Abstract
ROCK (Rho-kinase), an effector molecule of RhoA, phosphorylates the myosin binding subunit (MBS) of myosin phosphatase and inhibits the phosphatase activity. This inhibition increases phosphorylation of myosin light chain (MLC) of myosin II, which is suggested to induce RhoA-mediated assembly of stress fibers and focal adhesions. ROCK is also known to directly phosphorylate MLC in vitro; however, the physiological significance of this MLC kinase activity is unknown. It is also not clear whether MLC phosphorylation alone is sufficient for the assembly of stress fibers and focal adhesions.
We have developed two reagents with opposing effects on myosin phosphatase. One is an antibody against MBS that is able to inhibit myosin phosphatase activity. The other is a truncation mutant of MBS that constitutively activates myosin phosphatase. Through microinjection of these two reagents followed by immunofluorescence with a specific antibody against phosphorylated MLC, we have found that MLC phosphorylation is both necessary and sufficient for the assembly of stress fibers and focal adhesions in 3T3 fibroblasts. The assembly of stress fibers in the center of cells requires ROCK activity in addition to the inhibition of myosin phosphatase, suggesting that ROCK not only inhibits myosin phosphatase but also phosphorylates MLC directly in the center of cells. At the cell periphery, on the other hand, MLCK but not ROCK appears to be the kinase responsible for phosphorylating MLC. These results suggest that ROCK and MLCK play distinct roles in spatial regulation of MLC phosphorylation.
Keywords: myosin phosphatase, myosin phosphorylation, stress fibers, focal adhesions, RhoA
Introduction
Phosphorylation of regulatory light chain (MLC) of myosin II plays a critical role in controlling actomyosin contractility in both smooth muscle and nonmuscle cells (Kamm and Stull 1985; Moussavi et al. 1993; Somlyo and Somlyo 1994). MLC phosphorylation is regulated by the balance of two enzymatic activities, i.e., myosin light chain kinase(s) and myosin phosphatase. Myosin light chain kinase (MLCK) is regulated by Ca2+/calmodulin and is believed to be a major kinase in both smooth muscle and nonmuscle cells. Heterotrimeric myosin phosphatase is thought to be a major phosphatase in smooth muscle and perhaps in nonmuscle cells (Hartshorne et al. 1998).
The holoenzyme of myosin phosphatase consists of three subunits: a large subunit of ∼130 kD with myosin binding ability (called MBS here), a catalytic subunit of 38 kD (PP1cδ), and a small subunit of 20 kD of unknown function (Alessi et al. 1992; Shimizu et al. 1994; Shirazi et al. 1994). MBS is a critical subunit as it can bind both myosin and the catalytic subunit, thus targeting the substrate, myosin, with the phosphatase. Without MBS, the catalytic subunit, PP1cδ, shows rather weak phosphatase activity toward phosphorylated myosin (Alessi et al. 1992; Hirano et al. 1997; Johnson et al. 1997). The activity of MBS is regulated by phosphorylation with various kinases including protein kinase A, ROCK (Rho-kinase) and unidentified kinases (Ichikawa et al. 1996b; Ito et al. 1997; Totsukawa et al. 1999). For example, ROCK is shown to phosphorylate MBS in the COOH-terminal region including Thr 697 and Ser 854 (Kimura et al. 1996; Feng et al. 1999a; Kawano et al. 1999), resulting in the inhibition of phosphatase activity. This inhibition is suggested to be responsible for the RhoA-mediated Ca2+-sensitization process in smooth muscle (Somlyo and Somlyo 1994; Hartshorne et al. 1998).
The ROCK-mediated inhibition of myosin phosphatase is also suggested to cause RhoA-mediated assembly of stress fibers and focal adhesions in nonmuscle cells (Chrzanowska-Wodnicka and Burridge 1996; Kimura et al. 1996; Kawano et al. 1999). The inhibition of myosin phosphatase appears to account for an increase in MLC phosphorylation seen during serum stimulation of serum-starved 3T3 cells, and the resultant contractility of actomyosin is proposed to induce stress fibers and focal adhesions (Chrzanowska-Wodnicka and Burridge 1996). Indeed, BDM, an inhibitor of actomyosin contractility, was shown to inhibit assembly of stress fibers and focal adhesions during serum stimulation of 3T3 cells (Chrzanowska-Wodnicka and Burridge 1996). Furthermore, a constitutively active form of ROCK induced stress fibers and focal adhesions (Leung et al. 1996; Amano et al. 1997; Ishizaki et al. 1997), though the morphology of stress fibers is stellar and somewhat different from that of stress fibers induced by constitutively active Rho or lysophosphatidic acid. It remains to be elucidated, however, whether MLC phosphorylation alone is responsible for the induction of stress fibers and focal adhesions, because ROCK is known to phosphorylate other substrates including moesin, adducin and intermediate filament proteins (Kosako et al. 1997; Fukata et al. 1998; Kimura et al. 1998). In addition to the inhibition of myosin phosphatase, ROCK has also been reported to directly phosphorylate MLC in vitro (Amano et al. 1996). The physiological significance of this MLC kinase activity remains to be established. If ROCK indeed functions as an MLC kinase, it is then important to determine how the two MLC kinases, ROCK and MLCK regulate MLC phosphorylation in the same cell.
To investigate the physiological functions of myosin phosphorylation in RhoA-mediated assembly of stress fibers and focal adhesions, we have generated two reagents with opposing effects on myosin phosphatase activities. One is an antibody against MBS that is able to inhibit the phosphatase activity of myosin phosphatase. The other is a constitutively active mutant of MBS. Because the mutant lacks the COOH-terminal region that contains the regulatory phosphorylation sites, it constitutively activates myosin phosphatase. Microinjection of these two reagents followed by immunofluorescence with an antibody specific to phosphorylated MLC has allowed us to evaluate MLC phosphorylation with single cells and to examine its roles in microfilament assembly. We have found that MLC phosphorylation is essential and sufficient for the formation of stress fibers and focal adhesions in 3T3 fibroblasts. Our results with the combined use of the inhibitory antibody and an inhibitor of ROCK indicate that ROCK is likely to phosphorylate MLC directly. This MLC kinase activity and the inhibition of myosin phosphatase are both essential for the assembly of stress fibers in the center of cells. MLCK, on the other hand, is involved in microfilament assembly in the cell periphery, indicating that ROCK and MLCK have distinct roles in spatial regulation of MLC phosphorylation.
Materials and Methods
Reagents, Proteins, and Antibodies
The polyclonal antibody against MBS (termed M130Ab) was raised in rabbits against the bacterially expressed truncated protein of chicken MBS containing residues 1–296 (Ichikawa et al. 1996a), and affinity purified using the same truncated protein. A monoclonal antibody against Ser 19-phosphorylated MLC was described previously (Sakurada et al. 1998). A monoclonal antibody against vinculin was purchased from Sigma-Aldrich. A monoclonal antibody against paxillin and a monoclonal antibody against FAK were purchased from Transduction Laboratories. A polyclonal goat antibody against MLCK (a kind gift by Dr. de Lanerolle, University of Illinois, Chicago, IL) was prepared and affinity purified as described previously (de Lanerolle et al. 1987). Y-27632, a specific inhibitor of ROCK, was kindly provided by Yoshitomi Pharmaceutical Industries, Ltd. (Osaka, Japan) and ML-9 was purchased from Calbiochem-Novabiochem. Y-27632 and ML-9 were stored as 3 mM stock solution in DMSO in −80°C before use.
