Abstract
Improvements are made to our gas chromatography-mass spectrometry based assay for quantifying low levels of DNA-uracil. Folate deficiency leads to increased deoxyuridine monophosphate/thymidylate (dUMP/dTMP) ratios and uracil misincorporation into DNA, which may increase cancer risk. Vitamin B6 (B6) deficiency might also result in increased DNA-uracil because B6 is a cofactor for serine hydroxymethyltransferase (SHMT), which catalyzes the methylation of tetrahydrofolate (THF) to methylene-THF, the folate form that is required to convert dUMP to dTMP. However, the low baseline levels of DNA-uracil in healthy human lymphocytes are difficult to measure accurately. This version of the assay (Uracil assay V3) has an approximately ten-fold increase in signal strength over the previous method, and a ten-fold lower detection limit (0.2 pg uracil). Five micrograms of DNA, the amount in about 1 ml of human blood, is a suitable amount for this assay. Using this improved assay, DNA-uracil was measured in lymphocytes from twelve healthy smoking or nonsmoking young men and women who consumed a B6 restricted diet (0.7 mg B6/day, or ≈ half the RDA) for 28 days. DNA-uracil concentration was not significantly related to B6 status or smoking. More severe and/or prolonged vitamin B6 deficiency may be necessary to detect significant changes in DNA-uracil in humans. The average concentration of DNA-uracil from these subjects was found to be approximately 3,000 uracils per diploid lymphocyte, which is comparable to steady state levels of one of the oxidative adducts of DNA, 8-oxoguanine.
Keywords: folate, folic acid, DNA damage, DNA-uracil, pyridoxal phosphate, uracil, vitamin B6
Introduction
Folate deficiency leads to increased uracil misincorporation into DNA, with consequent nicks in DNA and chromosome breaks [1]. This DNA damage may contribute to the increased risk of cancer associated with folate deficiency [1; 2; 3; 4]. In folate deficiency, low cytosolic levels of N5,N10-methylene tetrahydrofolate (mnTHF), the folate cofactor for thymidylate synthase, which converts deoxyuridine monophosphate (dUMP) to thymidylate (dTMP), leads to lowered (dTMP) synthesis and a higher dUMP/dTMP ratio [5]. This higher ratio leads to an increased misincorporation of deoxyuridine triphosphate (dUTP) into DNA by DNA polymerase [6; 7; 8; 9]. In human and cell culture studies, it has been shown that DNA-uracil content rises with folate deficiency [5; 6; 7; 8]. We hypothesized that vitamin B6 (B6) deficiency may similarly lead to increased DNA-uracil because B6, as pyridoxal phosphate, is a required cofactor for serine hydroxymethyltransferase (SHMT), which catalyzes the methylation of tetrahydrofolate (THF) to form mnTHF [4; 10].
During repair of uracil in DNA, transient nicks are formed when uracil is excised by uracil-DNA glycosylase followed by cleavage of the sugar-phosphate backbone by an AP (apurinic/apyrimidinic) endonuclease. DNA polymerase β then fills in the missing base [11]. In addition to single strand breaks, folate deficiency leads to double strand breaks, which are more difficult to repair, if two nicks occur close to each other (within 14 base pairs) on opposite strands [12]. The presence of DNA nicks due to other causes increases the probability of double strand breaks by increasing the frequency of total DNA nicks. For example, another source of DNA nicks is glycosylase removal of oxidant-damaged bases such as 8-oxoguanine. Double strand DNA breaks may lead to acentric chromosomes fragments and micronuclei.
Although it has been established that folate deficiency leads to increased DNA-uracil and that there is an association between folate deficiency and increased DNA damage or micronuclei formation [1; 3; 13; 14], it is unknown whether B6 deficiency causes a similar effect. We used the improved uracil assay described here to measure lymphocyte DNA-uracil in healthy humans who were marginally B6 deficient.
Vitamin B6 is comprised of a group of related vitamers: pyridoxal, pyridoxine, and pyridoxamine, and their phosphorylated forms pyridoxal phosphate (PLP), pyridoxine phosphate, and pyridoxamine phosphate, respectively [15]. Plasma PLP is the most commonly used index for determining vitamin B6 status [15; 16]. The current recommended daily allowance (RDA) for adults (19-50 years of age) is 1.3 mg vitamin B6/d [16]. In the United States, about 28% of female and 7% of male adults are below the estimated average requirement (EAR) [17]. (The EAR is used to estimate the prevalence of inadequate population and intakes [17] are defined as 2 standard deviations below the RDA). Although this indicates that many people have an inadequate intake of vitamin B6, it is not clear what level is optimal for long-term health [18].
Some studies have found that smoking decreases vitamin folate status [19; 20] and other vitamins such as vitamin C [21], and increases DNA damage [22; 23], although these relationships are not definitive. In the current study, we included both smokers and nonsmokers as subjects for B6 and DNA-uracil assessment.
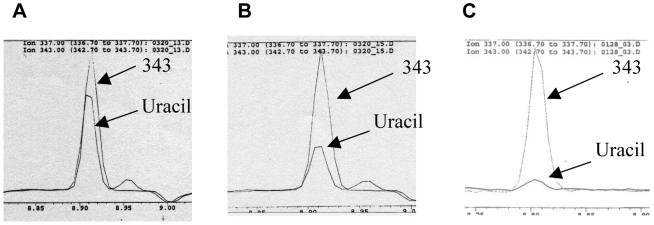
Previously, we described a set of improvements that were made to a gas chromatography-mass spectrometry (GC-MS) assay for uracil in DNA in order to analyze more accurately the DNA-uracil content of cells grown in tissue culture under folate-deficient conditions [24]. This assay, referred to hereafter as “Uracil assay V2,” was used in tissue culture studies that examined the effects of folate deficiency on the proliferation and DNA-uracil content of primary, cultured human lymphocytes [24]. However, preliminary studies indicated that DNA-uracil levels in freshly harvested lymphocytes from healthy (vitamin sufficient) humans were much lower than those found in lymphocytes grown in tissue culture with a concentration of 12 nM folic acid, a level that approximates adequate folate levels in vivo (Figure 1). This apparent discrepancy is probably caused by the fact that folic acid, the oxidized form of folate commonly used in supplements and tissue culture studies, is different from the folate forms naturally found in food, i.e., 5-methyltetrahydrofolate (5-MeTHF) and 10-formyltetrahydrofolate (10-formylTHF). Folic acid, is much less efficiently transported into the cells of most tissues via the reduced folate carrier than the fully reduced forms, 5-MeTHF and 10-formylTHF [2; 25]. Therefore, the concentrations of folate in the tissue culture experiments were effectively equivalent to a much lower in vivo level and DNA-uracil levels in these cultured cells were easier to detect.
