Abstract
Histone variant macroH2A1 (macroH2A1) contains an NH2-terminal domain that is highly similar to core histone H2A and a larger COOH-terminal domain of unknown function. MacroH2A1 is expressed at similar levels in male and female embryonic stem (ES) cells and adult tissues, but a portion of total macroH2A1 protein localizes to the inactive X chromosomes (Xi) of differentiated female cells in concentrations called macrochromatin bodies. Here, we show that centrosomes of undifferentiated male and female ES cells harbor a substantial store of macroH2A1 as a nonchromatin-associated pool. Greater than 95% of centrosomes from undifferentiated ES cells contain macroH2A1. Cell fractionation experiments confirmed that macroH2A1 resides at a pericentrosomal location in close proximity to the known centrosomal proteins γ-tubulin and Skp1. Retention of macroH2A1 at centrosomes was partially labile in the presence of nocodazole suggesting that intact microtubules are necessary for accumulation of macroH2A1 at centrosomes. Upon differentiation of female ES cells, Xist RNA expression became upregulated and monoallelic as judged by fluorescent in situ hybridization, but early Xist signals lacked associated macroH2A1. Xi acquired macroH2A1 soon thereafter as indicated by the colocalization of Xist RNA and macroH2A1. Accumulation of macroH2A1 on X chromosomes occurred with a corresponding loss of centrosomal macroH2A1. Our results define a sequence for the loading of macroH2A1 on the Xi and place this event in the context of differentiation and Xist expression. Furthermore, these results suggest a role for the centrosome in the X inactivation process.
Keywords: Xist, stem cells, chromatin, microtubule, dosage compensation
Introduction
Recent interest has focused on the histone variant macro H2A1 because it is specifically enriched on inactive X chromosomes (Xi) of female mammalian cells. MacroH2A1 forms macrochromatin bodies (MCBs), which are discrete macroH2A1-containing accumulations that colocalize with inactive, but not active, X chromosomes (Costanzi and Pehrson 1998; Costanzi et al. 2000). However, macroH2A1 is likely required for functions in addition to X inactivation because overall macroH2A1 expression is similar for male and female adult and embryonic cells, at the level of RNA (Rasmussen et al. 1999) and protein (Mermoud et al. 1999). The nonsex chromosome-specific portion of the macroH2A1 intracellular pool is characterized by diffuse, low-level nuclear staining that is distinct from MCBs. The association of macroH2A1 with the Xi becomes undetectable in differentiated cells that lack Xist RNA (a known component of Xi). This indicates that the localization of macroH2A1 to the Xi may require Xist RNA in some fashion (Csankovszki et al. 1999).
Histone macroH2A1 has an unusual domain structure that may account for its nonuniform distribution. The NH2-terminal domain contains a region that is colinear with and 64% identical to a normal core H2A histone (Pehrson and Fried 1992). MacroH2A1 differs from a conventional core H2A molecule due to the presence of a large nonhistone domain of unknown function that lies COOH-terminal to the H2A core domain (Pehrson and Fried 1992; Vijay-Kumar et al. 1995). The nonhistone domain of macroH2A1 contains two alternative leucine zippers generated by alternative splicing of primary transcripts (Costanzi and Pehrson 1998; Rasmussen et al. 1999) suggesting that the nonhistone domain may function in protein–protein interactions.
In the differentiated cells of female mammals, one of two X chromosomes is transcriptionally silenced so that the dosage of X-linked gene expression is similar to that in males. Dosage compensation occurs through an ordered process that insures that only a single X chromosome remains active for each diploid set of autosomes. Proper X inactivation requires the action of the Xist gene, which is expressed from the X-inactivation center (XIC) of the Xi (Brockdorff et al. 1991; Brown et al. 1991a,Brown et al. 1991b). Targeted mutation of Xist results in failed X inactivation and dosage compensation both in cell culture (Penny et al. 1996) and in vivo (Marahrens et al. 1997), but continued Xist expression is not required for maintenance of the inactive state (Brown and Willard 1994; Rack et al. 1994; Csankovszki et al. 1999).
Female ES cells provide a useful cell culture model for the X inactivation process because they undergo the X inactivation process upon differentiation (Martin et al. 1978; Keohane et al. 1996; Lee et al. 1996; Mermoud et al. 1999). Undifferentiated female ES cells contain two transcriptionally active X chromosomes and exhibit biallelic expression of Xist consisting of two pinpoints of unstable Xist RNA that mark the sites of transcription. Upon differentiation, one of two X chromosomes is chosen for inactivation and Xist expression is silenced from the Xist allele present on the future active X chromosome. The Xi is characterized by vastly upregulated expression of Xist RNA that spreads to coat the Xi. Inactivated X chromosomes are therefore marked by a large cloud of stable Xist RNA. Recently, it has been shown that a single macro H2A1-containing body (referred to as an MCB) can be detected in undifferentiated ES cells of both sexes (Mermoud et al. 1999). These bodies fail to colocalize with Xist RNA signals in cells entering differentiation, but after prolonged differentiation, macro H2A1-staining bodies and Xist RNA signals become colocalized.
Here, we report the unexpected finding that macro H2A1 exists in undifferentiated ES cells as prominent focal accumulations centered on centrosomes. This association is labile in the presence of the microtubule-disrupting drug nocodazole in a dose-dependent fashion. Upon differentiation, Xist RNA is first upregulated on the future Xi, and soon thereafter macroH2A1 is recruited to form MCBs. This process occurs in conjunction with a diminution of centrosomal-associated macroH2A1. Therefore, macroH2A1 localization exhibits a sequential shift from a centrosomal to an inactive X chromosomal location during the differentiation and X inactivation processes.