Trimeric myosin phosphatase was purified from chicken gizzard according to the method of Alessi et al. 1992. Regulatory light chain of myosin II (MLC) was purified from bovine lung myosin II as described (Sellers 1991). Bacterial expression of truncated MBS proteins, residues 1–296 (called MBS296) and residues 278–415 (MBS278-415), was performed essentially as described except that guanidine hydrochloride was omitted (Hirano et al. 1997; Totsukawa et al. 1999). As the MBS296 protein tended to aggregate during storage, microinjection experiments were performed within 2–3 d after purification.
Cell Culture, Microinjection, and Immunofluorescence
Balb/c 3T3 fibroblasts were maintained in DME containing 10% calf serum in an atmosphere of 5% CO2 and 95% air at 37°C. Epithelial type normal rat kidney cells (NRK, ATCC CRL 1571) and REF-2A cells (SV-40–transformed REF52 cells) were maintained in DME containing 10% newborn calf serum. Serum-starved 3T3 cells were prepared by culturing subconfluent Balb/c 3T3 cells for 3–5 d in DME without serum. To prepare serum-starved 3T3 cells that were well separated from each other, starved 3T3 cells were replated as follows: cells were detached by trypsinization and trypsin was neutralized with 0.5 mg/ml soybean trypsin inhibitor (Sigma-Aldrich) and 1% BSA. After washing with DME, cells were replated on poly-l-lysine-coated coverslips, and allowed to attach to coverslips for 2 h before microinjection.
Microinjection was performed as described (Yamakita et al. 1990). The antibody (M130Ab, 5 mg/ml) and the truncated proteins (MBS296, 1 mg/ml; MBS278-415, 2 mg/ml) were dialyzed against injection buffer (10 mM potassium phosphate and 70 mM KCl, pH 7.2). FITC-dextran (Molecular Probes) was comicroinjected to identify injected cells. In some experiments, antibody-injected cells were detected by Alexa 350–conjugated anti-rabbit secondary antibody (Molecular Probes). After microinjection of the antibody, serum-starved cells were incubated for 30 min to 2 h before fixation unless otherwise mentioned. In the microinjection experiments with truncated MBS mutants, microinjected cells were incubated for 30 min followed by serum stimulation for 10 min before fixation. In some experiments, 10 μM Y-27632 or 10 μM ML-9 was added 30 min before microinjection. Microinjection was also performed with 3T3 cells growing exponentially in the presence of serum. Each microinjection experiment was performed at least three times.
Immunofluorescence was performed as described previously (Matsumura et al. 1998). Rhodamine-conjugated phalloidin was used for F-actin staining. Phase and fluorescence images were taken with an AT200 cooled CCD camera (Photometric) and processed with a MicroTome image processing software (VayTek; Fairfield).
Myosin Phosphatase Assay
Trimeric myosin phosphatase (38 nM) was first incubated with various concentrations of M130Ab for 30 min on ice and then phosphatase activities were assayed at room temperature for 5 min with 32P-labeled MLC (5 μM) in 30 mM Tris-HCl (pH 7.5), 0.1 M KCl, 2 mM MgCl2, and 0.1 mg/ml BSA. The reaction was terminated by the addition of trichloroacetic acid and BSA to final concentrations of 10% and 3 mg/ml, respectively. After centrifugation, the radioactivities of the supernatants were determined by Cerenkov counting. 32P-labeled phosphorylase was also used as a substrate instead of MLC.
Other Procedures
Immunoblotting was performed as described previously (Totsukawa et al. 1999). Immunoreactive bands were detected with peroxidase-conjugated secondary antibody using a chemiluminescence method (NEN). SDS-PAGE was performed as described by Blatter et al. (Blatter et al. 1972) using 12.5% polyacrylamide gel and the Laemmli buffer system (Laemmli 1970). Protein concentrations were determined by the method of Bradford (Bradford 1976) using BSA as standard.
Results
Induction of Stress Fibers and Focal Adhesions by Inhibition of Myosin Phosphatase
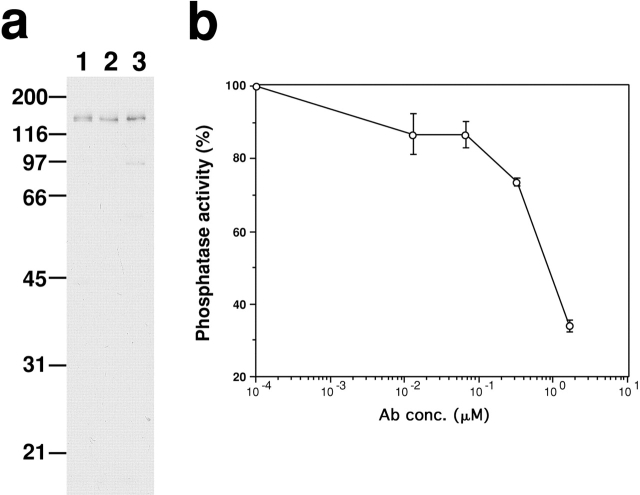
To generate an antibody that is able to inhibit myosin phosphatase, we chose the NH2-terminal region (1–296) of chick MBS as an antigen. This region is critical for the functions of MBS because it is responsible for the binding to both the catalytic subunit of PP1cδ and the substrate, myosin (Hirano et al. 1997). As Fig. 1 a shows, the antibody (termed M130Ab) specifically reacts with MBS from a variety of nonmuscle cells including REF2A cells (lane 1), 3T3 cells (lane 2) and NRK cells (lane 3). Fig. 1 b shows the effect of M130Ab on myosin phosphatase activity using phosphorylated MLC as a substrate. The antibody, at a concentration of 1.7 μM, is able to inhibit myosin phosphatase activity to less than 34% of that in the absence of the antibody. When phosphorylated phosphorylase is used as a substrate, the antibody does not show any inhibition (data not shown).
Figure 1.
M130Ab inhibits myosin phosphatase activity in vitro. (a) Specificity of M130Ab. Immunoblot showing that M130Ab raised against the NH2-terminal region (1–296) of chick MBS specifically recognizes 130-kD MBS in a variety of nonmuscle cells. Total cell lysates were separated by SDS-PAGE followed by immunoblotting using M130Ab. Lane 1, REF 2A cells; lane 2, Balb/c 3T3 cells; lane 3, NRK cells. (b) Inhibition of myosin phosphatase activity by M130Ab. Myosin phosphatase was first incubated with M130Ab, then myosin phosphatase activity was determined using 32P-labeled MLC as a substrate. The values shown are means ± SEM from three independent experiments.