Figure 1. DNA-uracil concentrations in cultured, “folate-sufficient” lymphocytes are higher than those in lymphocytes freshly harvested from healthy humans.
Typical chromatograms produced using the Uracil assay V2 using 50 pg of internal standard (“343”) and the following amounts of sample DNA: (A) 3 μg of DNA from human lymphocytes grown in tissue culture with 12 nM folic acid; (B) 3 μg of DNA from lymphocytes grown in 30 nM folic acid; (C) 10 μg of DNA from freshly collected healthy human lymphocytes
A wide range of DNA-uracil levels has been reported in the literature ranging from 0.2 to 129.0 pg uracil / μg DNA [1; 24; 26; 27; 28; 29; 30; 31]. Although the health and vitamin status of study subjects might greatly affect DNA-uracil levels, the wide range of reported values is probably partly due to the technical difficulty of accurately measuring DNA-uracil content, especially in samples where levels are low. Table 1 shows some measurements that have been reported from both animal and human studies. In order to detect more accurately low baseline lymphocyte DNA-uracil content of healthy human DNA, further modifications were made to the uracil assay; this most recent version of the assay will hereafter be referred to as “Uracil assay V3.” This assay was also used to measure DNA-uracil in lymphocytes from healthy humans (both smokers and nonsmokers) who were marginally B6 deficient.
Table 1. DNA-uracil concentrations reported in the literature.
A broad range of DNA-uracil concentrations from fresh or cultured cells has been reported by different researchers. Some of these concentrations are shown in the table above. “U” = uracil, “deficiency” = type of vitamin deficiency investigated for association with increased DNA-uracil.
| pg U/μg DNA | 1000 U/ diploid cell |
||||
|---|---|---|---|---|---|
| Reference | min | max | Min | max | organism / cell type / deficiency |
| Mashiyama et al. 2004 (24) | 0.33 | 6.35 | 10 | 195 | human / cultured primary lymphocytes / folate |
| Choi et al. 2003 (31) | 3.89 | 5.09 | 119 | 156 | rat / colon cells / folate |
| Ren et al. 2002 (30) | 0.20 | 1.94 | 6 | 60 | human / lymphocytes / folate |
| Crott et al. 2001 (29) | 0.76 | 4.84 | 23 | 149 | human / cultured primary lymphocytes / folate |
| Koury et al. 1997 (28) | 0.43 | 1.77 | 13 | 54 | mouse / erythroblasts / folate |
| Blount et al. 1997 (1) | 1.66 | 129.02 | 51 | 4000 | Human / cells from whole blood / folate |
| Ramsahoye et al. 1996 (27) | 41.41 | 42.93 | 1271 | 1318 | human / bone marrow cells / cobalamin |
| Blount et al. 1994 (26) | 0.70 | 3.00 | 21 | 92 | rat / hepatocytes / folate |
Materials and methods
Uracil assay improvement
The uracil assay was carried out as described [24] except for the following changes: Use of more concentrated enzyme: Cloning and purification of E. coli UDG Using smaller amounts of enzyme resulted in higher signals and that this was likely due to lessening glycerol from the enzyme storage buffer, as glycerol is known to be a good nucleophile and thus competes with uracil for the derivatization reagent. In a control experiment, 10 μl of UDG diluted to 1:10, 1:100, and 1:1000 were added to oligos containing 100 pg of uracil (in duplicate): 343 abundances were (mean ± STDEV) 2,007 ± 14; 7,656 ± 1,460; and 9,622 ± 257, respectively.
In order to obtain a higher concentration of UDG for the assay, so that sufficient enzyme could be used in the assay in smaller volumes, the E. coli UDG (uracil DNA glycosylase; also called “UNG”) gene was cloned (NCBI Entrez Nucleotide website at http://www.ncbi.nlm.nih.gov, accession number D13169) and the protein purified using standard molecular biology techniques. This sequence was used to design primers for amplifying the UDG gene and cloning into the pTYB1 expression vector included in the IMPACT-CN kit (New England Biolabs, Ipswich, MA). The primer sequences were: Forward (5' to 3'): CGGTCGCATATGGCTAACGAATTAACCTGG; reverse (5' to 3'): CGGTCGGCTCTTCCGCACTCACTCTCTGCCGGTAATAC. The culture from which the plasmid (verified by DNA sequencing) had been obtained was cryopreserved by adding autoclaved glycerol to a final concentration of 20% glycerol and storing in aliquots at −80 °C.
For protein expression and purification, the manufacturer's instructions for the IMPACT kit were followed using the clarified extracts from 2 liters of cells on each column. One important change to the IMPACT protocol was to add about 3 mg of DNase 1 (Sigma) to each cell pellet before sonication. The DNase addition made the cell pellets more compact after centrifugation, with a cleaner and less viscous supernatant, which prevented clogging of the column. Also, the column buffer was made to a final concentration of 2.5 M NaCl as the lower concentration of salt suggested in the instructions led to very inefficient elution of protein at the end of the process. The yield was about 16 ml of 1 mg/ml protein. An equal volume of glycerol was added to each enzyme preparation and stored at −20 °C for a final concentration of about 0.5 mg protein/ml.
One unit of the UDG enzyme was defined as the amount that could completely digest uracil from synthetic oligonucleotides containing 100 pg of uracil assayed using the uracil assay (described in this paper). A 1:500 dilution of the enzyme preparation yielded a concentration of about 1 U/μl.
Improved drying step
Previously, for the Uracil assay V2 [24], we noted found that speed-vac drying samples for a shorter period of time (2 hours instead of overnight as in the original protocol) and capping the tubes loosely or using caps that had been punched with a needle greatly decreased contamination problems. In an experiment in which samples were dried for 12 hours in a speed vac in duplicate, tubes with UDG and 15 pg ISTD had a 337/343 ratio (mean ± STDEV) of 3.40 ± 0.84 (43.2 ± 10.7 pg uracil) when dried with no caps and 0.33 ± 0.04 (4.0 ± 0.5 pg uracil) when dried with punched caps. In contrast, six replicate tubes with ISTD and buffer only had a 337/343 ratio of 0.02 ± 0.01 when dried with loose caps for 2 hours. The amount of contamination from speed-vac drying could vary widely from run to run, and probably was related to the cleanliness of the speed vac and what sort of samples had been previously run.
For the current Uracil assay V3 protocol, we avoided the speed vac altogether by drying samples in a desiccator lined with Drierite. In another control experiment, tubes with 5 pg of ISTD and no uracil added had a 337/343 ratio (mean ± STDEV) of 0.14 ± 0.03 for duplicate tubes dried in the SV (2 h.) vs. 0.07 ± 0.01 for duplicated tubes dried with Drierite for 1 day.