Materials and Methods
Cell Culture
ES cells were grown without feeder cells in standard ES cell medium containing 1,000 U/ml leukemia inhibitory factor. Differentiation was induced by plating ES cells at low density in DME with 15% FCS without leukemia inhibitory factor in the presence of all-trans retinoic acid at a final concentration of 10−7 M as described previously (Wutz and Jaenisch 2000). ES cell lines J1 (male), 2-1, and 2-2 (female) were described previously (Rasmussen et al. 1999).
Centrosome Preparation
Centrosomes were prepared by the method of Mitchison and Kirschner 1986 with the modifications of Bornens et al. 1987. Cells harvested for centrosome preparation were pretreated with nocodazole at 10 μg/ml or 2 μg/ml and cytochalasin B at 5 μg/ml in ES cell medium for 1 h before harvest. Centrosome-containing lysates were cleared of cell debris by centrifugation at 2,750 rpm in a Beckman JS-13 rotor for 15 min at 4°C, and were then cleared of residual chromatin via DNase I digestion. The resulting preparations were layered onto sucrose step gradients containing 40, 50, and 70% sucrose fractions and were centrifuged at 27,000 rpm for 1 h in an SW-27 rotor. Gradients were eluted from the bottom by peristaltic pump and frozen at −80°C for later analysis.
Immunofluorescence, Quantitative Centrosome Immunofluorescence, and Fluorescent In Situ Hybridization Methods
Immunofluorescence on whole ES cells was performed using standard methods. Antibodies specific for macroH2A1 and Skp1 were described previously (Costanzi and Pehrson 1998; Freed et al. 1999). Antibodies specific for γ- and α-tubulins were purchased from Sigma-Aldrich (catalog nos T-6557 and T9026). Quantitative centrosome immunofluorescence (QCIF) was performed as described (Bornens et al. 1987). In brief, 15 μl samples from each sucrose gradient fraction (generated by the centrosome purification method outlined above) were mixed with 3 ml of buffer PE (10 mM Pipes, pH7.2, 1 mM EDTA, and 8 mM β-mercaptoethanol). Centrosomes were then quantitatively sedimented onto round poly-lysine–coated coverslips by centrifugation in a Beckman JS-13 swinging bucket rotor at 13,000 rpm for 15 min at 4°C. The number of centrosomes per 100× oil immersion microscopic field were then counted for 10 randomly chosen fields for each coverslip and subjected to statistical analysis to generate means and SDs based on Poisson distributed data. Images were collected by fluorescence microscopy and processed using OpenLab™ software. Fluorescent in situ hybridization (FISH) for Xist was performed as described (Panning et al. 1997). Combined Xist FISH and immunofluorescence was performed by first performing Xist FISH, a second round of fixation in paraformaldehyde, followed by standard immunofluorescence.
Results
MacroH2A1 Accumulation Is Centered on Centrosomes of Undifferentiated ES Cells
The intracellular localization of macroH2A1 in undifferentiated ES cells was investigated with indirect immunofluorescence using an affinity-purified polyclonal antibody (Costanzi and Pehrson 1998). In interphase cells, macro H2A1 protein was highly concentrated in single discrete focal accumulations that were distinct from a lower level diffuse particulate nuclear staining. Double immunofluorescence using macroH2A1 antibody and an antibody that marks γ-tubulin (an established marker for centrosomes (Stearns et al. 1991; Zheng et al. 1991) showed that the macroH2A1 concentrations are centered upon centrosomes in both male and female undifferentiated ES cells (Fig. 1 a). Centrosomal macroH2A1 was found in several other independently derived male and female ES cell lines (data not shown). MacroH2A1 remained associated with the two centrosomes present at the poles of mitotic spindles (Fig. 1 b). Greater than 95% of centrosomes (as identified by γ-tubulin immunofluorescence) showed substantial concentrations of macroH2A1. The affinity-purified antibody used in these studies (Costanzi and Pehrson 1998) identified a single band of 42-kD in protein extracted from undifferentiated ES cells (Fig. 1 c), which is the size predicted from mouse macroH2A1cDNAs (Rasmussen et al. 1999).
Figure 1.
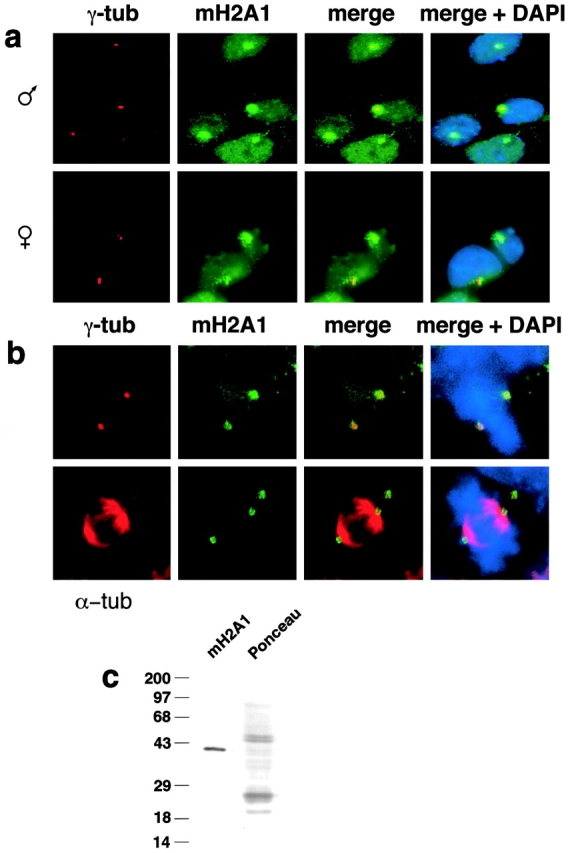
MacroH2A1 expression and intracellular location in undifferentiated male and female ES cells. a, MacroH2A1 localizes to centrosomes in interphase J1 male ES cells and 2-1 female ES cells. Centrosomes were marked with anti–γ-tubulin immunostaining and macro H2A1 was identified with an affinity-purified polyclonal antibody (Costanzi and Pehrson 1998). b, macroH2A1 remains associated with centrosomes in mitotic ES cells. Metaphase J1 ES cells stained with antibodies specific for either γ-tubulin or α-tubulin and antimacroH2A1 antibodies. c, Antimacro H2A1 immunoblot of total protein extracted from undifferentiated male J1 ES cells.
