Abstract
Although maturation/M phase promoting factor (MPF) can activate autonomously in Xenopus egg cytoplasm, indirect evidence suggests that nuclei and centrosomes may focus activation within the cell. We have dissected the contribution of these structures to MPF activation in fertilized eggs and in egg fragments containing different combinations of nuclei, centrosomes, and microtubules by following the behavior of Cdc2 (the kinase component of MPF), the regulatory subunit cyclin B, and the activating phosphatase Cdc25. The absence of the entire nucleus–centrosome complex resulted in a marked delay in MPF activation, whereas the absence of the centrosome alone caused a lesser delay. Nocodazole treatment to depolymerize microtubules through first interphase had an effect equivalent to removing the centrosome. Furthermore, microinjection of isolated centrosomes into anucleate eggs promoted MPF activation and advanced the onset of surface contraction waves, which are close indicators of MPF activation and could be triggered by ectopic MPF injection. Finally, we were able to demonstrate stimulation of MPF activation by the nucleus–centriole complex in vitro, as low concentrations of isolated sperm nuclei advanced MPF activation in cycling cytoplasmic extracts. Together these results indicate that nuclei and microtubule asters can independently stimulate MPF activation and that they cooperate to enhance activation locally.
Keywords: cell cycle, centrosome, maturation promoting factor, microtubule, nucleus, Xenopus
Introduction
Entry into mitosis in most, if not all, cells depends on the activation of maturation/M phase promoting factor (MPF), a complex containing the kinase Cdc2 and a regulatory cyclin B subunit (for reviews see Beckhelling and Ford 1998; Ohi and Gould 1999). MPF activation has been particularly well studied in Xenopus egg cytoplasm, where it is controlled by both the accumulation and periodic degradation of cyclin B (Murray and Kirschner 1989; Murray et al. 1989) and by the phosphorylation status of Cdc2. Inhibitory phosphorylations on Cdc2, catalyzed by the kinases Wee1/Myt1, are reversed by the phosphatase Cdc25 (Lew and Kornbluth 1996). MPF activation is probably amplified by a positive feedback loop involving Cdc25, which is itself activated by MPF (Gautier et al. 1991; Hoffmann et al. 1993) and polo-like kinases (Kumagai and Dunphy 1996; Abrieu et al. 1998; Nigg 1998). Active MPF triggers a cascade of phosphorylations that promote mitotic events including nuclear membrane breakdown, the formation of a bipolar mitotic spindle, and, ultimately, cyclin destruction by the anaphase promoting complex to terminate mitosis (Sudakin et al. 1995; Peters 1999).
Studies of cells from several different species have shown that Cdc2 kinase and various cyclin B isoforms, as well as other proteins involved in controlling MPF activity, move between different subcellular locations during the cell cycle (Ohi and Gould 1999; Pines 1999). In particular, cyclin B and Cdc2, along with certain Cdc25 isoforms, shift from predominantly cytoplasmic to predominantly nuclear locations at the beginning of mitosis (Seki et al. 1992; Lopez-Girona et al. 1999; Pines 1999). Within the cytoplasm, Cdc2 and cyclin B (B1 in human cells, B2 in Xenopus) associate with microtubules and centrosomes, particularly during late interphase and M phase (Bailly et al. 1989; Alfa et al. 1990; Pines and Hunter 1991; Bailly et al. 1992; Gallant and Nigg 1992; Jackman et al. 1995; Ookata et al. 1995). Polo-like kinases also concentrate at the centrosome (Llamazares et al. 1991; Golsteyn et al. 1995). Cyclin B2 and Myt1 colocalize with the ER and Golgi apparatus in mammalian cells (Jackman et al. 1995; Liu et al. 1997), whereas Wee1 is nuclear in human cells and yeast (Heald et al. 1993; Baldin and Ducommun 1995; Aligue et al. 1997). In eggs, distinct cortical and perinuclear localization has been reported for cyclin B in amphibians (Sakamoto et al. 1998) and Drosophila (Raff et al. 1990), and Cdc25 cortical localization has been observed in Caenorhabditis elegans eggs (Ashcroft et al. 1999). The significance of these various localizations is not yet clear. Activation and/or inactivation of MPF and its regulators, in association with particular subcellular structures, may be important to coordinate changes in intracellular organization, in particular those accompanying mitosis. The controlled compartmentalization of these molecules may also be important for the regulation of cell cycle progression itself (Ohi and Gould 1999; Pines 1999). There are clear examples of such spatial regulation being important in “checkpoint control” responses to DNA damage (Toyoshima et al. 1998; Lopez-Girona et al. 1999), but as yet there has been no direct evidence for a role during the normal process of MPF activation.
In amphibian oocytes, eggs and early embryos, MPF activation can clearly occur autonomously in the cytoplasm. It can occur in enucleated oocytes and eggs (Masui 1972; Newport and Kirschner 1984; Gautier 1987; Dabauvalle et al. 1988) as well as in the absence of microtubules (Gerhart et al. 1984; Kimelman et al. 1987). Despite the independence of MPF activation from nuclei and microtubules, a body of indirect evidence indicates that these structures may modulate the timing and/or the site of MPF activation. First, MPF activation in the intact egg is first detectable in the animal hemisphere (Iwao et al. 1993; Rankin and Kirschner 1997; Pérez-Mongiovi et al. 1998), where the nuclei and microtubule nucleating centers lie. Second, meiotic MPF activation is compromised in enucleated oocytes (Gautier 1987; Iwashita et al. 1998). Other evidence comes from analysis of the pairs of periodic cortical reorganizations, or “surface contraction waves” (SCWs), that accompany MPF activation and inactivation during each early cell cycle (Hara et al. 1980; Yoneda et al. 1982; Rankin and Kirschner 1997; Pérez-Mongiovi et al. 1998). SCW initiation is enhanced in the proximity of the nucleus–centrosome complex, whereas enucleation and disruption of microtubules delay the SCWs (Sakai and Kubota 1981; Shinagawa 1983, Shinagawa 1992; Shinagawa et al. 1989). Observations of locally regulated microtubule dynamics in maturing starfish oocytes (Barakat et al. 1994) and in mitotic ctenophore eggs (Houliston et al. 1993) also indicate that regionalized MPF activation occurs in relation to nuclear/spindle position.
The Xenopus egg is large and robust enough to permit physical separation of nuclei and centrosomes into different cytoplasmic fragments, allowing the role of nucleus and centrosome in MPF activation to be addressed directly. We have performed a series of experiments designed to create viable fragments with defined compositions (see Fig. 1), using the microtubule depolymerizing drug nocodazole to disrupt the microtubule network. Microinjection of supernumerary centrosomes into anucleate eggs permitted separation of the effect of the centrosomes from that of the nuclei. We exploited the relationship between MPF activation and the first SCW to aid analysis of the influence of injected centrosomes. Our in vivo experiments were complemented by analysis of cycling cytoplasmic extracts designed to address the role of nuclei in MPF activation in vitro to compare to previous studies, which reported that nuclei inhibited rather than favored MPF activation (Dasso and Newport 1990).
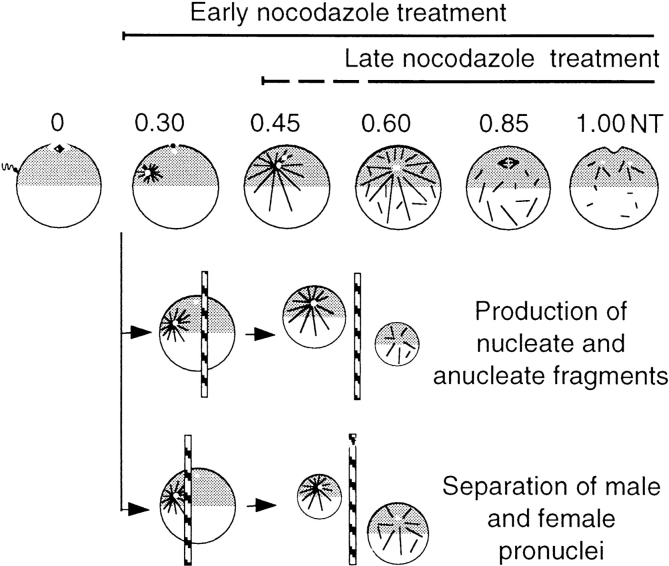
Figure 1.
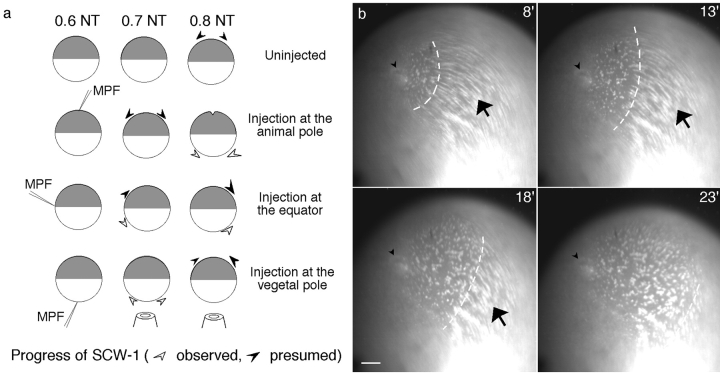
Dissection of the role of nuclei and microtubules in MPF activation in Xenopus eggs. Diagram showing the different experimental protocols used, in relation to the changing microtubule organization throughout the first cell cycle (Houliston and Elinson 1992). The centriole, brought by the sperm, recruits cytoplasmic components to form an active centrosome that nucleates a giant microtubule aster, which is first manifest by pigment accumulation around the sperm entry point in the animal cortex between 0.2 and 0.3 NT. The sperm aster mediates the migration of both male and female pronuclei to the center of the animal hemisphere where they are found closely apposed at ∼0.5 NT. At this time, general microtubule polymerization occurs throughout the egg. At ∼0.8 NT, the mitotic spindle forms in the animal hemisphere whereas cytoplasmic microtubules depolymerize. At 1.0 NT, the cleavage furrow is initiated at the animal pole. Early nocodazole treatments started between 0.2 and 0.3 NT to prevent sperm aster growth, and late treatments around 0.45 or 0.65 NT to eliminate microtubules at the time of mitosis. Nucleate and anucleate fragments were separated by placing glass rods to one side of the animal pole, thus generating a larger fragment containing the sperm aster and nuclei, and the smaller anucleate fragment. Alternatively, the rod was placed between the animal pole and the sperm entry point to produce one small fragment with the male pronucleus and attached centrosome, and a large fragment containing just the female pronucleus.
Materials and Methods
Ovulation and Fertilization
Female Xenopus laevis (Centre National de la Recherche Scientifique Rennes or Blades Biological), preinjected with 50 IU of pregnant mare serum (in some cases), were induced to ovulate by injection of 700 IU human chorionic gonadotropin (Organon). Testis minced in 80% Steinberg's solution (100% Steinberg's: 58 mM NaCl, 0.7 mM KCl, 0.8 mM MgSO4, 0.3 mM Ca[NO3]2, 5 mM Tris-HCl, pH 7.4) was used for insemination. Eggs were dejellied using 2.5% cysteine, pH 8.0, and cultured in 20% Steinberg's. Variations in ambient temperature affect the length of the first cell cycle (85–110 min), so a normalized time (NT) scale was used to express results. Insemination was 0 NT, and first cleavage (50% of control eggs showing a clear cleavage furrow at the animal pole) was 1.0 NT, with “time units” corresponding to the length of this period (Gerhart et al. 1984). Only batches in which the majority of eggs cleaved within a 5–10-min period were used.
