Abstract
We have found a new cell–cell adhesion system at cadherin-based cell–cell adherens junctions (AJs) consisting of at least nectin and l-afadin. Nectin is a Ca2+-independent homophilic immunoglobulin-like adhesion molecule, and l-afadin is an actin filament-binding protein that connects the cytoplasmic region of nectin to the actin cytoskeleton. Both the trans-interaction of nectin and the interaction of nectin with l-afadin are necessary for their colocalization with E-cadherin and catenins at AJs. Here, we examined the mechanism of interaction between these two cell–cell adhesion systems at AJs by the use of α-catenin–deficient F9 cell lines and cadherin-deficient L cell lines stably expressing their various components. We showed here that nectin and E-cadherin were colocalized through l-afadin and the COOH-terminal half of α-catenin at AJs. Nectin trans-interacted independently of E-cadherin, and the complex of E-cadherin and α- and β-catenins was recruited to nectin-based cell–cell adhesion sites through l-afadin without the trans-interaction of E-cadherin. Our results indicate that nectin and cadherin interact through their cytoplasmic domain–associated proteins and suggest that these two cell–cell adhesion systems cooperatively organize cell–cell AJs.
Keywords: immunoglobulin superfamily, afadin, ponsin, catenin, cell–cell adherens junctions
Introduction
We have recently identified a novel cell–cell adhesion system at cadherin-based cell–cell adherens junctions (AJs) consisting of at least nectin and l-afadin (Mandai et al. 1997; Takahashi et al. 1999). Nectin is a Ca2+-independent homophilic cell adhesion molecule that belongs to the immunoglobulin (Ig) superfamily (Takahashi et al. 1999). l-Afadin is an actin filament (F-actin)–binding protein with one PDZ domain, and connects the cytoplasmic region of nectin to the actin cytoskeleton (Mandai et al. 1997; Takahashi et al. 1999). Nectin consists of three extracellular Ig-like loops, a single transmembrane segment, and a cytoplasmic region. Nectin is identical to the poliovirus receptor–related protein (Eberlé et al. 1995; Lopez et al. 1995), and recently has been shown to serve as the α-herpes virus entry and cell–cell spread mediator (Geraghty et al. 1998; Warner et al. 1998; Cocchi et al. 2000). Nectin comprises a family consisting of at least three members: nectin-1, -2, and -3. Each member has two or three splicing variants: nectin-1 has two splicing variants, nectin-1α and -1β/HIgR (Lopez et al. 1995; Cocchi et al. 1998); nectin-2 also has two splicing variants, nectin-2α and -2δ (Morrison and Racaniello 1992; Aoki et al. 1994; Eberlé et al. 1995); and nectin-3 has three splicing variants, nectin-3α, -3β, and -3γ (Satoh-Horikawa et al. 2000). The extracellular regions of splicing variants of each member are identical, but their transmembrane segments and cytoplasmic regions are different. The cytoplasmic regions of nectin-1α, -2α, -2δ, -3α, and -3β, but not nectin-1β/HIgR and -3γ, have a COOH-terminal conserved motif of four amino acid (aa) residues (E/A-X-Y-V) that interacts with the PDZ domain of l-afadin (Takahashi et al. 1999). l-Afadin has a splicing variant, s-afadin (Mandai et al. 1997). Human s-afadin is identical to the AF-6 protein, the gene of which is originally found to be fused to the ALL-1 gene in acute leukemia (Prasad et al. 1993). It has been shown that the PDZ domain of the AF-6 protein/s-afadin also interacts with the Eph receptor tyrosine kinases (Hock et al. 1998; Buchert et al. 1999).
We have isolated another l-afadin–binding protein, named ponsin, which is colocalized with nectin and l-afadin at cadherin-based AJs (Mandai et al. 1999). Furthermore, ponsin binds to vinculin, which is known to interact with both F-actin and α-catenin (Burridge and Feramisco 1982; Menkel et al. 1994; Watabe-Uchida et al. 1998; Weiss et al. 1998; Imamura et al. 1999), but ponsin forms a binary complex with either l-afadin or vinculin and does not form a ternary complex (Mandai et al. 1999). Ponsin is a member of the family having three SH3 domains, including ArgBP2 (Wang et al. 1997), nArgBP2 (Kawabe et al. 1999), and vinexin (Kioka et al. 1999).
The cadherin/catenin system plays essential roles not only in the formation and maintenance of cell–cell AJs, but also in the organization of tight junctions (TJs; Takeichi 1991, Takeichi 1995; Gumbiner 1996, Gumbiner 2000). Cadherin consists of five extracellular tandemly repeated domains, EC1-EC5, a single transmembrane segment, and a cytoplasmic region (Takeichi 1991, Takeichi 1995; Gumbiner 1996, Gumbiner 2000). Cadherin shows through EC1 homophilic cis-dimerization and trans-interaction, which causes Ca2+-dependent cell–cell adhesion (Shapiro et al. 1995; Nagar et al. 1996; Tomschy et al. 1996; Tamura et al. 1998; Pertz et al. 1999). The cis-dimerization is essential for trans-interaction (Brieher et al. 1996; Tomschy et al. 1996; Tamura et al. 1998; Pertz et al. 1999). The cytoplasmic region of cadherin regulates the cis-dimerization and/or trans-interaction by interacting with many peripheral membrane proteins (Takeichi 1991, Takeichi 1995; Gumbiner 1996, Gumbiner 2000). The distal portion of the cytoplasmic region interacts with β-catenin, which in turn interacts with α-catenin (Nagafuchi and Takeichi 1989; Ozawa et al. 1989). α-Catenin interacts with F-actin (Rimm et al. 1995). Furthermore, α-catenin interacts with other F-actin–binding proteins, α-actinin and vinculin, through the NH2-terminal half (Knudsen et al. 1995; Nieset et al. 1997; Watabe-Uchida et al. 1998; Imamura et al. 1999) and ZO-1 through the COOH-terminal half (Itoh et al. 1997; Imamura et al. 1999). The juxtamembrane portion of cadherin interacts with p120ctn (Reynolds et al. 1994).
By analogy with cadherin, we have shown that nectin exhibits cis-dimerization, which may be a key regulatory step for its trans-interaction (Miyahara et al. 2000). The first and third Ig-like domains may be responsible for its trans-interaction and cis-dimerization, respectively (Miyahara et al. 2000). The interaction of nectin with l-afadin is not essential for its cis-dimerization or trans-interaction (Miyahara et al. 2000), but is essential for the colocalization of nectin and l-afadin with E-cadherin at cell–cell AJs (Takahashi et al. 1999). The interaction of nectin with l-afadin is furthermore necessary for their compact clustering at cell–cell adhesion sites (Miyahara et al. 2000). The colocalization of these two cell–cell adhesion systems at AJs, moreover, requires the trans-interaction of nectin (Miyahara et al. 2000). Studies on epithelial cells in afadin (−/−) mice and (−/−) embryoid bodies have shown that E-cadherin–based AJs are impaired in these mutant cells, suggesting that afadin plays a key role in the proper organization of E-cadherin–based cell–cell AJs (Ikeda et al. 1999). Thus, evidence is accumulating that the nectin/afadin system plays a key role at cadherin-based AJs. Furthermore, we have shown that the interaction of nectin-1α with l-afadin and their colocalization with the cadherin/catenin system are necessary for efficient cell–cell spread of herpes simplex virus type 1 (HSV1; Sakisaka, T., T. Taniguchi, H. Nakanishi, K. Takahashi, M. Miyahara, W. Ikeda, S. Yokoyama, Y.-F. Peng, K. Yamanishi, and Y. Takai, manuscript submitted for publication). However, it has not been understood which component(s) of the cadherin/catenin system is required for the colocalization with the nectin/afadin system. It has not been determined how these two cell adhesion systems are functionally correlated to each other. In this study, we have examined the mechanism of interaction between the nectin/afadin and cadherin/catenin systems by the use of α-catenin–deficient F9 cell lines and cadherin-deficient L cell lines stably expressing their various components.
Materials and Methods
Construction, Expression, and Purification
Mammalian expression vectors were constructed with pPGKIH (Miyahara et al. 2000), pEGFP-N1 (CLONTECH Laboratories, Inc.), pPGKIZ-HA, pEF-MC1neo (Visvader et al. 1992), pFLAG-CMV2 (Eastman Kodak Co), and pPGKIH-FLAG using standard molecular biology methods (Sambrook et al. 1989). pEGFP-N1 was designed to express a COOH-terminal green fluorescent protein (GFP)–tagged protein. pPGKIZ-HA was constructed as follows: pPGKIZ was first constructed by replacing the CAG promoter of pPCAGIZ (Niwa et al. 1998) with the murine phosphoglycerate kinase promoter (Adra et al. 1987). A cDNA fragment encoding the hemagglutinin (HA) epitope and multicloning site (5′-GAATTGTTAATACGACTCACTATAGGCTAGCGGTACC ATG GCT TAC CCA TAC GAT GTT CCA GAT TAC GCT AGC TTG GGT GGT GAA TTC CTC GAG ACG CGT GGT ACC TCT AGA GTC GAC CCG GGC GGC CGC TTC CCT TTA GTG AGG GTT AAT GCA ATT C-3′) was inserted into pPGKIZ to express an NH2-terminal HA-tagged protein. pFLAG-CMV2 was designed to express an NH2-terminal FLAG-tagged protein. pPGKIH-FLAG was constructed by inserting a cDNA fragment encoding the pre-protrypsin signal peptide and FLAG epitope of pFLAG-CMV1 (Eastman Kodak Co.) into pPGKIH as previously described (Takahashi et al. 1999; Miyahara et al. 2000; Satoh-Horikawa et al. 2000).