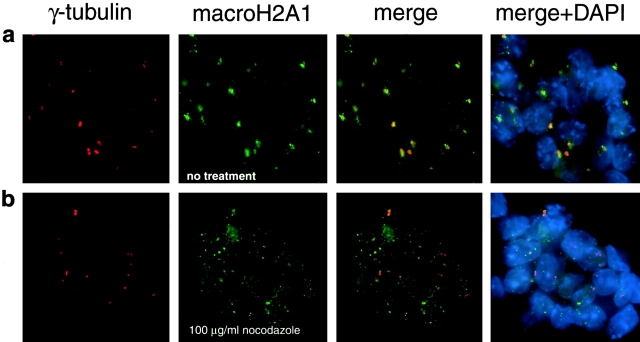
Because centrosomes are the nucleation centers for microtubule formation, undifferentiated ES cells were treated with a high concentration of nocodazole (100 μg/ml for 1 h) to see if focal centers of macroH2A1 accumulation require intact microtubules. This treatment resulted in a dramatic disaggregation of centrosomal macroH2A1 (Fig. 2), which caused macroH2A1 to assume a dispersed particulate distribution. This result indicated that intact microtubules are necessary for the retention of macro H2A1 at centrosomes in undifferentiated ES cells.
Figure 2.
Centrosomal accumulations of macroH2A1 are labile in the presence of the microtubule depolymerizing agent nocodazole. a, A colony of undifferentiated J1 ES cells immunostained for γ-tubulin (centrosomes) and macroH2A1. b, A colony of undifferentiated J1 ES cells treated for 1 h in the presence of nocodazole (100 μg/ml) before double immunofluorescence as in a.
Established methods for the purification of centrosomes (Mitchison and Kirschner 1986; Bornens et al. 1987) were used to corroborate the authenticity of the association of macroH2A1 with centrosomes in undifferentiated ES cells. These methods require pretreatment of cells with nocodazole and cytochalasin B to release centrosomes from their attachments to the nuclear membrane so that they can be isolated by sucrose gradient velocity centrifugation. Because centrosomal macroH2A1 accumulations are labile in the presence of a high concentrations of nocodazole (Fig. 2), we attempted centrosome purification at standard and reduced concentrations (10 μg/ml and 2 μg/ml, respectively) during pretreatment. Both pretreatment conditions allowed for the isolation of centrosomes with attached macroH2A1 (Fig. 3 a). Centrosomes isolated from cells pretreated with 2 μg/ml nocodazole retained far more macroH2A1 than centrosomes from cells pretreated with 10 μg/ml nocodazole. Centrosomes were easily identified by a characteristic paired structure (detected by γ-tubulin immunofluorescence) that results from the presence of two centrioles within each centrosome (Mitchison and Kirschner 1986; Bornens et al. 1987). In general, centrosomes from higher (less dense) sucrose fractions contained more associated macroH2A1 than centrosomes from lower fractions, though the amount of signal was greatly influenced by the degree of nocodazole pretreatment. The paired structures observed by γ-tubulin immunofluorescence were confirmed to be centrosomes by the colocalization of γ-tubulin with Skp1 immunostaining (Fig. 3 b). Skp1, like γ-tubulin, is a confirmed component of centrosomes (Freed et al. 1999).
Figure 3.
