Abstract
Membrane receptors are internalized either constitutively or upon ligand engagement. Whereas there is evidence for differential regulation of the two processes, little is known about the molecular machinery involved. Previous studies have shown that an unidentified kinase substrate is required for endocytosis of the epidermal growth factor receptor (EGFR), the prototypical ligand-inducible receptor, but not of the transferrin receptor (TfR), the prototypical constitutively internalized receptor. Eps15, an endocytic protein that is tyrosine phosphorylated by EGFR, is a candidate for such a function. Here, we show that tyrosine phosphorylation of Eps15 is necessary for internalization of the EGFR, but not of the TfR. We mapped Tyr 850 as the major in vivo tyrosine phosphorylation site of Eps15. A phosphorylation-negative mutant of Eps15 acted as a dominant negative on the internalization of the EGFR, but not of the TfR. A phosphopeptide, corresponding to the phosphorylated sequence of Eps15, inhibited EGFR endocytosis, suggesting that phosphotyrosine in Eps15 serves as a docking site for a phosphotyrosine binding protein. Thus, tyrosine phosphorylation of Eps15 represents the first molecular determinant, other than those contained in the receptors themselves, which is involved in the differential regulation of constitutive vs. regulated endocytosis.
Keywords: Eps15, phosphotyrosine, endocytosis, epidermal growth factor receptor, transferrin receptor
Introduction
Receptor-mediated endocytosis constitutes an important mechanism through which cells internalize membrane receptors, thereby regulating the uptake of nutrients or the permanence of signaling receptors at the plasma membrane. Two major classes of receptors are subjected to this process, constitutively internalized and ligand-inducible receptors, whose prototypes are the transferrin (Tf) receptor (TfR) and the EGF receptor (EGFR), respectively. Whereas the intracellular routing and the metabolic destiny of the two types of receptors are distinct (Dickson et al. 1983; Gorman and Poretz 1987; Jackle et al. 1991), the initial phases of endocytosis appear to proceed according to a common scheme. Specialized surface structures, called coated pits, function in both cases as recruitment and concentration centers for internalizing receptors, and then progressively invaginate until they are pinched off from the plasma membrane. Three major components constitute the hardware of a coated pit: clathrin, the AP2 complex, and dynamin. Clathrin provides the organizing framework of the pit, AP2 drives clathrin assembly and interacts with endocytic signals present on the receptors, and dynamin is required for fission of the vesicle from the plasma membrane (see Robinson 1994; Seaman et al. 1996; Pishvaee and Payne 1998; Kirchhausen 1999; Marsh and McMahon 1999).
Despite structural and molecular similarities, coated pits internalizing constitutive or ligand-inducible receptors must differ, as indicated by findings that endocytosis is a saturable process in which EGFRs compete with themselves, but not with TfRs (Hanover et al. 1984; Wiley 1988; Warren et al. 1997). It is now clear that one major difference resides in the receptors themselves. Constitutively internalized receptors continuously expose endocytic codes, which are responsible for interaction with the recruiting complex, AP2 (Nesterov et al. 1999). Ligand-induced receptors and, in particular, receptor tyrosine kinases (RTKs), possess cryptic codes that are unmasked by conformational changes after ligand engagement, receptor activation, and autophosphorylation (Nesterov et al. 1995a,Nesterov et al. 1995b). Thus, for receptors such as the EGFR, tyrosine kinase activity is required for internalization. Studies by Lamaze and Schmid 1995 demonstrated that a kinase substrate, other than the autophosphorylating receptor itself, is required for the efficient recruitment of EGFRs, but not of TfRs, into coated pits. Such a substrate might coincide with one of the several accessory proteins that are found in coated pits at substoichiometric ratios and are thought to constitute the regulatory software of the endocytic process (Pishvaee and Payne 1998; Marsh and McMahon 1999).
One such protein, Eps15, was originally identified as a substrate of the EGFR kinase (Fazioli et al. 1993) and subsequently implicated in endocytosis (Carbone et al. 1997; Benmerah et al. 1998, Benmerah et al. 1999). Eps15 is endowed with multiple binding activities. Through its COOH-terminal portion, it binds to the α subunit of the AP2 complex. In addition, the presence of three copies of the EH protein–protein interaction domain allows Eps15 to establish multiple interactions with endocytic/sorting proteins, including Epsin (Salcini et al. 1997; Chen et al. 1998). Eps15 is recruited to the plasma membrane upon EGFR activation (Torrisi et al. 1999) and localizes to coated pits (Tebar et al. 1996). In this study, we demonstrate that tyrosine phosphorylation of Eps15 is required for EGFR, but not for TfR, internalization. Thus, tyrosine phosphorylation of Eps15 constitutes the first example of a molecular event, affecting the endocytic machinery, which is involved in the differential regulation of ligand-induced and constitutive internalization.
Materials and Methods
Production of Recombinant Proteins and In Vitro Kinase Assays
GST fusion proteins were obtained by recombinant PCR using the murine Eps15 cDNA as a template. The EGFR kinase domain was purchased from Stratagene and in vitro kinase assays were performed according to the manufacturer's instructions, using 5 μg of GST-fusion proteins as substrates.