Ligation and Nocodazole Treatment
For “ligation” in vivo, fertilization envelopes were removed from dejellied eggs in 3–5% Ficoll/80% Steinberg's. The eggs were then transferred to 80% Steinberg's in dishes coated with 2% agar, and fragments were separated using 0.3-mm-diameter glass rods (Elinson and Palacek 1993) placed to produce big and small fragments (∼3/4 and 1/4, respectively, of egg volume) with defined compositions (Fig. 1). Fragments were selected for analysis that were completely separated by 0.65 NT, well before the entry into mitosis. The success of the ligation was assessed by examining cleavage patterns in groups of cultured fragments, and only experiments showing a high success (∼95%) were considered. Normal cleavage occurred in fragments containing nuclei and centrosomes; abortive “pseudocleavage furrows” occurred in fragments with the female nucleus but no organized centrosome; and no furrow was visible in anucleate fragments. For biochemical analysis, fragments were frozen using liquid nitrogen in the presence of RM (160 mM β-glycerophosphate, 40 mM EGTA, pH 7.5, 30 mM MgCl2, 4 mM DTT) including 0.1 mM NaF and protease inhibitors (10 μg/ml each of aprotinin, leupeptin, pepstatin, and 2 mM AEBSF; all from Sigma-Aldrich or ICN Biomedicals).
To depolymerize microtubules, fertilized eggs were transferred to 10 or 30 μM nocodazole (BioMol) in 20% Steinberg's at 0.2–0.3 NT (early treatments) or at 0.45–0.65 NT (late treatments). Fragments derived from fertilized eggs were ligated in the presence of nocodazole. No nocodazole-treated eggs cleaved or displayed abortive cleavage furrows.
MPF and Centrosome Injections
Fertilized eggs were microinjected in different regions with 4–8 nl of purified recombinant human Cdc2/cyclin B–GST expressed in baculovirus-infected insect cells (gift of J. Gautier, Columbia University, New York, NY), using a Drummond microinjection apparatus. This MPF was diluted to give histone H1 kinase activity equivalent to that measured in the same volume of mitotic egg cytoplasm. Eggs were immediately mounted and SCWs recorded (see below).
Centrosomes isolated from cultured human KE37 lymphocytes (Moudjou and Bornens 1994) were provided by M. Bornens (Institute Curie, Paris, France). They were injected into anucleate eggs obtained by removal of the metaphase II meiotic spindle of unfertilized eggs by aspiration of a small amount of cytoplasm around the second polar body with a fine glass pipette (Houliston and Elinson 1991). This “enucleation” usually caused simultaneous activation of the eggs. The success of enucleation can be assessed accurately by examining the occurrence of pseudocleavage at the time of first cleavage (Briggs and King 1953; Houliston and Elinson 1991). Successful enucleation could be anticipated in favorable egg batches by the absence of characteristic large, dark pigment accumulations near the animal pole of activated eggs. 10–15 nl of centrosome suspension in 10 mM Pipes, pH 7.0, estimated to contain an average of 15 centrosomes, was injected into the animal hemisphere 5–15 min after activation. In each experiment, anucleate noninjected eggs acted as negative controls, and nucleate prick-activated eggs, with or without centrosomes, as positive controls. All anucleate and nucleate eggs were activated within a 3-min period to ensure synchrony. For biochemical analysis, eggs from all groups were transferred simultaneously at 85–90 min after activation to ice-cold RM buffer containing inhibitors (see above) to stop the cell cycle.
Western Blotting
Frozen eggs or egg fragments were pooled and thawed on ice in the presence of RM buffer containing inhibitors (as above): 40 μl per egg, 30 μl per large fragment, and 10 μl per small fragment. In centrosome-injection experiments, single eggs were homogenized directly in 30 μl of ice-cold RM buffer containing inhibitors. Samples were homogenized and the supernatant was recovered after centrifugation at 15,000 rpm in an Eppendorf centrifuge for 5 min, mixed with 4× sample buffer and heated for 5 min at 95°C. Gel electrophoresis, electrophoretic transfer to nitrocellulose, and antibody detection were performed as described previously (Pérez-Mongiovi et al. 1998). Primary antibodies were anti-PSTAIR mAb (Sigma-Aldrich), affinity-purified anti–Xenopus cyclin B2 antibody from rabbit serum provided by M. Dorée (Centre de Recherches de Biochimie Macromoleculaire, Montpellier, France), and rabbit anti–Xenopus Cdc25C pAb provided by E. Shibuya (University of Alberta, Edmonton, Canada). Horseradish peroxidase goat anti–rabbit Ig (1:10,000; Zymed) or goat anti–mouse Ig (1:10,000; Amersham Pharmacia Biotech), were used and detected using the ECL system (Amersham Pharmacia Biotech).
In Vitro Experiments
Low speed egg extracts were prepared according to Hutchison et al. 1987, Hutchison et al. 1988, with modifications. Washed, dejellied eggs taken 20 min after ionophore activation (Lindsay et al. 1995) were packed for 60 s at 1,000 rpm in a Measuring and Scientific Equipment 2-L centrifuge. 100 μg/ml cytochalasin B added before centrifugation at 10,000 rpm for 10 min at 4°C in a Beckman L-2 ultracentrifuge (SW 50.1 rotor). The supernatant was mixed with 5 μg/ml cytochalasin B and 10 μg/ml aprotinin and then centrifuged a second time at 10,000 rpm for 10 min at 4°C to clarify the extract. Demembranated sperm heads were prepared as described (Hutchison et al. 1987) and resuspended in SuNaSp buffer (0.25 M sucrose, 75 mM NaCl, 0.15 mM spermine, 0.5 mM spermidine, 15 mM Hepes, pH 7.5) at 105 nuclei/ μl, then added to 200 μl aliquots of egg extracts to give 250–4,000 nuclei/μl of extract. Incubations were started by transfer to 21°C. At appropriate time points, the following samples were removed: for protein analysis, 2 μl of incubated extract was mixed with 2 volumes of 2× SDS sample buffer and 1 volume of water and heated for 3 min at 95°C; for histone kinase assays, 2 μl of extract was mixed with 48 μl of histone kinase buffer (see below) and frozen in liquid nitrogen at each time point.
Histone H1 kinase assays were carried out according to Felix et al. 1989, with the modifications of Lindsay et al. 1995. Extract aliquots were diluted 1:25 in histone kinase buffer (80 mM β-glycerophosphate, 20 mM EGTA, 15 mM MgCl2, 1 mM DTT, pH 7.3, 10 μg/ml aprotinin, 10 μg/ml leupeptin), and 10-μl duplicates were incubated with 10 μl of reaction cocktail (0.6 mM ATP, 2 mg/ml calf thymus histone H1; Sigma–Aldrich), 75 μCi/ml [γ-32P]ATP (Amersham Pharmacia Biotech) for 15 min at 21°C. The incubations were stopped by transfer to ice. 15 μl of reaction was spotted onto P81 paper (Whatman), washed three times with phosphoric acid (150 mM), and air dried before scintillation counting.
SCW Recordings
Mitochondrial aggregates associated with germ plasm were visualized by incubating dejellied eggs for 3 min in a 2.5-μg/ml solution of DiOC6(3) (Molecular Probes) in water and diluted immediately before use from a 2.5-mg/ml stock in ethanol (Savage and Danilchik 1993; Pérez-Mongiovi et al. 1998). Eggs were transferred to 3–5% Ficoll in 80% Steinberg's solution before removing the fertilization envelope, then mounted in 80% Steinberg's between a glass slide and coverslip using a silicone rubber spacer.
Time-lapse recordings were made using a cooled CCD camera on an Axiovert 100TV fluorescence microscope equipped with a Ludl motorized stage and shutters controlled by Metamorph software. This allowed simultaneous time-lapse recordings of several eggs to be made. Images were recorded at 30-s intervals. To demonstrate movements, images were averaged over 10 frames using NIH image software (http:/rsb.info.nih.gov/ nih-image).
Results
To assess the contribution of nuclei, centrosomes, and microtubules to MPF activation at first mitosis, we undertook a series of ligation experiments. Careful positioning of the glass rod used to bisect the eggs, in relation to the animal pole and the pigmented sperm entry point, before the time of pronuclear migration (Fig. 1), yielded defined fragments containing both pronuclei, no nuclei, only the male pronucleus (with associated centriole-containing centrosomes), or only the female pronucleus (which has some poorly organized microtubule nucleating material but no centriole). The behavior of these fragments at the time of first cleavage was used to assess the success of the ligations. Fragments containing nuclei and centrosomes cleave normally, whereas those containing only the female pronucleus exhibit abortive pseudocleavage furrows, and anucleate fragments show no visible furrowing (Briggs and King 1953; Houliston and Elinson 1991). MPF activation in groups of two or three fragments of different types derived from the same eggs frozen simultaneously at successive times was monitored by Western blotting using antibodies detecting cyclin B2 and Cdc2 as described previously in Pérez-Mongiovi et al. 1998, and/or Cdc25C. The three different antibodies were applied to parts of single blots corresponding to the appropriate molecular weight region, so that slight variations in loading could be assessed by comparing Cdc2 and Cdc25C levels between lanes. Activation of MPF is detected as the loss of slower migrating Cdc2 isoforms, corresponding to the subpopulation recruited to the preactive Cdc2–cyclin B complex (Solomon et al. 1990), accompanied by a marked shift in electrophoretic mobility of Cdc25 due to mitotic phosphorylation on multiple sites.
Nuclei Stimulate MPF Activation at First Mitosis
Comparison of the timing of detectable Cdc2 dephosphorylation and Cdc25 hyperphosphorylation in nucleate and anucleate fragments derived from the same eggs indicated that whereas activation of the cyclin B–Cdc2 population in nucleate fragments occurred at approximately the same time as in intact eggs, it was delayed in anucleate fragments by ∼0.2 time units (Fig. 2; see also Fig. 5 and Fig. 6, where experiments were performed seven times with similar results). The observed effect cannot be attributed to the smaller size of anucleate fragments, since the timing of MPF activation was equivalent in large and small nucleate fragments (below). Nor was there any evidence for globally reduced or delayed cyclin B2 accumulation in anucleate fragments; in fact, cyclin B2 went on to accumulate to higher levels than in nucleate fragments (Fig. 2). Furthermore, at the time of MPF activation in nucleate fragments, the phosphorylated inactive form of Cdc2 could be detected clearly in anucleate fragments, indicating that inactive Cdc2–cyclin B complex was available for activation. This suggests that the nucleus acts to trigger or amplify the activation of preformed Cdc2–cyclin B. The delay of ∼0.2 time units in MPF activation observed in anucleate fragments corresponds closely with that observed previously for SCWs (Shinagawa 1983, Shinagawa 1992). Thus, we have confirmed directly in vivo that in Xenopus early embryos, the nucleus acts to stimulate MPF activation.