Expression vectors for human nectin-1α and mouse nectin-2α (shown in Fig. 1) contained the following aa: pPGKIH-nectin-1α, aa 1–518 (full-length); pPGKIH-nectin-1α-ΔC, aa 1–514 (deletion of the COOH-terminal four aa residues); pPGKIH-FLAG-nectin-1α, aa 27–518; pPGKIH-FLAG-nectin-1α-ΔC, aa 27–514; pPGKIH-nectin-2α, aa 1–467 (full-length); and pPGKIH-nectin-2α-ΔC, aa 1–463 (deletion of the COOH-terminal four aa residues). An expression vector for mouse ponsin-2 (pEGFP-ponsin) contained aa 1–724 (full-length; see Fig. 1). An expression vector for l-afadin (pFLAG-CMV2-l-afadin) contained aa 1–1,829 (full-length; see Fig. 1). Expression vectors for mouse E-cadherin and α-catenin contained the following aa: pEF-tEC, aa 562–728 (cytoplasmic region and partial transmembrane segment of E-cadherin); pPGKIZ-HA-α-catenin, aa 1–906 (full-length); and pPGKIZ-HA-α-catenin-C, aa 509–906 (COOH-terminal half; see Fig. 1). pEF-tEC was designed to express the NH2-terminal T7 peptide (M-A-S-M-T-G-G-Q-Q-M-G)–tagged protein.
Figure 1.
Structures of various constructs of nectin, l-afadin, ponsin, E-cadherin, and α-catenin. CTM, COOH-terminal motif of four aa residues; GAL4AD, GAL4 activation domain; and CBD, catenin-binding domain.
Baculovirus transfer vectors were constructed with pFastBac1-Myc-His6 and -Msp-Fc. pFastBac1-Myc-His6 was constructed to express a COOH-terminal Myc- and His6-tagged protein by inserting a cDNA fragment encoding the Myc epitope and His6 (hexahistidine) tags of pcDNA3.1(−)/Myc-His (Invitrogen) into pFastBac1 (GIBCO BRL). pFastBac1-Msp-Fc was constructed to express a chimeric protein fused with the NH2-terminal honeybee melittin signal peptide and the COOH-terminal human IgG Fc by inserting cDNA fragments encoding the signal peptide (5′-ATG AAA TTC TTA GTC AAC GTT GCC CTT GTT TTT ATG GTC GTG TAC ATT TCT TAC ATC TAT GCG-3′; Tessier et al. 1991) and the IgG Fc into pFastBac1. Baculovirus transfer vectors for l-afadin and glycoprotein D (gD), an envelope component of HSV1, contained the following aa: pFastBac1-Myc-His6-l-afadin, aa 1–1,829 (full-length; see Fig. 1); and pFastBac1-Msp-Fc-gD, aa 1–285. A baculovirus bearing the l-afadin or gD cDNA was prepared with pFastBac1-Myc-His6-l-afadin or -Msp-Fc-gD, respectively, according to the manufacturer's protocol (GIBCO BRL). The Myc- and His6-tagged protein of l-afadin (Myc-His6-l-afadin) was expressed in High Five insect cells (Invitrogen) infected with the baculovirus bearing the l-afadin cDNA and purified by the use of TALON metal affinity beads (CLONTECH Laboratories, Inc.). The chimeric protein of gD fused with IgG Fc was expressed in High Five insect cells (Invitrogen) infected with the baculovirus bearing the gD cDNA and purified by the use of protein A–Sepharose beads (Amersham Pharmacia Biotech).
Glutathione S-transferase (GST) fusion vectors for α-catenin contained the following aa: GST-α-catenin, aa 1–906 (full-length; Itoh et al. 1997); and GST-α-catenin-C, aa 509–906 (COOH-terminal half; see Fig. 1). A GST fusion vector for ZO-1 (GST-ZO-1-N) contained aa 1–862 (NH2-terminal half; Itoh et al. 1997; Sakisaka et al. 1999). The GST fusion proteins were purified by the use of glutathione-Sepharose beads (Amersham Pharmacia Biotech).
Cell Culture and DNA Transfection
F9, L, EL, and COS7 cell lines were maintained in Dulbecco's modified Eagle's medium supplemented with 10% FCS. F9 cells were cultured in gelatin-coated (0.1%) culture dishes. α-Catenin–deficient F9 cells (F9Dα(−/−) cells) and F9Dα(−/−) cells reexpressing α-catenin (αF9Dα(−/−) cells) were obtained as previously described (Maeno et al. 1999). EL cells were cloned by the introduction of E-cadherin cDNA into L cells (Nagafuchi et al. 1987; see Fig. 1). The following L cell lines stably expressed the fusion molecules (see Fig. 1): nEα-L cells, the fusion molecule consisting of nonfunctional E-cadherin lacking its catenin-binding domain (aa 1–657) and full-length α-catenin (aa 1–906; Nagafuchi et al. 1994; Imamura et al. 1999); nEαN-L cells, the fusion molecule consisting of nonfunctional E-cadherin lacking its catenin-binding domain and the NH2-terminal half of α-catenin (aa 1–508; Nagafuchi et al. 1994; Imamura et al. 1999); and nEαC-L cells, the fusion molecule consisting of nonfunctional E-cadherin lacking its catenin-binding domain and the COOH-terminal half of α-catenin (aa 509–906; Nagafuchi et al. 1994; Imamura et al. 1999). nEα-L, nEαN-L, and nEαC-L cells were obtained as previously described (Nagafuchi et al. 1994; Imamura et al. 1999).
The following L and EL cell lines were cloned by the introduction of the pPGKIH and pPGKIH-FLAG vectors, respectively, as described previously (Takahashi et al. 1999; Miyahara et al. 2000; Satoh-Horikawa et al. 2000): nectin-1α-L cells, pPGKIH-nectin-1α (aa 1–518); nectin-1α-ΔC-L cells, pPGKIH-nectin-1α-ΔC (aa 1–514); nectin-2α-L cells, pPGKIH-nectin-2α (aa 1–467); nectin-2α-ΔC-L cells; pPGKIH-nectin-2α-ΔC (aa 1–463); nectin-1α-EL cells, pPGKIH-FLAG-nectin-1α (aa 27–518); and nectin-1α-ΔC-EL cells, pPGKIH-FLAG-nectin-1α-ΔC (aa 27–514). In brief, L and EL cells were transfected with the vectors described above using Lipofectamine reagent (GIBCO BRL) according to the manufacturer's protocol. The cells were cultured for 1 d, replated, and selected by culturing in the presence of 500 μg/ml of hygromycin (GIBCO BRL). To prepare nectin-1α-L and -2α-L cells, both of which transiently expressed α- and/or β-catenins, the cells were transfected with pPGKIZ-HA-α-catenin or pEF-tEC using Lipofectamine reagent. The cells were cultured for 1 d, replated, and cultured for 3 d.
Antibodies
One rabbit polyclonal anti–nectin-1α antibody (Ab) was raised against GST-nectin-1α-CPN (aa 379–438) as previously described (Takahashi et al. 1999; Satoh-Horikawa et al. 2000) and used as the polyclonal anti–nectin-1α Ab1. Another rabbit polyclonal anti–nectin-1α Ab was raised against the 19-mer synthetic peptide (corresponding to aa 450–468 of nectin-1α) as previously described (Satoh-Horikawa et al. 2000) and used as the polyclonal anti–nectin-1α Ab2. A rabbit polyclonal anti–nectin-2α Ab was prepared as described previously (Takahashi et al. 1999). A rat anti–nectin-2 mAb, which recognizes both nectin-2α and -2δ, was prepared as described previously (Aoki et al. 1997; Takahashi et al. 1999). A mouse anti–l-afadin mAb (Sakisaka et al. 1999) and a rabbit polyclonal antiponsin Ab (Mandai et al. 1999) were prepared as previously described. A rat anti–E-cadherin mAb (ECCD-2) was supplied from Dr. M. Takeichi (Kyoto University, Kyoto, Japan). A mouse antivinculin mAb was purchased from Sigma Chemical Co. A mouse anti-HA mAb was prepared as previously described (Yamochi et al. 1994). Mouse anti–β-catenin and anti-FLAG mAbs were purchased from Zymed and Eastman Kodak Co., respectively.
Yeast Two-Hybrid Interaction and β-Galactosidase Assay
The bait vectors, pBTM116-HA-α-catenin (full-length, aa 1–906), -HA-α-catenin-N (NH2-terminal half, aa 1–508), -HA-α-catenin-C (COOH-terminal half, aa 509–906), and -HA-nectin-2α-CP (cytoplasmic region, aa 387–467) were constructed by inserting cDNA fragments encoding the respective aa residues of α-catenin and nectin-2α into pBTM116-HA (see Fig. 1; Imamura et al. 1997). The prey vector, pGAD424-HA-l-afadin (aa 1–1,829, full-length), was constructed with pGAD424-HA (see Fig. 1). pGAD424-HA was constructed to express an HA-tagged protein fused with GAL4 activation domain by inserting a cDNA fragment encoding the HA epitope into pGAD424 (CLONTECH Laboratories, Inc.). The yeast two-hybrid β-galactosidase activity was measured according to the ONPG assay method (Guarente 1983).