A vacuum desiccator was prepared by lining with Drierite desiccant (W.A. Hammond Drierite Co. Ltd., Xenia, OH) and the desiccant allowed to settle for at least 24 hs to prevent contamination of samples by trace amounts of Drierite dust. Evacuation of the desiccator two times using a bench top vacuum source is also recommended before the first use of fresh desiccant. Recent trials indicate that use of several 10 g packets of silica gel (Minipax absorbent packets, Sigma-Aldrich, USA), aspirating trace dust from the packets and desiccator before use with a benchtop vacuum source, is more convenient than Drierite and also works well.
Sample, standard curve, and control tubes were made up as described [24] except that the standard curve was comprised of lower amounts of uracil and internal standard: 0, 0.2, 1, 2.5, and 5 pg of uracil (Sigma) in a volume of 10 μl for each tube. Five pg of internal standard (labeled uracil, MW=343: 13C4H4O2 15N2; Cambridge Isotope Laboratories, Andover, MA) was added in 10 μl to each tube as well as 5 μl of a 1:125 dilution of UDG (approximately 20 U) was next added. After bringing all tubes to a final volume of 45 μl with PIPPS buffer, the tubes were then capped, and the solution briefly spun down in a microcentrifuge (pulsing to 1,200 X g) to force all liquid to the bottom of the tubes, then incubated at 37 °C for 1 h in a dry incubator after wrapping the entire rack with sample tubes in aluminum foil.
After incubation, the tube caps were loosened 1/3 turn and placed in the vacuum desiccator and the chamber evacuated using a benchtop vacuum source for 30-60 sec. then sealed. The desiccator was then placed in the dark at room temperature for two days. After this time, air was let back into the chamber after first connecting (with rubber tubing) the dessicator inlet to a syringe in which two layers of small circles of filter paper had been inserted to the impede air flow. This allowed air to re-enter the chamber very slowly and prevented disturbance of the sample tube contents.
Concentration of the derivatized sample
We used a method to concentrate the sample at the isooctane extraction step that greatly increased signals. In a typical experiment using the Uracil assay V2 protocol [24] of adding 100 μl isooctane to derivatized samples, vortexing, then extracting off 85 μl, the mean 337 signal (mean ± STDEV) for duplicate tubes of 5 pg U standard was 575 ± 49.5. In contrast, in a typical experiment in which the V3 protocol was followed as described below, the mean 337 for duplicate tubes of 5 pg U standard was 18,3743 ± 1,613.6. The great increase in signal was attained without sacrificing reproducibility: 0 pg standards run in quadruplicate in this same experiment yielded a 337/343 ratio (mean ± STDEV) of 0.06 ± 0.01.
Derivatization of the samples was carried out as described [24] except that after derivatization, 50 μl of milliQ water and 205 μl of isooctane were added before vortexing and centrifugation. Next, 185 μl of the top isooctane layer was removed into GC vials and placed in a 70 °C heat block in the hood for approximately 1 h to concentrate the volume to about 20 μl. A standard plastic balance draft shield placed backwards was used to prevent dust from contaminating tubes during evaporation. The isooctane used was nano-pure and small bottles of the reagent were ordered as the concentration step can amplify small levels of background contamination. If background levels started to increase, a new bottle of isooctane was used. Vials were capped and run on the GC-MS in batches, keeping vials at −20 °C until analyzed to prevent evaporation. The GC-MS conditions used were the same as those described [24].
Cell culturing and/or collection
Fresh human mononuclear cells were obtained from AllCells (Berkeley, CA) for the low-folate culturing experiments and initial direct measurements (without culturing). Human lymphocytes were cultured in folate concentrations ranging from 0-30 nM before analysis as described [24]. Lymphocytes for the marginal B-6 deficiency studies were collected as described below in the section “Vitamin B6 deficiency study materials and methods.”
DNA from the pellets of human lymphocytes were extracted using a modification of the sodium-iodide “chaotropic” protocol included in the Wako DNA Extractor Kit (Wako Pure Chemical Industries, Ltd., Osaka, Japan) as described [32]. Some assay runs became contaminated, but it was discovered that the duplicate DNA pellets for these samples had been degraded. Therefore samples for these runs were extracted using duplicate cell pellets using a different extraction technique that might yield more stable duplicate DNA aliquots for the remaining precious cell samples. The alternate DNA extraction method was the QIAamp DNA Blood Maxi Kit Maxi kit (“Maxi kit”, Qiagen, Valencia, CA) for samples with up to 100 million cells, and used the optional RNase step. Using the Maxi kit, the DNA was eluted in 500 μl of PIPPS buffer (5 mM PIPPS, pH 8; PIPPS: piperazine N,N'-bis[3-propane sulfonic acid]; Calbiochem, San Diego, CA) and the eluant reapplied to the column membrane and the DNA re-eluted after a 5 min incubation at room temperature. The uracil assay was run on replicates of some samples that had been extracted using both methods to verify that similar DNA-uracil measurements were attained using either extraction method. For example, there were enough samples to run duplicates of DNA extracted with both techniques on all five timepoints for subject #LS-7. A set of five cell pellets was extracted with both the Qiagen and sodium-iodide techniques. Duplicates were run of samples with separate runs for the Qiagen-extracted and the sodium-iodide extracted DNA. The mean of duplicate measurements for each run was calculated, and then the standard deviation of between run means and the mean of the means between runs calculated to calculate inter-run variability The CV% of the mean of the means ranged from 5.1-18.8%.
Following either DNA extraction technique, DNA was quantified with Picogreen (Molecular Probes, Eugene, OR), then 50 μg of DNA for each sample was digested with RNase A and RNase T1 (0.5 U/μg DNA each, to remove possible residual RNA) at 37 °C for 1 h, then treated with Hind III (1 U/μg DNA) at 37 °C for 1 h. The DNA was then re-precipitated to remove the reaction buffer using modifications of the isopropanol/sodium-iodide method [32]. Briefly, 400 μl total volume of DNA was precipitated by adding 1 ml 100% isopropanol and inverting the tubes 30 times. Then, 600 μl of sodium iodide solution (11.4 g sodium iodide; 0.4 ml EDTA 0.5 M, pH 8; 0.4 ml 1 M Tris pH 8; and 6 ml of water for a total volume of 10 ml) was added to each tube and inverted 60 times. The DNA was pelleted by spinning at a maximum setting (12,000 ×g) in a microcentrifuge for 5 min, and the supernatant removed by aspiration. The DNA pellets were washed by adding 1 ml 80% ethanol, vortexing briefly, then centrifuging (12,000 × g) for 5 min. The wash was repeated with 1 ml 90% then 100% ethanol, with pellets transferred before the spin in 90% ethanol using a wide bore pipette tip to clean, DNase/RNase free tubes. For DNA in volumes up to 100 or 200 μl, volumes of reagents were scaled down to 25% or 50% of that listed above, except for the ethanol washes, which were 0.5 ml each. Following the 100% ethanol wash, spin, and aspiration, pellets were air-dried for 5 min, then PIPPS buffer was added to attain a DNA concentration of approximately 700 ng/μl and DNA incubated in the dark at room temperature for 30 min. After this time, DNA was vortexed for 30 sec., briefly spun down, and stored at 4 °C if the uracil assay was performed within a few days, or at −20 °C if the DNA was to be stored longer.