Mammalian Expression Vectors
An HA-tagged Eps15 (HA-Eps15) under the transcriptional control of the CMV promoter was generated by subcloning a fragment of the mouse Eps15 cDNA, obtained by PCR and encompassing positions 2–897, in the pcDNA-1-HA vector (a generous gift of S. Gutkind, National Institutes of Health, Bethesda, MD). Individual Tyr→Phe mutations were generated by recombinant PCR, followed by swapping of the appropriate fragments in the HA-Eps15 vector, to yield the HA-Y850F vector and the other vectors harboring mutations at Tyr 19, 22, 23, and 525. Wild-type Eps15 and the Y850F mutant were also cloned in the pEGFP vector (Clontech), to generate the GFP-fusion proteins GFP-Eps15 and GFP-Y850F. Details of the engineering strategies and sequences of the oligonucleotides used are available upon request. All fragments obtained by PCR were sequence-verified.
Transfection, Microinjection, and Biochemical Assays
B82L (a kind gift of G.N. Gill, University of California, San Diego, School of Medicine, La Jolla, CA) and Cos-7 cells were transfected with lipofectamine (GIBCO BRL) or by DEAE-dextran methods, respectively. For microinjection experiments CV-1 cells were processed as described previously (Carbone et al. 1997). Peptides were microinjected in the cytoplasm of the cells at a concentration of 800 μM.
Immunoprecipitation, immunoblotting and coimmunoprecipitations were performed as previously described (Salcini et al. 1997).
mAbs used were: a commercial antiphosphotyrosine (Upstate Biotech), an anti-α-adaptin (Sigma-Aldrich), the anti-EGFR mAb-1 (Oncogene Science), and the anti-HA 12CAS. A polyclonal anti-Numb serum (Salcini et al. 1997) was also used.
EGF and transferrin internalization assays were performed as previously described (Carbone et al. 1997). In brief, 24 h after transfection, cells were serum-starved for 16 h and then incubated for 1 h at 4°C with rhodamine-EGF (R-EGF, 500 ng/ml). Cells were then shifted to 37°C for 10 min to allow internalization. For transferrin internalization, cells were incubated in serum-free medium for 3 h and then with R-Tf (50 μg/ml) for 30 min at 37°C.
Immunofluorescence and Confocal Microscopy
Cells transfected with GFP-Eps15 or GFP-Y850F were fixed with 4% formaldehyde, followed by permeabilization with 0.1% Triton X-100. After blocking with 10% normal goat serum, cells were stained with anti-EGFR or mounted directly for confocal analysis. Cells transfected with HA-Eps15 or HA-Y850F cDNAs were similarly processed and stained with anti-HA antibodies. Bound antibodies were visualized with anti-mouse IgG-Cy3 (EGFR) or anti-mouse IgG-FITC (HA). Nuclear counterstaining was performed with DAPI (Sigma-Aldrich). Coverslips were analyzed with a Zeiss Axiophot epifluorescence microscope or a BioRad MRC 1024 confocal microscope. Images were processed using the Adobe PhotoShop software.
Electron Microscopy
For immunoelectron microscopy, 10% gelatin-embedded, 2.3 M sucrose-infused blocks of aldehyde-fixed growing B82L cells, transfected with either HA-Eps15 or HA-Y850F cDNAs, were frozen in liquid nitrogen. Ultra thin cryosections were obtained with a Reichert-Jung Ultracut E with FC4E cryoattachment and collected on copper formvar carbon-coated grids. Double immunogold localizations on ultrathin cryosections were performed as previously described (Schiaffino et al. 1999). In particular, sections were immunostained with mouse monoclonal anti-hEGFR (AB-5, Oncogene Science) or goat anti-hTfR (CD71 K-20, Santa Cruz Biotechnology, Inc.) antibodies, followed by appropriate rabbit anti–mouse or rabbit anti–goat bridging antibodies and 10-nm protein A–gold. After incubation with 1% glutaraldehyde in 0.1 M sodium phosphate buffer, to quench free antibody protein A binding sites, the sections were challenged with rabbit anti-HA antibody (Santa Cruz Biotechnology, Inc.), followed by 15-nm protein A–gold. Control sections were incubated with an unrelated antibody, or without primary antibodies. To determine quenching efficiency, sections incubated with anti-EGFR or anti-TfR antibodies were quenched with 1% glutaraldehyde, in 0.1 M sodium phosphate buffer, and challenged with protein A. In all control sections, low levels of labeling were detected (not shown). Sections were examined with Zeiss EM 902 or EM10C electron microscopes.
Results
Tyrosine 850 Is the Major Tyrosine Phosphorylation Site in Eps15
Previously, we have demonstrated that Eps15 can be phosphorylated in vitro by the EGFR (Fazioli et al. 1993). Thus, as an initial approach towards mapping of the tyrosine phosphorylation site of Eps15, we employed a purified EGFR kinase to phosphorylate recombinantly expressed and purified fragments of Eps15 (Fig. 1 A). An Eps15 COOH-terminal fragment, encompassing positions 751–874 and containing Tyr850, was efficiently phosphorylated, whereas other segments of the protein were poor substrates for the EGFR kinase in vitro (Fig. 1 B).
Figure 1.