Affinity Chromatography and Immunoprecipitation
Myc-His6-l-afadin (20 μg of protein) was immobilized on TALON metal affinity beads (CLONTECH Laboratories, Inc.) (100 μl of wet volume). GST-α-catenin and -α-catenin-C (100 μg of protein each) were separately applied to the Myc-His6-l-afadin–immobilized beads equilibrated with buffer A (20 mM Tris-Cl, pH 7.5, 150 mM NaCl, 0.1% Triton X-100, 10 μg/ml leupeptin, 1 mM PMSF, and 1 μg/ml pepstatin A). After the beads were extensively washed with buffer A, elution was performed with buffer A containing 100 mM imidazole chloride, pH 7.5. Each fraction was subjected to SDS-PAGE (10% polyacrylamide gel), followed by protein staining with Coomassie brilliant blue. Where indicated, GST-ZO-1-N and -α-catenin-C (100 μg of protein each) were mixed and subjected to affinity chromatography.
Immunoprecipitation was performed as described previously (Takahashi et al. 1999). In brief, COS7 cells transiently expressing FLAG-l-afadin and HA-α-catenin or -α-catenin-C were prepared with transfection of pFLAG-CMV2-l-afadin and pPGKIZ-HA-α-catenin or -HA-α-catenin-C, respectively, using Lipofectamine reagent. The cells were sonicated in buffer B (20 mM Tris-Cl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 10 μg/ml leupeptin, 1 mM PMSF, and 1 μg/ml pepstatin A), followed by centrifugation at 100,000 g for 30 min. The supernatant was incubated with the anti-FLAG or anti-HA mAb at 4°C for 2 h. Protein G–Sepharose beads (Amersham Pharmacia Biotech) were added to this sample, and incubation was further performed at 4°C for 1 h. After the beads were extensively washed with buffer B, the bound proteins were eluted by boiling the beads in an SDS sample buffer (60 mM Tris-Cl, pH 6.7, 3% SDS, 2% [vol/vol] 2-mercaptoethanol, and 5% glycerol), and subjected to SDS-PAGE, followed by Western blot analysis.
Coculture of L and EL Cells Stably Expressing Nectin
Nectin-1α-L, -1α-EL, and -1α-ΔC-EL cells were washed with PBS, incubated with 0.2% trypsin and 1 mM EDTA at 37°C for 5 min, and dispersed by gentle pipetting to obtain single cell suspensions. The cell number of each cell line was counted, and nectin-1α-EL or -1α-ΔC-EL cells were mixed with a 10-fold number of nectin-1α-L cells. These mixed cells were plated at 2 × 105 cells on a 35-mm culture dish and cultured for 2 d. Where indicated, gD was added to the mixed cell suspension to give a final concentration of 0.3 μM. The cells were further cultured with the same concentration of gD for 2 d.
Other Procedures
Immunofluorescence microscopy of cultured cells was done as described previously (Mandai et al. 1997, Mandai et al. 1999; Takahashi et al. 1999). Protein concentrations were determined with BSA as a reference protein (Bradford 1976). SDS-PAGE was done as previously described (Laemmli 1970).
Results
α-Catenin–dependent Colocalization of Nectin and l-Afadin with E-Cadherin
We first examined whether α-catenin is involved in the colocalization of nectin and l-afadin with E-cadherin. For this purpose, we used an α-catenin–deficient F9 cell line, F9Dα(−/−) cells (Maeno et al. 1999). F9 cells are mouse teratocarcinoma-derived embryonal carcinoma cells. F9Dα (−/−) cells were generated by a disruption of both α-catenin alleles with a targeting vector. As a control, we used αF9Dα(−/−) cells, which were generated by the introduction of an expression vector encoding full-length α-catenin into F9Dα(−/−) cells (Maeno et al. 1999). It has been shown that αF9Dα(−/−) cells reexpress α-catenin in an amount similar to that of wild-type F9 cells (Maeno et al. 1999). In αF9Dα(−/−) cells, E-cadherin was localized at beltlike cell–cell adhesion sites, where nectin-2 and l-afadin were colocalized (Fig. 2 A). In F9Dα(−/−) cells, E-cadherin showed the similar staining pattern, but nectin-2 and l-afadin were hardly concentrated at cell–cell adhesion sites between two cells, where E-cadherin was concentrated (Fig. 2 B). Nectin-2 and l-afadin were colocalized with E-cadherin only at spotlike cell–cell adhesion sites where >2 cells adhered to each other. Western blot analysis indicated that the protein levels of nectin-2α and l-afadin were similar between αF9Dα(−/−) and F9Dα(−/−) cells (data not shown). It has been shown that the protein levels of E-cadherin and β-catenin in αF9Dα(−/−) cells are comparable to those in F9Dα(−/−) cells (Maeno et al. 1999). Taken together, these results indicate that the colocalization of nectin and l-afadin with E-cadherin is dependent on α-catenin, but not on the cytoplasmic region of E-cadherin or β-catenin, in F9 cells.
Figure 2.

α-Catenin–dependent colocalization of nectin and l-afadin with E-cadherin. αF9Dα(−/−) and F9Dα(−/−) cells were double stained with various combinations of the anti–E-cadherin, anti–l-afadin, and anti–nectin-2 Abs. (arrows) Cell–cell adhesion sites between two cells; and (arrowheads) cell–cell adhesion sites where >2 cells adhere to each other. The results shown are representative of three independent experiments. Bars, 10 μm.
β-Catenin–independent Colocalization of Nectin and l-Afadin with E-Cadherin
We have previously shown that nectin-2 and l-afadin are colocalized with E-cadherin at cell–cell AJs in EL cells (Takahashi et al. 1999), which have been established as L cells stably expressing E-cadherin (Nagafuchi et al. 1987). In EL cells, ponsin and vinculin were colocalized and concentrated with E-cadherin at cell–cell AJs and focal contacts (data not shown). To further confirm the results obtained with the two F9 cell lines described above, we used L cells stably expressing a chimeric protein (nEα) of catenin-binding domain-deleted E-cadherin fused with full-length α-catenin (nEα-L cells). In the junctional level of nEα-L cells, nectin-2 and l-afadin were colocalized at cell–cell adhesion sites where the nEα molecule was localized (Fig. 3 A). Ponsin and vinculin were also colocalized with nEα there. In the basal level, nEα, nectin-2, or l-afadin was not concentrated at focal contacts (data not shown), whereas ponsin and vinculin were colocalized there (Fig. 3 B). These results are consistent with those obtained with the two F9 cell lines described above, and indicate that β-catenin is not essential for the colocalization of nectin, l-afadin, ponsin, and vinculin with E-cadherin at cell–cell adhesion sites.
Figure 3.
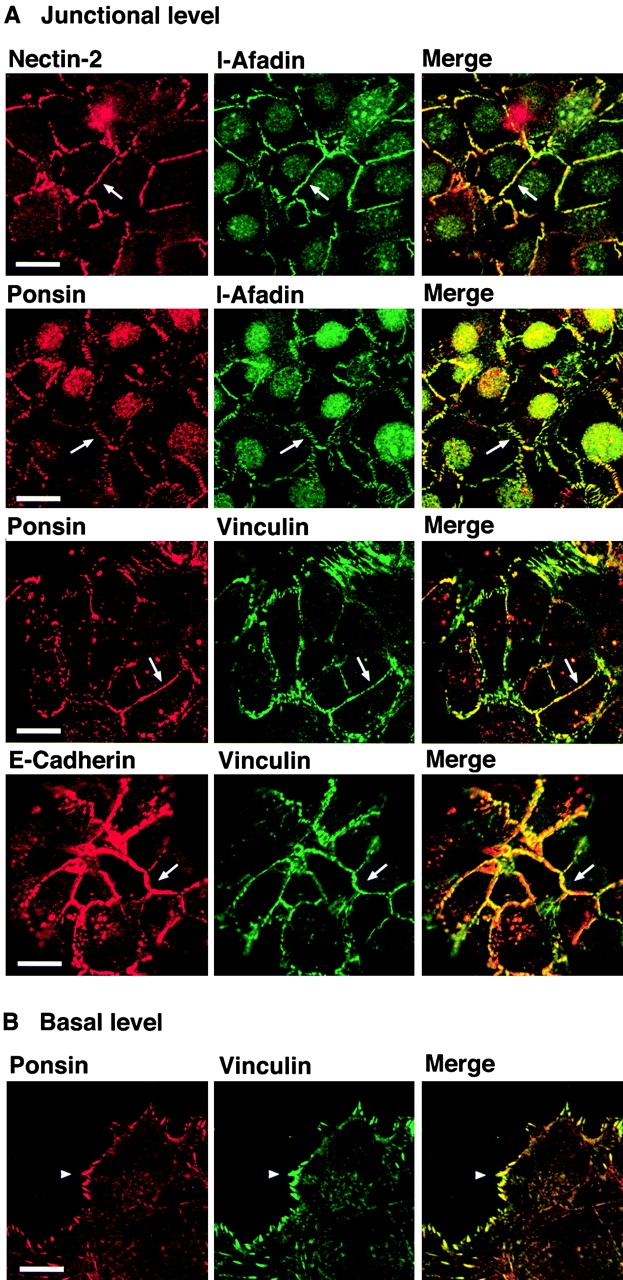
β-Catenin–independent colocalization of nectin, l-afadin, ponsin, and vinculin with E-cadherin. nEα-L cells were double stained with various combinations of the anti–nectin-2, anti–l-afadin, antiponsin, antivinculin, and anti–E-cadherin Abs. There was nuclear staining with the anti–l-afadin or antiponsin Ab, but the nuclear staining was not an artifact of second antibodies, although its significance is not known. (arrows) Cell–cell adhesion sites; and (arrowhead) focal contacts. The results shown are representative of three independent experiments. Bars, 10 μm.