We microinjected the antibody into serum-starved 3T3 cells to see whether the inhibition of myosin phosphatase results in increased MLC phosphorylation and whether an elevation of MLC phosphorylation (if any) induces the assembly of stress fibers and focal adhesions without serum stimulation. As a control, preimmune antibody was injected. As Fig. 2 shows, phalloidin staining reveals that cells microinjected with M130Ab form parallel or stellar stress fibers (Fig. 2, a–d) while the control injection with preimmune antibody has no effect (data not shown). Concomitantly, MLC phosphorylation of injected cells is found to be greatly increased (Fig. 2e and Fig. f). A monoclonal antibody specific to S19-phosphorylated MLC stains antibody-injected cells strongly while very weak staining is seen with uninjected cells. The induction of stress fibers and concomitant increase in MLC phosphorylation are observed in about 80% of cells injected with the M130Ab (n = 345).
Figure 2.
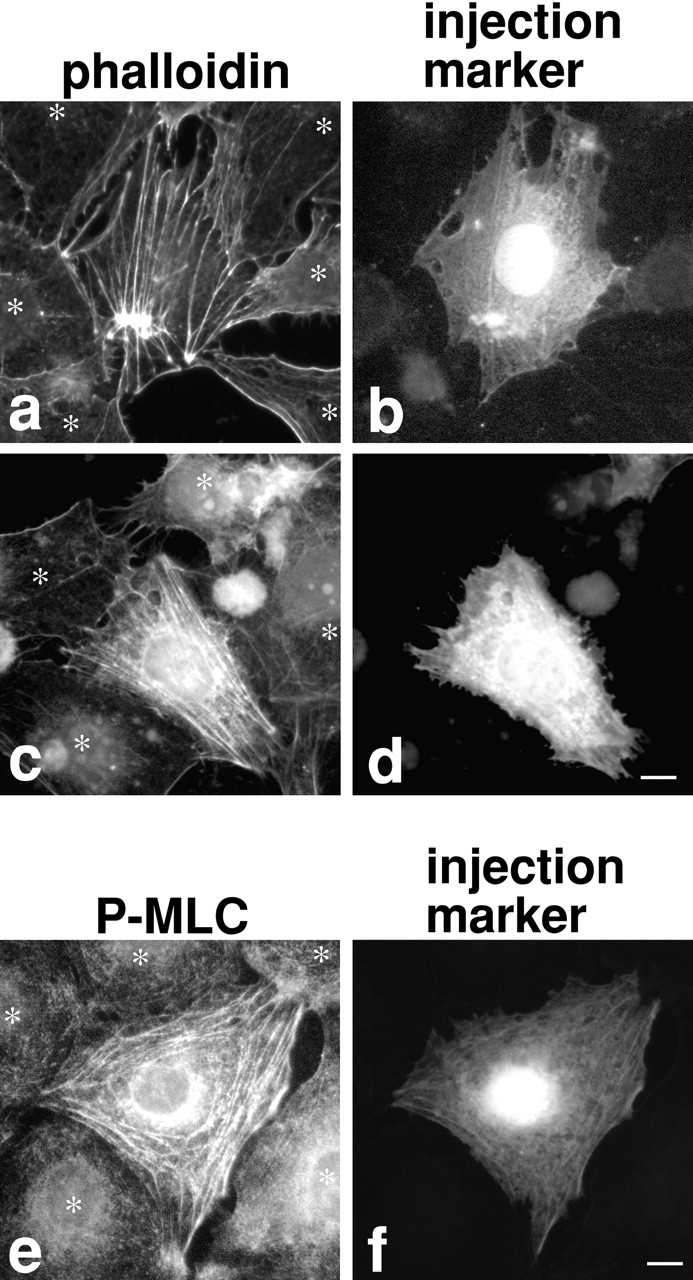
Microinjection of M130Ab induces stress fiber formation and increases MLC phosphorylation in serum-starved 3T3 cells. M130Ab (5 mg/ml) was microinjected into serum-starved 3T3 cells. FITC-dextran was coinjected to identify injected cells (b, d, and f). 2 h after injection, cells were fixed and stained with rhodamine-phalloidin (a–d), or anti–S19-phosphorylated MLC antibody (e and f). Asterisks indicated uninjected cells. Bars, 10 μm.
Two types of stress fibers are formed by M130Ab injection. Most (75%) of stress fibers are parallel (Fig. 2c and Fig. d), while the rest exhibit stellar stress fibers radiating from several foci (Fig. 2, a and b). When the concentration of M130Ab is doubled, more cells show stellar stress fibers, suggesting that the formation of stellar stress fibers depends on the extent of inhibition of myosin phosphatase. About 20–50% of MLC is phosphorylated in nonmuscle cells under normal conditions (Yamakita et al. 1994; Kolega and Kumar 1999). These cells have well developed stress fibers, indicating that partial MLC phosphorylation is sufficient for the formation of stress fibers. It is possible that the higher concentrations of M130Ab cause more complete inhibition of myosin phosphatase and thus increase MLC phosphorylation above the levels observed under normal conditions. This may result in the formation of stellar stress fibers. Similar stellar stress fibers were induced by overexpression of constitutively active ROCK (Leung et al. 1996; Amano et al. 1997; Ishizaki et al. 1997). It is likely that constitutively active ROCK would also lead to unusually high levels of MLC phosphorylation via extensive inhibition of myosin phosphatase.
The inhibition of myosin phosphatase by M130Ab injection also induces focal adhesion assembly (Fig. 3). About 80% of injected cells show higher staining with antibodies against components of focal adhesions including vinculin (Fig. 3, a and b; n = 190), paxillin (Fig. 3c and Fig. d; n = 114) and FAK (Fig. 3e and Fig. f; n = 132). Double staining with rhodamine-conjugated phalloidin (Fig. 3 h) and the anti-vinculin antibody (Fig. 3 i) reveals that vinculin staining is concentrated at the ends of or along stress fibers, indicating that focal adhesions are indeed formed (Fig. 3 j). These observations indicate that the inhibition of myosin phosphatase increases MLC phosphorylation, and suggest that the increase is sufficient to induce both stress fibers and focal adhesions. They also indicate that the heterotrimeric myosin phosphatase is a major phosphatase controlling MLC phosphorylation in 3T3 cells.
Figure 3.