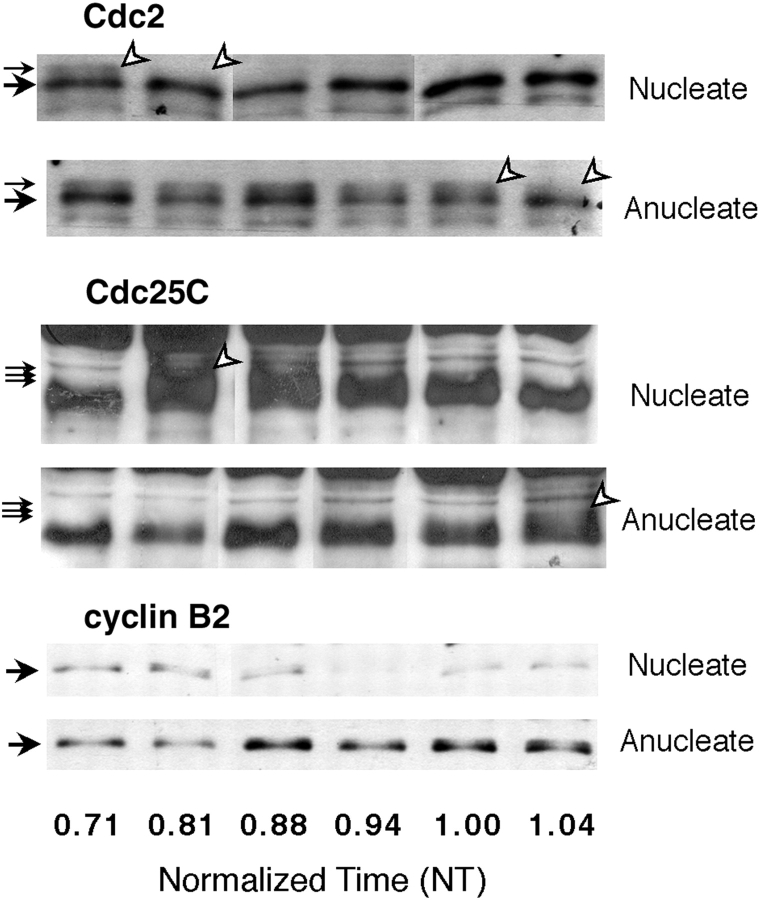
Figure 2.
MPF activation is delayed in anucleate egg fragments. Small groups of nucleate and anucleate fragments were frozen at different times, processed for Western blotting, and probed with antibodies to detect Cdc2, Cdc25C, and cyclin B2. Approximately 0.2 egg volume equivalents were loaded in each lane. For each blot shown (as well as for those in the figures following), different molecular weight regions of the same blot were probed with the appropriate antibody. Cdc2 was detected with anti-PSTAIR antibody. The small black arrows indicate the phosphorylated (upper, inactive) isoform and the large black arrows the lower isoforms of Cdc2. The lower bands include both the inactive non-T14/Y15–phosphorylated isoform and the active T14/Y15-dephosphorylated isoform. The white arrowheads indicate the maximal detected phosphorylation and the beginning of dephosphorylation of Cdc2 (MPF activation). For Cdc25C, the small arrows mark the phosphorylated isoforms, which appear upon Cdc25C activation (white arrowheads). Activation of both Cdc2 and Cdc25C was delayed by ∼0.25 time units in anucleate fragments. Cyclin B2 accumulated similarly in both fragments until ∼0.8 NT, after which time it was degraded in nucleate but not in anucleate fragments. Reaccumulation of cyclin B2 in the second cycle is first detectable in nucleate fragments at the time of cleavage.
Figure 5.
MPF activation depends on nuclei and centrosomes. Cdc2 and cyclin B2 proteins followed by Western blotting (see Fig. 2) in nucleate (Nuc) and anucleate (Anuc) fragments compared with fragments containing a single male or female pronucleus. The centrosome associated with the male pronucleus organizes a microtubule aster. Cdc2 dephosphorylation was first detectable in fragments containing both nuclei and asters, then in fragments containing the female pronucleus, and finally in anucleate fragments. Low cyclin B2 levels in the last lane of anucleate samples (left blot) partially reflects reduced loading levels (compare with Cdc2 band intensities).
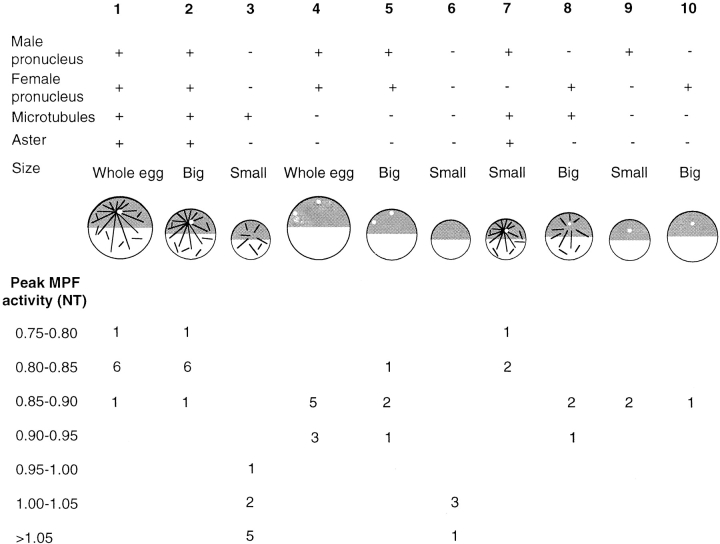
Figure 6.
Additive effects of microtubules, nuclei, and centrosomes in vivo. Combined data from ligation and nocodazole experiments in which the timing of MPF activation was assessed from blots of groups of fragments frozen at successive times with antibodies recognizing Cdc2, cyclin B2, and Cdc25C. The number of cases in which MPF activation was detected during each time interval is indicated for each category.
Stimulation of MPF Activation by Nuclei In Vitro
The extensive use of cytoplasmic extracts prepared from activated Xenopus eggs, which can undergo autonomous cycles of MPF activation and inactivation (Hutchison et al. 1988, Murray et al. 1989), has reinforced the idea that MPF activation in early amphibian embryos is controlled entirely by cytoplasmic factors. In such “cycling extracts,” neither nuclei nor microtubules are necessary for MPF activation to occur (Dasso and Newport 1990; Murray and Hunt 1993; Minshull et al. 1994), whereas exogenous nuclei can cause a delay in MPF activation. This latter effect results from the activation of a “replication checkpoint” by nuclei blocked in or still undergoing S phase (Dasso and Newport 1990). Our in vivo demonstration that nuclei can advance MPF activation raised a doubt as to whether cytoplasmic extracts fully reproduce the mechanisms of MPF regulation used in the intact egg. We thus tested the influence of nuclei on the time of MPF activation in cycling egg cytoplasmic extracts by adding different numbers of exogenous sperm nuclei. The kinetics of MPF activation was measured by histone H1 kinase activity (Fig. 3 a), confirmed by Western blotting (Fig. 3 b), and examination of chromatin condensation and nuclear envelope breakdown microscopically (not shown). Control experiments confirmed that no variation in the timing of histone H1 kinase activity peaks was observed between aliquots containing equivalent numbers of nuclei (data not shown). We found that low concentrations of nuclei (250 and 1,000 nuclei/μl of extract) advanced the time of peak MPF activation by 10–20 min at 21°C compared with that seen in extracts without nuclei (Fig. 3, similar results were obtained in three experiments), thus reproducing the effect seen in vivo. To ensure that MPF activation was not being promoted by active MPF or Cdc25C in the sperm preparation, we probed sperm nuclei for the presence of these proteins by Western blotting. No Cdc2, cyclin B2, or Cdc25 was detected in samples of 4,000 demembranated nuclei (data not shown). It is also unlikely that the preparation of nuclei stimulated cyclin B synthesis, since no consistent differences were observed between cyclin B levels in relation to the numbers of nuclei added. As reported previously (Dasso and Newport 1990), extracts containing higher concentrations of nuclei (4,000 nuclei/μl) showed delayed MPF activation. This indicates that with higher concentrations of sperm nuclei present, the replication machinery in the extracts was insufficient to allow complete replication by the time MPF activated in control extracts, triggering the replication checkpoint to delay mitosis.
Figure 3.
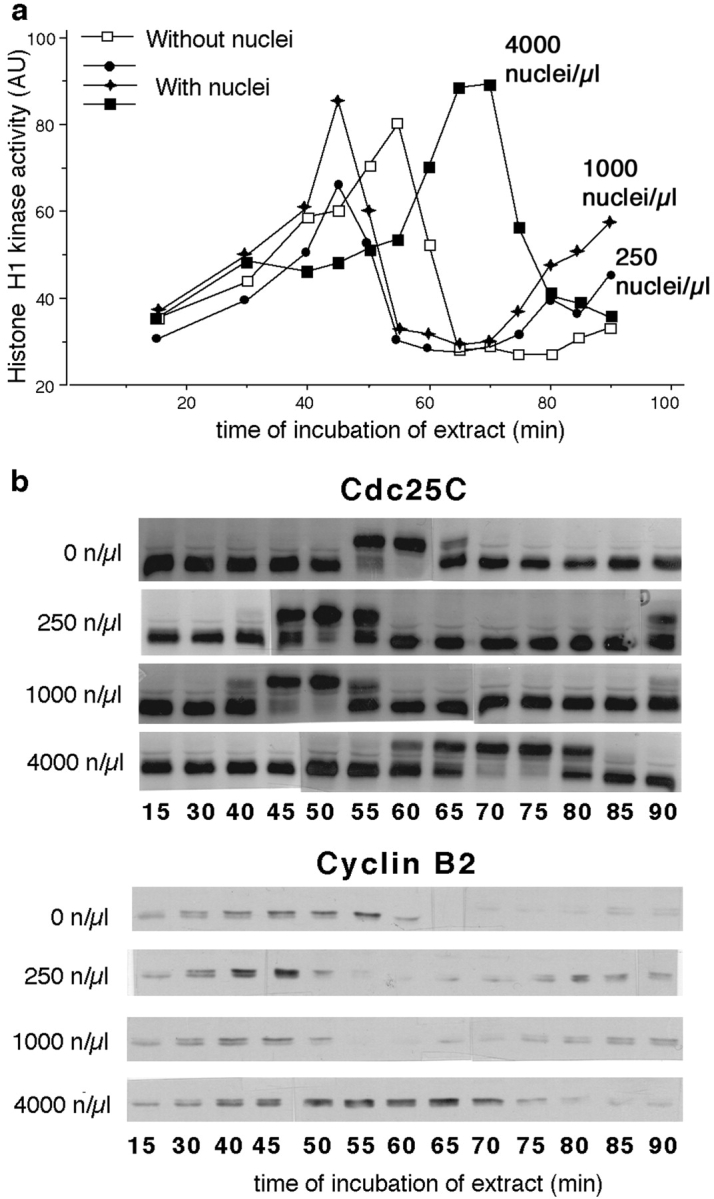
Nuclei stimulate MPF activation in vitro. (a) Histone H1 kinase activity during the first cell cycle of a Xenopus extract made 20 min after egg activation. Increasing volumes (0–8 μl) of nucleus suspension were added to 200 μl aliquots of the extract to give 0, 250, 1,000, and 4,000 nuclei/μl of extract, then divided into 10 μl aliquots, incubated at 21°C, and samples were taken at 5-min intervals over a 90-min period. 250 and 1,000 nuclei/μl advanced the peak of MPF activation by 10 min compared with extracts without nuclei. The delayed MPF activation in the presence of 4,000 nuclei/μl is explained by the activation of the DNA replication checkpoint (Dasso and Newport 1990). (b) Western blot performed with the same samples using anti-Cdc25C and anti-cyclin B2 antibodies. Maximum phosphorylation of Cdc25C and peak cyclin B2 coincided with peak H1 kinase activity.