Ponsin- and Vinculin-independent Colocalization of Nectin and l-Afadin with E-Cadherin
We next examined whether ponsin and vinculin are necessary for the colocalization of nectin and l-afadin with E-cadherin. For this purpose, we took advantage of two L cell lines. One cell line (nEαN-L) is an L cell clone stably expressing a chimeric protein (nEαN) of catenin-binding domain–deleted E-cadherin fused with the NH2-terminal half of α-catenin, which is capable of binding vinculin and α-actinin but not ZO-1 (Nagafuchi et al. 1994; Imamura et al. 1999). The other cell clone (nEαC-L) is an L cell clone stably expressing a chimeric protein (nEαC) of catenin-binding domain–deleted E-cadherin fused with the COOH-terminal half of α-catenin, which is capable of binding ZO-1, but not vinculin or α-actinin (Nagafuchi et al. 1994; Imamura et al. 1999). In the junctional level of nEαN-L cells, the nEαN molecule was localized at cell–cell adhesion sites where neither nectin-2 nor l-afadin was concentrated (Fig. 4 A). However, vinculin was colocalized with nEαN there. Ponsin appeared to be also colocalized with nEαN there, but the staining was not clear (data not shown). When a GFP-tagged protein of full-length ponsin (GFP-ponsin) was transiently expressed, it was clearly colocalized with nEαN at cell–cell adhesion sites (Fig. 4 A). In the basal level of nEαN-L cells, ponsin and vinculin were colocalized at focal contacts (Fig. 4 B).
Figure 4.

Nectin- and l-afadin–independent colocalization of ponsin and vinculin with E-cadherin. nEαN-L cells were double stained with various combinations of the anti–nectin-2, anti–l-afadin, antiponsin, antivinculin, and anti–E-cadherin Abs. Alternatively, nEαN-L cells were transfected with pEGFP-ponsin and stained with the anti–E-cadherin Ab. (arrows) Cell–cell adhesion sites; and (arrowhead) focal contacts. The results shown are representative of three independent experiments. Bars, 10 μm.
In the junctional level of nEαC-L cells, the nEαC molecule was localized at cell–cell adhesion sites, where nectin-2 and l-afadin were colocalized (Fig. 5 A). However, neither ponsin nor vinculin was concentrated with nEαC there (Fig. 5 A), although they were colocalized at focal contacts (Fig. 5 B). We have previously shown that the protein levels of nectin-1α and l-afadin are similar among nEα-L, nEαN-L, and nEαC-L cells (Sakisaka, T., T. Taniguchi, H. Nakanishi, K. Takahashi, M. Miyahara, W. Ikeda, S. Yokoyama, Y.-F. Peng, K. Yamanishi, and Y. Takai, manuscript submitted for publication). In addition, Western blot analysis indicated that the protein levels of nectin-2α, ponsin, and vinculin were also similar among these three L cell lines (data not shown). These results indicate that neither ponsin nor vinculin is necessary for the colocalization of nectin and l-afadin with E-cadherin at cell–cell adhesion sites, and that their colocalization is dependent on the COOH-terminal half of α-catenin, but not on the cytoplasmic region of E-cadherin or the NH2-terminal half of α-catenin.
Figure 5.
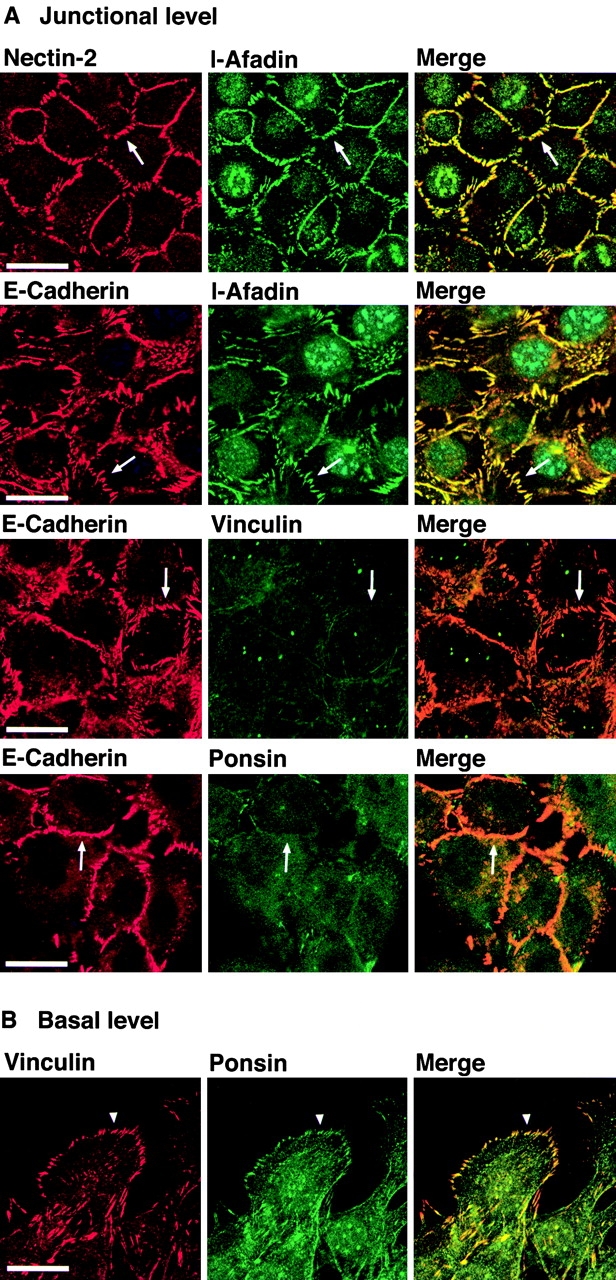
Ponsin- and vinculin-independent colocalization of nectin and l-afadin with E-cadherin. nEαC-L cells were double stained with various combinations of the anti–nectin-2, anti–l-afadin, antiponsin, antivinculin, and anti–E-cadherin Abs. (arrows) Cell–cell adhesion sites; and (arrowhead) focal contacts. The results shown are representative of three independent experiments. Bars, 10 μm.
Molecular Linkage between l-Afadin and α-Catenin
We have previously shown that l-afadin does not directly interact with α-catenin by affinity chromatography using the purified samples (Sakisaka et al. 1999). In these experiments, we used full-length proteins. It has been shown that α-catenin is homologous to vinculin (Nagafuchi et al. 1991; Herrenknecht et al. 1991; Pokutta and Weis 2000), and that vinculin shows the intramolecular association of its NH2-terminal and COOH-terminal halves (Johnson and Craig 1995). Therefore, we examined whether l-afadin directly interacts with the COOH-terminal half of α-catenin. The yeast two-hybrid analysis revealed that l-afadin (pGAD424-HA-l-afadin) interacted with the COOH-terminal half (pBTM-116-HA-α-catenin-C), but not with the full-length (pBTM-116-HA-α-catenin) or the NH2-terminal half (pBTM-116-HA-α-catenin-N; Table ). This yeast two-hybrid interaction of l-afadin with the COOH-terminal half of α-catenin was comparable to that of l-afadin with the cytoplasmic region of nectin-2α (pBTM-116-HA-nectin-2α-CP). We further confirmed the in vitro direct interaction of l-afadin with α-catenin by affinity chromatography. A GST fusion protein of the COOH-terminal half of α-catenin (GST-α-catenin-C) bound to a Myc- and His6-tagged protein of full-length l-afadin (Myc-His6-l-afadin) immobilized on metal affinity beads (Fig. 6), whereas a GST fusion protein of the full-length (GST-α-catenin) hardly bound to the beads (data not shown). However, the stoichiometry of the interaction of GST-α-catenin-C with Myc-His6-l-afadin was ∼0.1:1. It has been shown that the NH2-terminal half of ZO-1 directly interacts with the AF-6 protein/s-afadin (Yamamoto et al. 1997) and the COOH-terminal half of α-catenin (Itoh et al. 1997). To examine whether the NH2-terminal half of ZO-1 enhances the stoichiometry of the interaction of the COOH-terminal half of α-catenin with l-afadin, a GST fusion protein of the NH2-terminal half of ZO-1 (GST-ZO-1-N) was mixed with GST-α-catenin-C, and the mixture was subjected to the Myc-His6-l-afadin–immobilized affinity chromatography. However, GST-ZO-1-N did not enhance the stoichiometry of the interaction of GST-α-catenin-C with Myc-His6-l-afadin (data not shown). To further confirm the interaction of l-afadin with the COOH-terminal half of α-catenin in intact cells, a FLAG-tagged protein of full-length l-afadin (FLAG-l-afadin) and an HA-tagged protein of the COOH-terminal half of α-catenin (HA-α-catenin-C) were coexpressed in COS7 cells and immunoprecipitated by either the anti-FLAG or anti-HA Ab, but they were not coimmunoprecipitated to a significant extent (data not shown). However, negative results obtained from immunoprecipitation experiments do not necessarily reflect in vivo negative protein–protein interactions. Therefore, these results suggest that l-afadin directly interacts with α-catenin under appropriate conditions.
Table 1.