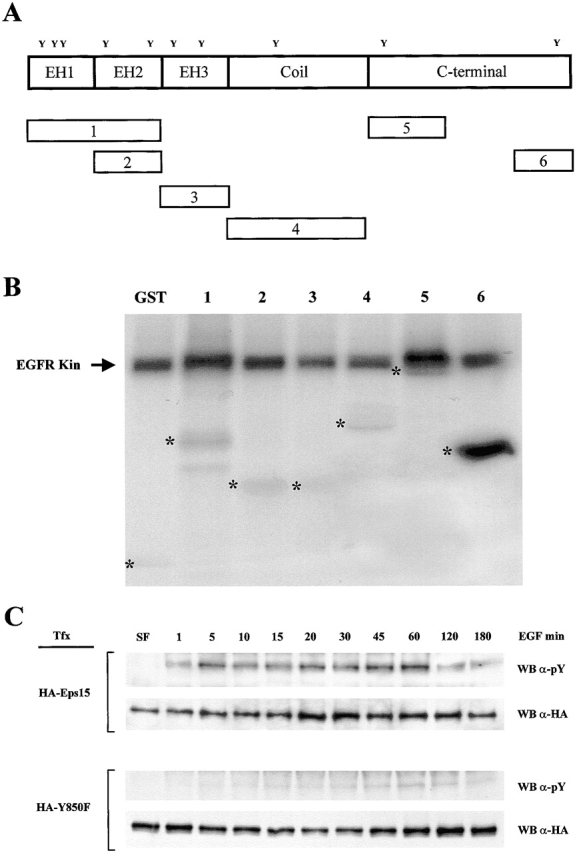
Tyrosine 850 is the major tyrosine phosphorylation site in Eps15. A, Schematic representation of Eps15 and of its fragments (numbered 1–6). The positions of the Tyr residues (Y), and the domains of Eps15, are indicated. B, Recombinant GST fragments of Eps15 were incubated with purified EGF-R kinase and γ[32P]ATP. Eps15 fragments are as in A: 1, aa 9–216; 2, aa 122–216; 3, aa 218–315; 4, aa 321–521; 5, aa 501–622; and 6, aa 751–874. Asterisks indicate the positions of the GST-Eps15 fragments, as determined by Coomassie blue staining. C, Cos-7 cells, transiently transfected (Tfx) with either HA-Eps15 or HA-Y850F were stimulated with EGF (100 ng/ml) for the indicated times. Total cellular proteins (5 mg) were immunoprecipitated with the anti-HA mAb, followed by immunoblotting with anti-pY (WB α-pY, 4/5 of the sample) or anti-HA (WB α-HA, 1/5 of the sample) mAbs.
We engineered a mutant in which Tyr850 was mutated to Phe, in the context of an HA-tagged full-length Eps15 (HA-Y850F). As controls, we also engineered mutants in which Tyr 19, 22, 23, and 525 were individually mutagenized to Phe, and an HA-tagged wild-type Eps15 (HA-Eps15). When the HA-Y850F was transfected in Cos-7 cells, little if any tyrosine phosphorylation could be detected upon EGF stimulation from one minute to three hours, whereas the HA-Eps15 was efficiently phosphorylated (Fig. 1 C). Tyrosine phosphorylation of the other Tyr→Phe mutants was indistinguishable from that of wild-type Eps15 (not shown). We concluded that Tyr 850 is the major tyrosine phosphorylation site in Eps15.
Lack of Phosphorylation Does Not Alter the Binding and Routing Properties of Eps15
Eps15 is endowed with multiple binding properties (reviewed in Santolini et al. 1999). We tested whether interactions established by Eps15 were affected by its phosphorylation status. The Y850F mutant retained intact abilities to bind to AP2 (Fig. 2 A) and to Numb, an EH-binding protein (Fig. 2 B), indicating that the mutation did not affect in a major way the conformation of the protein.
Figure 2.
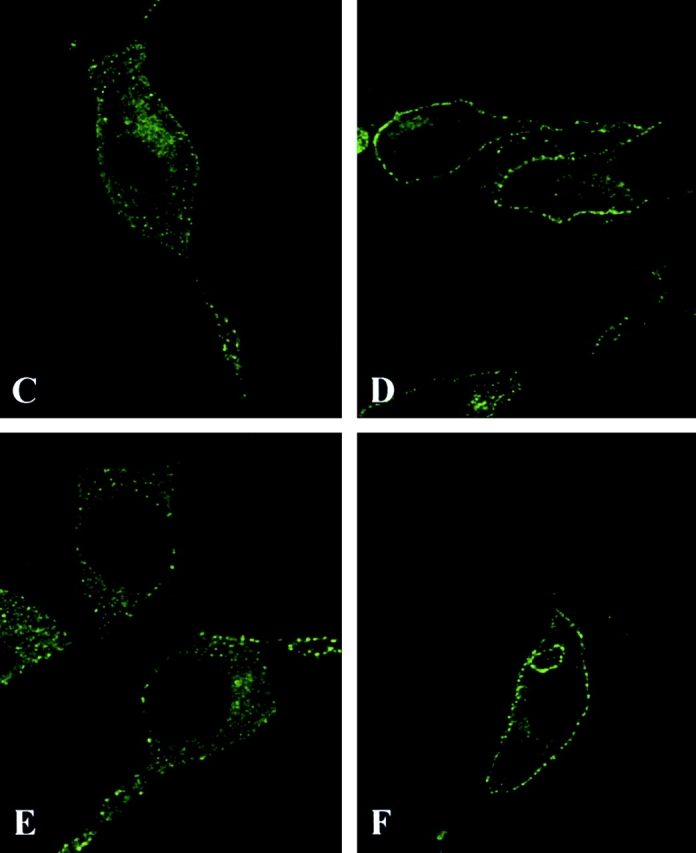
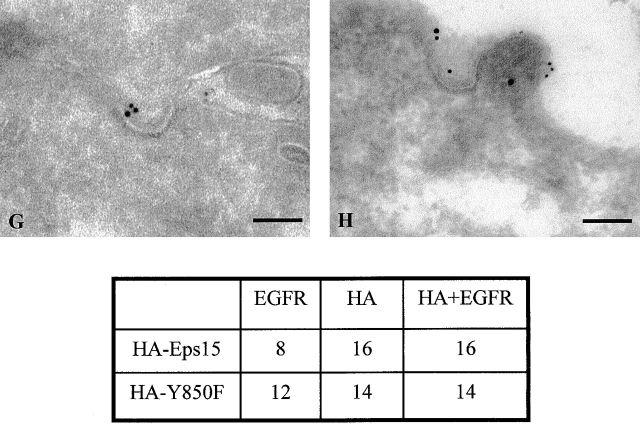
Mutation of Tyr850 of Eps15 does not alter the binding and routing properties of Eps15, but it interferes with EGFR trafficking. A, Cos-7 cells were transiently transfected (Tfx) with either HA-Eps15 or HA-Y850F and treated with EGF (100 ng/ml for 10 min, + lanes) or mock-treated (− lanes). Total cellular proteins (2 mg) were immunoprecipitated with the anti-HA mAb, followed by immunoblotting with anti-pY (WB α-pY) or anti-α-adaptin (WB