The restriction enzyme digest step was added because sodium iodide precipitated DNA is often extremely difficult to suspend in buffer if the pellet is over-dried. Working an over-dried DNA pellet into solution can take 30 min. or longer with a pipette, whereas an over-dried DNA pellet that had been pre-treated with restriction enzyme easily went into solution in seconds. Precipitation of DNA was used because large amounts of DNA from healthy human lymphocytes were originally necessary for use in the uracil assay, and also because experiments run during this time period involving tissue samples indicated a danger of large amounts of RNA contamination, and so we added an additional RNase step and re-precipitation was needed to remove RNase and RNase buffer. However, we subsequently found that for lymphocytes, RNA contamination is unlikely to be a problem and that lymphocyte DNA with or without secondary RNase treatment yielded similar DNA-uracil measurements (data not shown). Tissue samples may pose more of a problem, however, and secondary RNase treatment is recommended for DNA extracted from tissue samples.
If samples are extracted using sodium iodide precipitation, use of the restriction enzyme is recommended. Hind III was chosen to homogenize DNA samples because of its robust character (Hind III can be used at concentrations up to 200-fold without “star activity,” or cleavage of DNA at non-specific sites), low cost, and because it was predicted (based on computer analysis of the human genome and Hind III sites) to produce fragments long enough not to be lost in washing steps of the DNA pellet, but not so long as to become tangled and inhibit solubilization.
Vitamin B6 deficiency study materials and methods
Subject selection and experimental design
Subject recruitment, treatment, and blood sample collection were carried out by the Shultz group at Washington State University (WSU) following approval by the WSU Committee for the Protection of Human Subjects (WSU IRB approval #4113). Twelve healthy young subjects, 6 smokers (S) and 6 nonsmokers (NS), consumed a controlled diet for three successive 28-day (d) periods: depletion (0.7 mg B6/d), repletion 1 (1.4 mg B6/d, ≈RDA [16]), and repletion 2 (2.2 mg B6/d); followed by a 28-day self-selected diet supplemented with 10.3 mg B6/d. During the two repletion periods, additional vitamin B-6 was provided as a solution of pyridoxine hydrochloride (PN-HCL) given at breakfast. Supplemental PN-HCL, a highly bioavailable form, provided 62 and 76% of the total B-6 intake during these periods.
Male S (n=2) and NS (n=3), female S (n=4) and NS (n=3) were recruited from the local university community by advertisements in newspapers and announcements posted and circulated throughout the community. Subjects ranged in age from 21 to 44 y (mean ± SD: 28.6 ± 7.9). Smokers selected for the study smoked an average of 16 cigarettes/d (range: 9-25/d) and had been smoking for an average of 10 y (range: 3 mo-25 y). A urinary cotinine test was conducted once/mo to estimate cigarette smoking quantity and/or exposure to second-hand smoke. Screening of S urinary cotinine (NicAlert, Nymox Laboratories, Maywood, NJ) concentrations indicated that these subjects were heavy smokers with a concentration >2,000 ng/mL, while NS cotinine concentration revealed none or negligible environmental tobacco smoke exposure. Monthly urinary cotinine measurements during the study confirmed smoking status. Potential subjects were additionally screened by assessment of plasma folate concentration. Subjects were considered to have adequate folate status with a plasma concentration of ≥7 nmol/L (16). Subjects folate concentration (mean ± SD) at screening and baseline was 41.1 ± 16.5 nmol/L. Smoker and NS folate concentration was not significantly different at screening. To provide the Dietary Reference Intake RDA (16) for all nutrients except vitamin B-6, subjects were supplemented every other day with 400 μg folic acid (Rite Aid Corp., Harrisburg, PA). There were no significant differences between S and NS plasma folate concentrations at baseline and at the end of each experimental period. During the entire study, plasma folate concentrations averaged 43.7 ± 8.2 nmol/L.
All subjects met the following criteria for inclusion in the study: 1) general good health; 2) no hormonal therapy (i.e., oral contraceptives, estrogen, androgen, progesterone or glucocorticoids) or other drugs (i.e., isoniazid, penicillamine and others) which would influence vitamin B-6 [33] or folate [34] metabolism; 3) no use of vitamin or other nutritional supplements one month prior to the beginning of the study.
At the start of the study and at the end of each study period, whole blood was collected, plasma separated, and lymphocytes isolated using Lymphocyte Separation Medium (ICN Biochemical, Aurora, OH) according to the manufacturer's instructions. Plasma, lymphocyte, and erythrocyte PLP concentrations were measured as previously described [35; 36]. Lymphocyte pellets from 10-20 ml of whole blood were frozen at -80 °C and shipped on dry ice to the Ames laboratory, where they were stored at -80 °C until analyzed for DNA-uracil. A solid phase no boil Dualcount radioassay (Diagnostic Products Corp., Los Angeles, CA) was used to determine folic acid concentration.
Uracil analysis
The UC Berkeley Committee for the Protection of Human Subjects and the Institutional Review Board of CHORI approved the role of UC Berkeley and CHORI researchers, respectively, in measuring DNA-uracil in all the samples used in experiments described in this paper (lymphocytes from the B6 deficiency study, and lymphocytes from AllCells). Uracil analysis using 5-10 μg of DNA per sample was carried out using GC-MS as described above.
Data analysis
Data analysis was performed using GraphPad Prism software, version 4.00. Repeated measures one-way analysis of variance (ANOVA) was used to determine if a significant difference existed between means corresponding to different treatments (baseline and different vitamin B6 intake levels) with Dunnett's post-hoc test performed to determine significant differences between treatment groups and the marginally vitamin B6 deficient treatment. For the comparison of DNA-uracil levels in S vs. NS, an unpaired t-test was performed for each treatment. Pearson's correlation coefficients were calculated to determine relationships among vitamin B6 status measures and lymphocyte DNA-uracil concentrations. P < 0.05 was considered statistically significant. For the preliminary results of DNA-uracil in healthy individuals with low or high folate status, a Student's t-test was performed in Microsoft Excel to determine if there was a significant difference between the DNA-uracil concentrations of the two groups.