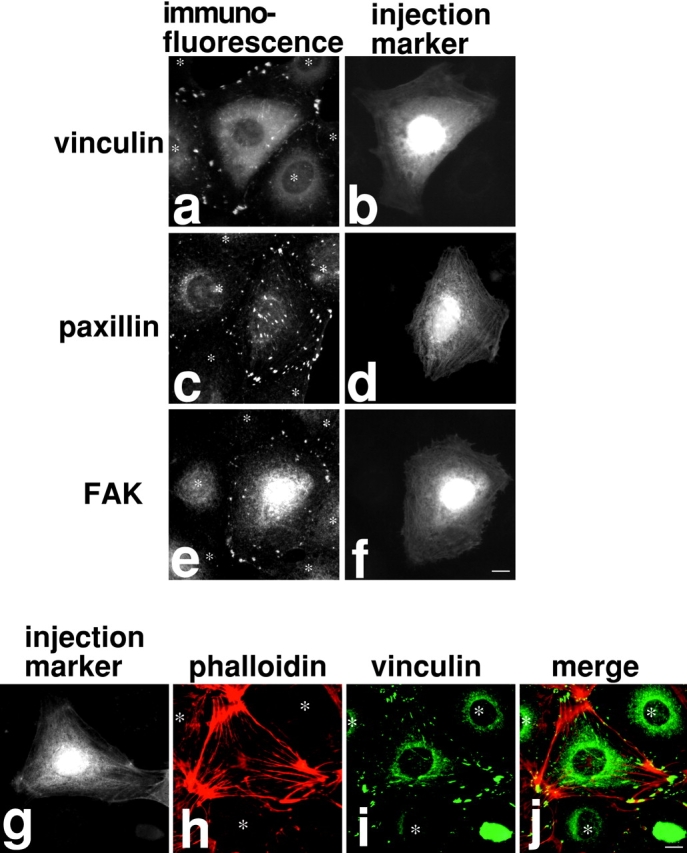
Microinjection of M130Ab induces focal adhesion formation in serum-starved 3T3 cells. a–f: M130Ab was microinjected into serum-starved 3T3 cells as in Fig. 2. Cells were fixed and stained with anti-vinculin antibody (a and b), anti-paxillin antibody (c and d), or anti-FAK antibody (e and f). FITC-dextran was coinjected to identify injected cells (b, d, and f). (g–j) M130Ab-injected cells were double stained with rhodamine-phalloidin (h, red) and anti-vinculin antibody (i, green). Injected cells were detected by anti-rabbit IgG secondary antibody (g). A merged image of rhodamine-phalloidin staining (red) and vinculin localization (green) is shown in j. Asterisks indicated uninjected cells. Bars, 10 μm.
Inhibition of the Formation of Stress Fibers and Focal Adhesions by Constitutive Activation of Myosin Phosphatase
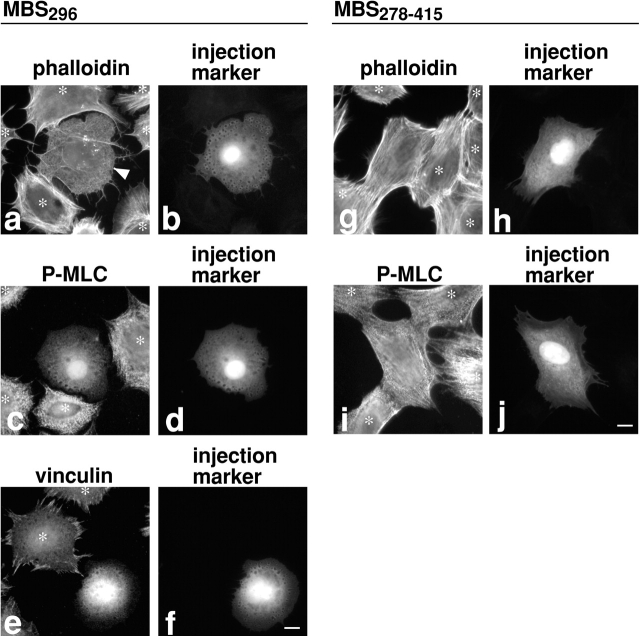
A constitutively active mutant of MBS would be a useful tool to examine whether the inhibition of MLC phosphorylation blocks the RhoA-mediated induction of stress fibers and focal adhesions. We speculated that the NH2 terminus of MBS spanning residues 1–296 (MBS296) would behave as a constitutively active mutant for the following reasons. First, MBS296 when combined with PP1cδ has increased myosin phosphatase activity compared with PP1cδ alone (Hirano et al. 1997). This reflects the binding of MBS296 to both myosin and PP1cδ. Second, MBS296 may be able to associate with PP1cδ because cells have a large pool of PP1cδ (Fernandez et al. 1992; Andreassen et al. 1998). Third and most importantly, MBS296 does not contain the inhibitory phosphorylation sites. While myosin phosphatase activity is inhibited by phosphorylation of MBS, the inhibitory phosphorylation sites so far reported are located towards the COOH terminus of MBS. For example, Rho-kinase phosphorylates Thr 697 and Ser 854 (Kawano et al. 1999) and recently phosphorylation at Thr 697 has been shown to be responsible for the inhibition of myosin phosphatase (Feng et al. 1999a). Thus MBS296 would not be phosphorylated following microinjection into cells and would not be subjected to regulation by ROCK.
We microinjected MBS296 into serum-starved 3T3 cells and then stimulated with serum to observe the effects on the assembly of stress fibers and focal adhesions. As Fig. 4 shows, MBS296 microinjection blocks serum-induced stress fiber formation though a network of microfilaments is formed (Fig. 4, a and b; n = 195). Concomitantly, MLC phosphorylation stays very low in injected cells (Fig. 4c and Fig. d; n = 187) while noninjected cells show greatly increased MLC phosphorylation upon serum stimulation (indicated by asterisks). Focal adhesion assembly is also blocked (Fig. 4e and Fig. f; n = 131). As a control, we injected the central region of MBS, residues 278–415, and found no effect on stress fiber formation (Fig. 4g and Fig. h; n = 89) or MLC phosphorylation (Fig. 4i and Fig. j; n = 84). These observations, together with the results of the M130Ab injection, indicate that MLC phosphorylation is both necessary and sufficient for the induction of stress fibers and focal adhesions. The formation of the network of microfilaments in the MBS296-injected cells (arrowhead in Fig. 4 a) indicates that serum-induced actin polymerization is not blocked by MBS296. This observation is consistent with the report by Watanabe et al. 1999 demonstrating that mDia, another RhoA effector, is responsible for the RhoA-induced actin polymerization.
Figure 4.
MBS296 inhibits MLC phosphorylation, stress fiber assembly, and focal adhesion formation of serum-stimulated 3T3 cells. MBS296 (1 mg/ml) was microinjected into serum-starved 3T3 cells 30 min before serum stimulation. Because subconfluent serum-starved cells were difficult to examine for focal adhesion assembly due to their overlaps, individually separated cells were prepared by replating as described in Materials and Methods. Cells were stimulated with serum for 10 min, then fixed and stained with rhodamine-phalloidin (a and b), anti–S19-phophorylated MLC antibody (c and d), or anti–vinculin antibody (e and f). Note that stress fibers of the uninjected cell at the top of panel a are not obvious because of out of focus. As a control, MBS278–415 was microinjected into serum-starved 3T3 cells, and cells were stimulated with serum in the same way. The injection of MBS278–415 showed no effect when examined by rhodamine-phalloidin staining (g and h) or by anti–S19-phophorylated MLC antibody (i and j). It is noticed that MBS296-injected cells show prominent vesicles distributed circumferentially around the cells. The identity of these vesicles is currently unknown. Injected cells were detected by coinjection of FITC-dextran (b, d, f, h, and j). Asterisks indicate uninjected cells. Bars, 10 μm.