Yeast Two-Hybrid Interaction of l-Afadin with α-Catenin
| α-Catenin | Nectin-2α | ||||
|---|---|---|---|---|---|
| Control(pBTM116-HA) | Full-length(pBTM116-HA-α-catenin) | COOH-terminal(pBTM116-HA-α-catenin-C) | NH2-terminal(pBTM116-HA-α-catenin-N) | Cytoplasmic region(pBTM116-HA-nectin-2α-CP) | |
| Control (pGAD424-HA) | <1 | <1 | <1 | <1 | <1 |
| l-Afadin (pGAD424-HA-l- afadin) | 1 ± 0.5 | <1 | 30 ± 2 | <1 | 41 ± 2 |
The data list units of β-galactosidae activity of yeast strains harboring the respective bait and prey plasmids. The values are means ± SEM of three independent experiments.
Figure 6.
Interaction of l-afadin with α-catenin on affinity chromatography. GST-α-catenin-C was applied to metal affinity beads on which Myc-His6-l-afadin was immobilized. After the beads were extensively washed, elution was performed with 100 mM imidazole chloride, pH 7.5. Each fraction was subjected to SDS-PAGE (10% polyacrylamide gel), followed by protein staining with Coomassie brilliant blue. The results shown are representative of three independent experiments.
l-Afadin–dependent Recruitment of α-Catenin to Nectin-based Cell–Cell Adhesion Sites in the Absence of E-Cadherin
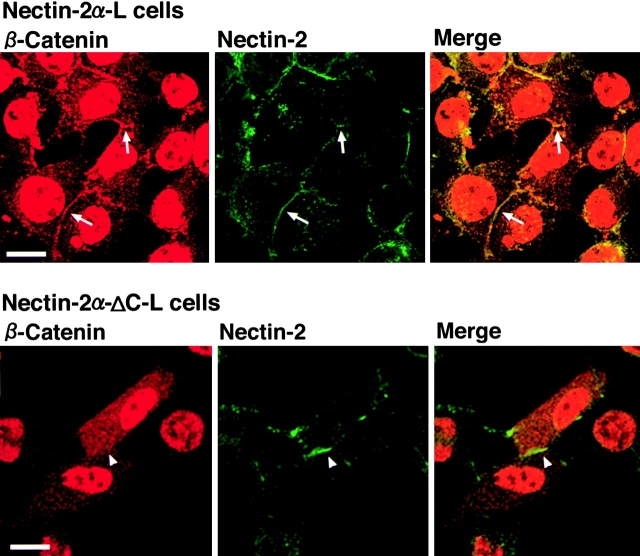
In the next series of experiments, we examined whether α-catenin and β-catenin are recruited through l-afadin to nectin-based cell–cell adhesion sites in the absence of E-cadherin. For this purpose, we used L cells stably expressing full-length nectin-2α (nectin-2α-L cells) or the COOH-terminal four aa-deleted nectin-2α (nectin-2α-ΔC-L cells). We have previously shown that nectin-2α-L and -2α-ΔC-L cells show similar cell adhesion activity, as estimated by a cell aggregation assay (Miyahara et al. 2000). The protein levels of nectin-2α and l-afadin in nectin-2α-L cells are similar to those in nectin-2α-ΔC-L cells, respectively. However, in nectin-2α-L cells, l-afadin is concentrated at nectin-2α–based cell adhesion sites, whereas in nectin-2α-ΔC-L cells, l-afadin is not recruited to nectin-2α-ΔC–based cell adhesion sites (Miyahara et al. 2000). We first confirmed this result (Fig. 7). When an HA-tagged protein of full-length α-catenin (HA-α-catenin) was transiently expressed in nectin-2α-L cells, HA-α-catenin was concentrated at nectin-2α-based cell–cell adhesion sites (Fig. 8, top). However, when HA-α-catenin was transiently expressed in nectin-2α-ΔC-L cells, HA-α-catenin was not concentrated at nectin-2α-ΔC–based cell–cell adhesion sites (Fig. 8, bottom). Similar results were obtained with L cells stably expressing full-length nectin-1α (nectin-1α-L cells) or the COOH-terminal four aa-deleted nectin-1α (nectin-1α-ΔC-L cells; data not shown). These results indicate that α-catenin is recruited through l-afadin to nectin-based cell–cell adhesion sites in the absence of E-cadherin.
Figure 7.

Localization of nectin and l-afadin in nectin-2α-L and -2α-ΔC-L cells. Nectin-2α-L and -2α-ΔC-L cells were double stained with the anti–nectin-2 and anti–l-afadin Abs. (arrows) Nectin-2α–based cell–cell adhesion sites; and (arrowheads) nectin-2α-ΔC–based cell–cell adhesion sites. The results shown are representative of three independent experiments. Bar, 10 μm.
Figure 8.
l-Afadin–dependent recruitment of α-catenin to nectin-based cell–cell adhesion sites in the absence of E-cadherin. Nectin-2α-L and -2α-ΔC-L cells were transfected with pPGKIZ-HA-α-catenin and double stained with the anti–nectin-2 and anti-HA Abs. (arrow) Nectin-2α–based cell–cell adhesion sites; and (arrowhead) nectin-2α-ΔC–based cell–cell adhesion sites. The results shown are representative of three independent experiments. Bars, 10 μm.
l-Afadin–dependent Recruitment of β-Catenin to Nectin-based Cell–Cell Adhesion Sites without the trans-Interaction of E-Cadherin
In L cells, endogenous β-catenin was reduced to a practically undetectable level, but the amount of β-catenin as well as α-catenin was increased upon expression of a T7 peptide–tagged protein of the cytoplasmic region and partial transmembrane segment of E-cadherin (tEC; data not shown). In nectin-2α-L cells transiently expressing tEC, β-catenin was recruited to nectin-2α–based cell–cell adhesion sites (Fig. 9, top). In contrast, in nectin-2α-ΔC-L cells transiently expressing tEC, β-catenin was not concentrated at nectin-2α-ΔC–based cell–cell adhesion sites (Fig. 9, bottom). The amounts of increased endogenous β-catenin were similar between these two types of cells (data not shown). These results indicate that β-catenin is recruited through l-afadin to nectin-based cell–cell adhesion sites without the trans-interaction of E-cadherin.
Figure 9.
l-Afadin–dependent recruitment of β-catenin to nectin-based cell–cell adhesion sites without the trans-interaction of E-cadherin. Nectin-2α-L and -2α-ΔC-L cells were transfected with pEF-tEC and double stained with the anti–nectin-2 and anti–β-catenin Abs. (arrows) Nectin-2α–based cell–cell adhesion sites; and (arrowhead) nectin-2α-ΔC–based cell–cell adhesion sites. The results shown are representative of three independent experiments. Bars, 10 μm.
l-Afadin–dependent Recruitment of E-Cadherin to Nectin-based Cell–Cell Adhesion Sites without the trans-Interaction of E-Cadherin
In the last set of experiments, we examined whether E-cadherin is recruited to nectin-based cell–cell adhesion sites without the trans-interaction of E-cadherin. For this purpose, we cocultured nectin-1α-L cells with EL cells stably expressing a FLAG-tagged protein of full-length nectin-1α (nectin-1α-EL cells) or a FLAG-tagged protein of the COOH-terminal four aa-deleted nectin-1α (nectin-1α-ΔC-EL cells). When nectin-1α-L cells were cocultured with nectin-1α-EL cells, nectin-1α was concentrated at adhesion sites between the same type of cells and between two different types of cells (Fig. 10, top). E-Cadherin was concentrated at adhesion sites between two nectin-1α-EL cells. In addition, E-cadherin was concentrated at adhesion sites between nectin-1α-L and -1α-EL cells. When nectin-1α-L cells were cocultured with nectin-1α-ΔC-EL cells, nectin-1α and -1α-ΔC were also concentrated at adhesion sites between the same type of cells and between the two different types of cells (Fig. 10, middle). Consistent with our previous report that the interaction of nectin with l-afadin is necessary for its clustering (Miyahara et al. 2000), the concentration of nectin-1α and -1α-ΔC at adhesion sites between nectin-1α-L and -1α-ΔC-EL cells was weaker than that of nectin-1α at adhesion sites between nectin-1α-L and -1α-EL cells. Moreover, the recruitment of E-cadherin to adhesion sites between nectin-1α-L and -1α-ΔC-EL cells was significantly reduced when compared with that between nectin-1α-L and -1α-EL cells. Western blot analysis indicated that the protein levels of nectin-1α or -1α-ΔC among these three cell lines were similar (data not shown). The polyclonal anti–nectin-1α Ab2 recognized two or three protein bands. Their relationship is not clear, but this may be due to different levels of the posttranslational modifications such as glycosylation.
Figure 10.

l-Afadin–dependent recruitment of E-cadherin to nectin-based cell–cell adhesion sites without the trans-interaction of E-cadherin. Nectin-1α-L cells were cocultured with nectin-1α-EL or -1α-ΔC-EL cells in the presence or absence of gD and double stained with the anti–nectin-1α Ab1 and the anti–E-cadherin Ab. In the absence of gD, E-cadherin was concentrated at ∼40% of the adhesion sites between nectin-1α-L and -1α-EL cells. The recruitment of E-cadherin was observed in ∼20% of the adhesion sites between nectin-1α-L and -1α-ΔC-EL cells. In the presence of gD, the recruitment of E-cadherin was observed in ∼8% of the adhesion sites between nectin-1α-L and -1α-EL cells. (arrows) Cell–cell adhesion sites between two nectin-1α-L cells or between two nectin-1α-ΔC-L cells; (arrowheads) cell–cell adhesion sites between nectin-1α-L and -1α-EL or -1α-ΔC-EL cells; and (double arrowheads) cell–cell adhesion sites between two nectin-1α-EL cells or between two nectin-1α-ΔC-EL cells. The results shown are representative of three independent experiments.