α-AP2) mAbs. An aliquot (1/5) of the immunoprecipitates was immunoblotted with the anti-HA mAb (WB α-HA) to check for equal loading. B, Cos-7 cells were transiently transfected with either HA-Eps15 or HA-Y850F or the corresponding empty vector (Ctr) and treated with EGF 100 ng/ml for 10 min, (+ lanes) or mock-treated (− lanes). Total cellular proteins (4 mg) were immunoprecipitated (ip) with anti-Numb serum or with control serum (Ctr), followed by immunoblotting with anti-HA (top) or anti-Numb (bottom) mAbs. C–F, B82L cells were transiently transfected with either GFP-Eps15 (C and D) or GFP-Y850F (E and F). After serum starvation, cells were either mock-treated (C and E) or treated with EGF (100 ng/ml) at 4°C for 1 h (D and F) and analyzed by confocal microscopy. A representative horizontal section is shown. G and H, Cryosections of B82L cells transfected with either HA-Eps15 (G) or HA-Y850F (H) were labeled with anti-HA and anti-EGFR mAbs, as described in Materials and Methods. HA, 15-nm gold; EGFR, 10-nm gold. Bars: (G) 0.12 μm; (H) 0.14 μm. Morphometric data: 40 labeled coated pits were counted in each of the two experimental conditions. Data express the number of pits where EGFR colocalize with HA-Eps15 or HA-Y850F (HA+EGFR) and where only HA or EGFR staining is detectable.
We have previously shown that, upon EGF stimulation, a pool of Eps15 is recruited to the plasma membrane (Torrisi et al. 1999). We investigated whether tyrosine phosphorylation of Eps15 is responsible for this phenomenon. B82L cells, transfected with either GFP-Y850F or GFP-Eps15, were stimulated with EGF at 4°C, to prevent receptor internalization, and analyzed by confocal microscopy. Both wild-type and mutant Eps15 were similarly translocated to the plasma membrane (Fig. 2, C–F). EM analysis revealed that both HA-Eps15 and HA-Y850F were properly targeted to coated pits containing either activated EGFR (Fig. 2G and Fig. H) or TfR (not shown). Thus, tyrosine phosphorylation of Eps15 is not required for its membrane translocation after EGF treatment or for its targeting to coated pits. However, when cells loaded with EGF at 4°C were shifted to 37°C, to allow internalization, reduced internalization of the EGFR was visible in GFP-Y850F, with respect to GFP-Eps15 transfectants (Fig. 3 A). This finding was further corroborated by EM experiments that showed a clear accumulation on the plasma membrane of the EGFR in HA-Y850F transfectants, when compared with HA-Eps15 transfectants (Fig. 3 B). This suggested that the Y850F mutant could function as a dominant negative on the internalization process.
Figure 3.