Distinct Roles of ROCK (Rho-Kinase) and MLCK in Spatial Regulation of MLC Phosphorylation
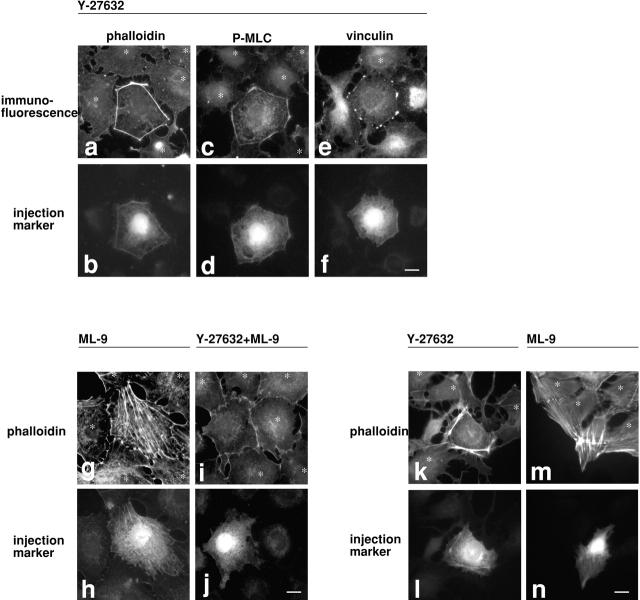
The increase of MLC phosphorylation by inhibition of myosin phosphatase (Fig. 2e and Fig. f) indicates the presence of active MLC kinase(s) in serum-starved cells. MLCK and ROCK are possible candidates. To determine which kinase(s) is involved, serum-starved 3T3 cells were treated, before M130Ab injection, with kinase inhibitors, Y-27632 or ML-9, to inhibit ROCK (Uehata et al. 1997) or MLCK (Saitoh et al. 1987), respectively. As Fig. 5 shows, these inhibitors produce distinctive effects on the microfilament organization in M130Ab-injected cells. The ROCK inhibitor, Y-27632, blocks the formation of stress fibers in the center of injected cells (Fig. 5, a and b; n = 178). Interestingly, however, thick cortical actin bundles are induced along the cell periphery of M130Ab-injected cells (Fig. 5 a). These fibers are stained with the monoclonal antibody against S19-phosphorylated MLC (Fig. 5c and Fig. d; n = 139), and contain focal adhesions as revealed by vinculin staining (Fig. 5e and Fig. f; n = 107). These observations suggest that ROCK is responsible for MLC phosphorylation in the center of cells but not at the cell periphery. Phosphorylation by ROCK of other of its substrates (including moesin, adducin, and intermediate filament proteins) is not required for focal adhesion assembly.
Figure 5.
Effects of ROCK or MLCK inhibitors on M130Ab-induced stress fiber and focal adhesion assembly. Serum-starved 3T3 cells were first treated for 30 min with 10 μM Y-27632 alone (a–f), 10 μM ML-9 alone (g and h) or both 10 μM Y-27632 and 10 μM ML-9 (i and j). M130Ab (5 mg/ml) was then microinjected together with FITC-dextran. 2 h after injection, cells were fixed and stained with rhodamine-phalloidin (a, b, and g–j), anti S19-phosphorylated MLC antibody (c and d), or anti-vinculin antibody (e and f). In k–n, 3T3 cells grown with 10% serum were pretreated with 10 μM Y-27632 (k and l) or 10 μM ML-9 (m and n) for 30 min, and microinjected with M130Ab. 2 h after injection, cells were fixed and stained with rhodamine-phalloidin. Injected cells were detected by coinjected FITC-dextran (b, d, f, h, j, l and n). Uninjected cells are indicated by asterisks. Bars, 10 μm.
The MLCK inhibitor, ML-9, on the other hand, does not inhibit the formation of M130Ab-induced stress fibers (Fig. 5g and Fig. h; n = 121). The stress fibers are again stained strongly with the monoclonal antibody against S19-phosphorylated MLC (data not shown). These results suggest that MLCK is not a major kinase responsible for stress fiber assembly in the center of 3T3 cells. MLCK, however, seems to be involved in the phosphorylation and assembly of peripheral microfilament bundles. When the two inhibitors are added simultaneously, the peripheral microfilament bundles that are formed in the presence of Y-27632 alone are not induced (Fig. 5i and Fig. j; n = 141).
Similar spatially differentiated regulation by these two kinases is also observed with 3T3 cells growing in the presence of serum. Exponentially growing cells were first treated with either Y-27632 or ML-9, and then M130Ab was injected. As reported previously (Uehata et al. 1997), the treatment with Y-27632 disassembles stress fibers (Fig. 5 k, see uninjected cells indicated by asterisks). Inhibition of myosin phosphatase by microinjection of M130Ab induces the formation of similar peripheral microfilament bundles, but no stress fibers are found in the center (Fig. 5k and Fig. l; n = 150). Unlike Y-27632, treatment with ML-9 does not markedly affect the assembly of stress fibers of 3T3 cells growing in the presence of serum (Fig. 5 m, see uninjected cells indicated by asterisks). Microinjection of M130Ab, however, frequently produces stellar stress fibers in the center of cells and injected cells seem to be contracted (Fig. 5m and Fig. n; n = 175). This is probably due to an increased inhibition of myosin phosphatase (by M130Ab) in addition to the inhibition induced by ROCK in the presence of serum.
If MLCK is responsible for the formation of peripheral microfilament bundles, MLCK should be localized in such bundles. Serum-starved 3T3 cells were again treated with Y-27632 and injected with the M130Ab to induce peripheral microfilament bundles. To examine the localization of MLCK in 3T3 cells injected with the M130Ab (rabbit antibody), the goat anti-MLCK antibody (de Lanerolle et al. 1987) was used. As Fig. 6, a–c, shows, the peripheral microfilament bundles (arrowheads in b, rhodamine-phalloidin) were stained with the anti-MLCK antibody (arrowheads in c; n = 123). Similar localization of MLCK was found in 3T3 cells grown in the presence of serum (Fig. 6, d–f, n = 95). The anti-MLCK antibody stained peripheral microfilament bundles (e, rhodamine-phalloidin; f, MLCK) though the background of MLCK staining was higher than that seen in the absence of serum. These results support that MLCK is responsible for the phosphorylation of MLC at the cell periphery.
Figure 6.