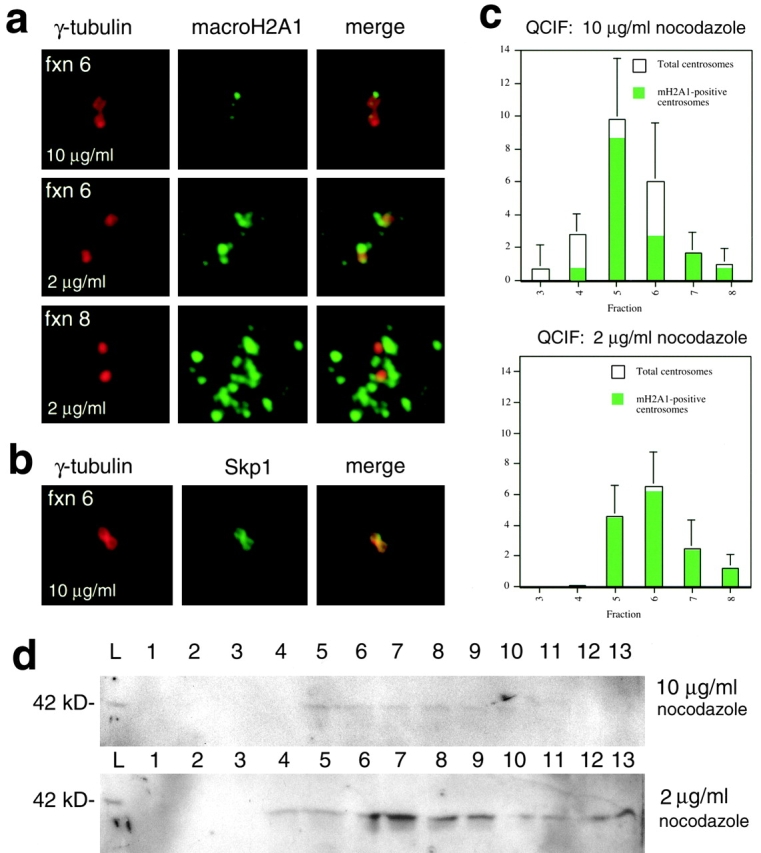
Quantitative analysis of centrosomes obtained by cell fractionation of undifferentiated ES cells. a, Typical examples of isolated centrosomes analyzed by γ-tubulin/macroH2A1 double immunofluorescence from fraction 6 (10 μg/ml or 2 μg/ml nocodazole pretreatment) and fraction 8 (2 μg/ml nocodazole pretreatment). b, Typical example of centrosome isolated from fraction 6 (10 μg/ml nocodazole pretreatment) immunostained for γ-tubulin and Skp1 (both established components of centrosomes). c, QCIF (Bornens et al. 1987; Mitchison and Kirschner 1986) of undifferentiated ES cells pretreated with nocodazole for 1 h at 10 μg/ml and 2 μg/ml. Identical quantities of input cells were used in both fractionations. Total centrosomes from 15 μl samples from each sucrose fraction were quantitatively sedimented onto round coverslips and the mean number of centrosomes (detected by γ-tubulin immunofluorescence) per 100× oil immersion field was determined. Green areas within bars represent the relative proportions of centrosomes that contained macroH2A1 anchored to centrosomes. Data from fractions 3–8 (fractions with significant numbers of centrosomes) are shown. Lower fraction numbers correspond to higher sucrose concentrations. d, Immunoblot of macroH2A1 present in sucrose fractions. Fractions are numbered from 1 (bottom of gradient) to 13. The lanes marked L contain samples of equal volume from the initial lysates later subjected to sucrose ultracentrifugation. Equal numbers of input cells were used for both fractionations (10 μg/ml and 2 μg/ml nocodazole pretreatments).
The relative quantities of centrosomes in each sucrose fraction were determined with QCIF, (Mitchison and Kirschner 1986; Bornens et al. 1987). Centrosomes from each sucrose fraction were quantitatively sedimented onto round coverslips. Coverslips were then subjected to double immunofluorescence with anti–γ-tubulin and antimacroH2A1 antibodies. The relative numbers of centrosomes present in each fraction was determined by counting centrosomes on 10 randomly chosen 100× oil immersion fields (Fig. 3 c). More centrosomes were isolated from cells pretreated with 10 μg/ml nocodazole, but a lower proportion of these centrosomes retained associated macroH2A1. Though the absolute yield of centrosomes from cells pretreated with 2 μg/ml nocodazole was slightly reduced, virtually all of these centrosomes contained associated macroH2A1. Samples of each sucrose fraction were subjected to immunoblotting using the macroH2A1 antibody (Fig. 3 d). The distribution of macroH2A1 detected by immunoblotting of sucrose fractions corresponded well with the relative quantities of centrosomes as judged by QCIF. The absolute yield of macroH2A1 detected by Western analysis was greatly enhanced by the reduced pretreatment with nocodazole (Fig. 3 d).
Recruitment of MacroH2A1 to the Inactive X Chromosome
We were surprised to find macroH2A1, a protein previously thought to associate only with chromatin, concentrated in a focal center around the centrosome, an organelle devoid of chromatin. We therefore carried out an analysis of macroH2A1 localization in female ES cells using retinoic acid-induced differentiation (Wutz and Jaenisch 2000), which causes cells to undergo differentiation and X inactivation. Differentiating cells were assayed for changes in the relative positions of centrosomes (detected by γ-tubulin immunofluorescence), Xist RNA expression (detected by FISH) and macroH2A1 location (detected by immunofluorescence). We subjected such differentiating cultures to three independent tests: γ-tubulin immunofluorescence combined with Xist FISH; macroH2A1 immunofluorescence combined with Xist FISH; and γ-tubulin and macroH2A1 double immunofluorescence. These experiments revealed the timing of association of macro H2A1 with the Xi during the X inactivation process.
As expected, biallelic pinpoints of expression of Xist RNA were detected from both X chromosomes in undifferentiated female ES cells, a pattern of Xist expression indicative of a preinactivation state for X chromosomes (Panning et al. 1997). The positions of Xist signals and centrosomes (γ-tubulin staining) were spatially separate in these cells (Fig. 4 a). MacroH2A1 accumulation was also spatially separate from the location of Xist RNA signals, but macroH2A1 immunostaining invariably overlapped the positions of centrosomes as visualized by γ-tubulin immunofluorescence (Fig. 4 a). We termed such undifferentiated cells class 1, characterized by centrosomal macro H2A1 and biallelic double pinpoint Xist signals.
Figure 4.