Participation of Microtubules in MPF Activation
The experiments described above allowed us to show clearly both in vivo and in vitro that low concentrations of sperm-derived nuclei advance the time of MPF activation. Since sperm-derived nuclei have tightly associated centrioles, we reasoned that microtubule asters nucleated around this structure may contribute to the stimulatory effect. The sperm-derived centriole provides the dominant microtubule nucleating center in the egg during the first 20–40 min after fertilization, such that the vast majority of egg microtubules at this time form a rapidly expanding “sperm aster” around it (Houliston and Elinson 1992). During the second half of the first interphase period, more generalized microtubule polymerization occurs throughout the egg. Then, just before mitosis, the duplicated centrosome nucleates small prophase asters, which separate to form the mitotic spindle poles, whereas cytoplasmic microtubules depolymerize elsewhere (Fig. 1). To test the influence of these different microtubule structures on the timing of MPF activation, eggs were treated with 10 or 30 μM nocodazole at different times after fertilization. We verified that 10 μM nocodazole caused depolymerization of the vast majority of egg microtubules within 10–15 min of treatment as assessed by antitubulin immunofluorescence (data not shown). Cleavage was blocked in all nocodazole-treated groups. In seven experiments in which nocodazole treatment began between 0.2 and 0.3 NT, Western blot analysis indicated that Cdc2 dephosphorylation and Cdc25C hyperphosphorylation were delayed by ∼0.1 time units compared with untreated controls (Fig. 4 and see Fig. 6). However, early nocodazole treatment consistently delayed Cdc2 dephosphorylation and Cdc25C hyperphosphorylation. Later treatments, beginning at 0.45 or 0.65 NT, did not (Fig. 4). Since it takes 10–15 min for the drug to penetrate the egg and cause microtubule network disassembly, the cutoff time for the stimulatory role of microtubules effectively lies between 0.40 and 0.55 NT. This corresponds to the time at which the sperm aster is replaced by a more widespread microtubule network (Fig. 1; Houliston and Elinson 1992).
Figure 4.
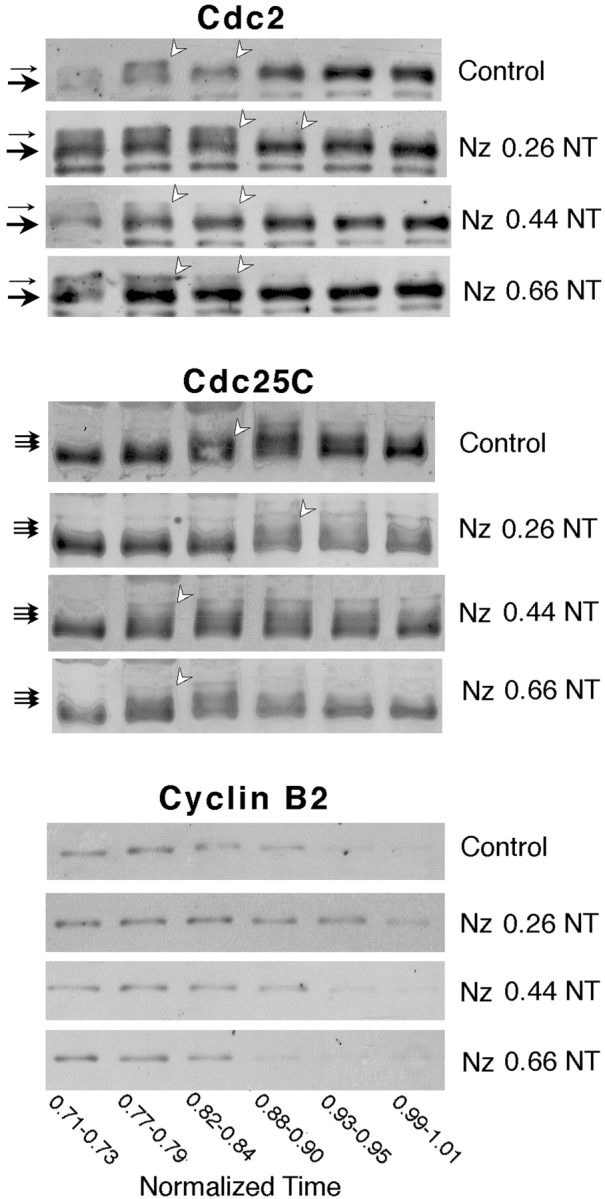
Early nocodazole treatment delays MPF activation. Western blots (details and annotations as in the legend to Fig. 2) showing the behavior of Cdc2, Cdc25C, and cyclin B2 during first mitosis after treatment at different times with 10 mM nocodazole. Treatments were started at 0.26, 0.44, and 0.66 NT as indicated. The times below each lane show the period of collection and freezing of control and treated eggs. Eggs treated at 0.26 NT showed a delay in Cdc2 and Cdc25C activation of ∼0.1 time units, whereas treatments beginning at 0.44 and 0.66 NT did not. Degradation of cyclin B2 was delayed in the early nocodazole-treated eggs and appeared to degrade more slowly.
Although the accumulation of cyclin B2 was similar in treated and nontreated eggs, in some experiments it appeared to be slightly enhanced in the presence of nocodazole (three out of seven early treatments, and one out of four later treatments). A variable inhibitory effect of nocodazole on subsequent cyclin B degradation was also observed in 6 of the 11 treated groups. A generalized role for microtubules in encouraging cyclin B2 degradation has been demonstrated previously in unfertilized eggs (Thibier et al. 1997). Increased cyclin B accumulation through the first cell cycle could explain the slight advance in the onset of MPF activation and the delay of inactivation detected in eggs fertilized in the presence of nocodazole (Gerhart et al. 1984). It may also partially offset the stimulatory effect of the sperm aster to produce the more irregular broad MPF activation peak at first mitosis in such eggs described by Gerhart et al. 1984.
Taken together, our nocodazole experiments indicate that although microtubules are not required for MPF activation in Xenopus eggs and may generally oppose MPF activation by favoring cyclin B degradation, the microtubules of the sperm aster stimulate the activation process at first mitosis. The sperm aster may act by reorganizing components in the animal cytoplasm involved later in the MPF activation process (see Discussion).
Additive Effects of Microtubule Asters and Nuclei to Stimulate MPF Activation
The experiments described above showed that elimination of sperm aster microtubules, like enucleation, can delay Cdc2 dephosphorylation during the first cell cycle. Since the delay provoked by early nocodazole treatment was less than that observed in the absence of a nucleus, the absence of the sperm aster in enucleate eggs is unlikely to fully account for the observed delay, suggesting that the nucleus itself contributes to the activation process. The relative contributions of the nucleus, centrosome, and microtubules were assessed by comparing the timing of MPF activation in various types of egg fragments produced, as shown in Fig. 1, with or without nocodazole treatment. An example of an experiment comparing the effect of different combinations of nuclei and centrioles is shown in Fig. 5, and the results obtained from the various experiments are combined in Fig. 6. Note that as shown previously for SCWs (Shinagawa 1992), the time of Cdc2 dephosphorylation was not affected by the size of the fragments. It occurred in parallel in whole eggs, large fragments (∼3/4 volume of a whole egg), and small fragments (∼1/4 volume of a whole egg) as long as nuclei, centrosomes, and microtubules were present. The same was true for different sized fragments containing nuclei but no microtubules.
Fragments lacking both nuclei and sperm asters showed a consistent delay in Cdc2 dephosphorylation of ∼0.2 time units, compared with shorter delays of ∼0.1 time units for nocodazole-treated eggs or nocodazole-treated fragments containing nuclei (Fig. 6, compare columns 3 and 6 with 4, 5, 9, and 10). Furthermore, the presence of either pronucleus or the zygote nucleus was sufficient to advance MPF activation in the absence of microtubules (Fig. 6, compare columns 5, 9, and 10 with 6). It is also informative that the time of Cdc2 dephosphorylation in fragments lacking a centrosome (anucleate, columns 3 and 6; or with female pronucleus only, columns 8 and 10) was not affected by nocodazole treatment, indicating that microtubules only have an effect if organized as an aster. The importance of the aster is highlighted by the observation that fragments containing the male pronucleus and centrosome activate MPF in advance of those containing the female pronucleus lacking an organized centrosome (Fig. 5 and Fig. 6).
Taken together, the results of these experiments show that both nuclei and the sperm aster act to stimulate MPF activation in the Xenopus egg. The centrosome-nucleated sperm aster seems to enhance the stimulatory effect of the nucleus alone.
Centrosomes Injected into Anucleate Eggs Advance SCWs
To test whether microtubule asters could affect MPF activation in the absence of a nucleus, we injected purified centrosomes into anucleate eggs obtained by removal of the meiotic spindle at the time of activation. Successful elimination of the female pronucleus was judged by failure to show pseudofurrows at the time of mitosis (Briggs and King 1953; Houliston and Elinson 1991), which varied between egg batches but comprised at least 60% in the experiments used (5 experiments, with 10–20 eggs followed in each). Centrosome solutions were diluted sufficiently to produce multiple cortical pigment accumulations in most injected eggs during first interphase, indicating the presence of microtubule asters. Eggs showing these pigment patterns reliably underwent multiple pseudofurrows, indicating the presence of active centrosomes (Heidemann and Kirschner 1975).
Analysis of MPF activation after enucleation and centrosome microinjection is compromised by the variable reliability of these procedures. To circumvent this difficulty, we first chose to monitor the time of MPF activation by videomicroscopy of the SCWs during the mitotic period beyond the time of pseudocleavage in prick-activated controls. This method has the advantage of allowing the status of each egg to be determined definitively at the time of cleavage. In addition, it does not depend on sample collection at a single time point. A close correlation has been demonstrated between SCW progression and MPF activation propagating across the egg (Rankin and Kirschner 1997; Pérez-Mongiovi et al. 1998). Both are blocked in isolated vegetal egg fragments and delayed by equivalent amounts in anucleate fragments (Pérez-Mongiovi et al. 1998). To further validate the use of SCWs as reporters of MPF activation, we injected human recombinant MPF into different regions of the egg before mitosis (∼0.65 NT). Videomicroscopy of mitochondrial island displacement (Savage and Danilchik 1993; Pérez-Mongiovi et al. 1998) (Fig. 7) showed that vegetal or equatorial MPF injections provoked ectopic cortical waves, similar to the first SCW, which propagated across the vegetal hemisphere from the injection site (Fig. 7 b). These were 40–50% slower than endogenous SCWs, but still fell within the range of SCW speeds reported previously (Savage and Danilchik 1993; Pérez-Mongiovi et al. 1998). Subsequent cleavage furrows sometimes deviated around the injected region, perhaps because of impaired degradation of exogenous human Cdc2/cyclinB–GST locally. Injections into the animal hemisphere provoked premature waves indistinguishable from normal SCWs, and premature cleavage furrows, indicating that a premature endogenous MPF activation and inactivation cycle had been triggered. Injections of heat-denatured MPF had no effect.
Figure 7.