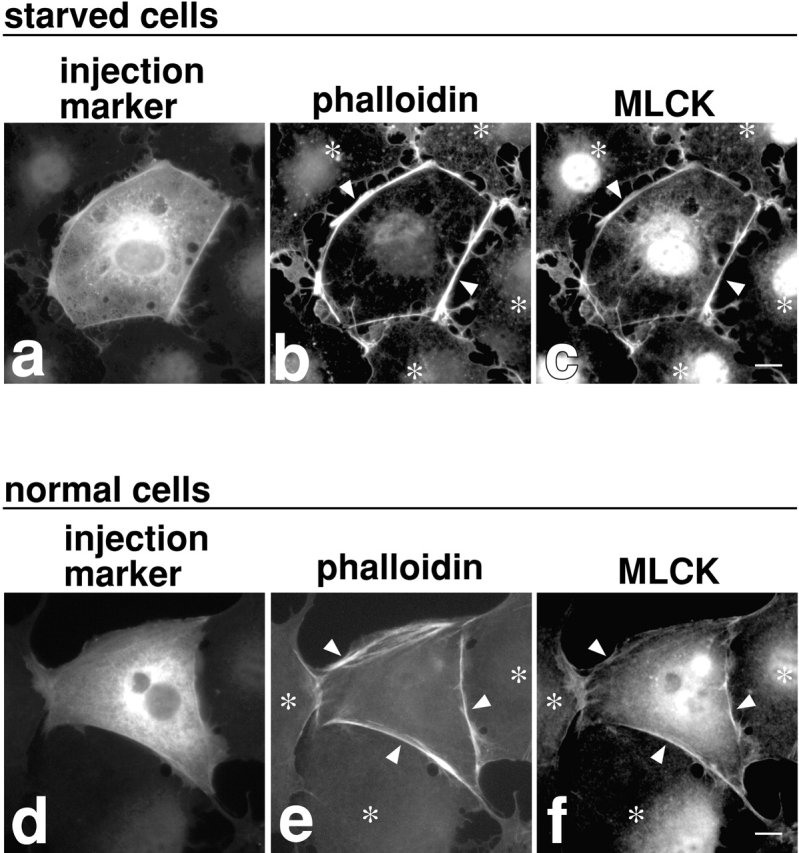
Localization of MLCK in cortical actin bundles induced by M130Ab injection in the presence of Y-27632. Serum-starved 3T3 cells (a–c) or 3T3 cells grown with 10% serum (d–f) were first treated for 30 min with 10 μM Y-27632, and M130Ab (5 mg/ml) was then microinjected. 2 h after injection, cells were fixed and double stained with rhodamine-phalloidin (b and e) or anti-MLCK antibody (c and f). Injected cells were detected by anti-rabbit IgG secondary antibody (a and d). Arrowheads indicate cortical actin bundles. Asterisks indicate uninjected cells. Bars, 10 μm.
Discussion
Using the two newly developed reagents with the opposing effects on myosin phosphatase, we have demonstrated that MLC phosphorylation is not only necessary but also sufficient to induce the assembly of stress fibers and focal adhesions. This result strongly supports the model for the assembly of stress fibers and focal adhesions proposed earlier by Burridge and coworkers (Chrzanowska-Wodnicka and Burridge 1996) that myosin II–based contractility is the force that drives the assembly of stress fibers and focal adhesions. The contractility is essential because simple actin bundling by an actin bundling protein such as fascin failed to produce stress fibers when injected into serum-starved 3T3 cells (data not shown).
It is worthy of note that focal adhesion assembly can be induced by MLC phosphorylation alone even when the RhoA/ROCK (Rho-kinase) activity is blocked. Focal adhesions are formed along the peripheral microfilament bundles that are induced by M130Ab injection in the presence of Y-27632 (Fig. 5, a–f). Similar peripheral bundles and focal adhesions are formed in the presence of Toxin B, a bacterial toxin that blocks Rho activity (Just et al. 1994). These results suggest that other RhoA/ROCK-mediated factors (such as phosphorylation of other substrates by ROCK or an elevation of PIP2 levels) may not be essential for focal adhesion assembly. Perhaps, vinculin in serum-starved cells is in a PIP2-bound state. Microfilaments in serum-starved cells are thus likely to stay associated with vinculin and other focal adhesion components but be dispersed due to the lack of active myosin. Activation of myosin would simply bring such microfilaments together to form focal adhesions.
Our results suggest that ROCK's ability to directly phosphorylate MLC is significant in 3T3 cells. This is based on the observation that, when ROCK is blocked by Y-27632, inhibition of myosin phosphatase via microinjection of M130Ab did not form stress fibers in the center of cells (though peripheral microfilament bundles were formed, see Fig. 5). The simplest interpretation of this observation is that the MLC kinase activity of ROCK and its known function of inhibiting myosin phosphatase by phosphorylating MBS are both essential for stress fiber assembly in the center of cells. We can not exclude the possibility, however, that, under different conditions or in other types of cells, MLCK can play a more significant role in MLC phosphorylation than ROCK. This appears to be the case in smooth muscle cells: ROCK functions mainly as an inhibitor of myosin phosphatase under physiological conditions (Iizuka et al. 1999).
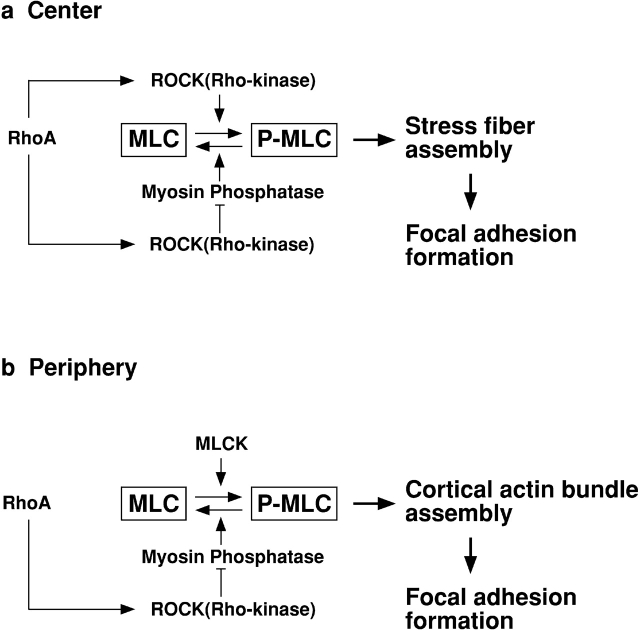
Interestingly, we have found that the two different MLC kinases of ROCK and MLCK function at different cellular locations to induce assembly of distinct sets of microfilament bundles in 3T3 cells. As illustrated in Fig. 7, ROCK is involved in the formation of stress fibers in the center of cells, while MLCK functions at the periphery. In the center of cells (a), ROCK controls not only MLC phosphorylation (by direct phosphorylation) but also MLC dephosphorylation (by inhibiting myosin phosphatase). MLCK appears not to be involved in the MLC phosphorylation. At the cell periphery (b), on the other hand, the major role of ROCK is to control MLC dephosphorylation through inhibiting myosin phosphatase. MLCK is likely to be the kinase responsible for MLC phosphorylation. Because the ROCK inhibitor does not affect the assembly of peripheral microfilament bundles, the Rho/ROCK pathway does not appear to regulate MLCK. It should be noted, however, that the spatially differentiated regulation of MLC phosphorylation by these two kinases may vary depending on cell types and contractile activities.