Results
Uracil assay V3
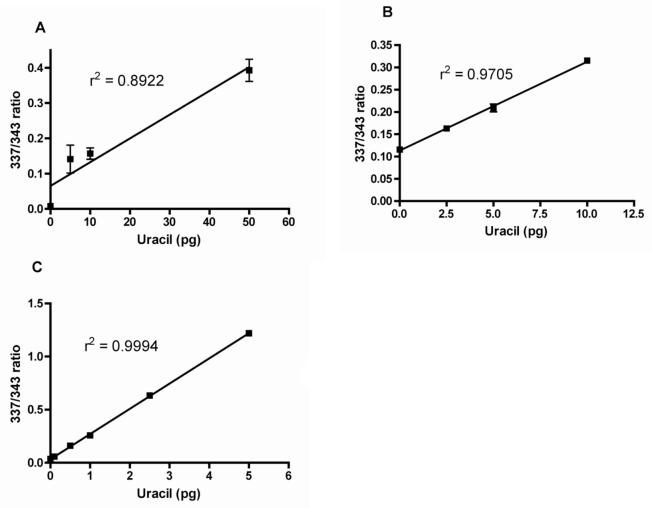
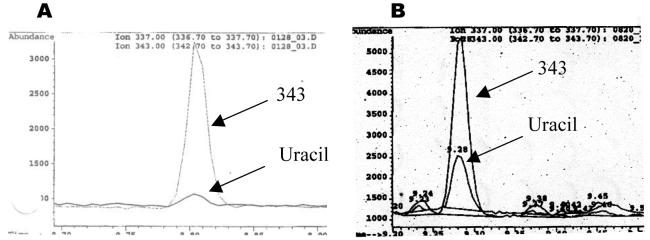
Figure 2 (A, B, C) shows a comparison of typical standard curves produced using the Uracil assay V1, V2, V3. Shown in Figure 3 is a comparison of the GC-MS chromatograms obtained using directly collected (uncultured) human lymphocytes from AllCells processed using Uracil assay V2, (Figure 3A) vs. V3 (Figure 3B).
Figure 2. Comparison of typical standard curves.
Comparison of typical standard curves produced using the uracil assay (A) original protocol, (B) V2, or (C) V3. Progressively lower ranges of uracil can be accurately detected with each subsequent protocol version.
Figure 3. Uracil assay chromatography signals were amplified by concentrating the isooctane extract.
Signals from the internal standard (“343,” mass-to-charge ratio) and uracil are indicated on the GC-MS chromatograms. (A) Typical chromatogram produced following the previous Uracil assay (V2) protocol; 10 μg of DNA and 50 pg of internal standard (“343”) were used. (B) Typical chromatogram produced following the new Uracil assay (V3) protocol; 10 μg of DNA and 5 pg of internal standard were used.
Improved linearity test
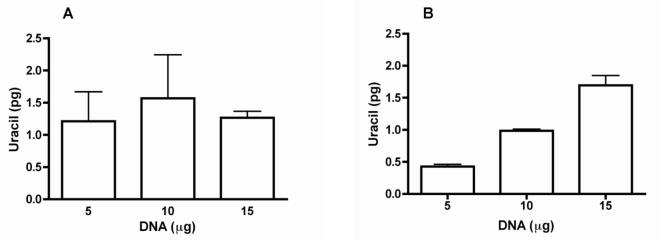
Using the previous version of the Uracil assay (V2), a standard curve of the amount of uracil vs. the amount of DNA from healthy humans, which contains very low levels of DNA-uracil, often produced non-linear results because of lack of sufficient accuracy of the assay in measuring samples with very low DNA-uracil content (Figure 4A). However, using the V3 protocol, linear results were produced as shown in Figure 4B.
Figure 4. Improved linearity test using low uracil-content DNA from lymphocytes of healthy humans.
Representative results from running linearly increasing amounts of DNA from healthy human lymphocytes in duplicate through the uracil assay using (A) the previous protocol, Uracil assay V2, or (B) the new protocol, Uracil assay V3. Values represented as mean +/− SEM.
Intra- and inter-assay variability
The study of marginal vitamin B6 deficiency and lymphocyte DNA-uracil incorporation yielded 59 lymphocyte samples (five different timepoints for each of twelve donors, except for one donor for whom there were four time point samples). There was enough material from some of these samples to assay DNA-uracil in duplicate one or more times. For same-day duplicates, each DNA sample was prepared for the uracil assay in two vials, and each pair of duplicate vials run on GC-MS the same day. Intra-assay variability was calculated using the same-day measurements of 53 sets of duplicates. These samples had an average intra-assay coefficient of variation % (CV%) of 5.0% (ranging from 0.1-23.1%). For the inter-assay variability, samples were measured in duplicate on two different days (e.g., DNA was prepared for the uracil assay as detailed above for the same-day duplicate measurements, and the duplicates assayed for uracil; then the entire process was repeated, starting on a different day for a second pair of duplicates). There was sufficient DNA for twelve sets of these two-day duplicates. Average inter-assay CV% of the mean of each day's duplicates over two days was 17.9% (ranging from 4.5-41.8%). Because of the low levels of uracil detected, there were some measurements that had higher variability, but the overall assay performance was at a level of less than 20% variability, which is generally considered acceptable.
Marginal vitamin B6 deficiency in humans
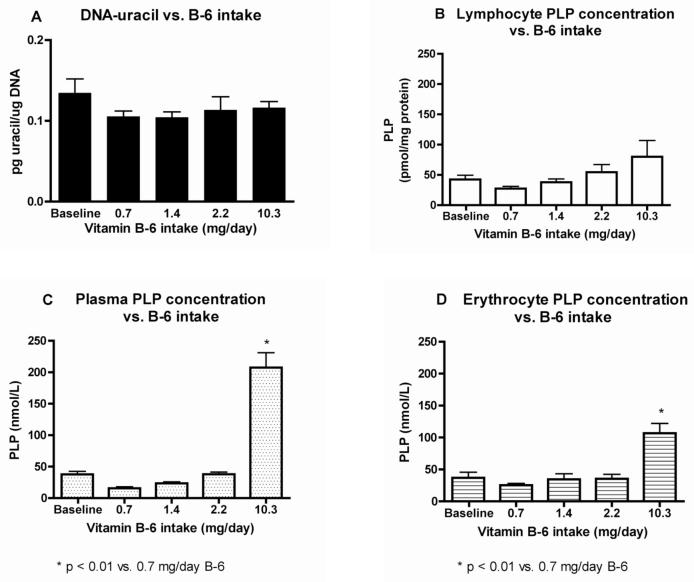
Figure 5A shows that DNA-uracil remained relatively constant at all vitamin B6 intake levels in this study, and that marginal B6 deficiency for 4 wks at approximately half the RDA (0.7 mg B6/day) did not significantly increase DNA-uracil concentrations. Consumption of the marginally deficient vitamin B6 diet (0.7 mg B6/day) reduced mean lymphocyte and plasma PLP by 35 and 58%, respectively, but these differences were not statistically significant when compared with baseline (Figures 5B and C). Plasma and erythrocyte PLP concentrations appeared to be more responsive than lymphocytes to the highest vitamin B6 intake (Figures 5C and D).