MPF injection provokes waves of cortical reorganization. (a) Diagram summarizing the effects of precocious injection of active MPF (human recombinant Cdc2–cyclin B) into different sites of fertilized eggs during interphase (0.6 NT). Animal pole injections (n = 2) provoked premature SCWs and cleavage furrow formation. Injection in the equatorial region (n = 2) and vegetal pole (n = 8) produced waves propagating from the injection point that were similar in appearance, although not identical to the first SCW. (b) Superimposed sequences of 10 consecutive images from 1 recording showing the wave of cortical reorganization produced by vegetal injection of MPF. White stained dots are mitochondrial islands labeled with DiOC6(3). These translocate in a coordinate fashion indicating the direction of the cortical rotation relative to the cytoplasm (arrow). The cortical rotation was progressively stopped by the passage of a wave, similar to the first SCW, propagating from the point of injection (arrowhead). The time after injection in minutes is indicated. This ectopic wave is visible as a front of stationary germ plasm islands (dashed line). Bar, 100 μm.
To assess the effect of centrosomes, simultaneous recordings were made of SCW progression in centrosome-injected anucleate–activated eggs, anucleate eggs without centrosomes, nucleate prick-activated eggs, and nucleate centrosome-injected eggs (all activated within a 3-min period, Fig. 8 a). The timing of SCWs in the five different experiments performed is illustrated in Fig. 8 b. In anucleate eggs (A), SCWs in the vegetal hemisphere were delayed; typical SCWs coinciding with the end of the cortical rotation were observed in only two of five experiments. In the anucleate eggs in the other three experiments, accelerated aggregation of the mitochondrial islands towards the vegetal pole was observed after the cortical rotation had finished, but before the SCWs arrived. Similar aggregation movements in a prolonged gap between the cortical rotation and the SCWs were observed in some anucleate fragments produced by ligation (Pérez-Mongiovi, D., unpublished observations). However, in all nine “anucleate” eggs injected with centrosomes (A+C, Fig. 8), characteristic SCWs coincided with the end of the cortical rotation and appeared clearly in advance compared with uninjected anucleate eggs. Note that whereas the status of anucleate and activated eggs could be attributed definitively at the end of the recordings by examining cleavage patterns, the status of anucleate eggs injected with centrosomes could not be verified, since either a residual female pronucleus or injected centrosomes can cause furrowing activity. This introduces an error into the data in Fig. 8A+C, such that 25–40% of these eggs may have possessed a nucleus (and/or injected centrosome) based on our success at enucleation.
Figure 8.
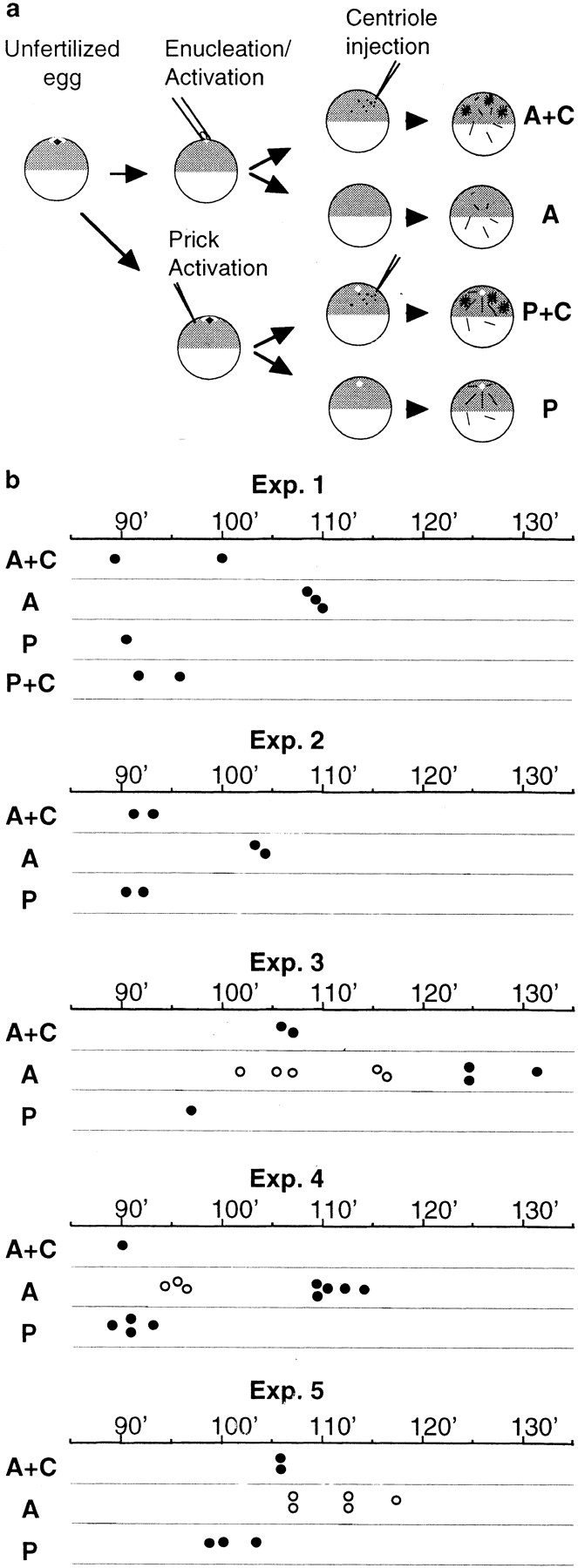
Centrosome injection into anucleate eggs advances SCWs. (a) Experimental design. Definitive assignment of egg categories was made at the end of each experiment based on cleavage pattern (see text). A, anucleate eggs; A+C, anucleate egg with centrosomes; P, prick-activated eggs; P+C, prick-activated eggs with centrosomes. (b) Comparison of timing of the first SCW in different egg categories in five separate experiments. Filled circles indicate the time (minutes after activation) that the first SCW crossed the vegetal hemisphere. Open circles represent the time of exaggerated germ plasm aggregation starting at the end of the cortical rotation seen in anucleate eggs in three experiments, which preceded characteristic SCWs in two of these experiments. Enucleation success for experiments (Exp.) 1, 2, 3, and 4 was 60%, and for experiment 5 was 75% (assessed from groups of 10–20 eggs cultured in parallel).
We conclude from these experiments that microtubule asters can restore and/or advance the timing of typical SCWs to anucleate eggs. They thus indicate that centrosomes can stimulate MPF activation in the absence of nuclei.
Centrosome Injection into Anucleate Eggs Stimulates MPF Activation
To obtain biochemical confirmation that the SCWs observed in anucleate eggs injected with centrioles indeed marked precocious MPF activation, we repeated the experiment outlined in Fig. 8 a and harvested eggs for Cdc2, cyclin B, and Cdc25 C blots. The time of sample collection was chosen as 85–90 min after activation. From the timing of SCWs in the vegetal hemisphere (Fig. 8 b), we expected that at this time anucleate eggs would tend to contain inactive pre-MPF, activated eggs to have completed mitosis, and centrosome-injected eggs to be in intermediate states. Note that MPF activation in the animal cytoplasm precedes vegetal SCWs by 15–20 min (Pérez-Mongiovi et al. 1998). Since eggs used for biochemistry cannot be scored for behavior at the time of cleavage, errors of attribution could not be corrected as they could in the SCW experiments. This introduced variation that made initial experiments hard to interpret. We subsequently used only batches of eggs in which pigment patterns favored selection of anucleate eggs before mitosis, and discarded eggs with ambiguous pigment patterns. Although fewer eggs were available for analysis in each experiment, this stringent selection considerably reduced errors in the A and A+C categories.
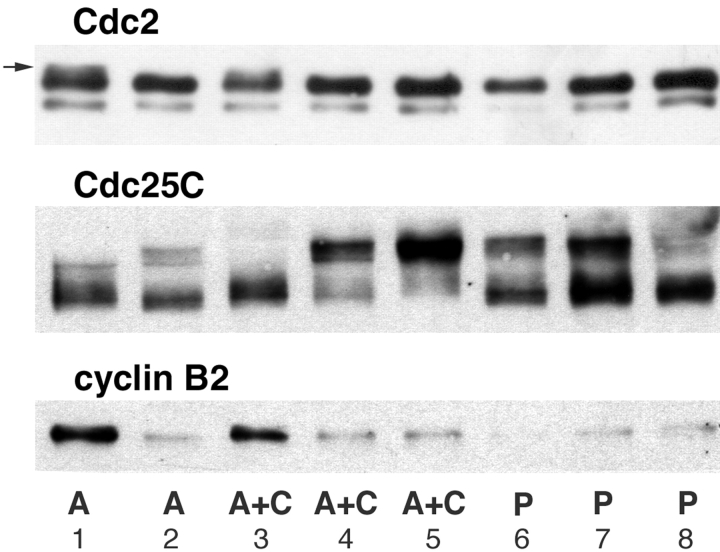
An example of one experiment is shown in Fig. 9. At the time of sample preparation, all three prick-activated eggs (P) were clearly postmitotic, as indicated by the dephosphorylation of Cdc25C and absence of cyclin B2, whereas one of the two A-category eggs was clearly premitotic, with cyclin B2 and inactive Cdc2 isoforms detectable. Enucleation in the second A-category egg was probably unsuccessful since its protein profile was equivalent to those of prick-activated eggs. In contrast, two of the three A+C eggs had blot profiles clearly distinct from both A and P eggs. They contained fully active MPF as shown by hyperphosphorylation of Cdc25C. Enucleation was almost certainly successful in all three A+C eggs, since MPF activation was clearly delayed with respect to the prick-activated eggs. Centrosome injection does not appear to interfere with MPF activation, since P+C and P eggs harvested simultaneously had similar blot profiles (not shown). Thus, we can conclude that centrosomes advanced the cell cycle in two of three anucleate eggs injected in this experiment. The third A+C egg was in a premitotic state, expected if the injected centrosomes were not active or were unable to provoke detectable activation at this time.
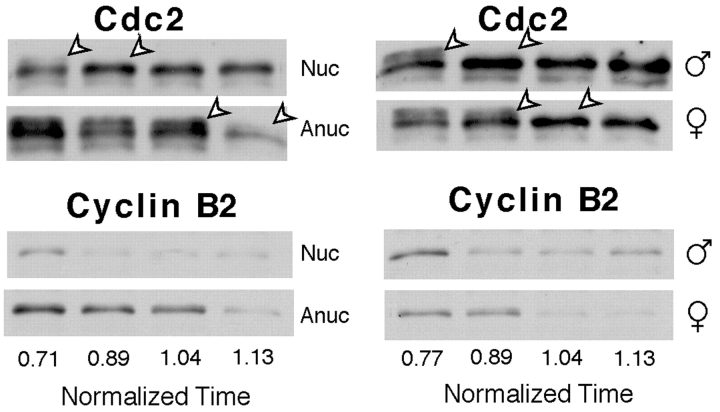
Figure 9.
Centrosome injection into anucleate eggs stimulates MPF activation. Cdc2/Cdc25C/Cyclin B2 blots (details and annotations as in the legend to Fig. 2) of single eggs harvested simultaneously 88 min after activation from an equivalent experiment to that described in the legend to Fig. 8. At this time, the “anucleate” (A) egg in track 1 contained preactive MPF as shown by the presence of inactive Cdc2 isoforms (arrow) and cyclin B2. The “anucleate” centriole-injected (A+C) egg in lane 3 was in an equivalent premitotic state; however, the other two were strongly mitotic, with no detectable inactive, phosphorylated Cdc2, and highly phosphorylated Cdc25C. In contrast, the three prick-activated (P) eggs (lanes 6–8) have all passed through mitosis, as indicated by the partial dephosphorylation of Cdc25C and absence of cyclin B2. The migration of the three proteins in the A egg in lane 2 resembles the activated eggs, suggesting that enucleation was probably unsuccessful.