Figure 7.
Model of spatially differentiated regulation of MLC phosphorylation by ROCK and MLCK. (a) in the center of cells; (b) at the cell periphery. See the text for detail.
What mechanism could make the two MLC kinases function at different cellular locations? One possibility is that the upstream signals to activate each kinase may work at different locations. For example, if calcium concentration is high at the cell periphery, then MLCK would become preferentially active in such a location. In addition, the difference in their intracellular localizations partly explains the spatially differentiated regulation. ROCK is diffusely localized in the center (or perinuclear) region of cells. While MLCK was reported to be localized on stress fibers (de Lanerolle et al. 1981; Guerriero et al. 1981), a close examination of the work by Guerriero et al. 1981 has revealed that MLCK is localized more prominently on cortical microfilament bundles at the cell periphery of 3T3 cells.
The spatially differentiated regulation by the two MLC kinases is likely to provide a mechanism to regulate MLC phosphorylation in localized regions of the cell and by the different upstream stimuli. In addition, this system would be able to confer very different contractile activities on actomyosin depending on which kinase is responsible. According to the kinetic data (Amano et al. 1996; Feng et al. 1999b), MLCK phosphorylates the substrate 2–7 times more rapidly than does ROCK while the Km of ROCK appears to be 15–20 times lower than that of MLCK. This suggests that MLCK would cause rapid contraction though the higher Km value may require close contact between MLCK and myosin. ROCK, on the other hand, would be able to induce contraction (though more slowly) even if the concentrations of ROCK and myosin are low. Indeed, ROCK is diffusely present throughout the cell. The contractility of peripheral microfilament bundles is thus predicted to be more responsive to the upstream stimuli than that of stress fibers. Future studies should be directed toward elucidating how the two kinases regulate, both in space and time, complex contractile processes such as cell division, neurite retraction and cell migration.
Acknowledgments
We thank Yoshitomi Pharmaceutical Industries, Ltd. for providing Y-27632; Drs. K. Aktories and F. Hofmann (Institute for Pharmakologie and Ocikologie) for Toxin B; Dr. P. de Laneroll (University of Illinois) for anti-MLCK antibody; and Dr. F. Deis (Rutgers) for critical reading of this manuscript.
This work is supported by the National Institutes of Health grants CA42742 to F. Matsumura and HL23615 to D.J. Hartshorne. F. Matsumura is a member of the Cancer Institute of New Jersey. G. Totsukawa is supported by a fellowship from the Leukemia Research Foundation.
Footnotes
Abbreviations used in this paper: MBS, myosin binding subunit of myosin phosphatase; MLC, regulatory light chain; MLCK, myosin light chain kinase; PP1c, catalytic subunit of type 1 protein phosphatase; ROCK, Rho-associated kinase.
References
- Alessi D., MacDougall L.K., Sola M.M., Ikebe M., Cohen P. The control of protein phosphatase-1 by targetting subunits. The major myosin phosphatase in avian smooth muscle is a novel form of protein phosphatase-1. Eur. J. Biochem. 1992;210:1023–1035. doi: 10.1111/j.1432-1033.1992.tb17508.x. [DOI] [PubMed] [Google Scholar]
- Amano M., Ito M., Kimura K., Fukata Y., Chihara K., Nakano T., Matsuura Y., Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J. Biol. Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Amano M., Chihara K., Kimura K., Fukata Y., Nakamura N., Matsuura Y., Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- Andreassen P.R., Lacroix F.B., Villa-Moruzzi E., Margolis R.L. Differential subcellular localization of protein phosphatase-1 alpha, gamma1, and delta isoforms during both interphase and mitosis in mammalian cells. J. Cell Biol. 1998;141:1207–1215. doi: 10.1083/jcb.141.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatter D.P., Garner F., Van Slyke K., Bradley A. Quantitative electrophoresis in polyacrylamide gels of 2-40% J. Chromatogr. 1972;64:147–155. [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M., Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lanerolle P., Adelstein R.S., Feramisco J.R., Burridge K. Characterization of antibodies to smooth muscle myosin kinase and their use in localizing myosin kinase in nonmuscle cells. Proc. Natl. Acad. Sci. USA. 1981;78:4738–4742. doi: 10.1073/pnas.78.8.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lanerolle P., Nishikawa M., Felsen R., Adelstein R.S. Immunological properties of myosin light-chain kinases. Biochim. Biophys. Acta. 1987;914:74–82. doi: 10.1016/0167-4838(87)90163-4. [DOI] [PubMed] [Google Scholar]
- Feng J., Ito M., Ichikawa K., Isaka N., Nishikawa M., Hartshorne D.J., Nakano T. Inhibitory phosphorylation site for Rho-associated kinase on smooth muscle myosin phosphatase J. Biol. Chem. 274 1999. 37385 37390a [DOI] [PubMed] [Google Scholar]
- Feng J., Ito M., Kureishi Y., Ichikawa K., Amano M., Isaka N., Okawa K., Iwamatsu A., Kaibuchi K., Hartshorne D.J., Nakano T. Rho-associated kinase of chicken gizzard smooth muscle J. Biol. Chem. 274 1999. 3744 3752b [DOI] [PubMed] [Google Scholar]
- Fernandez A., Brautigan D.L., Lamb N.J. Protein phosphatase type 1 in mammalian cell mitosischromosomal localization and involvement in mitotic exit. J. Cell Biol. 1992;116:1421–1430. doi: 10.1083/jcb.116.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata Y., Kimura K., Oshiro N., Saya H., Matsuura Y., Kaibuchi K. Association of the myosin-binding subunit of myosin phosphatase and moesindual regulation of moesin phosphorylation by Rho-associated kinase and myosin phosphatase. J. Cell Biol. 1998;141:409–418. doi: 10.1083/jcb.141.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerriero V., Jr., Rowley D.R., Means A.R. Production and characterization of an antibody to myosin light chain kinase and intracellular localization of the enzyme. Cell. 1981;27:449–458. doi: 10.1016/0092-8674(81)90386-x. [DOI] [PubMed] [Google Scholar]
- Hartshorne D.J., Ito M., Erdodi F. Myosin light chain phosphatasesubunit composition, interactions and regulation. J. Muscle Res. Cell Motil. 1998;19:325–341. doi: 10.1023/a:1005385302064. [DOI] [PubMed] [Google Scholar]
- Hirano K., Phan B.C., Hartshorne D.J. Interactions of the subunits of smooth muscle myosin phosphatase. J. Biol. Chem. 1997;272:3683–3688. doi: 10.1074/jbc.272.6.3683. [DOI] [PubMed] [Google Scholar]