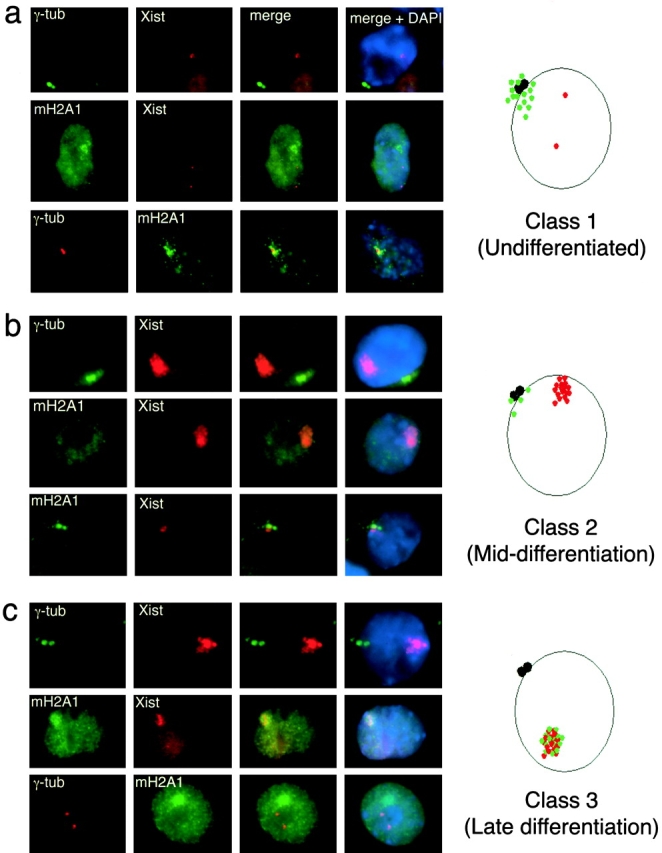
Analysis of macroH2A1 and X inactivation in differentiating 2-1 female ES cells. a, Undifferentiated female ES cells (Class 1, day 0). Xist RNA exhibited early biallelic expression characteristic of the pre–X-inactivation state. MacroH2A1 was localized in focal accumulations centered on centrosomes. MacroH2A1 and Xist occupied separate locations. b, Differentiating female ES cells (Class 2, days 3–6 of differentiation). Stable Xist was observed as a distinctive nuclear cloud that was separated in space from centrosomes. Xist RNA signals lacked associated macroH2A1, though both signals were often in close proximity. Two representative examples of immuno-FISH for macroH2A1 and Xist RNA are shown. c, Differentiated ES cells (Class 3, days 3–12). Xist RNA and macroH2A1 colocalized in MCBs that were distinct from centrosomes.
After three days of differentiation, the cells acquired a fibroblast-like morphology and no longer formed colonies like undifferentiated ES cells (data not shown). No class 1 cells were detected and most cells contained intense monoallelic Xist FISH signals indicative of X chromosomes undergoing the X inactivation process (Fig. 4 b). Most cells contained robust Xist FISH signals that were distinct from centrosomes and lacked associated macro H2A1concentrations (Fig. 4 b). Centrosomal macroH2A1 became increasingly difficult to detect, but when observed, centrosomal macroH2A1 immunostaining was markedly less intense as compared with that observed in undifferentiated ES cells and often occurred in close proximity to Xist signals. We termed differentiating cells that contain intense monoallelic Xist FISH signals that are devoid of macroH2A1 concentrations class 2.
At day 3 and later, we began to observe a significant proportion of cells that contained well defined Xist signals that had acquired concentrations of macroH2A1. These cells, therefore, contained bona fide MCBs as evidenced by a confirmed association of macroH2A1 with X chromosomes. We termed these cells class 3 (Fig. 4 c).
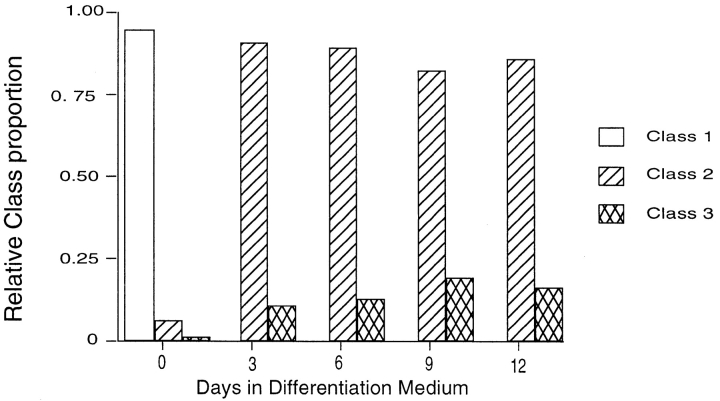
Though all combinations of immunofluorescence and FISH using two antibodies (directed against macroH2A1 and γ-tubulin), and one FISH probe (directed against Xist RNA) were performed, class 1, 2, and 3 cells could be easily distinguished from one another by Xist FISH combined with macroH2A1 immunofluorescence. This allowed us to quantitate the relative proportions of cells in each of the three classes during the course of retinoic acid-induced differentiation (Fig. 5). 200 cells were typed as class 1, 2, or 3 from female ES cells undergoing retinoic acid induced differentiation for 0, 3, 6, 9, and 12 d. Cells were ascertained by first looking for Xist expression (Cy3 channel), and then analyzed for their macroH2A1 distribution (FITC channel). The relative proportions of class 1, 2, and 3 cells were determined for each time point on slides whose identity was blinded, and after counting was complete, the results were plotted versus time in differentiation medium (Fig. 5). The proportion of class 3 cells increased at the expense of class 2 cells during the differentiation process.
Figure 5.
Quantitation of changes in localization of macroH2A1 with respect to the centrosome and the Xi during retinoic acid induced differentiation and X inactivation. Quantitation of relative proportions of class 1, 2, and 3 cells in differentiating female ES cell cultures. At least 200 cells were counted from each sample. Class 1, Biallelic expression of Xist RNA with centrosomic macroH2A1. Class 2, monoallelic cloud-like Xist RNA expression without macroH2A1 colocalization. Class 3, monoallelic cloud-like Xist RNA expression with discrete macroH2A1 colocalization.