Figure 5. DNA-uracil is not significantly affected by marginally deficient vitamin B-6 status.
The vitamin B-6 (B-6) Recommended Dietary Allowance is 1.3 mg/day. A) Lymphocyte DNA-uracil content vs. B-6 intake levels. B) Lymphocyte PLP concentration vs. B-6 intake. C) Plasma PLP concentration vs. B-6 intake. D) Erythrocyte PLP concentration vs. B-6 intake. Values are represented as mean ± SEM, n=12.
For the entire group, including both smokers (S) and non-smokers (NS), plasma, lymphocyte, and erythrocyte PLP were not significantly correlated with lymphocyte DNA-uracil. Between S and NS, DNA-uracil also did not significantly differ at any of the vitamin B6 intake levels. DNA-uracil (mean ± SEM, pg uracil/μg DNA) concentrations for S vs. NS was: 0.124 ± 0.015 vs. 0.145 ± 0.033 (baseline); 0.111 ± 0.013 vs. 0.100 ± 0.007 (depletion); 0.113 ± 0.011 vs. 0.095 ± 0.007 (repletion 1); 0.129 ± 0.032 vs. 0.097 ± 0.008 (repletion 2); and 0.120 ± 0.009 vs. 0.112 ± 0.014 (supplementation). Smoker plasma PLP was 52% (P<0.05) lower than NS at baseline, but not significantly different during the experimental periods.
Discussion
This paper describes the most recent set of improvements to the uracil assay (Uracil assay V3). The earlier versions: the original assay [26] and the Uracil assay V2 [24], were suitable for measuring moderately high levels of DNA-uracil found in the DNA of severely folate-deficient humans or tissue culture cells that had been grown under folate-deficient conditions. However, the assay needed to be improved further for measuring baseline or mildly vitamin-deficient lymphocyte DNA-uracil in humans.
For Uracil assay V3, three major changes were made to the previously described [24] Uracil assay V2 protocol. The first improvement was to use smaller volumes of a more concentrated enzyme by producing our own UDG in-house. Because the glycerol in the storage buffer of enzymes decreases GC-MS signal strength, using smaller amounts of the enzyme resulted in higher overall signals.
The second improvement was to replace the speed vac in the dry-down step with a standard laboratory glass vacuum desiccator lined with desiccant (Drierite or packets of silica gel). Previously [24], we explained that modifications to the dry-down step after enzyme reaction with DNA greatly reduced cross-contamination of uracil from one tube to another in the speed vac and lowered to threshold of detection to 2.5 pg of uracil. In the current method, the threshold is further lowered to 0.2 pg, and this increase in sensitivity was in large part due to modified dry-down step. Although processing time was increased considerably, this step was critical for reducing contamination to the point that lower limits of uracil that can be detected reproducibly.
In a third major change, signal strength was increased by about ten-fold by adding a concentration step after the isooctane extraction. Tests showed that evaporating the extract at 70 °C neither affected the 337/343 ratio nor introduced artifactual uracil signals (data not shown).
In comparison with Uracil assay V2, Uracil assay V3 has an approximately ten-fold increase in signal strength and a ten-fold lower detection limit of 0.2 pg uracil. Using this method, five micrograms of DNA is a suitable amount for measuring baseline levels of DNA-uracil in healthy, well-nourished humans. With the previous methods, even using large amounts of DNA often produced variable results when DNA-uracil levels were very low, likely because of low signal-to-noise ratios. This improved protocol may be useful in human studies where DNA-uracil levels are likely to be low and small differences in DNA-uracil levels may be important.
Marginal vitamin B6 deficiency in humans
As shown in Figure 5A lymphocyte DNA-uracil was not significantly affected by the short-term marginal vitamin B6 restriction during the 4 wk time period, suggesting that the cell has a robust system for protecting DNA from uracil incorporation. Lymphocyte DNA-uracil (mean ± SEM) concentrations for all subjects were 0.134 ± 0.018, 0.105 ± 0.007, 0.104 ± 0.007, 0.113 ± 0.017, and 0.116 ± 0.008 pg uracil/μg DNA for baseline, depletion, repletion 1, repletion 2, and supplementation periods, respectively.
Lymphocyte PLP concentrations (Figure 5B) show an increasing dose response to vitamin B6 intake, with a three-fold higher mean lymphocyte PLP concentration (± SEM) at supplementation vs. depletion (80.1 ± 27.0 vs. 27.4 ± 3.8 pmol/mg protein); however, there was a lack of significant difference between the marginal lymphocyte PLP concentration and any of the baseline or repletion/supplementation periods. This may be due to variability in the subjects' response to vitamin B6 intake and/or the small sample size (n = 12). Increased B vitamin intake, such as vitamin B6, over the RDA raises the coenzyme level, e.g. PLP, considerably over the level present at the RDA [37], as is also shown here.
Plasma and erythrocyte PLP concentrations (as shown in Figures 5C and D) were more responsive to the vitamin B6 supplementation period with a significant difference (p < 0.01 for both plasma and erythrocyte PLP) when compared with the marginal B6 treatment. Plasma PLP (mean ± SEM) was 15.6 ± 2.4 nM at depletion vs. 207.3 ± 23.8 nM at supplementation; the fifteen-fold increase in vitamin B6 intake resulted in a corresponding thirteen-fold increase in plasma PLP. This shows that vitamin B6 taken orally enters the bloodstream rapidly and efficiently. Erythrocyte PLP (mean ± SEM) increased from 25.2 ± 0.9 nM at depletion to 106.7 ± 15.3 nM at supplementation. This four-fold increase in PLP concentration due to a 15-fold increase in vitamin B6 intake was significant, but not as dramatic as for plasma PLP. The increase in PLP concentration in lymphocytes (a three-fold increase) due to the same fifteen-fold increase in vitamin B6 intake was lower than for erythrocytes, indicating that lymphocytes may have more tightly controlled uptake than erythrocytes.
Even the highest supplementation level of 10.3 mg B6/day, or about 8 times the RDA, did not result in significantly lower DNA-uracil concentrations compared to baseline measurements in these subjects. The low baseline concentrations of DNA-uracil indicate that the donors were likely to be healthy individuals who were well-nourished before beginning the study. This postulation is supported by the fact that individual B6 intake of subjects before the study, calculated from 3-d diet records averaged 1.6 ± 0.2 mg/d (mean ± SEM; data not shown), an intake greater than the RDA. Results of this study suggest that intakes greater than the RDA for B6 do not reduce DNA-uracil concentrations. During the study, subjects were well-nourished except for marginal B6 intakes over the “depletion” period, consuming approximately 100% of the RDA for all other nutrients (with the exception of vitamin C for smokers).