- Ichikawa K., Hirano K., Ito M., Tanaka J., Nakano T., Hartshorne D.J. Interactions and properties of smooth muscle myosin phosphatase Biochemistry 35 1996. 6313 6320a [DOI] [PubMed] [Google Scholar]
- Ichikawa K., Ito M., Hartshorne D.J. Phosphorylation of the large subunit of myosin phosphatase and inhibition of phosphatase activity J. Biol. Chem. 271 1996. 4733 4740b [DOI] [PubMed] [Google Scholar]
- Iizuka K., Yoshii A., Samizo K., Tsukagoshi H., Ishizuka T., Dobashi K., Nakazawa T., Mori M. A major role for the rho-associated coiled coil forming protein kinase in G-protein-mediated Ca2+ sensitization through inhibition of myosin phosphatase in rabbit trachea. Br. J. Pharmacol. 1999;128:925–933. doi: 10.1038/sj.bjp.0702864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki T., Naito M., Fujisawa K., Maekawa M., Watanabe N., Saito Y., Narumiya S. p160ROCK, a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. FEBS Lett. 1997;404:118–124. doi: 10.1016/s0014-5793(97)00107-5. [DOI] [PubMed] [Google Scholar]
- Ito M., Feng J., Tsujino S., Inagaki N., Inagaki M., Tanaka J., Ichikawa K., Hartshorne D.J., Nakano T. Interaction of smooth muscle myosin phosphatase with phospholipids. Biochemistry. 1997;36:7607–7614. doi: 10.1021/bi9702647. [DOI] [PubMed] [Google Scholar]
- Johnson D., Cohen P., Chen M.X., Chen Y.H., Cohen P.T. Identification of the regions on the M110 subunit of protein phosphatase 1M that interact with the M21 subunit and with myosin. Eur. J. Biochem. 1997;244:931–939. doi: 10.1111/j.1432-1033.1997.00931.x. [DOI] [PubMed] [Google Scholar]
- Just I., Fritz G., Aktories K., Giry M., Popoff M.R., Boquet P., Hegenbarth S., von Eichel-Streiber C. Clostridium difficile toxin B acts on the GTP-binding protein Rho. J. Biol. Chem. 1994;269:10706–10712. [PubMed] [Google Scholar]
- Kamm K.E., Stull J.T. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu. Rev. Pharmacol. Toxicol. 1985;25:593–620. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- Kawano Y., Fukata Y., Oshiro N., Amano M., Nakamura T., Ito M., Matsumura F., Inagaki M., Kaibuchi K. Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J. Cell Biol. 1999;147:1023–1038. doi: 10.1083/jcb.147.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K., Ito M., Amano M., Chihara K., Fukata Y., Nakafuku M., Yamamori B., Feng J., Nakano T., Okawa K., Iwamatsu A., Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Kimura K., Fukata Y., Matsuoka Y., Bennett V., Matsuura Y., Okawa K., Iwamatsu A., Kaibuchi K. Regulation of the association of adducin with actin filaments by Rho-associated kinase (Rho-kinase) and myosin phosphatase. J. Biol. Chem. 1998;273:5542–5548. doi: 10.1074/jbc.273.10.5542. [DOI] [PubMed] [Google Scholar]
- Kolega J., Kumar S. Regulatory light chain phosphorylation and the assembly of myosin II into the cytoskeleton of microcapillary endothelial cells. Cell Motil. Cytoskeleton. 1999;43:255–268. doi: 10.1002/(SICI)1097-0169(1999)43:3<255::AID-CM8>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Kosako H., Amano M., Yanagida M., Tanabe K., Nishi Y., Kaibuchi K., Inagaki M. Phosphorylation of glial fibrillary acidic protein at the same sites by cleavage furrow kinase and Rho-associated kinase. J. Biol. Chem. 1997;272:10333–10336. doi: 10.1074/jbc.272.16.10333. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leung T., Chen X.Q., Manser E., Lim L. The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol. Cell Biol. 1996;16:5313–5327. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura F., Ono S., Yamakita Y., Totsukawa G., Yamashiro S. Specific localization of serine 19 phosphorylated myosin II during cell locomotion and mitosis of cultured cells. J. Cell Biol. 1998;140:119–129. doi: 10.1083/jcb.140.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussavi R.S., Kelley C.A., Adelstein R.S. Phosphorylation of vertebrate nonmuscle and smooth muscle myosin heavy chains and light chains. Mol. Cell. Biochem. 1993;127–128:219–227. doi: 10.1007/BF01076773. [DOI] [PubMed] [Google Scholar]
- Saitoh M., Ishikawa T., Matsushima S., Naka M., Hidaka H. Selective inhibition of catalytic activity of smooth muscle myosin light chain kinase. J. Biol. Chem. 1987;262:7796–7801. [PubMed] [Google Scholar]
- Sakurada K., Seto M., Sasaki Y. Dynamics of myosin light chain phosphorylation at Ser19 and Thr18/Ser19 in smooth muscle cells in culture. Am. J. Physiol. 1998;274:C1563–C1572. doi: 10.1152/ajpcell.1998.274.6.C1563. [DOI] [PubMed] [Google Scholar]
- Sellers J.R. Regulation of cytoplasmic and smooth muscle myosin. Curr. Opin. Cell Biol. 1991;3:98–104. doi: 10.1016/0955-0674(91)90171-t. [DOI] [PubMed] [Google Scholar]
- Shimizu H., Ito M., Miyahara M., Ichikawa K., Okubo S., Konishi T., Naka M., Tanaka T., Hirano K., Hartshorne D.J., Nakano T. Characterization of the myosin-binding subunit of smooth muscle myosin phosphatase. J. Biol. Chem. 1994;269:30407–30411. [PubMed] [Google Scholar]
- Shirazi A., Iizuka K., Fadden P., Mosse C., Somlyo A.P., Somlyo A.V., Haystead T.A. Purification and characterization of the mammalian myosin light chain phosphatase holoenzyme. The differential effects of the holoenzyme and its subunits on smooth muscle. J. Biol. Chem. 1994;269:31598–31606. [PubMed] [Google Scholar]
- Somlyo A.P., Somlyo A.V. Signal transduction and regulation in smooth muscle [published erratum appears in Nature 372:812] Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- Totsukawa G., Yamakita Y., Yamashiro S., Hosoya H., Hartshorne D.J., Matsumura F. Activation of myosin phosphatase targeting subunit by mitosis-specific phosphorylation. J. Cell Biol. 1999;144:735–744. doi: 10.1083/jcb.144.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehata M., Ishizaki T., Satoh H., Ono T., Kawahara T., Morishita T., Tamakawa H., Yamagami K., Inui J., Maekawa M., Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Kato T., Fujita A., Ishizaki T., Narumiya S. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat. Cell Biol. 1999;1:136–143. doi: 10.1038/11056. [DOI] [PubMed] [Google Scholar]
- Yamakita Y., Yamashiro S., Matsumura F. Microinjection of nonmuscle and smooth muscle caldesmon into fibroblasts and muscle cells. J. Cell Biol. 1990;111:2487–2498. doi: 10.1083/jcb.111.6.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakita Y., Yamashiro S., Matsumura F. In vivo phosphorylation of regulatory light chain of myosin II during mitosis of cultured cells. J. Cell Biol. 1994;124:129–137. doi: 10.1083/jcb.124.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]