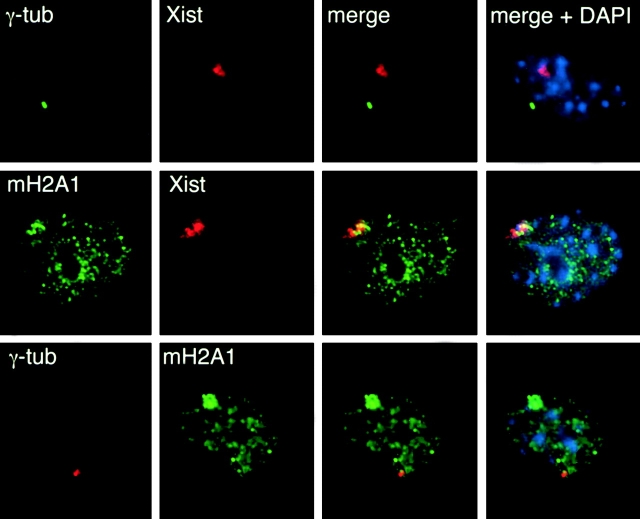
We wished to investigate whether or not class 3 cells resembled differentiated female somatic cells. Mouse embryonic fibroblasts (MEFs) from female E13.5 mouse embryos were analyzed for their content of macroH2A1, Xist RNA, and γ-tubulin. Female MEFs resembled class 3 differentiated female ES cells as judged by the presence of MCBs characterized by colocalized Xist RNA and macro H2A1 staining (Fig. 6). The positions of MCBs were independent of the positions of centrosomes, similar to late class 3 cells. MCBs also occurred in fibroblasts derived from adult female mouse ears and in a mouse mammary carcinoma cell line called X3 with a stable XXX karyotype (Wutz and Jaenisch 2000). X3 cells contained 2 MCBs per cell, indicating dosage compensation for an additional X chromosome. We conclude that class 3 differentiated female ES cells resemble female somatic cells differentiated in vivo.
Figure 6.
Analysis of MCBs and centrosomes in female MEFs. Fibroblasts were derived from a day 13.5 female embryo and subjected to γ-tubulin immunofluorescence combined with Xist FISH (top), macroH2A1 immunofluorescence combined with Xist FISH (middle), and γ-tubulin and macroH2A1 double immunofluorescence (bottom). Representative cells from each treatment are shown.
Discussion
Centrosomal Centers of MacroH2A1 Accumulation
Our results detail the unexpected finding that macroH2A1 accumulates around centrosomes in undifferentiated ES cells. This is surprising since chromatin, the usual site of histone accumulation, is not thought to be directly associated with centrosomes. MacroH2A1 is an unusual histone, however, and the outstanding feature of macroH2A1 is the presence of a large COOH-terminal extension: the nonhistone domain. This domain may be responsible for the unusual distribution of macroH2A1. The nonhistone domain could include a site for centrosome docking. Alternatively, the nonhistone domain might contain sites for microtubule attachment. If so, microtubule-associated motor proteins might be responsible for accumulation and/or retention of macroH2A1 at the centrosome.
Several lines of evidence support the conclusion that macroH2A1 is truly associated with centrosomes in undifferentiated ES cells. The affinity-purified antibody used in these studies to detect macroH2A1 is highly specific for macroH2A1 as judged by Western analysis (Fig. 1 c). We detected macroH2A1 (42 kD) by Western blotting in fractions highly enriched for purified centrosomes (Fig. 3 d). The distribution of macroH2A1 in these fractions agreed well with the distribution of centrosomes in these fractions. Western blotting showed that the yield of macro H2A1 in fractions containing centrosomes was greatly enhanced at a lower level of nocodazole pretreatment (Fig. 3 d). This finding parallels the nocodazole lability of the macroH2A1 centrosomal signal observed by immunofluorescence (Fig. 2). MacroH2A1 cosedimented with Skp1 and γ-tubulin, both established components of centrosomes (Fig. 3, a, b, c). Centrosomal staining of macroH2A1 diminished and eventually disappeared upon differentiation, whereas the signal of macroH2A1 at Xi increased.
Numerous studies have shown that centrosomes are the major cellular centers for nucleation of microtubules. The effect of nocodazole on the retention of macroH2A1 suggests a mechanism for the retention of macroH2A1 at centrosomes. Nocodazole is a specific inhibitor of microtubule polymerization. Since the centrosomal accumulation of macroH2A1 is labile in the presence of nocodazole, it seems reasonable that it is retained at centrosomes by virtue of microtubular associations. The amount of macro H2A1 present at centrosomes responds to nocodazole in a dose-dependent fashion and the degree of nocodazole pretreatment had a profound effect on the amount of macroH2A1 detected around purified centrosomes by immunofluorescence (Fig. 2 and Fig. 3). A reduced treatment with nocodazole allowed us to purify centrosomes that retained an extensive network of associated macroH2A1 (Fig. 3 a). Centrosomes with extensive arrays of macro H2A1 exhibited reduced mobility during velocity gradient centrifugation in sucrose. Under these pretreatment conditions, virtually 100% of purified centrosomes retained associated macroH2A1. In contrast, centrosomes isolated from cells pretreated with 10 μg/ml nocodazole had reduced levels of associated macroH2A1 (Fig. 3), and many compact centrosomes in fractions 3 and 4 were stripped of detectable macroH2A1 (Fig. 3 c). These results strongly suggest that intact microtubules are necessary for the retention of macroH2A1 at centrosomes.