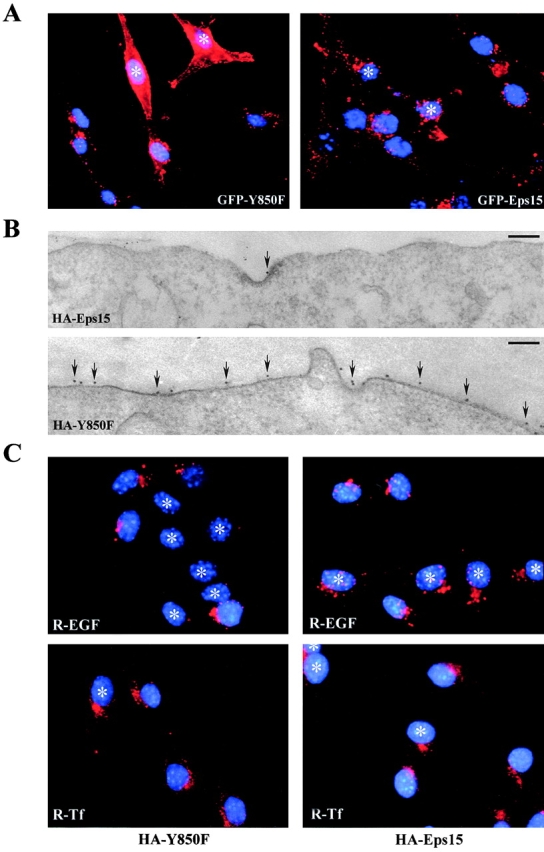
The Y850F tyrosine phosphorylation mutant of Eps15 inhibits endocytosis of the EGFR, but not of the TfR. A, B82L cells were transiently transfected with either GFP-Eps15 or GFP-Y850F. After serum starvation cells were treated with EGF (100 ng/ml) for 1 h at 4°C, followed by shift at 37°C for 10 min, to allow internalization. Cells were stained with the anti-EGFR mAb. Transfected cells, identified by GFP epifluorescence, are marked with asterisks. Nuclei are counterstained in blue with DAPI. B, Surface EGFR immunostaining on HA-Eps15 or HA-Y850F transfected B82L cells. EGFR (10-nm gold particles) was revealed by preembedding immunogold staining of PFA fixed cells. 5-nm gold labeled BSA was used as tracer. Micrographs show two examples of portions of cell profiles of HA-Eps15 (top) and HA-Y850F (bottom) transfected cells. Arrows point to 10-nm gold particles. The number of 10-nm gold particles per cell profile was counted (20 cell profiles per experimental condition), and yielded the following results (number of gold particles per cell profile). HA-Eps15 transfected B82L cells, 44.14 particles, Bar, 0.19 μm. HA-Y850F transfected B82L cells, 121.71 particles. Bar, 0.16 μm. C, B82L cells were transfected with either HA-Y850F (left) or HA-Eps15 (right), and subsequently incubated with R-EGF (500 ng/ml) or R-Tf (50 μg/ml) as described in Materials and Methods. Transfected cells were identified with the anti-HA mAb and marked with asterisks. Nuclei are counterstained in blue with DAPI.
Tyrosine Phosphorylation of Eps15 Is Required for Endocytosis of the EGFR, but Not of the TfR
To test the relevance of tyrosine phosphorylation of Eps15 in the process of receptor-mediated endocytosis, we transfected the HA-Y850F mutant and the control HA-Eps15 in B82L cells, which overexpress the EGFR, and measured the uptake of fluorochrome-conjugated EGF or Tf. B82L-HA-Y850F displayed an almost complete inhibition (>80% of the expressing cells) of EGF uptake, whereas Tf internalization was unaffected (Fig. 3 C). Conversely, B82L-HA-Eps15 cells did not show any perturbation of the internalization of either EGF or Tf (Fig. 3 C). Similar results were obtained in Cos-7 cells (not shown).
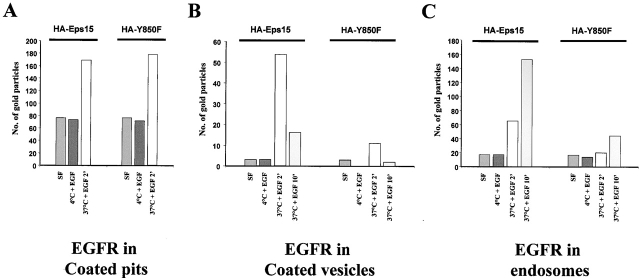
EM analysis of the distribution of the EGFR in endocytic compartments, in HA-Y850F vs. HA-Eps15 transfectants, further confirmed the block in EGFR internalization and provided insight as to the mechanisms involved. In both transfectants, EGFRs were comparably recruited to coated pits, once cells loaded with EGF at 4°C were allowed to undergo endocytosis by temperature shift at 37°C for two minutes (Fig. 4 A). However, whereas EGFRs in HA-Eps15 transfectants were able to progress to the intracellular stations of the endocytic route (coated vesicles and endosomes), they were severely impaired in doing so in HA-Y850F transfectants (Fig. 4B and Fig. C). Thus, it appears as if the block in EGFR endocytosis induced by the HA-Y850F mutant is at a level subsequent to the recruitment of the receptor in the forming pit.
Figure 4.
EGFRs are efficiently recruited into coated pits, but are not further trafficked in cells expressing HA-Y850F. Morphometric analysis of EGFR localization, as detected by immunoelectron microscopy in HA-Eps15 vs. HA-Y850F transfected cells. 24 h after transfection, B82L cells were serum-starved for 16 h (SF), loaded with EGF (100 ng/ml) for 1 h at 4°C (4°C + EGF), and were then shifted at 37°C for 2 min (37°C + EGF 2') and 10 min (37°C + EGF 10') to allow endocytosis. Staining and fixing were as in Fig. 3 B. Numbers of gold particles counted are the mean of 10 cell profiles per experimental condition in the three compartments: A, coated pits; B, coated vesicles; C, endosomes.