The average DNA-uracil content of these donors over all timepoints of the study was about 3,000 uracils per diploid cell, or approximately 1 U for every million thymines (data not shown). This is in the range of that reported by the most recent report on DNA-uracil concentration of human lymphocytes that were not pre-cultured in folate-deficient conditions before measurement [30]. The level of vitamin B6 deficiency used in this study for the relatively short period of 4 wks did not lead to increased lymphocyte DNA-uracil concentrations. Tissue culture studies have shown that while folate deficiency may not result in increased DNA-uracil content, cell repair response to oxidative insults can be impaired and it takes longer for DNA repair to occur [3]. In a similar way, marginal vitamin B6 deficiency may not result in a measurably higher steady-state level of DNA-uracil, but the capacity of the cell to respond to stress, such as that induced by deficiencies of other vitamins or oxidative damage may be compromised under marginal vitamin deficiencies such as B6 [18]. DNA-uracil also did not significantly differ between smoking and non-smoking at any of the vitamin B6 intake levels.
In conclusion, while extreme experimental conditions show that the phenomenon of increased DNA-uracil content under folate-deficient conditions undoubtedly occurs [24; 26; 29], and is likely to lead to lowered DNA-repair capacity under stress and increased long-term risk for DNA damage, it is more difficult than previously thought to measure this increase in normal, healthy humans with physiological vitamin B6 deficiency.
Acknowledgments
Financial support provided by NIH grants NCCAM K05 AT001323 and R21 AT001918, NCMHD P60 MD00222, and NFCR grant M2661 to BNA, and R03 CA89722 to TDS.
Abbreviations
- B6
vitamin B6
- BTFMBzBr
N1,N3-(3,5-bis(trifluoromethyl) benzylbromide)
- dTMP
thymidylate
- dUMP
deoxyuridine monophosphate
- dUTP
deoxyuridine triphosphate EAR, Estimated Average Requirement
- formylTHF
10-formyltetrahydrofolate
- GC-MS
gas chromatography-mass spectrometry
- MeTHF
5-methyltetrahydrofolate
- mnTHF
N5,N10-methylene tetrahydrofolate
- NS
nonsmokers
- PIPPS buffer
piperazine N,N'-bis (3-propane sulfonic acid)
- PLP
pyridoxal phosphate
- RDA
Recommended Dietary Allowance
- S
smokers
- SHMT
serine hydroxymethyltransferase
- THF
tetrahydrofolate
- UDG
E. coli uracil DNA-glycosylase
- UNG
human uracil DNA-glycosylase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blount BC, Mack MM, Wehr CM, Macgregor JT, Hiatt RA, Wang G, Wickramasinghe SN, Everson RB, Ames BN. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: Implications for cancer and neuronal damage. Proc Natl Acad Sci U S A. 1997;94:3290–3295. doi: 10.1073/pnas.94.7.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey LB, Moyers S, Gregory JF., III . Chapter 21: Folate, Present Knowledge in Nutrition. ILSI Press; Washington, D.C.: 2001. pp. 214–229. [Google Scholar]
- 3.Duthie SJ, Hawdon A. DNA instability (strand breakage, uracil misincorporation, and defective repair) is increased by folic acid depletion in human lymphocytes in vitro. Faseb J. 1998;12:1491–7. [PubMed] [Google Scholar]
- 4.Ames BN, Wakimoto P. Are vitamin and mineral deficiencies a major cancer risk? Nat Rev Cancer. 2002;2:694–704. doi: 10.1038/nrc886. [DOI] [PubMed] [Google Scholar]
- 5.Das KC, Herbert V. In vitro DNA synthesis by megaloblastic bone marrow: effect of folates and cobalamins on thymidine incorporation and de novo thymidylate synthesis. Am J Hematol. 1989;31:11–20. doi: 10.1002/ajh.2830310103. [DOI] [PubMed] [Google Scholar]
- 6.Wickramasinghe SN, Fida S. Bone marrow cells from vitamin B12- and folate-deficient patients misincorporate uracil into DNA. Blood. 1994;83:1656–61. [PubMed] [Google Scholar]
- 7.Goulian M, Bleile B, Tseng BY. Methotrexate-induced misincorporation of uracil into DNA. Proc Natl Acad Sci U S A. 1980;77:1956–60. doi: 10.1073/pnas.77.4.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reidy JA. Role of deoxyuridine incorporation and DNA repair in the expression of human chromosomal fragile sites. Mutat Res. 1988;200:215–20. doi: 10.1016/0027-5107(88)90085-1. [DOI] [PubMed] [Google Scholar]
- 9.Luzzatto L, Falusi AO, Joju EA. Uracil in DNA in megaloblastic anemia. N Engl J Med. 1981;305:1156–7. doi: 10.1056/NEJM198111053051918. [DOI] [PubMed] [Google Scholar]
- 10.Voet D, Voet JG. Biochemistry. John Wiley & Sons; New York: 1990. [DOI] [PubMed] [Google Scholar]
- 11.Lindahl T. Suppression of spontaneous mutagenesis in human cells by DNA base excision-repair. Mutat Res. 2000;462:129–35. doi: 10.1016/s1383-5742(00)00024-7. [DOI] [PubMed] [Google Scholar]
- 12.Dianov GL, Timchenko TV, Sinitsina OI, Kuzminov AV, Medvedev OA, Salganik RI. Repair of uracil residues closely spaced on the opposite strands of plasmid DNA results in double-strand break and deletion formation. Mol Gen Genet. 1991;225:448–52. doi: 10.1007/BF00261686. [DOI] [PubMed] [Google Scholar]
- 13.Kim YI, Pogribny IP, Basnakian AG, Miller JW, Selhub J, James SJ, Mason JB. Folate deficiency in rats induces DNA strand breaks and hypomethylation within the p53 tumor suppressor gene. Am J Clin Nutr. 1997;65:46–52. doi: 10.1093/ajcn/65.1.46. [DOI] [PubMed] [Google Scholar]
- 14.Crott JW, Mashiyama ST, Ames BN, Fenech M. The effect of folic acid deficiency and MTHFR C677T polymorphism on chromosome damage in human lymphocytes in vitro. Cancer Epidemiol Biomarkers Prev. 2001;10:1089–96. [PubMed] [Google Scholar]
- 15.Leklem JE. Chapter 18: Vitamin B-6. In: Ziegler EE, Filer LJ Jr., editors. Present Knowledge in Nutrition. ILSI Press; Washington, D.C: 1996. pp. 191–205. [Google Scholar]