Possible Involvement of Centrosomes in the X Inactivation Process
We can envision two possible explanations for the finding that macroH2A1 resides at centrosomes in undifferentiated ES cells. It is possible that macroH2A1 has some as yet unidentified function at the centrosome, per se. Alternatively, the centrosome may represent a storage site for macroH2A1 in undifferentiated ES cells. We favor the second possibility for a number of reasons. Our results show that macroH2A1 accumulated as MCBs that are associated with Xist RNA signals upon differentiation and concomitant X inactivation. This process occurred at the expense of centrosomal macroH2A1. Therefore, if macro H2A1 has a direct role in centrosome function, this role must be restricted to the undifferentiated state. It seems more likely that macroH2A1 is stored at centrosomes before its incorporation into core nucleosomes of the inactive X. We can envision two potential mechanisms for transfer of macroH2A1 from centrosomes to Xi: macro H2A1 might be transported to the X chromosome during interphase by a novel mechanism; or macroH2A1 might transfer to X chromosomes via microtubules during mitosis when the nuclear membrane is disassembled and microtubules directly connect centrosomes to chromosomes. This second mechanism would not preclude a role for cytoplasmic microtubules in the transport or concentration of macroH2A1 at centrosomes. Future investigations of such potential mechanisms may yield insights into the processes by which chromatin components are targeted to the nucleus. In addition, our results suggest a novel role for centrosomes in nuclear organization.
The timing of acquisition of macroH2A1 by inactivating X chromosomes gives clues as to the possible function of macroH2A1 in the X inactivation process. X inactivation is complete in somatic female cells such as fibroblasts. In such cells macroH2A1 is present as MCBs that colocalize with the inactive X, but not the active X chromosome (Costanzi and Pehrson 1998). The situation in undifferentiated ES cells (those before the onset of X inactivation) is markedly different. In undifferentiated male and female ES cells, macroH2A1 is detected as a discrete focal accumulation (previously referred to as an MCB) that is not associated with X chromosomes (Mermoud et al. 1999). Our work showed that single focal centers of macroH2A1 staining in undifferentiated ES cells consist of centrosomal concentrations of macroH2A1 and are not true MCBs. During the course of female ES cell differentiation, Xist RNA becomes detectable as a discrete cloud that is initially devoid of macroH2A1 (Mermoud et al. 1999; this study). Only later does the Xist cloud (which marks the inactivating X chromosome) acquire macroH2A1, thus forming an MCB. The macroH2A1 focal accumulation in male ES cells gradually dissipates upon differentiation (Mermoud et al. 1999; our unpublished results). Recently, male ES cells have been developed that harbor an inducible autosomal Xist trans-gene. After three days of continuous Xist expression in differentiating ES cells, Xist-mediated gene silencing switches from a reversible to an irreversible state (Wutz and Jaenisch 2000). This is the time at which we observed the initial formation of MCBs using identical differentiation methods. Although this is only a temporal correlation, our observations raise the possibility that acquisition of macroH2A1 may be involved in the locking in of the inactive state. Therefore, macroH2A1 may be involved in the establishment of the inactive state, but it seems unlikely that it is required for maintenance of X inactivation. This is because X-linked genes remain silenced in cells that lack Xist and MCBs as a consequence of deletion of Xist (Csankovszki et al. 1999).
Acknowledgments
We wish to thank Peter Jackson for providing Skp1 antibodies and Cathrin Brisken for X3 cells. We thank Anton Wutz, Gyorgyi Csankovzski, and Joost Gribnau for critical readings of this work. This work was conducted using the W.M. Keck Foundation Biological Imaging Facility at the Whitehead Institute.
T. Rasmussen was supported by the National Institutes of Health fellowship GM19510. A. Eden was supported by the European Molecular Biology Organization fellowship ALTF 43-1999. R. Jaenisch was supported by National Institutes of Health/National Cancer Institute grant 5-R35-CA44339.
Footnotes
Abbreviations used in this paper: ES cell, embryonic stem cell; FISH, fluorescent in situ hybridization; MCB, macrochromatin body; MEF, mouse embryonic fibroblast; QCIF, quantitative centrosome immunofluorescence; Xi, inactive X chromosomes.