Tyrosine Phosphorylation of Eps15 Creates a Docking Site for a pY-binding Protein
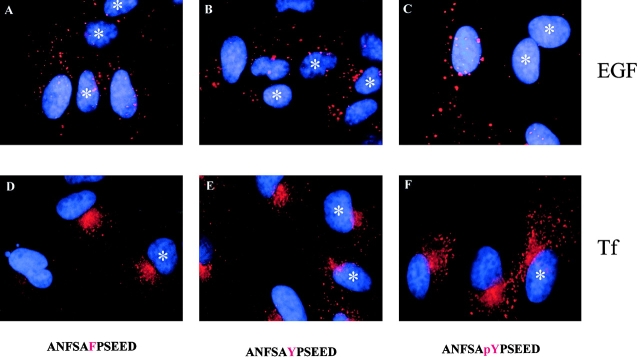
Two models can be envisioned to account for the role of tyrosine phosphorylation of Eps15. In one model, phosphorylation might induce a conformational change responsible for the transition of Eps15 from an inactive to an active state. In the other, the pY residue might serve as a docking site for a pY-binding protein, which in turn might be responsible for some specific function in the endocytosis of the EGFR. If the latter model were true, then a phosphopeptide corresponding to the phosphorylated sequence of Eps15 might titer out in vivo, the hypothetical pY-binding protein, resulting in inhibition of EGFR endocytosis. A peptide, ANFSApYPSEED, was synthesized, which corresponds to position 845–855 of mouse Eps15, and was microinjected into CV-1 cells. This peptide led to a complete block in endocytosis of EGFR in >80% of the microinjected cells, leaving Tf endocytosis unaltered (Fig. 5). Control peptides, in which the pY was replaced by a Tyr or a Phe (Fig. 5, ANFSAYPSEED and ANFSAFPSEED), did not interfere with either EGF or Tf internalization. We concluded that tyrosine phosphorylation of Eps15 most likely creates a docking site for an as yet unidentified pY-binding protein.
Figure 5.
A phosphopeptide corresponding to the phosphorylated sequence of Eps15 inhibits endocytosis of the EGFR, but not of the TfR. CV-1 cells were microinjected with the peptides indicated at the bottom, together with mouse IgG, as a marker, and then incubated with rhodamine-EGF (A–C) or rhodamine-Tf (D–F). Internalizing ligands are visible in red. Injected cells were identified using an FITC-conjugated goat anti-mouse IgG (not shown) and are marked by asterisks.
Discussion
In this study, we establish tyrosine phosphorylation of Eps15 as the first known molecular determinant, other than those contained in the receptors themselves, which is required in the differential regulation of endocytosis of ligand-inducible vs. cargo receptors. When viewed in the context of existing knowledge, our findings reveal a complex picture. Previous studies suggested that Eps15 has a general role in receptor-mediated endocytosis. Indeed, expression of dominant negative mutants of Eps15 or microinjection of neutralizing antibodies inhibited endocytosis of both EGFR and TfR (Carbone et al. 1997; Benmerah et al. 1998). Results obtained with dominant negative mutants should be evaluated with caution, in that the effects might be indirect and due to titering out in vivo of AP2, to which Eps15 is known to bind stoichiometrically (Benmerah et al. 1995; Tebar et al. 1996; Iannolo et al. 1997). However, results obtained with neutralizing antibodies are not prone to the same interpretative caveat. Anti-Eps15 antibodies could only inhibit AP2 molecules stably bound to Eps15. However, there is a vast excess of AP2 over Eps15 in the cell, since most AP2 is not bound to Eps15, whereas most, if not all, Eps15 is complexed with AP2 (Tebar et al. 1996; Cupers et al. 1998). Thus, the great majority of the AP2 pool should be insensitive to neutralization with anti-Eps15 antibodies. Therefore, it seems reasonable to assume that Eps15 has a general function in endocytosis.
Our results, however, show that tyrosine phosphorylation of Eps15 is required exclusively in the process of ligand-induced receptor internalization. Two hypotheses allow to reconcile this observation with previous findings. It is possible that Eps15 is endowed with a dual function: one connected with the protein itself that is of general relevance to endocytosis, and one connected with its tyrosine phosphorylation that is specific for the internalization of RTKs. Alternatively, there may be just one function that is optimized by tyrosine phosphorylation when there is need for efficient removal of receptors from the surface, as it might be required after ligand stimulation.
What is the role of tyrosine phosphorylation of Eps15? Our results allow us to exclude that this posttranslational modification is required for any of the known protein–protein interactions of Eps15. Furthermore, tyrosine phosphorylation does not seem to be required for proper relocalization of Eps15 to the plasma membrane, or for its targeting to coated pits. Finally, it appears that phosphorylation-impaired mutants of Eps15 do not interfere with EGFR recruitment to pits, but rather with subsequent phases of the internalization process. Thus, the point of action of phospho-Eps15 might be at the level of vesiculation. Such a function could be linked to the scaffolding properties of Eps15, and possibly to the function of dynamin, as indirectly suggested by the localization of Eps15 at the rim of coated pits (Tebar et al. 1996); also, by the site of dynamin localization and by our findings of genetic interaction between the C. elegans homologues of dynamin and Eps15 (Salcini, A.E., P.P. Di Fiore, and P. Bazzicalupo, unpublished results).
