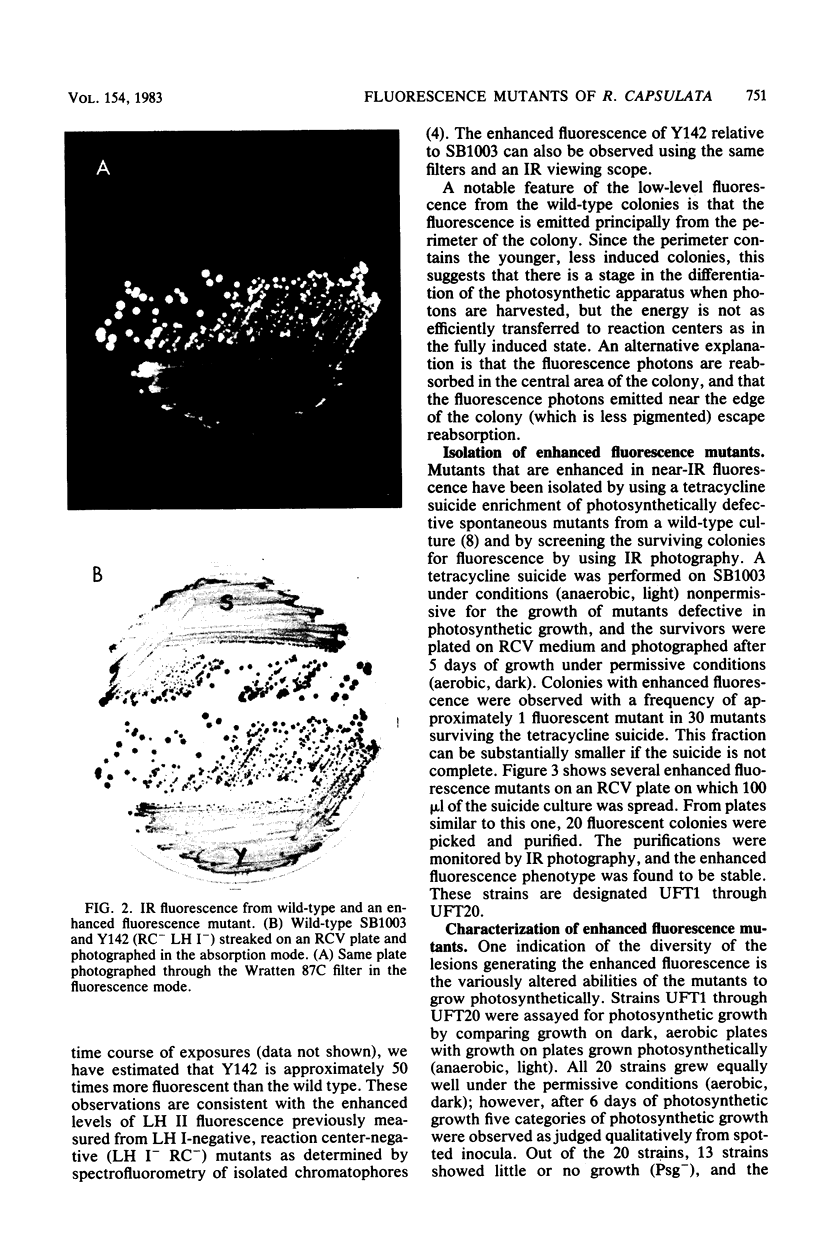
Abstract
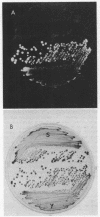
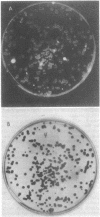
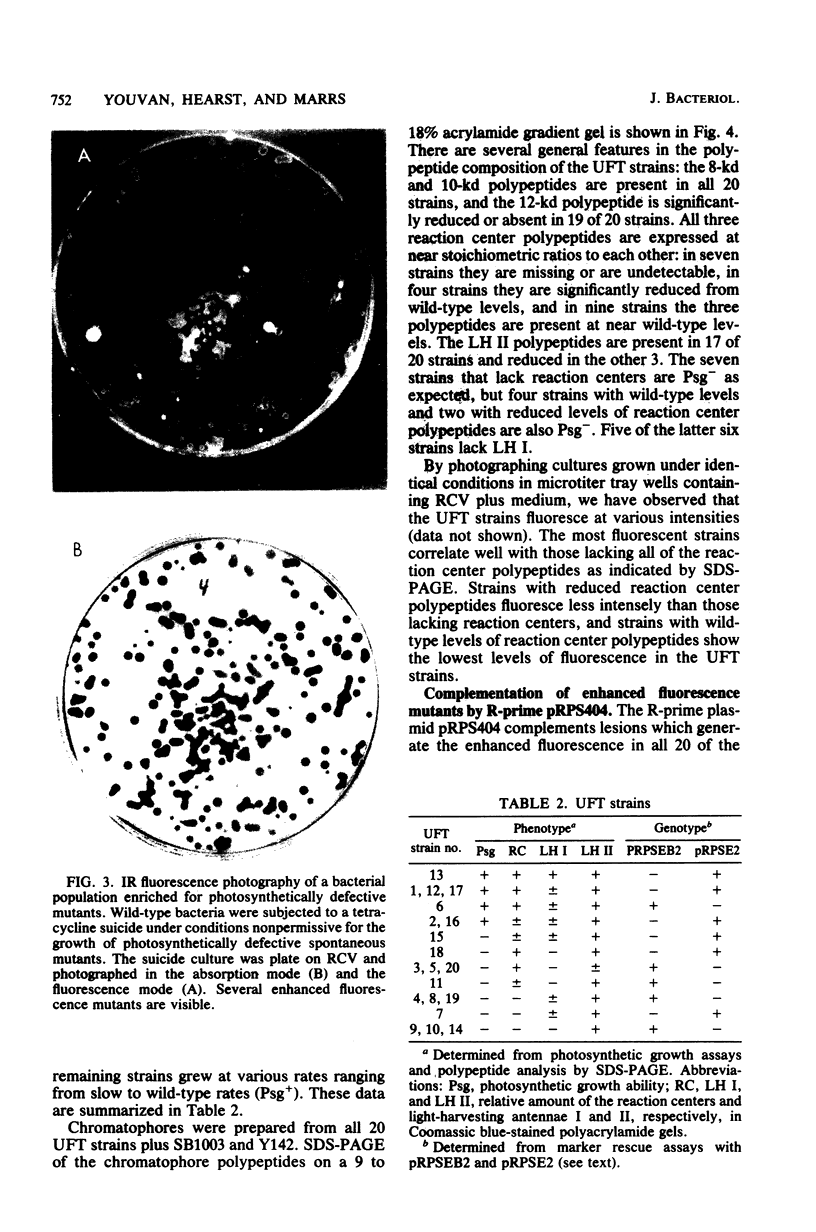
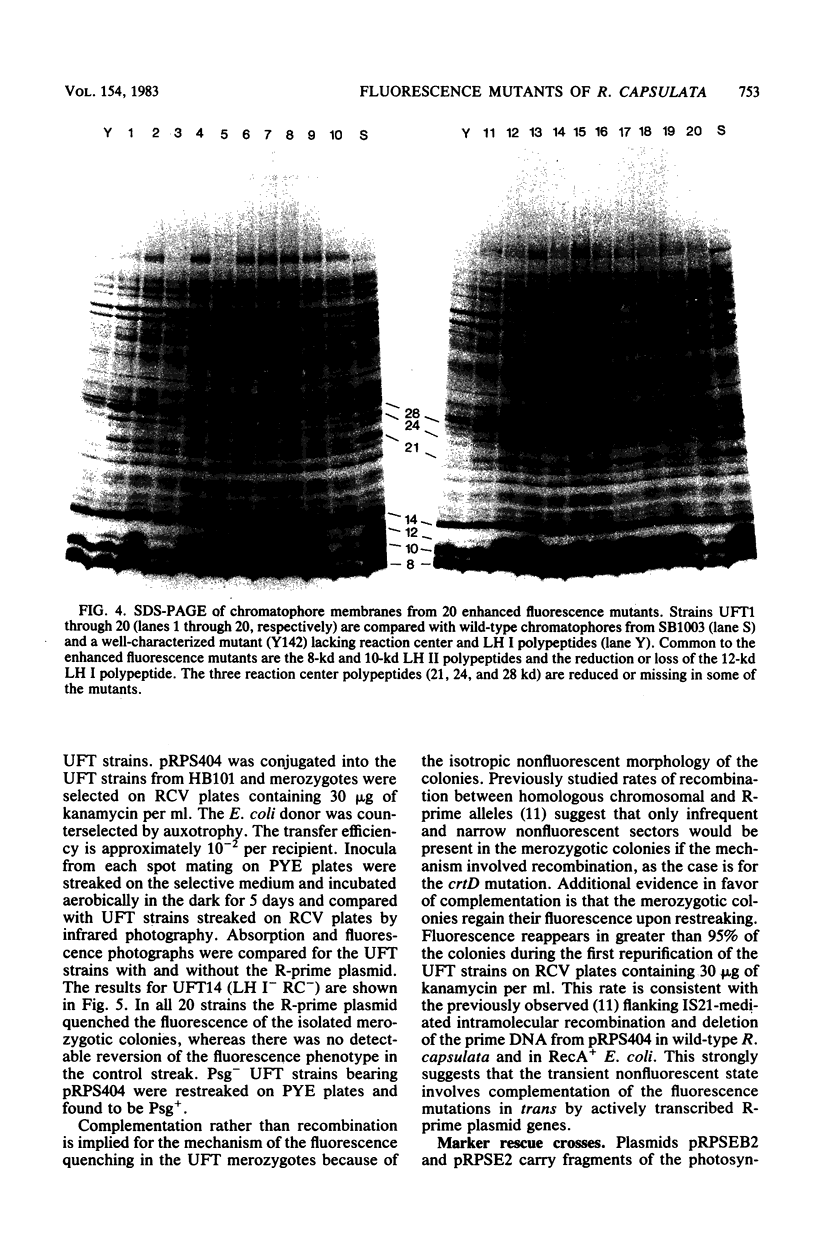
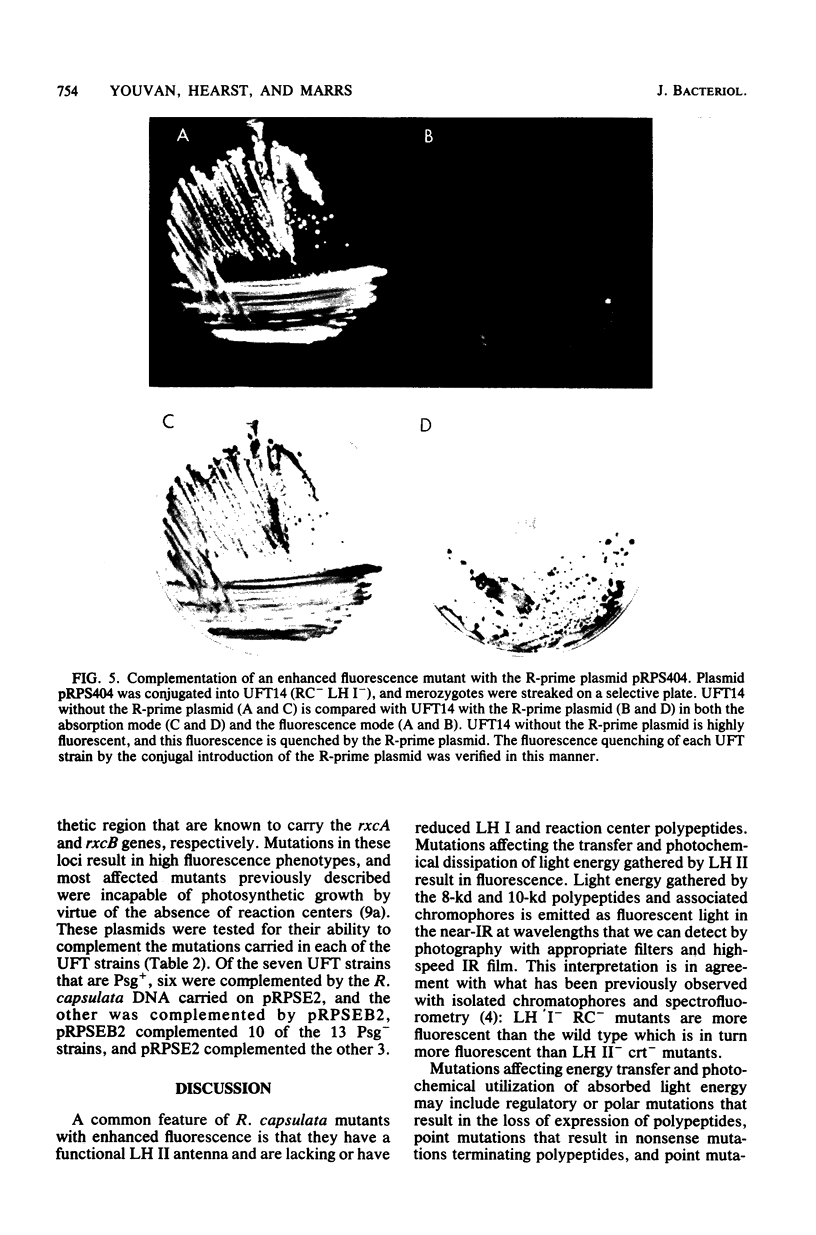
After enrichment by a tetracycline suicide under conditions nonpermissive for the growth of mutants defective in photosynthesis, colonies were screened for enhanced fluorescence in near-infrared light by using high-speed infrared photography. Twenty mutants were isolated, and the chromatophore membranes were analyzed by a new, rapid microprocedure that revealed many different phenotypes among the mutants. The enhanced fluorescence mutants typically possessed a functional light-harvesting II antenna, but showed reduced or absent light-harvesting I. Twelve isolates were also defective in reaction center polypeptides. An R-prime plasmid that bears 50 kilobases of Rhodopseudomonas capsulata DNA coding for components of the photosynthetic apparatus (B. L. Marrs, J. Bacteriol. 