- 16.Institute of Medicine . DRI Reference Intakes for Thiamine, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. National Academy Press; Washington, DC: 1998. [PubMed] [Google Scholar]
- 17.Moshfegh A, Goldman J, Cleveland L. What We Eat in America, NHANES 2001-2002: Usual Nutrient Intakes from Food Compared to Dietary Reference Intakes. U.S. Department of Agriculture, Agricultural Research Service; 2005. [Google Scholar]
- 18.Ames BN. Low micronutrient intake may accelerate the degenerative diseases of aging through allocation of scarce micronutrients by triage. Proc Natl Acad Sci U S A. 2006;103:17589–94. doi: 10.1073/pnas.0608757103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozerol E, Ozerol I, Gokdeniz R, Temel I, Akyol O. Effect of smoking on serum concentrations of total homocysteine, folate, vitamin B12, and nitric oxide in pregnancy: a preliminary study. Fetal Diagn Ther. 2004;19:145–8. doi: 10.1159/000075139. [DOI] [PubMed] [Google Scholar]
- 20.Wallock LM, Tamura T, Mayr CA, Johnston KE, Ames BN, Jacob RA. Low seminal plasma folate concentrations are associated with low sperm density and count in male smokers and nonsmokers. Fertil Steril. 2001;75:252–9. doi: 10.1016/s0015-0282(00)01697-6. [DOI] [PubMed] [Google Scholar]
- 21.Lykkesfeldt J, Christen S, Wallock LM, Chang HH, Jacob RA, Ames BN. Ascorbate is depleted by smoking and repleted by moderate supplementation: a study in male smokers and nonsmokers with matched dietary antioxidant intakes. Am J Clin Nutr. 2000;71:530–6. doi: 10.1093/ajcn/71.2.530. [DOI] [PubMed] [Google Scholar]
- 22.Fracasso ME, Doria D, Franceschetti P, Perbellini L, Romeo L. DNA damage and repair capacity by comet assay in lymphocytes of white-collar active smokers and passive smokers (non- and ex-smokers) at workplace. Toxicol Lett. 2006;167:131–41. doi: 10.1016/j.toxlet.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Sepaniak S, Forges T, Gerard H, Foliguet B, Bene MC, MonnierBarbarino P. The influence of cigarette smoking on human sperm quality and DNA fragmentation. Toxicology. 2006;223:54–60. doi: 10.1016/j.tox.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Mashiyama ST, Courtemanche C, Elson-Schwab I, Crott J, Lee BL, Ong CN, Fenech M, Ames BN. Uracil in DNA, determined by an improved assay, is increased when deoxynucleosides are added to folate-deficient cultured human lymphocytes. Anal Biochem. 2004;330:58–69. doi: 10.1016/j.ab.2004.03.065. [DOI] [PubMed] [Google Scholar]
- 25.Antony AC. Folate receptors. Annu Rev Nutr. 1996;16:501–21. doi: 10.1146/annurev.nu.16.070196.002441. [DOI] [PubMed] [Google Scholar]
- 26.Blount BC, Ames BN. Analysis of uracil in DNA by gas chromatographymass spectrometry. Analytical Biochemistry. 1994;219:195–200. doi: 10.1006/abio.1994.1257. [DOI] [PubMed] [Google Scholar]
- 27.Ramsahoye BH, Burnett AK, Taylor C. Nucleic acid composition of bone marrow mononuclear cells in cobalamin deficiency. Blood. 1996;87:2065–70. [PubMed] [Google Scholar]
- 28.Koury MJ, Horne DW, Brown ZA, Pietenpol JA, Blount BC, Ames BN, Hard R, Koury ST. Apoptosis of late-stage erythroblasts in megaloblastic anemia: association with DNA damage and macrocyte production. Blood. 1997;89:4617–23. [PubMed] [Google Scholar]
- 29.Crott JW, Mashiyama ST, Ames BN, Fenech MF. Methylenetetrahydrofolate reductase C677T polymorphism does not alter folic acid deficiency-induced uracil incorporation into primary human lymphocyte DNA in vitro. Carcinogenesis. 2001;22:1019–25. doi: 10.1093/carcin/22.7.1019. [DOI] [PubMed] [Google Scholar]
- 30.Ren J, Ulvik A, Refsum H, Ueland PM. Uracil in human DNA from subjects with normal and impaired folate status as determined by high-performance liquid chromatography-tandem mass spectrometry. Anal Chem. 2002;74:295–9. doi: 10.1021/ac010556k. [DOI] [PubMed] [Google Scholar]
- 31.Choi SW, Friso S, Dolnikowski GG, Bagley PJ, Edmondson AN, Smith DE, Mason JB. Biochemical and molecular aberrations in the rat colon due to folate depletion are age-specific. J Nutr. 2003;133:1206–12. doi: 10.1093/jn/133.4.1206. [DOI] [PubMed] [Google Scholar]
- 32.Beckman KB, Saljoughi S, Mashiyama ST, Ames BN. A simpler, more robust method for the analysis of 8-oxoguanine in DNA. Free Radical Biology & Medicine. 2000;29:357–367. doi: 10.1016/s0891-5849(00)00316-6. [DOI] [PubMed] [Google Scholar]
- 33.Kretsch MJ, Sauberlich HE, Skala JH, Johnson HL. Vitamin B-6 requirement and status assessment: young women fed a depletion diet followed by a plant- or animal-protein diet with graded amounts of vitamin B-6. Am J Clin Nutr. 1995;61:1091–101. doi: 10.1093/ajcn/61.4.1091. [DOI] [PubMed] [Google Scholar]
- 34.Gregory JF., 3rd Chemical and nutritional aspects of folate research: analytical procedures, methods of folate synthesis, stability, and bioavailability of dietary folates. Adv Food Nutr Res. 1989;33:1–101. doi: 10.1016/s1043-4526(08)60126-6. [DOI] [PubMed] [Google Scholar]
- 35.Hansen CM, Shultz TD, Kwak HK, Memon HS, Leklem JE. Assessment of vitamin B-6 status in young women consuming a controlled diet containing four levels of vitamin B-6 provides an estimated average requirement and recommended dietary allowance. J Nutr. 2001;131:1777–86. doi: 10.1093/jn/131.6.1777. [DOI] [PubMed] [Google Scholar]
- 36.Kwak HK, Hansen CM, Leklem JE, Hardin K, Shultz TD. Improved vitamin B-6 status is positively related to lymphocyte proliferation in young women consuming a controlled diet. J Nutr. 2002;132:3308–13. doi: 10.1093/jn/132.11.3308. [DOI] [PubMed] [Google Scholar]
- 37.Ames BN, Elson-Schwab I, Silver EA. High-dose vitamin therapy stimulates variant enzymes with decreased coenzyme binding affinity (increased K(m)): relevance to genetic disease and polymorphisms. Am J Clin Nutr. 2002;75:616–58. doi: 10.1093/ajcn/75.4.616. [DOI] [PubMed] [Google Scholar]