We have previously shown that gD, an envelope component of HSV1, specifically inhibits nectin-1α–mediated cell adhesion activity as estimated by a cell aggregation assay (Sakisaka, T., T. Taniguchi, H. Nakanishi, K. Takahashi, M. Miyahara, W. Ikeda, S. Yokoyama, Y.-F. Peng, K. Yamanishi, and Y. Takai, manuscript submitted for publication). When gD was added to the coculture system of nectin-1α-L and -1α-EL cells, nectin-1α was hardly concentrated at adhesion sites between the same type of cells or between two different types of cells (Fig. 10, bottom). The recruitment of E-cadherin to adhesion sites between nectin-1α-L and -1α-EL cells was remarkably reduced when compared with that in the absence of gD. However, the concentration of E-cadherin at adhesion sites between two nectin-1α-EL cells was not affected. These results indicate that E-cadherin is recruited through l-afadin to nectin-based cell–cell adhesion sites without the trans-interaction of E-cadherin, and that nectin, of which trans-interaction is inhibited, is not recruited to cadherin-based cell–cell adhesion sites.
Discussion
Our previous series of studies have shown that l-afadin is essential for the colocalization and compact clustering of nectin with E-cadherin at cell–cell AJs and proper organization of E-cadherin-based cell–cell AJs (Ikeda et al. 1999; Takahashi et al. 1999; Miyahara et al. 2000). Extending these earlier observations, we have shown here that the COOH-terminal half of α-catenin is, furthermore, essential for this colocalization. This colocalization is not mediated through the cytoplasmic region of E-cadherin or β-catenin. α-Catenin directly interacts with vinculin and α-actinin through the NH2-terminal half (Knudsen et al. 1995; Nieset et al. 1997; Watabe-Uchida et al. 1998; Imamura et al. 1999) and with ZO-1 through the COOH-terminal half (Itoh et al. 1997; Imamura et al. 1999). All of these α-catenin–binding proteins directly interact with F-actin (Burridge and Feramisco 1982; Menkel et al. 1994; Johnson and Craig 1995; Itoh et al. 1997; Fanning et al. 1998). The present results show that the NH2-terminal half of α-catenin is not essential for the colocalization of nectin and l-afadin with E-cadherin, indicating that neither vinculin, α-actinin, nor ponsin is necessary for this colocalization. This result is consistent with our previous observation that ponsin forms a binary complex with either l-afadin or vinculin, and does not form a ternary complex (Mandai et al. 1999).
It is of crucial importance to clarify the molecular linkage between l-afadin and the COOH-terminal half of α-catenin. We have previously shown that l-afadin does not directly interact with α-catenin as estimated by affinity chromatography using the purified samples (Sakisaka et al. 1999). Therefore, we assumed that there might be a protein that interacts with both l-afadin and α-catenin and connects them. On the basis of this assumption, we have been attempting to isolate an l-afadin–binding protein in the presence of α-catenin or an α-catenin–binding protein in the presence of l-afadin by using various methods currently available, including yeast two-hybrid, affinity chromatography, immunoprecipitation, and blot overlay. We have not yet obtained any candidate proteins, but we have found, by the yeast two-hybrid method, that full-length l-afadin directly interacts with the COOH-terminal half of α-catenin. We have confirmed this interaction by affinity chromatography, although the stoichiometry of this interaction is small. We have attempted to increase this small stoichiometry by the use of the NH2-terminal or COOH-terminal half of l-afadin. The COOH-terminal half directly interacted with the COOH-terminal half of α-catenin, but the stoichiometry did not increase (data not shown). We have added the NH2-terminal half of ZO-1 because it has been shown to directly interact with the AF-6 protein/s-afadin (Yamamoto et al. 1997) and with the COOH-terminal half of α-catenin (Itoh et al. 1997), but the NH2-terminal half of ZO-1 does not increase the stoichiometry. We have not yet obtained the conditions where the stoichiometry is increased. Moreover, l-afadin and the COOH-terminal half of α-catenin overexpressed in COS7 cells are not coimmunoprecipitated to a significant extent. Thus, our results concerning the interaction of l-afadin with the COOH-terminal half of α-catenin are apparently inconsistent depending on the methods used: one positive, one semi-positive, and one negative. However, the negative results obtained from the immunoprecipitation experiments do not necessarily reflect in vivo negative protein–protein interactions, because coimmunoprecipitation of two proteins is sometimes affected by an extraction buffer used in experiments, and sometimes is not observed when they form a very complicated multicomplex. Taken together, l-afadin may directly interact with α-catenin under appropriate conditions, but the molecular linkage between these two proteins may not be so simple and another factor and/or posttranslational modifications of l-afadin and α-catenin may be necessary for this linkage.
Several other possible mechanisms of the linkage between the nectin/afadin and cadherin/catenin systems are conceivable. One possibility is that l-afadin indirectly interacts with α-catenin through ZO-1, because it has been shown that ZO-1 directly interacts in vitro with the AF-6 protein/s-afadin (Yamamoto et al. 1997) and with the COOH-terminal half of α-catenin (Itoh et al. 1997). However, we have previously shown that the stoichiometry of the interaction of l-afadin with ZO-1 is negligible as estimated by affinity chromatography (Sakisaka et al. 1999). We have shown here that ZO-1 does not affect the interaction of l-afadin with the COOH-terminal half of α-catenin. Therefore, this possibility is unlikely. The second possibility is that l-afadin indirectly interacts with α-catenin through vinculin, because it has been shown that the COOH-terminal half of α-catenin directly interacts with vinculin in vitro (Weiss et al. 1998) and that vinculin interacts with ponsin (Mandai et al. 1999). However, consistent with previous reports (Watabe-Uchida et al. 1998; Imamura et al. 1999), we have shown by the use of nEαC-L and nEαN-L cells that vinculin is colocalized with the NH2-terminal half of α-catenin (nEαN), but not with the COOH-terminal half (nEαC) or l-afadin. Therefore, this possibility is unlikely either. The third possibility is that F-actin is involved in the linkage between l-afadin and α-catenin, because l-afadin (Mandai et al. 1997) and the COOH-terminal half of α-catenin (Rimm et al. 1995) directly interact with F-actin. This possibility cannot be excluded, but the in vivo interaction of α-catenin with F-actin remains unknown.
It may be noted that nectin has a potency to recruit α- and β-catenins and E-cadherin through l-afadin at nectin-based cell–cell adhesion sites in the absence of E-cadherin or without its trans-interaction. It is likely that α-catenin is recruited there by the direct or indirect interaction with l-afadin, and that β-catenin is recruited there by the direct interaction with α-catenin. E-cadherin may be recruited there by the direct interaction with the α- and β-catenin complex through l-afadin. However, it remains unknown whether E-cadherin, which is concentrated at adhesion sites between L and EL cells stably expressing nectin, forms a cis-dimer or not.
In contrast to the recruitment of the cadherin-catenin complex to nectin-based cell–cell adhesion sites without the trans-interaction of E-cadherin, we have shown that nectin-1α, of which trans-interaction is inhibited by gD, is not recruited to E-cadherin–based cell–cell adhesion sites between two nectin-1α-EL cells. Thus, nectin trans-interacts independently of the trans-interaction of E-cadherin, and nectin, of which trans-interaction is inhibited or which lacks the ability to interact with l-afadin, does not appear to be recruited to E-cadherin–based cell–cell adhesion sites. However, it remains unresolved whether the trans-interaction of E-cadherin is dependent on the trans-interaction of nectin, because nectin has three isoforms, and cell adhesion activities of all the isoforms cannot be inhibited simultaneously at this time (Satoh-Horikawa et al. 2000).
On the basis of these present and previous observations, we propose here at least two models for the formation of cell–cell AJs. One model is that nectin and E-cadherin independently form the respective trans-interactions and the nectin/afadin and cadherin/catenin systems recruit each other to form compact cell–cell AJs. The other model is that nectin first forms a trans-interaction that recruits, through l-afadin, the cadherin-catenin system in which E-cadherin does not trans-interact, followed by the trans-interaction of E-cadherin at nectin-based cell–cell adhesion sites, finally leading to the formation of compact cell–cell AJs. It is currently unknown which is the case, but it is likely that the nectin/afadin system plays a key role in the organization of cell–cell AJs in cooperation with the cadherin-catenin system.
We have previously shown by the use of epithelial cells in afadin (−/−) mice and (−/−) embryoid bodies that not only cadherin-based AJs, but also claudin/occludin-based TJs are impaired in these mutant cells (Ikeda et al. 1999). Claudin and occludin are Ca2+-independent homophilic cell adhesion molecules at TJs, of which cytoplasmic domains interact with ZO-1, -2, and -3 (Tsukita and Furuse 1999; Tsukita et al. 1999). ZO-1 and -2 are F-actin–binding proteins that connect the cytoplasmic regions of claudin and occludin to the actin cytoskeleton (Tsukita et al. 1999). Moreover, we have shown that behavior of nectin and l-afadin is different from that of E-cadherin and similar to that of ZO-1 during the formation of TJs in cultured MDCK cells (Sakisaka et al. 1999; Asakura et al. 1999). These results suggest that the nectin/afadin system plays a key role in proper organization of not only cadherin-based cell–cell AJs, but also claudin/occludin-based TJs. It remains unknown how the nectin/afadin system organizes TJs properly, but l-afadin may also indirectly connect nectin to the component of TJs through an unidentified factor. It is of crucial importance to clarify the molecular linkages among these three different cell–cell adhesion systems.