146:1003-1012, 1981), pRPS404, complemented all 20 enhanced fluorescence mutants as demonstrated by the quenching of fluorescence in mutants that had received the R-prime plasmid by conjugation. Fluorescence was regained upon loss of the 50-kilobase insert. Complementation of the fluorescent lesions implies that most or all of the genes necessary for the expression of the reaction center and the light-harvesting antennae are carried by the R-prime plasmid and that these genes are actively transcribed in the homologous organism. All 20 mutants are complemented by one of two pBR322 subclones of the R-prime plasmid, pRPSEB2 or pRPSE2. pRPSEB2 bears a 4.5-kilobase fragment of R. capsulata DNA including the rxcA locus, and pRPSE2 is a pBR322 derivative bearing a 7.5-kilobase R. capsulata DNA fragment bearing the rxcB locus. These fragments therefore carry sequences necessary for the normal synthesis of the light-harvesting and reaction center polypeptide complexes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Drews G., Dierstein R., Schumacher A. Genetic transfer of the capacity to form bacteriochlorophyll-protein complexes in Rhodopseudomonas capsulata. FEBS Lett. 1976 Sep 15;68(1):132–136. doi: 10.1016/0014-5793(76)80421-8. [DOI] [PubMed] [Google Scholar]
- Feick R., van Grondelle R., Rijgersberg C. P., Drews G. Fluorescence emission by wild-type- and mutant-strains of Rhodopseudomonas capsulata. Biochim Biophys Acta. 1980 Dec 3;593(2):241–253. doi: 10.1016/0005-2728(80)90062-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marrs B. Mobilization of the genes for photosynthesis from Rhodopseudomonas capsulata by a promiscuous plasmid. J Bacteriol. 1981 Jun;146(3):1003–1012. doi: 10.1128/jb.146.3.1003-1012.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stüber D., Bujard H. Organization of transcriptional signals in plasmids pBR322 and pACYC184. Proc Natl Acad Sci U S A. 1981 Jan;78(1):167–171. doi: 10.1073/pnas.78.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. P., Cohen S. N., Clark W. G., Marrs B. L. Alignment of genetic and restriction maps of the photosynthesis region of the Rhodopseudomonas capsulata chromosome by a conjugation-mediated marker rescue technique. J Bacteriol. 1983 May;154(2):580–590. doi: 10.1128/jb.154.2.580-590.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen H. C., Marrs B. Growth of Rhodopseudomonas capsulata under anaerobic dark conditions with dimethyl sulfoxide. Arch Biochem Biophys. 1977 Jun;181(2):411–418. doi: 10.1016/0003-9861(77)90246-6. [DOI] [PubMed] [Google Scholar]
- Youvan D. C., Elder J. T., Sandlin D. E., Zsebo K., Alder D. P., Panopoulos N. J., Marrs B. L., Hearst J. E. R-prime site-directed transposon Tn7 mutagenesis of the photosynthetic apparatus in Rhodopseudomonas capsulata. J Mol Biol. 1982 Nov 25;162(1):17–41. doi: 10.1016/0022-2836(82)90160-7. [DOI] [PubMed] [Google Scholar]