References
- Bornens M., Paintrand M., Berges J., Marty M.C., Karsenti E. Structural and chemical characterization of isolated centrosomes. Cell Motil. Cytoskel. 1987;8:238–249. doi: 10.1002/cm.970080305. [DOI] [PubMed] [Google Scholar]
- Brockdorff N., Ashworth A., Kay G.F., Cooper P., Smith S., McCabe V.M., Norris D.P., Penny G.D., Patel D., Rastan S. Conservation of position and exclusive expression of mouse Xist from the inactive X chromosome. Nature. 1991;351:329–331. doi: 10.1038/351329a0. [DOI] [PubMed] [Google Scholar]
- Brown C.J., Willard H.F. The human X-inactivation centre is not required for maintenance of X-chromosome inactivation. Nature. 1994;368:154–156. doi: 10.1038/368154a0. [DOI] [PubMed] [Google Scholar]
- Brown C.J., Ballabio A., Rupert J.L., Lafreniere R.G., Grompe M., Tonlorenzi R., Willard H.F. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome Nature 349 1991. 38 44a [DOI] [PubMed] [Google Scholar]
- Brown C.J., Lafreniere R.G., Powers V.E., Sebastio G., Ballabio A., Pettigrew A.L., Ledbetter D.H., Levy E., Craig I.W., Willard H.F. Localization of the X inactivation centre on the human X chromosome in Xq13 Nature 349 1991. 82 84b [DOI] [PubMed] [Google Scholar]
- Costanzi C., Pehrson J.R. Histone macroH2A1 is concentrated in the inactive X chromosome of female mammals. Nature. 1998;393:599–601. doi: 10.1038/31275. [DOI] [PubMed] [Google Scholar]
- Costanzi C., Stein P., Worrad D.M., Schultz R.M., Pehrson J.R. Histone macroH2A1 is concentrated in the inactive X chromosome of female preimplantation mouse embryos. Development. 2000;127:2283–2289. doi: 10.1242/dev.127.11.2283. [DOI] [PubMed] [Google Scholar]
- Csankovszki G., Panning B., Bates B., Pehrson J.R., Jaenisch R. Conditional deletion of Xist disrupts histone macroH2A localization but not maintenance of X inactivation. Nat. Genet. 1999;22:323–324. doi: 10.1038/11887. [DOI] [PubMed] [Google Scholar]
- Freed E., Lacey K.R., Huie P., Lyapina S.A., Deshaies R.J., Stearns T., Jackson P.K. Components of an SCF ubiquitin ligase localize to the centrosome and regulate the centrosome duplication cycle. Genes Dev. 1999;13:2242–2257. doi: 10.1101/gad.13.17.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keohane A.M., O'Neill L P., Belyaev N.D., Lavender J.S., Turner B.M. X-Inactivation and histone H4 acetylation in embryonic stem cells. Dev. Biol. 1996;180:618–630. doi: 10.1006/dbio.1996.0333. [DOI] [PubMed] [Google Scholar]
- Lee J.T., Strauss W.M., Dausman J.A., Jaenisch R. A 450 kb transgene displays properties of the mammalian X-inactivation center. Cell. 1996;86:83–94. doi: 10.1016/s0092-8674(00)80079-3. [DOI] [PubMed] [Google Scholar]
- Marahrens Y., Panning B., Dausman J., Strauss W., Jaenisch R. Xist-deficient mice are defective in dosage compensation but not spermatogenesis. Genes Dev. 1997;11:156–166. doi: 10.1101/gad.11.2.156. [DOI] [PubMed] [Google Scholar]
- Martin G.R., Epstein C.J., Travis B., Tucker G., Yatziv S., Martin D.W., Jr., Clift S., Cohen S. X-chromosome inactivation during differentiation of female teratocarcinoma stem cells in vitro. Nature. 1978;271:329–333. doi: 10.1038/271329a0. [DOI] [PubMed] [Google Scholar]
- Mermoud J.E., Costanzi C., Pehrson J.R., Brockdorff N. Histone macroH2A1.2 relocates to the inactive X chromosome after initiation and propagation of X-inactivation. J. Cell Biol. 1999;147:1399–1408. doi: 10.1083/jcb.147.7.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T.J., Kirschner M.W. Isolation of mammalian centrosomes. Methods Enzymol. 1986;134:261–268. doi: 10.1016/0076-6879(86)34094-1. [DOI] [PubMed] [Google Scholar]
- Panning B., Dausman J., Jaenisch R. X chromosome inactivation is mediated by Xist RNA stabilization. Cell. 1997;90:907–916. doi: 10.1016/s0092-8674(00)80355-4. [DOI] [PubMed] [Google Scholar]
- Pehrson J.R., Fried V.A. MacroH2A, a core histone containing a large nonhistone region. Science. 1992;257:1398–1400. doi: 10.1126/science.1529340. [DOI] [PubMed] [Google Scholar]
- Penny G.D., Kay G.F., Sheardown S.A., Rastan S., Brockdorff N. Requirement for Xist in X chromosome inactivation. Nature. 1996;379:131–137. doi: 10.1038/379131a0. [DOI] [PubMed] [Google Scholar]
- Rack K.A., Chelly J., Gibbons R.J., Rider S., Benjamin D., Lafreniere R.G., Oscier D., Hendriks R.W., Craig I.W., Willard H.F. Absence of the XIST gene from late-replicating isodicentric X chromosomes in leukaemia. Hum. Mol. Genet. 1994;3:1053–1059. doi: 10.1093/hmg/3.7.1053. [DOI] [PubMed] [Google Scholar]
- Rasmussen T.P., Huang T., Mastrangelo M.A., Loring J., Panning B., Jaenisch R. Messenger RNAs encoding mouse histone macroH2A1 isoforms are expressed at similar levels in male and female cells and result from alternative splicing. Nucleic Acids Res. 1999;27:3685–3689. doi: 10.1093/nar/27.18.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns T., Evans L., Kirschner M. Gamma-tubulin is a highly conserved component of the centrosome. Cell. 1991;65:825–836. doi: 10.1016/0092-8674(91)90390-k. [DOI] [PubMed] [Google Scholar]
- Vijay-Kumar S., Chandra N., Dharia C., Pehrson J.R. Crystallization and preliminary X-ray crystallographic studies of nonhistone region of macroH2A.1. Proteins. 1995;22:290–292. doi: 10.1002/prot.340220311. [DOI] [PubMed] [Google Scholar]
- Wutz A., Jaenisch R. A shift from reversible to irreversible X inactivation is triggered during ES cell differentiation. Mol. Cell. 2000;5:695–705. doi: 10.1016/s1097-2765(00)80248-8. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Jung M.K., Oakley B.R. Gamma-tubulin is present in Drosophila melanogaster and Homo sapiens and is associated with the centrosome. Cell. 1991;65:817–823. doi: 10.1016/0092-8674(91)90389-g. [DOI] [PubMed] [Google Scholar]