While the molecular mechanisms of action of phospho-Eps15 remain to be clarified, an interesting avenue of investigation is opened by our findings that tyrosine phosphorylation of Eps15 might create a docking site for an as yet unidentified pY-binding protein. Many SH2- or PTB/PID-containing proteins are recruited to the EGFR after receptor activation, some of which have also been implicated in endocytosis (Okabayashi et al. 1996; Wang and Moran 1996; Wang et al. 1996; Sanchez et al. 1998). The identification of binding partners for phospho-Eps15 might help to elucidate the exact molecular mechanism(s) through which Eps15 regulates the endocytic process.
Acknowledgments
We thank E. Frittoli, G. Giardina, and P. Luzzi for their valuable technical assistance; and S. Fré, G. Iannolo, E. Santolini, and G. Scita for helpful discussion. The microinjector Axiovert 100 (ZEISS) was donated by the Lattanzio family.
This work was supported by grants from Associazione Italiana Ricerca sul Cancro, Istituto Superiore della Sanita' (AIDS 1999), Telethon-Italy (Grant No. D.90), Consiglio Nazionale delle Ricerche (CNR; Target project Biotechnology), and the Ministry of University and Scientific and Technological Research (MURST); from the Armenise-Harvard Foundation to P.P. Di Fiore, and from Telethon-Italy (Grant No. E0942), CNR (Target project Biotechnology), and MURST to C. Tachetti. S. Confalonieri is the recipient of a fellowship from FIRC, the Fondazione Italiana Ricerca sul Cancro.
Footnotes
Abbreviations used in this paper: EGFR, epidermal growth factor receptor; RTKs, receptor tyrosine kinases; Tf, transferrin; TfR, transferrin receptor.
References
- Benmerah A., Gagnon J., Begue B., Megarbane B., Dautry-Varsat A., Cerf-Bensussan N. The tyrosine kinase substrate eps15 is constitutively associated with the plasma membrane adaptor AP-2. J. Cell Biol. 1995;131:1831–1838. doi: 10.1083/jcb.131.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmerah A., Lamaze C., Begue B., Schmid S.L., Dautry-Varsat A., Cerf-Bensussan N. AP-2/Eps15 interaction is required for receptor-mediated endocytosis. J. Cell Biol. 1998;140:1055–1062. doi: 10.1083/jcb.140.5.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmerah A., Bayrou M., Cerf-Bensussan N., Dautry-Varsat A. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 1999;112:1303–1311. doi: 10.1242/jcs.112.9.1303. [DOI] [PubMed] [Google Scholar]
- Carbone R., Fre S., Iannolo G., Belleudi F., Mancini P., Pelicci P.G., Torrisi M.R., Di Fiore P.P. eps15 and eps15R are essential components of the endocytic pathway. Cancer Res. 1997;57:5498–5504. [PubMed] [Google Scholar]
- Chen H., Fre S., Slepnev V.I., Capua M.R., Takei K., Butler M.H., Di Fiore P.P., De Camilli P. Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature. 1998;394:793–797. doi: 10.1038/29555. [DOI] [PubMed] [Google Scholar]
- Cupers P., Jadhav A.P., Kirchhausen T. Assembly of clathrin coats disrupts the association between Eps15 and AP-2 adaptors. J. Biol. Chem. 1998;273:1847–1850. doi: 10.1074/jbc.273.4.1847. [DOI] [PubMed] [Google Scholar]
- Dickson R.B., Hanover J.A., Willingham M.C., Pastan I. Prelysosomal divergence of transferrin and epidermal growth factor during receptor-mediated endocytosis. Biochemistry. 1983;22:5667–5674. doi: 10.1021/bi00293a033. [DOI] [PubMed] [Google Scholar]
- Fazioli F., Minichiello L., Matoskova B., Wong W.T., Di Fiore P.P. eps15, a novel tyrosine kinase substrate, exhibits transforming activity. Mol. Cell. Biol. 1993;13:5814–5828. doi: 10.1128/mcb.13.9.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman R.M., Poretz R.D. Resolution of multiple endosomal compartments associated with the internalization of epidermal growth factor and transferrin. J. Cell Physiol. 1987;131:158–164. doi: 10.1002/jcp.1041310204. [DOI] [PubMed] [Google Scholar]
- Hanover J.A., Willingham M.C., Pastan I. Kinetics of transit of transferrin and epidermal growth factor through clathrin-coated membranes. Cell. 1984;39:283–293. doi: 10.1016/0092-8674(84)90006-0. [DOI] [PubMed] [Google Scholar]
- Iannolo G., Salcini A.E., Gaidarov I., Goodman O.B., Jr., Baulida J., Carpenter G., Pelicci P.G., Di Fiore P.P., Keen J.H. Mapping of the molecular determinants involved in the interaction between eps15 and AP-2. Cancer Res. 1997;57:240–245. [PubMed] [Google Scholar]
- Jackle S., Runquist E.A., Miranda-Brady S., Havel R.J. Trafficking of the epidermal growth factor receptor and transferrin in three hepatocytic endosomal fractions. J. Biol. Chem. 1991;266:1396–1402. [PubMed] [Google Scholar]
- Kirchhausen T. Adaptors for clathrin-mediated traffic. Annu. Rev. Cell Dev. Biol. 1999;15:705–732. doi: 10.1146/annurev.cellbio.15.1.705. [DOI] [PubMed] [Google Scholar]