Acknowledgments
We thank Dr. M. Takeichi (Kyoto University, Kyoto, Japan) for helpful discussions and for providing us with the anti–E-cadherin mAb. We also thank Drs. J. Miyazaki and H. Niwa (Osaka University, Osaka, Japan) for providing us with the pPCAGIZ vector, and Dr. T. Nakano for pEF-MC1neo (Osaka University, Osaka, Japan).
The work at Osaka University was supported by grants-in-aid for scientific research and for cancer research from the Ministry of Education, Science, Sports and Culture, Japan (1999, 2000).
Footnotes
Abbreviations used in this paper: aa, amino acid(s); Ab, antibody; AJ, adherens junction; F-actin, actin filament; gD, glycoprotein D; GFP, green fluorescent protein; GST, glutathione-S-transferase; HA, hemagglutinin; His6, hexa-histidine; HSV1, herpes simplex virus type 1; Ig, immunoglobulin; TJ, tight junction.
References
- Adra C.N., Boer P.H., McBurney M.W. Cloning and expression of the mouse pgk-1 gene and the nucleotide sequence of its promoter. Gene. 1987;60:65–74. doi: 10.1016/0378-1119(87)90214-9. [DOI] [PubMed] [Google Scholar]
- Aoki J., Koike S., Ise I., Sato-Yoshida Y., Nomoto A. Amino acid residues on human poliovirus receptor involved in interaction with poliovirus. J. Biol. Chem. 1994;269:8431–8438. [PubMed] [Google Scholar]
- Aoki J., Koike S., Asou H., Ise I., Suwa H., Tanaka T., Miyasaka M., Nomoto A. Mouse homolog of poliovirus receptor-related gene 2 product, mPRR2, mediates homophilic cell aggregation. Exp. Cell Res. 1997;235:374–384. doi: 10.1006/excr.1997.3685. [DOI] [PubMed] [Google Scholar]
- Asakura T., Nakanishi H., Sakisaka T., Takahashi K., Mandai K., Nishimura M., Sasaki T., Takai Y. Similar and differential behavior between the nectin-afadin-ponsin and cadherin-catenin systems during the formation and disruption of the polarized junctional alignment in epithelial cells. Genes Cells. 1999;4:573–581. doi: 10.1046/j.1365-2443.1999.00283.x. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brieher V.M., Yap A.S., Gumbiner B.M. Lateral dimerization is required for the homophilic binding activity of C-cadherin. J. Cell Biol. 1996;135:487–496. doi: 10.1083/jcb.135.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchert M., Scheider S., Maskenaite V., Adams M.T., Canaani E., Baechi T., Moelling K., Hovens C.M. The junction-associated protein AF-6 interacts and clusters with specific Eph receptor tyrosine kinases at specialized sites of cell–cell contact in the brain. J. Cell Biol. 1999;144:361–371. doi: 10.1083/jcb.144.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Feramisco J.R. α-Actinin and vinculin from non-muscle cellscalcium sensitive interactions with actin. Cold Spring Harbor Symp. Quant. Biol. 1982;46:587–597. doi: 10.1101/sqb.1982.046.01.055. [DOI] [PubMed] [Google Scholar]
- Cocchi F., Menotti L., Mirandola P., Lopez M., Campaddelli-Fiume G. The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attribute of a bona fide receptor for herpes simplex virus type 1 and 2 in human cells. J. Virol. 1998;72:9992–10002. doi: 10.1128/jvi.72.12.9992-10002.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi F., Menotti L., Dubreuil P., Lopez M., Campadelli-Fiume G. Cell-to-cell spread of wild-type herpes simplex virus type 1, but not of syncytial strains, is mediated by the immunoglobulin-like receptors that mediate virion entry, nectin1 (PRR1/HveC/HIgR) and nectin2 (PRR2/HveB) J. Virol. 2000;74:3909–3917. doi: 10.1128/jvi.74.8.3909-3917.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberlé F., Dubreuil P., Mattei M.-G., Devilard E., Lopez M. The human PRR2 gene, related to the poliovirus receptor gene (PVR), is the true homolog of the murine MPH gene. Gene. 1995;159:267–272. doi: 10.1016/0378-1119(95)00180-e. [DOI] [PubMed] [Google Scholar]
- Fanning A.S., Jameson B.J., Jesaitis L.A., Anderson J.M. The tight junction protein establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- Geraghty R.J., Krummenacher C., Cohen G.H., Eisenberg R.J., Spear P.G. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280:1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- Guarente L. Yeast promotes and lacZ fusion designed to study expression of cloned genes in yeast. Methods Enzymol. 1983;101:181–191. doi: 10.1016/0076-6879(83)01013-7. [DOI] [PubMed] [Google Scholar]
- Gumbiner B.M. Cell adhesionthe molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Gumbiner B.M. Regulation of cadherin adhesive activity. J. Cell Biol. 2000;148:399–403. doi: 10.1083/jcb.148.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrenknecht K., Ozawa M., Eckerskorn C., Lottspeich F., Lenter M., Kemler R. The uvomorulin-anchorage protein catenin is a vinculin homologue. Proc. Natl. Acad. Sci. USA. 1991;88:9156–9160. doi: 10.1073/pnas.88.20.9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock B., Böhme B., Karn T., Yamamoto T., Kaibuchi K., Holtrich U., Holland S., Pawson T., Rübsamen-Waigmann H., Strebhardt K. PDZ-domain-mediated interaction of the eph-related receptor tyrosine kinase EphB3 and the ras-binding protein AF6 depends on the kinase activity of the receptor. Proc. Natl. Acad. Sci. USA. 1998;95:9779–9784. doi: 10.1073/pnas.95.17.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda W., Nakanishi H., Miyoshi J., Mandai K., Ishizaki H., Tanaka M., Togawa A., Takahashi K., Nishioka H., Yoshida H. Afadina key molecule essential for structural organization of cell–cell junctions of polarized epithelia during embryogenesis. J. Cell Biol. 1999;146:1117–1131. doi: 10.1083/jcb.146.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura H., Tanaka K., Hihara T., Umikawa M., Kamei T., Takahashi K., Sasaki T., Takai Y. Bni1p and Bnr1pdownstream targets of the Rho family small G-proteins which interact with profilin and regulate actin cytoskeleton in Saccharomyces cerevisiae . EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:2745–2755. doi: 10.1093/emboj/16.10.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura Y., Itoh M., Maeno M., Tsukita S., Nagafuchi A. Functional domains of α-catenin required for the strong state of cadherin-based cell adhesion. J. Cell Biol. 1999;144:1311–1322. doi: 10.1083/jcb.144.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M., Nagafuchi A., Moroi S., Tsukita S. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to α catenin and actin filaments. J. Cell Biol. 1997;138:181–192. doi: 10.1083/jcb.138.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R.P., Craig S.W. F-actin binding site masked by the intramolecular association of vinculin head and tail domains. Nature. 1995;373:261–264. doi: 10.1038/373261a0. [DOI] [PubMed] [Google Scholar]
- Kawabe H., Hata Y., Takeuchi M., Ide N., Mizoguchi A., Takai Y. nArgBP2, a novel neural member of ponsin/ArgBR2/vinexin family that interacts with synapse-associated protein 90/postsynaptic density-95-associated protein (SAPAP) J. Biol. Chem. 1999;274:30914–30918. doi: 10.1074/jbc.274.43.30914. [DOI] [PubMed] [Google Scholar]
- Kioka N., Sakata S., Kawauchi T., Amachi T., Akiyama S.K., Okazaki K., Yaen C., Yamada K.M., Aota S. Vinexina novel vinculin-binding protein with multiple SH3 domains enhances actin cytoskeletal organization. J. Cell Biol. 1999;144:59–69. doi: 10.1083/jcb.144.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen K.A., Soler A.P., Johnson K.R., Wheelock M.J. Interaction of α-actinin with the cadherin/catenin cell–cell adhesion complex via α-catenin. J. Cell Biol. 1995;130:67–77. doi: 10.1083/jcb.130.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lopez M., Eberlé F., Mattei M.-G., Gabert J., Birg F., Birdin F., Maroc C., Dubreuil P. Complementary DNA characterization and chromosomal localization of a human gene related to the poliovirus receptor-encoding gene. Gene. 1995;155:261–265. doi: 10.1016/0378-1119(94)00842-g. [DOI] [PubMed] [Google Scholar]
- Maeno Y., Moroi S., Nagashima H., Noda T., Shiozaki H., Monden M., Tsukita S., Nagafuchi A. α-Catenin-deficient F9 cells differentiate into signet ring cells. Am. J. Pathol. 1999;154:1323–1328. doi: 10.1016/s0002-9440(10)65385-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai K., Nakanishi H., Satoh A., Obaishi H., Wada M., Nishioka H., Itoh M., Mizoguchi A., Aoki T., Fujimoto T. Afadina novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. J. Cell Biol. 1997;139:517–528. doi: 10.1083/jcb.139.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai K., Nakanishi H., Satoh A., Takahashi K., Satoh K., Nishioka H., Mizoguchi A., Takai Y. Ponsin/SH3P12an l-afadin– and vinculin-binding protein localized at cell–cell and cell–matrix adherens junctions. J. Cell Biol. 1999;144:1001–1017. doi: 10.1083/jcb.144.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menkel A.R., Kroemker M., Bubeck P., Ronsiek M., Nikolai G., Jockusch B.M. Characterization of an F-actin–binding domain in the cytoskeletal protein vinculin. J. Cell Biol. 1994;126:1231–1240. doi: 10.1083/jcb.126.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahara M., Nakanishi H., Takahashi K., Satoh-Horikawa K., Tachibana K., Takai Y. Interaction of nectin with afadin is necessary for its clustering at cell-cell contact sites but not for its cis dimerization or trans interaction. J. Biol. Chem. 2000;275:613–618. doi: 10.1074/jbc.275.1.613. [DOI] [PubMed] [Google Scholar]
