 receptors: multiple sites and multiple receptors. Trends Pharmacol Sci. 1998;19:99–107. doi: 10.1016/s0165-6147(98)01170-5. [DOI] [PubMed] [Google Scholar]
- Bombeli T, Karsan A, Tait JF, Harlan JM. Apoptotic vascular endothelial cells become procoagulant. Blood. 1997;89:2429–2442. [PubMed] [Google Scholar]
- Brown SL, Lundgren CH, Nordt T, Fujii S. Stimulation of migration of human aortic smooth muscle cells by vitronectin: implications for atherosclerosis. Cardiovasc Res. 1994;28:1815–1820. doi: 10.1093/cvr/28.12.1815. [DOI] [PubMed] [Google Scholar]
- Byzova TV, Plow EF. Networking in the hemostatic system. Integrin αIIbβ3binds prothrombin and influences its activation. J Biol Chem. 1997;272:27183–27188. doi: 10.1074/jbc.272.43.27183. [DOI] [PubMed] [Google Scholar]
- Charo IF, Nannizzi L, Phillips DR, Hsu MA, Scarborough RM. Inhibition of fibrinogen binding to GP IIb-IIIa by a GP IIIa peptide. J Biol Chem. 1991;266:1415–1421. [PubMed] [Google Scholar]
- Cheresh DA. Human endothelial cells synthesize and express an Arg-Gly-Asp-directed adhesion receptor involved in attachment to fibrinogen and von Willebrand factor. Proc Natl Acad Sci USA. 1987;84:6471–6475. doi: 10.1073/pnas.84.18.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti G, Dominguez-Jimenez C, Zanetti A, Gimbrone MA, Jr, Cremona O, Marchisio PC, Dejana E. Human endothelial cells express integrin receptors on the luminal aspect of their membrane. Blood. 1992;80:437–446. [PubMed] [Google Scholar]
- Cooray P, Yuan Y, Schoenwaelder SM, Mitchell CA, Salem HH, Jackson SP. Focal adhesion kinase (pp125FAK) cleavage and regulation by calpain. Biochem J. 1996;318:41–47. doi: 10.1042/bj3180041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza SE, Byers-Ward VJ, Gardiner EE, Wang H, Sung S-S. Identification of an active sequence within the first immunoglobulin domain of intercellular cell adhesion molecule-1 (ICAM-1) that interacts with fibrinogen. J Biol Chem. 1996;271:24270–24277. doi: 10.1074/jbc.271.39.24270. [DOI] [PubMed] [Google Scholar]
- Danilov YN, Juliano RL. Phorbol ester modulation of integrin-mediated cell adhesion: a postreceptor event. J Cell Biol. 1989;108:1925–1933. doi: 10.1083/jcb.108.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwel GO, Hogervorst F, Sonnenberg A. Cleavage of the α6A subunit is essential for activation of the α6Aβ1integrin by phorbol 12-myristate 13-acetate. J Biol Chem. 1996;271:7293–7296. doi: 10.1074/jbc.271.13.7293. [DOI] [PubMed] [Google Scholar]
- Detmers PA, Wright SD, Olsen E, Kimball B, Cohn ZA. Aggregation of complement receptors on human neutrophils in the absence of ligand. J Cell Biol. 1987;105:1137–1145. doi: 10.1083/jcb.105.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Saido TC, Tsubuki S, Indig FE, Williams MJ, Ginsberg MH. Calpain cleavage of the cytoplasmic domain of the integrin beta 3 subunit. J Biol Chem. 1995;270:26146–26151. doi: 10.1074/jbc.270.44.26146. [DOI] [PubMed] [Google Scholar]
- Farrell DH, Thiagarajan P, Chung DW, Davie EW. Role of fibrinogen alpha and gamma chain sites in platelet aggregation. Proc Natl Acad Sci USA. 1992;89:10729–10732. doi: 10.1073/pnas.89.22.10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faull RJ, Kovach NL, Harlan JM, Ginsberg MH. Affinity modulation of integrin α5β1: regulation of the functional response by soluble fibronectin. J Cell Biol. 1993;121:155–162. doi: 10.1083/jcb.121.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felding-Habermann B, Cheresh DA. Vitronectin and its receptors. Curr Opin Cell Biol. 1993;5:864–868. doi: 10.1016/0955-0674(93)90036-p. [DOI] [PubMed] [Google Scholar]