- Lamaze C., Schmid S.L. Recruitment of epidermal growth factor receptors into coated pits requires their activated tyrosine kinase. J. Cell Biol. 1995;129:47–54. doi: 10.1083/jcb.129.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M., McMahon H.T. The structural era of endocytosis. Science. 1999;285:215–220. doi: 10.1126/science.285.5425.215. [DOI] [PubMed] [Google Scholar]
- Nesterov A., Kurten R.C., Gill G.N. Association of epidermal growth factor receptors with coated pit adaptins via a tyrosine phosphorylation-regulated mechanism J. Biol. Chem 270 1995. 6320 6327a [DOI] [PubMed] [Google Scholar]
- Nesterov A., Wiley H.S., Gill G.N. Ligand-induced endocytosis of epidermal growth factor receptors that are defective in binding adaptor proteins Proc. Natl. Acad. Sci. USA 92 1995. 8719 8723b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesterov A., Carter R.E., Sorkina T., Gill G.N., Sorkin A. Inhibition of the receptor-binding function of clathrin adaptor protein AP-2 by dominant-negative mutant mu2 subunit and its effects on endocytosis. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:2489–2499. doi: 10.1093/emboj/18.9.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabayashi Y., Sugimoto Y., Totty N.F., Hsuan J., Kido Y., Sakaguchi K., Gout I., Waterfield M.D., Kasuga M. Interaction of Shc with adaptor protein adaptins. J. Biol. Chem. 1996;271:5265–5269. doi: 10.1074/jbc.271.9.5265. [DOI] [PubMed] [Google Scholar]
- Pishvaee B., Payne G.S. Clathrin coatsthreads laid bare. Cell. 1998;95:443–446. doi: 10.1016/s0092-8674(00)81611-6. [DOI] [PubMed] [Google Scholar]
- Robinson M.S. The role of clathrin, adaptors and dynamin in endocytosis. Curr. Opin. Cell Biol. 1994;6:538–544. doi: 10.1016/0955-0674(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Salcini A.E., Confalonieri S., Doria M., Santolini E., Tassi E., Minenkova O., Cesareni G., Pelicci P.G., Di Fiore P.P. Binding specificity and in vivo targets of the EH domain, a novel protein–protein interaction module. Genes Dev. 1997;11:2239–2249. doi: 10.1101/gad.11.17.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez P., De Carcer G., Sandoval I.V., Moscat J., Diaz-Meco M.T. Localization of atypical protein kinase C isoforms into lysosome-targeted endosomes through interaction with p62. Mol. Cell. Biol. 1998;18:3069–3080. doi: 10.1128/mcb.18.5.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santolini E., Salcini A.E., Kay B.K., Yamabhai M., Di Fiore P.P. The EH network. Exp. Cell Res. 1999;253:186–209. doi: 10.1006/excr.1999.4694. [DOI] [PubMed] [Google Scholar]
- Schiaffino M.V., d'Addio M., Alloni A., Baschirotto C., Valetti C., Cortese K., Puri C., Bassi M.T., Colla C., De Luca M. Ocular albinismevidence for a defect in an intracellular signal transduction system. Nat. Genet. 1999;23:108–112. doi: 10.1038/12715. [DOI] [PubMed] [Google Scholar]
- Seaman M.N., Burd C.G., Emr S.D. Receptor signalling and the regulation of endocytic membrane transport. Curr. Opin. Cell Biol. 1996;8:549–556. doi: 10.1016/s0955-0674(96)80034-2. [DOI] [PubMed] [Google Scholar]
- Tebar F., Sorkina T., Sorkin A., Ericsson M., Kirchhausen T. Eps15 is a component of clathrin-coated pits and vesicles and is located at the rim of coated pits. J. Biol. Chem. 1996;271:28727–28730. doi: 10.1074/jbc.271.46.28727. [DOI] [PubMed] [Google Scholar]
- Torrisi M.R., Lotti L.V., Belleudi F., Gradini R., Salcini A.E., Confalonieri S., Pelicci P.G., Di Fiore P.P. Eps15 is recruited to the plasma membrane upon epidermal growth factor receptor activation and localizes to components of the endocytic pathway during receptor internalization. Mol. Biol. Cell. 1999;10:417–434. doi: 10.1091/mbc.10.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Moran M.F. Requirement for the adapter protein GRB2 in EGF receptor endocytosis. Science. 1996;272:1935–1939. doi: 10.1126/science.272.5270.1935. [DOI] [PubMed] [Google Scholar]
- Wang Z., Tung P.S., Moran M.F. Association of p120 ras GAP with endocytic components and colocalization with epidermal growth factor (EGF) receptor in response to EGF stimulation. Cell. Growth Differ. 1996;7:123–133. [PubMed] [Google Scholar]
- Warren R.A., Green F.A., Enns C.A. Saturation of the endocytic pathway for the transferrin receptor does not affect the endocytosis of the epidermal growth factor receptor. J. Biol. Chem. 1997;272:2116–2121. doi: 10.1074/jbc.272.4.2116. [DOI] [PubMed] [Google Scholar]
- Wiley H.S. Anomalous binding of epidermal growth factor to A431 cells is due to the effect of high receptor densities and a saturable endocytic system. J. Cell Biol. 1988;107:801–810. doi: 10.1083/jcb.107.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]