- Morrison M.E., Racaniello V.R. Molecular cloning and expression of a murine homolog of the human poliovirus receptor gene. J. Virol. 1992;66:2807–2813. doi: 10.1128/jvi.66.5.2807-2813.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A., Takeichi M. Transmembrane control of cadherin-mediated cell adhesiona 94kDa protein functionally associated with a specific region of the cytoplasmic domain of E-cadherin. Cell Regul. 1989;1:3679–3684. doi: 10.1091/mbc.1.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A., Shirayoshi Y., Okazaki K., Yasuda K., Takeichi M. Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature. 1987;329:341–343. doi: 10.1038/329341a0. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A., Takeichi M., Tsukita S. The 102 kD cadherin-associated proteinsimilarity to vinculin and posttranscriptional regulation of expression. Cell. 1991;65:849–857. doi: 10.1016/0092-8674(91)90392-c. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A., Ishihara S., Tsukita S. The roles of catenins in the cadherin-mediated cell adhesionfunctional analysis of E-cadherin–α-catenin fusion molecules. J. Cell Biol. 1994;127:235–247. doi: 10.1083/jcb.127.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagar B., Overduin M., Ikura M., Rini J.M. Structural basis of calcium-induced E-cadherin rigidification and dimerization. Nature. 1996;380:360–364. doi: 10.1038/380360a0. [DOI] [PubMed] [Google Scholar]
- Nieset J.E., Redfield A.R., Jin F., Knudson K.A., Johnson K.R., Wheelock M.J. Characterization of the interactions of α-catenin with α-actinin and β-catenin/plakoglobin. J. Cell Sci. 1997;110:1013–1022. doi: 10.1242/jcs.110.8.1013. [DOI] [PubMed] [Google Scholar]
- Niwa H., Burdon T., Chambers I., Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 1998;12:2048–2060. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M., Barbault H., Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO (Eur. Mol. Biol. Organ.) J. 1989;8:1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertz O., Bozic D., Koch A.W., Fauser C., Brancaccio A., Engel J. A new crystal structure, Ca2+ dependence and mutational analysis reveal molecular details of E-cadherin homoassociation. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:1738–1747. doi: 10.1093/emboj/18.7.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokutta S., Weis W. Structure of the dimerization and β-catenin-binding region of α-catenin. Mol. Cell. 2000;5:533–543. doi: 10.1016/s1097-2765(00)80447-5. [DOI] [PubMed] [Google Scholar]
- Prasad R., Gu Y., Alder H., Nakamura T., Canaani O., Saito H., Huebner K., Gale R.P., Nowell P.C., Kuriyama K. Cloning of the ALL-1 fusion partner, the AF-6 gene, involved in acute myeloid leukemias with the t(6;11) chromosome translocation. Cancer Res. 1993;53:5624–5628. [PubMed] [Google Scholar]
- Reynolds A.B., Daniel J., McCrea P., Wheelock M.J., Wu J., Zhang Z. Identification of a new cateninthe tyrosine kinase substrate p120ctn associates with E-cadherin complexes. Mol. Cell. Biol. 1994;14:8333–8342. doi: 10.1128/mcb.14.12.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimm D.L., Koslov E.R., Kebriaei P., Cianci C.D., Morrow J.S. α1(E)-Catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc. Natl. Acad. Sci. USA. 1995;92:8813–8817. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakisaka T., Nakanishi H., Takahashi K., Mandai K., Miyahara M., Satoh A., Takaishi K., Takai Y. Different behavior of l-afadin and neurabin-II during the formation and destruction of cell-cell adherens junction. Oncogene. 1999;18:1609–1617. doi: 10.1038/sj.onc.1202451. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E.F., Maniatis T. Molecular CloningA Laboratory Manual Second Edition 1989. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: pp. 545 [Google Scholar]
- Satoh-Horikawa K., Nakanishi H., Takahashi K., Miyahara M., Nishimura M., Tachibana K., Takai Y. Nectin-3a new member of immunoglobulin-like cell adhesion molecules that shows homophilic and heterophilic cell-cell adhesion activities. J. Biol. Chem. 2000;275:10291–10299. doi: 10.1074/jbc.275.14.10291. [DOI] [PubMed] [Google Scholar]
- Shapiro L., Fannon A.M., Kwong P.D., Thompson A., Lehmann M.S., Grubel G., Legrand J.F., Als-Nielsen J., Colman D.R., Hendrickson W.A. Structural basis of cell-cell adhesion by cadherins. Nature. 1995;374:327–337. doi: 10.1038/374327a0. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Nakanishi H., Miyahara M., Mandai K., Satoh K., Satoh A., Nishioka H., Aoki J., Nomoto A., Mizoguchi A., Takai Y. Nectin/PRRan immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junction through interaction with afadin, a PDZ domain-containing protein. J. Cell Biol. 1999;145:539–549. doi: 10.1083/jcb.145.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Morphogenetic roles of classic cadherins. Curr. Opin. Cell Biol. 1995;7:619–627. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- Tamura K., Shan W.S., Hendrickson W.A., Colman D.R., Shapiro L. Structure-function analysis of cell adhesion by neural (N-)cadherin. Neuron. 1998;20:1153–1163. doi: 10.1016/s0896-6273(00)80496-1. [DOI] [PubMed] [Google Scholar]
- Tessier D.C., Thomas D.Y., Khouri H.E., Laliberte F., Vernet T. Enhanced secretion from insect cells of a foreign protein fused to the honeybee melttin signal peptide. Gene. 1991;98:177–183. doi: 10.1016/0378-1119(91)90171-7. [DOI] [PubMed] [Google Scholar]
- Tomschy A., Fauser C., Landwehr R., Engel J. Homophilic adhesion of E-cadherin occurs by a co-operative two-step interaction of N-terminal domains. EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:3507–3514. [PMC free article] [PubMed] [Google Scholar]
- Tsukita S., Furuse M. Occludin and claudin in tight junction strandsleading or supporting players? Trends Cell Biol. 1999;9:268–273. doi: 10.1016/s0962-8924(99)01578-0. [DOI] [PubMed] [Google Scholar]
- Tsukita S., Furuse M., Itoh M. Structural and signalling molecules come together at tight junctions. Curr. Opin. Cell Biol. 1999;11:628–633. doi: 10.1016/s0955-0674(99)00016-2. [DOI] [PubMed] [Google Scholar]
- Visvader J.F., Elefanty A.G., Strasser A., Adams J.M. GATA-1 but not SCL induces megakaryocytic differentiation in an early myeloid line. EMBO (Eur. Mol. Biol. Organ.) J. 1992;11:4557–4564. doi: 10.1002/j.1460-2075.1992.tb05557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M., Uchida N., Imamura Y., Nagafuchi A., Fujimoto K., Uemura T., Vermeulen S., van Roy F., Adamson E.D., Takeichi M. α-Catenin–vinculin interaction functions to organize the apical junctional complex in epithelial cells. J. Cell Biol. 1998;142:847–857. doi: 10.1083/jcb.142.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Golemis E.A., Kruh G.D. ArgBP2, a multiple Src homology 3 domain-containing, Arg/Abl-interacting protein, is phosphorylated in v-Abl-transformed cells and localized in stress fibers and cardiocyte Z-disks. J. Biol. Chem. 1997;272:17542–17550. doi: 10.1074/jbc.272.28.17542. [DOI] [PubMed] [Google Scholar]
- Warner M.S., Geraghty R.J., Martinez W.M., Montgomery R.I., Whitbeck J.C., Xu R., Eisenberg R.J., Cohen G.H., Spear P.G. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. J. Virol. 1998;246:179–189. doi: 10.1006/viro.1998.9218. [DOI] [PubMed] [Google Scholar]
- Weiss E.E., Kroemker M., Rudiger A.H., Jockusch B.M., Rudiger M. Vinculin is part of the cadherin–catenin junctional complexcomplex formation between α-catenin and vinculin. J. Cell Biol. 1998;142:847–857. doi: 10.1083/jcb.141.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Harada N., Kano K., Taya S., Canaani E., Matsuura Y., Mizoguchi A., Ide C., Kaibuchi K. The Ras target AF-6 interacts with ZO-1 and serves as a peripheral component of tight junctions in epithelial cells. J. Cell Biol. 1997;139:785–795. doi: 10.1083/jcb.139.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamochi W., Tanaka K., Nonaka H., Maeda A., Musha T., Takai Y. Growth site localization of Rho1 small GTP-binding protein and its involvement in bud formation in Saccharomyces cerevisiae . J. Cell Biol. 1994;125:1077–1093. doi: 10.1083/jcb.125.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]