- Glass II, W.F., and J.I. Kreisberg. Regulation of integrin-mediated adhesion at focal contacts by cyclic AMP. J Cell Physiol. 1993;157:296–306. doi: 10.1002/jcp.1041570212. [DOI] [PubMed] [Google Scholar]
- Hato T, Pampori N, Shattil SJ. Complementary roles for receptor clustering and conformational change in the adhesive and signaling functions of integrin αIIbβ3 . J Cell Biol. 1998;141:1685–1695. doi: 10.1083/jcb.141.7.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton MWC, Southward SMR, Serebrin SD, Kulczycky M, Blajchman MA. Catabolism of rabbit prothrombin in rabbits: uptake of prothrombin by the aorta wall before and after a de-endothelializing injury in vivo. J Lab Clin Med. 1995;126:521–529. [PubMed] [Google Scholar]
- Huttenlocher A, Palecek SP, Lu Q, Zhang W, Mellgren RL, Lauffenburger DA, Ginsberg MH, Horwitz AF. Regulation of cell migration by the calcium-dependent protease calpain. J Biol Chem. 1997;272:32719–32722. doi: 10.1074/jbc.272.52.32719. [DOI] [PubMed] [Google Scholar]
- Jordan, R.E., M.A. Mascelli, M.T. Nakada, and H.F. Weisman. 1997. Pharmacology and clinical development of abciximab (c7E3 Fab, ReoPro): a monoclonal antibody inhibitor of GPIIb/IIIa and αVβ3 In New Therapeutic Agents in Thrombosis and Thrombolysis. A.A. Sasahara and J. Loscalzo, editors. Marcel Dekker, Inc., New York. 291–313.
- Kishimoto A, Mikawa K, Hashimoto K, Yasuda I, Tanaka S, Tominaga M, Kuroda T, Nishizuka Y. Limited proteolysis of protein kinase C subspecies by calcium-dependent neutral protease (calpain) J Biol Chem. 1989;264:4088–4092. [PubMed] [Google Scholar]
- Kobayashi E, Nakano H, Morimoto M, Tamaoki T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1989;159:548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- Kucik DF, Dustin ML, Miller JM, Brown EJ. Adhesion-activating phorbol ester increases the mobility of leukocyte integrin LFA-1 in cultured lymphocytes. J Clin Invest. 1996;97:2139–2144. doi: 10.1172/JCI118651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Languino LR, Plescia J, Duperray A, Brian AA, Plow EF, Geltosky JE, Altieri DC. Fibrinogen mediates leukocyte adhesion to vascular endothelium through an ICAM-1-dependent pathway. Cell. 1993;73:1423–1434. doi: 10.1016/0092-8674(93)90367-y. [DOI] [PubMed] [Google Scholar]
- Marcus AJ, Broekman MJ, Drosopoulos JHF, Islam N, Alyonycheva TN, Safier LB, Hajjar KA, Posnett DN, Schoenborn MA, Schooley KA, et al. The endothelial cell ecto-ADPase responsible for inhibition of platelet function is CD39. J Clin Invest. 1997;99:1351–1360. doi: 10.1172/JCI119294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masumoto A, Hemler ME. Multiple activation states of VLA-4. Mechanistic differences between adhesion to CS1/fibronectin and to vascular cell adhesion molecule-1. J Biol Chem. 1993;268:228–234. [PubMed] [Google Scholar]
- Mazurov AV, Khaspekova SG, Byzova TV, Tikhomirov OY, Berndt MC, Steiner B, Kouns WC. Stimulation of platelet glycoprotein IIb-IIIa (αIIbβ3-integrin) functional activity by a monoclonal antibody to the N-terminal region of glycoprotein IIIa. FEBS Lett. 1996;391:84–88. doi: 10.1016/0014-5793(96)00709-0. [DOI] [PubMed] [Google Scholar]
- McBane II, R.D., R.S. Miller, N.L. Hassinger, J.H. Chesebro, Y. Nemerson, and W.G. Owen. Tissue prothrombin. Universal distribution in smooth muscle. Arterioscler Thromb Vasc Biol. 1997;17:2430–2436. doi: 10.1161/01.atv.17.11.2430. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Akiyama SK, Yamada KM. Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science. 1995;267:883–885. doi: 10.1126/science.7846531. [DOI] [PubMed] [Google Scholar]