A second experiment gave very similar results: four of six A+C eggs contained fully active MPF, whereas four of five P eggs were postmitotic, and two of the four A eggs were premitotic (one mitotic and one postmitotic). Thus, whereas MPF activation in anucleate eggs is clearly delayed with respect to eggs containing nuclei, albeit to variable degrees, the addition of centrosomes tends to reduce this delay. These data are entirely consistent with the SCW analyses, confirming that SCWs are reliable markers for MPF activation. In addition, they lend biochemical support that centrosomes can stimulate MPF activation in the absence of a nucleus.
Discussion
MPF activation at first mitosis in Xenopus eggs begins at a site within the animal hemisphere (Rankin and Kirschner 1997; Pérez-Mongiovi et al. 1998). We have been able to demonstrate by a combination of approaches that both nuclei and microtubule asters can stimulate MPF activation and are likely to contribute to this localized effect. To assess the role of centrosomes in anucleate eggs, we used SCWs as an indirect indicator of MPF activity. The close spatiotemporal correspondence between the progress of the SCWs and the waves of MPF activation and inactivation and the identical response of SCWs and MPF activation in ligation and enucleation experiments (Shinagawa 1985; Pérez-Mongiovi et al. 1998; this study) suggested that MPF activation and SCWs were closely related. We show further evidence of a causal relationship by triggering ectopic SCWs by localized MPF injection. We are confident in using SCW initiation as a marker for the site of MPF activation, and can interpret the comprehensive series of findings of Shinagawa concerning SCWs (Shinagawa 1983, Shinagawa 1985, Shinagawa 1992; Shinagawa et al. 1989) as revealing stimulatory effects of the nucleus, centrosomes, and microtubules on MPF activation. Taken together, the results from Shinagawa's group and our current study show that nuclei and microtubule asters can separately enhance MPF activation. We propose that these structures act independently, and cooperate to focus MPF activation within the intact egg.
Enhancement of MPF Activation by Structural Components In Vivo and In Vitro
The effect of the nucleus–centrosome complex on MPF activation in vivo was revealed as a clear delay in detectable Cdc2 activation and Cdc25 hyperphosphorylation in anucleate egg fragments compared with nucleate fragments. Surprisingly, we were also able to reproduce the stimulatory effect of the nucleus–centrosome complex in cytoplasmic extracts from activated eggs. Low concentrations of sperm-derived nuclei incubated in these extracts advanced MPF activation by 10–20 min, an interval similar to that seen in egg fragments. Our inability to accelerate MPF activation further by adding more nuclei probably reflects the requirement for cyclin B to accumulate to a sufficient level to allow MPF activation (Murray and Kirschner 1989; Hartley et al. 1996). It may also partly depend on a limit imposed by the time necessary for S phase (Blow et al. 1989), since a DNA-replication checkpoint ensures that mitosis is delayed until replication is complete. Cycling extracts supplemented with nuclei were originally used to demonstrate this checkpoint: MPF activation was delayed above a high threshold nuclear concentration and the effect was related to the presence of incompletely replicated DNA (Dasso and Newport 1990). MPF activation was not directly compared in samples with and without low numbers of nuclei in previous studies (Dasso and Newport 1990; Minshull et al. 1994).
Now that we have clearly established the role of the nucleus and microtubule asters in MPF activation by in vivo experiments, it may be possible to dissect the molecular basis of their action by manipulating egg cytoplasmic extracts.
The Nucleus and MPF Activation
The stimulation of MPF activation by nuclei was detectable in the absence of microtubules, implying that nuclear factors participate directly in the activation process. The known partitioning of MPF regulators between the cytoplasm and the nucleus, for instance the nuclear retention of phosphorylated cyclin B, is likely to favor initiation and/or amplification of MPF activation within the nucleus (see Introduction). In fission yeast, MPF activation in the nucleus is predominant, and mitosis can be blocked in response to DNA damage by exclusion of Cdc25 from the nucleus (Lopez-Girona et al. 1999). In Xenopus and other multicellular eukaryotes, where cytoplasmic activation is more significant, it is not clear where MPF first activates. In maturing amphibian oocytes, evidence favors initial cytoplasmic activation whereas nuclear factors aid amplification (Gautier 1987; Iwashita et al. 1998). The nuclear translocation of cyclin B1 that occurs before meiosis (Li et al. 1997; Yang et al. 1998, Yang et al. 1999; Hagting et al. 1999) is not sufficient to induce MPF activation (Pines 1999). Likewise, in starfish oocytes MPF activation appears to precede nuclear import of cyclin B (Ookata et al. 1992). In mammalian cells, the cytoplasmically localized B isoform of Cdc25 activates before the predominantly nuclear Cdc25C. Cdc25B is thought to act as an initial MPF activator, and Cdc25C to participate in amplification (Gabrielli et al. 1996, Gabrielli et al. 1997; Nishijima et al. 1997; Lammer et al. 1998; Karlsson et al. 1999). Our data indicate that in the fertilized Xenopus egg, both nuclear and cytoplasmic factors contribute to MPF activation, and that the nucleus is important in initiating and/or amplifying MPF activation locally within the animal half of the egg.
Centrioles, Asters, and MPF Activation
We have been able to demonstrate that isolated centrosomes introduced shortly after egg activation can advance SCWs and stimulate MPF activation in the absence of nuclei. We also observed that nocodazole treatment and/or the absence of a centriole caused a delayed MPF activation in the first cell cycle, albeit less than the delay seen in the absence of the nucleus–centrosome complex. Similarly, microtubule depolymerization has been reported to delay MPF activation in fertilized sea urchin eggs (Walker et al. 1997) and to prevent it in fission yeast (Alfa et al. 1990). We propose that active centrosomes, by nucleating microtubule asters, can organize cytoplasmic components in such a way as to favor local MPF activation in the perinuclear region. The period during which microtubules were required to enhance MPF activation in Xenopus eggs coincides with the presence of the sperm aster, a giant aster that forms around the sperm centriole shortly after fertilization. This implicates the sperm aster in promoting MPF activation. The relatively small prophase asters that form at the beginning of mitosis may also stimulate MPF activation. A local effect of prophase asters on overall MPF activation may be insufficient to be detected, or may be masked, by a general antagonizing effect of microtubules on MPF activation (see below).
Previous studies showed that MPF-activation cycles in Xenopus eggs are microtubule independent (Gerhart et al. 1984; Kimelman et al. 1987). In contrast to our findings and to those concerning SCWs (Shinagawa 1983), a slight advance in the onset of MPF activation was reported in eggs fertilized and cultured in nocodazole (Gerhart et al. 1984). Activation of MPF, assayed by its ability to promote maturation when microinjected into oocytes, then proceeded with relatively slow and uneven kinetics. This may partly explain the apparent discrepancy. Cdc2 dephosphorylation and Cdc25 hyperphosphorylation can only be detected once MPF amplification is well underway, whereas the “rounding-up” monitored by Shinagawa 1983 coincides with MPF inactivation (Rankin and Kirschner 1997). A weak premature rise in MPF levels in nocodazole-treated eggs would be undetectable by these latter methods. Such kinetics may arise because polymerized microtubules generally favor MPF inactivation by encouraging cyclin B degradation. Such an effect has been demonstrated clearly in unfertilized Xenopus (Thibier et al. 1997) and mouse (Kubiak et al. 1993) eggs. Consistent with this, we observed retarded cyclin B degradation in some nocodazole experiments (see Fig. 4). Mitosis in sea urchin eggs is likewise prolonged when microtubules are depolymerized (Sluder 1979; Hunt et al. 1992). We propose that in Xenopus eggs, cyclin B accumulates more quickly in the presence of nocodazole due to decreased cyclin B turnover, resulting in generally elevated MPF activity. However, amplification steps are compromised because regulatory factors are not previously concentrated in a central region by the sperm aster. The opposing actions of microtubules may cancel each other out, depending on the timing of the treatment. Gerhart et al. 1984 preincubated and fertilized eggs in the presence of nocodazole rather than adding it during first interphase, which likely resulted in elevated cyclin B levels during the first cell cycle (Thibier et al. 1997).
The sperm aster might favor precocious MPF activation in the central region of the animal hemisphere by concentrating regulatory molecules via minus end–directed transport or association with astral microtubules, or possibly by stimulating cyclin B synthesis. Candidate regulatory factors include Cdc2, cyclin B, Cdc25, and polo-like kinases, all of which associate with microtubules and/or the centrosome or have been observed in the perinuclear region in prophase (see Introduction). Perinuclear accumulation of cyclin B is known to be microtubule dependent in Drosophila (Raff et al. 1990). Raising cyclin B levels locally could promote MPF activation by accelerating complex formation. Polo-like kinases and Cdc25 would then actively participate in MPF amplification, the former by antagonizing the phosphatase PP2A in the two-step activation of Cdc25 (Karaïskou et al. 1998, Karaïskou et al. 1999). We favor the possibility that a regulator of MPF activity, rather than locally increased levels of cyclin B, is responsible for the sperm aster's effect because cyclin B2 accumulates to elevated levels before activation in certain types of egg fragments and in nocodazole-treated eggs (Pérez-Mongiovi et al. 1998; this study).
Cooperation between Asters and Nuclei to Stimulate MPF Activation
Taken together, our results indicate that nuclei and microtubule asters have independent but additive effects on MPF activation, and suggest that these structures cooperate to trigger MPF activation within the egg. If MPF activation is initiated within the nucleus, the centrosome-nucleated aster could act to relay the state of the cytoplasm, concentrating molecules such as cyclin B in the perinuclear region and so favoring their transfer into the nucleus for activation. Since we could demonstrate stimulation of cytoplasmic activation by centrosomes in anucleate eggs, we favor the possibility that MPF activates first in the centrosomal region, before translocation to the nucleus.
Regulatory molecules have been the focus of attention in cell cycle research over the past years, and it is becoming increasingly clear that their spatial organization is of great importance (Pines 1999). Thus, whereas MPF can activate in the absence of nuclei, centrosomes, and microtubules in Xenopus eggs, we have shown that these structural components are not merely effectors, but active protagonists in controlling cell cycle progression.
Acknowledgments
We thank M. Dorée and E. Shibuya for generously supplying antibodies; J. Gautier for MPF; M. Bornens and C. Celati for providing isolated centrosomes, and F. Tournier for advice on their microinjection. We also thank Jean Gautier and our colleagues at Villefranche for useful discussions, Christian Rouvière for help with imaging, Sebastien Motreuil for frog care, and the Café de Turin for excellent seafood.
This study was financed by the Centre National de la Recherche Scientifique, Association Nationale pour la Recherche contre le Cancer contract 9144 to E. Houliston, Cancer Research Campaign funding to C.C. Ford, and an Alliance Franco-British cooperation programme. C. Beckhelling was supported by the Medical Research Council and D. Pérez-Mongiovi by Association Nationale pour la Recherche contre le Cancer.
Footnotes
D. Pérez-Mongiovi's present address is Instituto de Biologia Molecular e Celular, Universidade do Porto, Rua do Campo Alegre 823, 4150-180 Porto, Portugal.
Abbreviations used in this paper: MPF, maturation/M phase promoting factor; NT, normalized time; SCW, surface contraction wave.
References
- Abrieu A., Brassac T., Galas S., Fisher D., Labbé J.C., Dorée M. The Polo-like kinase Plx1 is a component of the MPF amplification loop at the G2/M-phase transition of the cell cycle in Xenopus eggs. J. Cell Sci. 1998;111:1751–1757. doi: 10.1242/jcs.111.12.1751. [DOI] [PubMed] [Google Scholar]
- Alfa C.E., Ducommun B., Beach D., Hyams J.S. Distinct nuclear and spindle pole body population of cyclin-cdc2 in fission yeast. Nature. 1990;347:680–682. doi: 10.1038/347680a0. [DOI] [PubMed] [Google Scholar]
- Aligue R., Wu L., Russell P. Regulation of Schizosaccharomyces pombe Wee1 tyrosine kinase. J. Biol. Chem. 1997;272:13320–13325. doi: 10.1074/jbc.272.20.13320. [DOI] [PubMed] [Google Scholar]
- Ashcroft N.R., Srayko M., Kosinski M.E., Mains P.E., Golden A. RNA-mediated interference of a cdc25 homolog in Caenorhabditis elegans results in defects in the embryonic cortical membrane, meiosis, and mitosis. Dev. Biol. 1999;206:15–32. doi: 10.1006/dbio.1998.9135. [DOI] [PubMed] [Google Scholar]
- Bailly E., Doree M., Nurse P., Bornens M. p34cdc2 is located in both nucleus and cytoplasm; part is centrosomally associated at G2/M and enters vesicles at anaphase. EMBO (Eur. Mol. Biol. Organ.) J. 1989;8:3985–3995. doi: 10.1002/j.1460-2075.1989.tb08581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly E., Pines J., Hunter T., Bornens M. Cytoplasmic accumulation of cyclin B1 in human cellsassociation with a detergent-resistant compartment and with the centrosome. J. Cell Sci. 1992;101:529–545. doi: 10.1242/jcs.101.3.529. [DOI] [PubMed] [Google Scholar]
- Baldin V., Ducommun B. Subcellular localization of human wee1 kinase is regulated during the cell cycle. J. Cell Sci. 1995;108:2425–2432. doi: 10.1242/jcs.108.6.2425. [DOI] [PubMed] [Google Scholar]
- Barakat H., Geneviere-Garrigues A.M., Schatt P., Picard P. Subcellular distribution of aster-nucleated microtubule lengtha more or less mitotic status of cytoplasmic areas during meiosis I of starfish oocytes. Biol. Cell. 1994;81:205–213. [Google Scholar]
- Beckhelling C., Ford C. Maturation promoting factor activation in early amphibian embryostemporal and spatial control. Biol. Cell. 1998;90:467–476. [PubMed] [Google Scholar]
- Blow J.J., Sheehan M.A., Watson J.V., Lanskey R.A. Nuclear structure and the control of DNA replication in the Xenopus embryo. J. Cell Sci. Suppl. 1989;12:183–195. doi: 10.1242/jcs.1989.supplement_12.16. [DOI] [PubMed] [Google Scholar]
- Briggs R., King T.J. Factors affecting the transplantability of nuclei of frog embryonic cells. J. Exp. Zool. 1953;122:485–506. [Google Scholar]
- Dabauvalle M.C., Doree M., Bravo R., Karsenti E. Role of nuclear material in the early cell cycle of Xenopus embryos. Cell. 1988;52:525–533. doi: 10.1016/0092-8674(88)90465-5. [DOI] [PubMed] [Google Scholar]
- Dasso M., Newport J.W. Completion of DNA replication is monitored by a feedback system that controls the initiation of mitosis in vitrostudies in Xenopus . Cell. 1990;61:811–823. doi: 10.1016/0092-8674(90)90191-g. [DOI] [PubMed] [Google Scholar]
- Elinson R.P., Palacek J. Independence of microtubule systems in fertilized frog eggsthe sperm aster and the vegetal parallel array. Roux's Arch. Dev. Biol. 1993;202:224–232. doi: 10.1007/BF02427883. [DOI] [PubMed] [Google Scholar]
- Felix M.A., Pines J., Hunt T., Karsenti E. Temporal regulation of cdc2 mitotic kinase activity and cyclin degradation in cell-free extracts of Xenopus eggs. J. Cell Sci. Suppl. 1989;12:99–116. doi: 10.1242/jcs.1989.supplement_12.9. [DOI] [PubMed] [Google Scholar]
- Gabrielli B., De Souza C., Tonks I., Clark J., Hayward N. Cytoplasmic accumulation of cdc25B phosphatase in mitosis triggers centrosomal microtubule nucleation in HeLa cells. J. Cell Sci. 1996;109:1081–1093. doi: 10.1242/jcs.109.5.1081. [DOI] [PubMed] [Google Scholar]
- Gabrielli B.G., Clark J.M., McCormack A.K., Ellem K.A.O. Hyperphosphorylation of the N-terminal domain of Cdc25 regulates activity toward cyclin B1/Cdc2 but not cyclin A/Cdk2. J. Biol. Chem. 1997;272:28607–28614. doi: 10.1074/jbc.272.45.28607. [DOI] [PubMed] [Google Scholar]
- Gallant P., Nigg E.A. Cyclin B2 undergoes cell cycle–dependent nuclear translocation and, when expressed as a non-destructible mutant, causes mitotic arrest in HeLa cells. J. Cell Biol. 1992;117:213–224. doi: 10.1083/jcb.117.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier J. The role of the germinal vesicle for the appearance of Maturation-Promoting Factor activity in the axolotl oocyte. Dev. Biol. 1987;123:483–486. [Google Scholar]
- Gautier J., Solomon M.J., Booher R.N., Bazan J.F., Kirschner M.W. cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991;67:197–211. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- Gerhart J.C., Wu M., Kirschner M. Cell-cycle dynamics of an M-phase-specific cytoplasmic factor in Xenopus laevis oocytes and eggs. J. Cell Biol. 1984;98:1247–1255. doi: 10.1083/jcb.98.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golsteyn R.M., Mundt K.E., Fry A.M., Nigg E.A. Cell cycle regulation of the activity and subcellular localization of Plk1, a human protein kinase implicated in mitotic spindle function. J. Cell Biol. 1995;129:1617–1628. doi: 10.1083/jcb.129.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagting A., Jackman M., Simpson K., Pines J. Translocation of cyclin B1 to the nucleus at prophase requires a phosphorylation-dependent nuclear import signal. Curr. Biol. 1999;9:680–689. doi: 10.1016/s0960-9822(99)80308-x. [DOI] [PubMed] [Google Scholar]
- Hara K., Tydeman P., Kirschner M. A cytoplasmic clock with the same period as the division cycle in Xenopus eggs. Proc. Natl. Acad. Sci. USA. 1980;77:462–466. doi: 10.1073/pnas.77.1.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley R.S., Rempel R.E., Malles J.L. In vivo regulation of the early embryonic cell cycle in Xenopus . Dev. Biol. 1996;173:408–419. doi: 10.1006/dbio.1996.0036. [DOI] [PubMed] [Google Scholar]
- Heald R., McLoughlin M., McKeon F. Human Wee1 maintains mitotic timing by protecting the nucleus from cytoplasmically activated Cdc2 kinase. Cell. 1993;74:463–474. doi: 10.1016/0092-8674(93)80048-j. [DOI] [PubMed] [Google Scholar]
- Heidemann S.R., Kirschner M.W. Aster formation in eggs of Xenopus laevis. Induction by isolated basal bodies. J. Cell Biol. 1975;67:105–117. doi: 10.1083/jcb.67.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann I., Clarke P.R., Marcote M.J., Karsenti E., Draetta G. Phosphorylation and activation of human Cdc25C by Cdc2-cyclin B and its involvement in the self-amplification of MPF at mitosis. EMBO (Eur. Mol. Biol. Organ.) J. 1993;12:53–63. doi: 10.1002/j.1460-2075.1993.tb05631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houliston E., Elinson R.P. Patterns of microtubule polymerization relating to cortical rotation in Xenopus laevis eggs. Development. 1991;112:107–117. doi: 10.1242/dev.112.1.107. [DOI] [PubMed] [Google Scholar]
- Houliston E., Elinson R.P. Microtubules and cytoplasmic reorganization in the frog egg. Curr. Top. Dev. Biol. 1992;26:53–70. doi: 10.1016/s0070-2153(08)60440-8. [DOI] [PubMed] [Google Scholar]
- Houliston E., Carré D., Johnston J.A., Sardet C. Axis establishment and microtubule-mediated waves prior to first cleavage in Beroe ovata . Development. 1993;117:75–87. doi: 10.1242/dev.117.1.75. [DOI] [PubMed] [Google Scholar]
- Hunt T., Luca F.C., Ruderman J.V. The requirements for protein synthesis and degradation, and the control of destruction of cyclins A and B in the meiotic and mitotic cell cycles of the clam embryo. J. Cell Biol. 1992;116:707–724. doi: 10.1083/jcb.116.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison C.J., Cox R., Drepaul R.S., Gomperts M., Ford C.C. Periodic DNA synthesis in cell-free extracts of Xenopus eggs. EMBO (Eur. Mol. Biol. Organ.) J. 1987;6:2003–2010. doi: 10.1002/j.1460-2075.1987.tb02464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison C.J., Cox R., Ford C.C. The control of DNA replication in a cell-free extract that recapitulates a basic cell cycle in vitro. Development. 1988;103:553–566. doi: 10.1242/dev.103.3.553. [DOI] [PubMed] [Google Scholar]
- Iwao Y., Sakamoto N., Takahara K., Yamashita M., Nagahama Y. The egg nucleus regulates the behaviour of sperm nuclei as well as cycling of MPF in physiologically polyspermic newt eggs. Dev. Biol. 1993;160:15–27. doi: 10.1006/dbio.1993.1282. [DOI] [PubMed] [Google Scholar]
- Iwashita J., Hayano Y., Sagata N. Essential role of germinal vesicle material in the meiotic cell cycle of Xenopus oocytes. Proc. Natl. Acad. Sci. USA. 1998;95:4392–4397. doi: 10.1073/pnas.95.8.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman M., Firth M., Pines J. Human cyclins B1 and B2 are localized to strikingly different structuresB1 to microtubules, B2 primarily to the Golgi apparatus. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:1646–1654. doi: 10.1002/j.1460-2075.1995.tb07153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaïskou A., Cayla X., Haccard O., Jessus C., Ozon R. MPF amplification in Xenopus oocyte extracts depends on a two-step activation of cdc25 phosphatase. Exp. Cell Res. 1998;244:491–500. doi: 10.1006/excr.1998.4220. [DOI] [PubMed] [Google Scholar]
- Karaïskou A., Jessus C., Brassac T., Ozon R. Phosphatase 2A and polo kinase, two antagonistic regulators of cdc25 activation and MPF auto-amplification. J. Cell Sci. 1999;112:3747–3756. doi: 10.1242/jcs.112.21.3747. [DOI] [PubMed] [Google Scholar]
- Karlsson C., Katich S., Hagting A., Hoffmann I., Pines J. Cdc25B and Cdc25C differ markedly in their properties as initiators of mitosis. J. Cell Biol. 1999;146:573–584. doi: 10.1083/jcb.146.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelman D., Kirschner M., Scherson T. The events of midblastula transition in Xenopus are regulated by changes in the cell cycle. Cell. 1987;48:399–407. doi: 10.1016/0092-8674(87)90191-7. [DOI] [PubMed] [Google Scholar]
- Kubiak J.Z., Weber M., Pennart H., Winston N.J., Maro B. The metaphase II arrest in mouse oocytes is controlled through microtubule-dependent destruction of cyclin B in the presence of CSF. EMBO (Eur. Mol. Biol. Organ.) J. 1993;12:3773–3778. doi: 10.1002/j.1460-2075.1993.tb06055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A., Dunphy W.G. Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science. 1996;273:1377–1380. doi: 10.1126/science.273.5280.1377. [DOI] [PubMed] [Google Scholar]
- Lammer C., Wagerer S., Saffrich R., Mertens D., Ansorge A., Hoffmann I. The cdc25B phosphatase is essential for the G2/M phase in human cells. J. Cell Sci. 1998;111:2445–2453. doi: 10.1242/jcs.111.16.2445. [DOI] [PubMed] [Google Scholar]
- Lew D.J., Kornbluth S. Regulatory roles of cyclin dependent kinase phosphorylation in cell cycle control. Curr. Opin. Cell Biol. 1996;8:795–804. doi: 10.1016/s0955-0674(96)80080-9. [DOI] [PubMed] [Google Scholar]
- Li J., Meyer A.N., Donoghue D.J. Nuclear localization of cyclin B1 mediates its biological activity and is regulated by phosphorylation. Proc. Natl. Acad. Sci. USA. 1997;94:502–507. doi: 10.1073/pnas.94.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay H.D., Whitaker M.J., Ford C.C. Calcium requirements during mitotic cdc2 kinase activation and cyclin degradation in Xenopus egg extracts. J. Cell Sci. 1995;108:3557–3568. doi: 10.1242/jcs.108.11.3557. [DOI] [PubMed] [Google Scholar]
- Liu F., Stanton J.J., Wu Z., Piwnica-Worms H. The human Myt1 kinase preferentially phosphorylates Cdc2 on threonine 14 and localizes to the endoplasmic reticulum and Golgi complex. Mol. Cell. Biol. 1997;17:571–583. doi: 10.1128/mcb.17.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llamazares S., Moreira A., Tabares A., Girdham C., Spruce B.A., Gonzalez C., Karess R.E., Glover D.M., Sunkel C.E. Polo encodes a protein kinase homolog required for mitosis in Drosophila . Genes Dev. 1991;5:2153–2165. doi: 10.1101/gad.5.12a.2153. [DOI] [PubMed] [Google Scholar]
- Lopez-Girona A., Furnari B., Montdesert O., Russell P. Nuclear localisation of Cdc25 is regulated by DNA damage and a 14-3-3 protein. Nature. 1999;397:172–175. doi: 10.1038/16488. [DOI] [PubMed] [Google Scholar]
- Masui Y. Distribution of cytoplasmic activity inducing germinal vesicle breakdown in frog oocytes. J. Exp. Zool. 1972;179:365–377. [Google Scholar]
- Minshull J., Sun H., Tonks N.K., Murray A.W. A MAP kinase-dependent spindle assembly checkpoint in Xenopus egg extracts. Cell. 1994;79:475–486. doi: 10.1016/0092-8674(94)90256-9. [DOI] [PubMed] [Google Scholar]
- Moudjou, M., and M. Bornens. 1994. Isolation of centrosomes from cultured animal cells. Cell Biology: A Laboratory Handbook. Vol. 1. Julio E. Celis, editor. Academic Press, Aarhus, Denmark. 595–604.