- Mould AP, Akiyama SK, Humphries MJ. Regulation of integrin α5β1-fibronectin interactions by divalent cations—evidence for distinct classes of binding sites for Mn2+, Mg2+, and Ca2+ . J Biol Chem. 1995;270:26270–26277. doi: 10.1074/jbc.270.44.26270. [DOI] [PubMed] [Google Scholar]
- Moulder K, Roberts K, Shevach EM, Coligan JE. The mouse vitronectin receptor is a T cell activation antigen. J Exp Med. 1991;173:343–347. doi: 10.1084/jem.173.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy KS, Makhlouf GM. Coexpression of ligand-gated P2X and G protein-coupled P2Y receptors in smooth muscle. Preferential activation of P2Y receptors coupled to phospholipase C (PLC)-β1 via Gαq/11 and to PLC-β3 via Gβγi3 . J Biol Chem. 1998;273:4695–4704. doi: 10.1074/jbc.273.8.4695. [DOI] [PubMed] [Google Scholar]
- Nurden P, Savi P, Heilmann E, Bihour C, Herbert J-M, Maffrand J-P, Nurden A. An inherited bleeding disorder linked to a defective interaction between ADP and its receptor on platelets. Its influence on glycoprotein IIb-IIIa complex function. J Clin Invest. 1995;95:1612–1622. doi: 10.1172/JCI117835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier AJ, Kunicki T, Quaranta V. Activation of the integrin αvβ3involves a discrete cation-binding site that regulates conformation. J Biol Chem. 1996;271:1364–1370. doi: 10.1074/jbc.271.3.1364. [DOI] [PubMed] [Google Scholar]
- Plow EF, Srouji AH, Meyer D, Marguerie G, Ginsberg MH. Evidence that three adhesive proteins interact with common recognition site on activated platelets. J Biol Chem. 1984;259:5388–5391. [PubMed] [Google Scholar]
- Pober JS, Cotran RS. The role of endothelial cells in inflammation. Transplantation. 1990;50:537–544. doi: 10.1097/00007890-199010000-00001. [DOI] [PubMed] [Google Scholar]
- Pytela R, Pierschbacher MD, Ginsberg MH, Plow EF, Ruoslahti E. Platelet membrane glycoprotein IIb/IIIa: Member of a family of Arg-Gly-Asp-specific adhesion receptors. Science. 1986;231:1559–1562. doi: 10.1126/science.2420006. [DOI] [PubMed] [Google Scholar]
- Qi W, Loh E, Vilaire G, Bennett JS. Regulation of αIIbβ3function in human B lymphocytes. J Biol Chem. 1998;273:15271–15278. doi: 10.1074/jbc.273.24.15271. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- Sadhu C, Masinovsky B, Staunton DE. Differential regulation of chemoattractant-stimulated β2, β3 and β7integrin activity. J Immunol. 1998;160:5622–5628. [PubMed] [Google Scholar]
- Savage B, Bottini E, Ruggeri ZM. Interaction of integrin αIIbβ3with multiple fibrinogen domains during platelet adhesion. J Biol Chem. 1995;270:28812–28817. doi: 10.1074/jbc.270.48.28812. [DOI] [PubMed] [Google Scholar]
- Savage B, Saldívar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. 1996;84:289–297. doi: 10.1016/s0092-8674(00)80983-6. [DOI] [PubMed] [Google Scholar]
- Savill J, Dransfield I, Hogg N, Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 1990;343:170–173. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- Scarpati EM, Sadler JE. Regulation of endothelial cell coagulant properties. Modulation of tissue factor, plasminogen activator inhibitors, and thrombomodulin by phorbol 12-myristate 13-acetate and tumor necrosis factor. J Biol Chem. 1989;264:20705–20713. [PubMed] [Google Scholar]
- Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- Seiffert D, Smith JW. The cell adhesion domain in plasma vitronectin is cryptic. J Biol Chem. 1997;272:13705–13710. doi: 10.1074/jbc.272.21.13705. [DOI] [PubMed] [Google Scholar]
- Shattil SJ, Brass LF. Induction of the fibrinogen receptor on human platelets by intracellular mediators. J Biol Chem. 1987;262:992–1000. [PubMed] [Google Scholar]
- Shattil SJ, Ginsberg MH. Perspective series: cell adhesion in vascular biology. Integrin signaling in vascular biology. J Clin Invest. 1997;100:S91–S95. doi: 10.1172/JCI119500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EB, Staples EM. Haemostatic factors in human aortic intima. Lancet. 1981;1:1171–1174. doi: 10.1016/s0140-6736(81)92346-1. [DOI] [PubMed] [Google Scholar]
- Smith JW, Ruggeri ZM, Kunicki TJ, Cheresh DA. Interaction of integrins αvβ3and glycoprotein IIb-IIIa with fibrinogen. J Biol Chem. 1990a;265:12267–12271. [PubMed] [Google Scholar]
- Smith JW, Vestal DJ, Irwin SV, Burke TA, Cheresh DA. Purification and functional characterization of integrin αvβ5. An adhesion receptor for vitronectin. J Biol Chem. 1990b;265:11008–11013. [PubMed] [Google Scholar]
- Smith JW, Piotrowicz RS, Mathis D. A mechanism for divalent cation regulation of β3-integrins. J Biol Chem. 1994;269:960–967. [PubMed] [Google Scholar]
- Smith JW, Cheresh DA. Integrin (αvβ3)-ligand interaction. J Biol Chem. 1990;265:2168–2172. [PubMed] [Google Scholar]
- Stern DM, Esposito C, Gerlach H, Gerlach M, Ryan J, Handley D, Nawroth P. Endothelium and regulation of coagulation. Diabetes Care. 1991;14:160–166. doi: 10.2337/diacare.14.2.160. [DOI] [PubMed] [Google Scholar]
- Stubbs MT, Bode W. A player of many parts: the spotlight falls on thrombin structure. Thromb Res. 1993;69:1–58. doi: 10.1016/0049-3848(93)90002-6. [DOI] [PubMed] [Google Scholar]
- Suehiro K, Smith JW, Plow EF. The ligand recognition specificity of β3integrins. J Biol Chem. 1996;271:10365–10371. doi: 10.1074/jbc.271.17.10365. [DOI] [PubMed] [Google Scholar]
- Suehiro K, Gailit J, Plow EF. Fibrinogen is a ligand for integrin α5β1on endothelial cells. J Biol Chem. 1997;272:5360–5366. doi: 10.1074/jbc.272.8.5360. [DOI] [PubMed] [Google Scholar]
- Sueishi K, Ichikawa K, Nakagawa K, Kato K, Elsayed YA, Namoto M. Procoagulant properties of atherosclerotic aortas. Ann NY Acad Sci. 1995;748:185–192. doi: 10.1111/j.1749-6632.1994.tb17318.x. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Saido TC, Hirai S. Modulation of cellular signals by calpain. Ann NY Acad Sci. 1992;674:218–227. doi: 10.1111/j.1749-6632.1992.tb27490.x. [DOI] [PubMed] [Google Scholar]
- Taubman MB. Tissue factor regulation in vascular smooth muscle: a summary of studies performed using in vivo and in vitro models. Am J Cardiol. 1993;72:55C–60C. doi: 10.1016/0002-9149(93)90256-c. [DOI] [PubMed] [Google Scholar]
- Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- Tsujinaka T, Kajiwara Y, Kambayashi J, Sakon M, Higuchi N, Tanaka T, Mori T. Synthesis of a new cell penetrating calpain inhibitor (calpeptin) Biochem Biophys Res Commun. 1988;153:1201–1208. doi: 10.1016/s0006-291x(88)81355-x. [DOI] [PubMed] [Google Scholar]
- Vijayalakshmi J, Padmanabhan KP, Mann KG, Tulinsky A. The isomorphous structures of prethrombin2, hirugen-, and PPACK-thrombin: changes accompanying activation and exosite binding to thrombin. Protein Sci. 1994;3:2254–2271. doi: 10.1002/pro.5560031211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuori K, Ruoslahti E. Activation of protein kinase C precedes α5β1integrin-mediated cell spreading on fibronectin. J Biol Chem. 1993;268:21459–21462. [PubMed] [Google Scholar]