- Murray A., Kirschner M. Cyclin synthesis drives the early embryonic cell cycle. Nature. 1989;339:275–280. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
- Murray A.W., Hunt T. The Cell Cycle, an Introduction 1993. W.H. Freeman; New York: pp. 251 [Google Scholar]
- Murray A.W., Solomon M.J., Kirschner M.W. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989;339:280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- Newport J., Kirschner M. Regulation of the cell cycle during early Xenopus development. Cell. 1984;37:731–742. doi: 10.1016/0092-8674(84)90409-4. [DOI] [PubMed] [Google Scholar]
- Nigg E.A. Polo-like kinasespositive regulators of cell division from start to finish. Curr. Biol. 1998;10:776–783. doi: 10.1016/s0955-0674(98)80121-x. [DOI] [PubMed] [Google Scholar]
- Nishijima H., Nishitani H., Seki T., Nishimoto T. A dual specificity phosphatase cdc25 is an unstable protein and triggers p34cdc2/cyclinB activation in hamster BHK21 cells arrested with hydroxyurea. J. Cell Biol. 1997;138:1105–1116. doi: 10.1083/jcb.138.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi R., Gould K.L. Regulating the onset of mitosis. Curr. Opin. Cell Biol. 1999;11:267–273. doi: 10.1016/s0955-0674(99)80036-2. [DOI] [PubMed] [Google Scholar]
- Ookata K., Hisanaga S., Bulinski J.C., Murofushi H., Aizawa H., Itoh T.J., Hotani H., Okumura E., Tachibana K., Kishimoto T. Cyclin B interaction with microtubule-associated protein 4 (MAP4) targets p34cdc2 kinase to microtubules and is a potential regulator of M-phase microtubule dynamics. J. Cell Biol. 1995;128:849–862. doi: 10.1083/jcb.128.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ookata K., Hisanaga S., Okano T., Tachibana K., Kishimoto T. Relocation and distinct subcellular localization of p34cdc2-cyclin B complex at meiosis reinitiation in starfish oocytes. EMBO (Eur. Mol. Biol. Organ.) J. 1992;11:1763–1772. doi: 10.1002/j.1460-2075.1992.tb05228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Mongiovi D., Chang P., Houliston E. A propagated wave of MPF activation accompanies surface contraction waves at first mitosis in Xenopus . J. Cell Sci. 1998;111:385–393. doi: 10.1242/jcs.111.3.385. [DOI] [PubMed] [Google Scholar]
- Peters J.M. Subunits and substrates of the anaphase-promoting complex. Exp. Cell Res. 1999;248:339–349. doi: 10.1006/excr.1999.4443. [DOI] [PubMed] [Google Scholar]
- Pines J. Four-dimensional control of the cell cycle. Nature Cell Biol. 1999;1:E73–E79. doi: 10.1038/11041. [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T. Human cyclins A and B1 are differentially located in the cell and undergo cell cycle–dependent nuclear transport. J. Cell Biol. 1991;115:1–17. doi: 10.1083/jcb.115.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff J.W., Whitfield W.G., Glover D.M. Two distinct mechanisms localise cyclin B transcripts in syncytial Drosophila embryos. Development. 1990;110:1249–1261. doi: 10.1242/dev.110.4.1249. [DOI] [PubMed] [Google Scholar]
- Rankin S., Kirschner M.W. The surface contraction waves of Xenopus eggs reflect the metachronous cell-cycle state of the cytoplasm. Curr. Biol. 1997;7:451–454. doi: 10.1016/s0960-9822(06)00192-8. [DOI] [PubMed] [Google Scholar]
- Sakai M., Kubota H.Y. Cyclic surface changes in the non-nucleate fragment of Xenopus laevis . Dev. Growth Differ. 1981;23:41–49. doi: 10.1111/j.1440-169X.1981.00041.x. [DOI] [PubMed] [Google Scholar]
- Sakamoto I., Takahara K., Yamashita M., Iwao Y. Changes in cyclin B during oocyte maturation and early embryonic cell cycle in the newt, Cynops pyrrhogasterrequirement of germinal vesicle for MPF activation. Dev. Biol. 1998;195:60–69. doi: 10.1006/dbio.1997.8835. [DOI] [PubMed] [Google Scholar]
- Savage R., Danilchik M. Dynamics of germ plasm localization and its inhibition by ultraviolet irradiation in early cleavage Xenopus embryos. Dev. Biol. 1993;157:371–382. doi: 10.1006/dbio.1993.1142. [DOI] [PubMed] [Google Scholar]
- Seki T., Katumi Y., Hideo N., Takagi T., Russell P., Takeharu N. Chromosome condensation caused by loss of RCC1 function requires the cdc25C protein that is located in the cytoplasm. Mol. Biol. Cell. 1992;3:1373–1388. doi: 10.1091/mbc.3.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinagawa A. The interval of the cytoplasmic cycle observed in non-nucleate egg fragments is longer than that of the cleavage cycle in normal eggs of Xenopus laevis . J. Cell Sci. 1983;64:147–162. doi: 10.1242/jcs.64.1.147. [DOI] [PubMed] [Google Scholar]
- Shinagawa A. Localization of the factors producing the periodic activities responsible for synchronous cleavage in Xenopus embryos. J. Embryol. Exp. Morph. 1985;85:33–46. [PubMed] [Google Scholar]
- Shinagawa A. Relative timing of stiffening with various combinations of nucleate and enucleated egg fragments of Xenopus laevis . Dev. Growth Differ. 1992;34:419–425. doi: 10.1111/j.1440-169X.1992.00419.x. [DOI] [PubMed] [Google Scholar]
- Shinagawa A., Konno S., Yoshimoto Y., Hiramoto Y. Nuclear involvement in localisation of the initiation site of surface contraction waves in Xenopus eggs. Dev. Growth Differ. 1989;31:249–255. doi: 10.1111/j.1440-169X.1989.00249.x. [DOI] [PubMed] [Google Scholar]
- Sluder G. Role of spindle microtubules in the control of cell cycle timing. J. Cell Biol. 1979;80:674–691. doi: 10.1083/jcb.80.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M.J., Glotzer M., Lee T.H., Philippe M., Kirschner M.W. Cyclin activation of p34cdc2. Cell. 1990;63:1013–1024. doi: 10.1016/0092-8674(90)90504-8. [DOI] [PubMed] [Google Scholar]
- Sudakin V., Ganoth D., Dahan A., Heller H., Herrshko J., Luca F., Ruderman J.V., Hershko A. The cyclosome, a large complex containing cyclin-selective ubiquitin ligase activity targets cyclins for destruction at the end of mitosis. Mol. Biol. Cell. 1995;6:185–198. doi: 10.1091/mbc.6.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibier C.V., De Smedt R., Poulhe D., Huchon C., Jessus, Ozon R. In vivo regulation of cytostatic activity in Xenopus metaphase II-arrested oocytes. Dev. Biol. 1997;185:55–66. doi: 10.1006/dbio.1997.8543. [DOI] [PubMed] [Google Scholar]
- Toyoshima F., Moriguchi T., Wada A., Fukuda M., Nishida E. Nuclear export of cyclin B1 and its possible role in the DNA damage-induced G2 checkpoint. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:2728–2735. doi: 10.1093/emboj/17.10.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G.R., Shuster C.B., Burgess D.R. Microtubule-entrained kinase activities associated with the cortical cytoskeleton during cytokinesis. J. Cell Sci. 1997;110:1373–1386. doi: 10.1242/jcs.110.12.1373. [DOI] [PubMed] [Google Scholar]
- Yang J., Bardes E.S.G., Moore J.D., Brennan J., Powers M.A., Kornbluth S. Control of cyclin B1 localisation through regulated binding of the nuclear export factor CRM1. Genes Dev. 1998;12:2131–2143. doi: 10.1101/gad.12.14.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Winkler K., Yoshida M., Kornbluth S. Maintenance of G2 arrest in the Xenopus oocytea role for 14-3-3-mediated inhibition of Cdc25 nuclear import. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:2174–2183. doi: 10.1093/emboj/18.8.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda M., Kobayakawa Y., Kubota H., Sakai M. Surface contraction waves in amphibian eggs. J. Cell Sci. 1982;54:35–46. doi: 10.1242/jcs.54.1.35. [DOI] [PubMed] [Google Scholar]