The composition of the extracellular matrix (ECM) surrounding cells is key to their behavior: it modulates their ability to proliferate, differentiate, and migrate (Giancotti and Ruoslahti 1999). ECM components can signal directly to cells through transmembrane receptors such as integrins, and can also present soluble cytokines and growth factors to cells. The morphology of cells varies greatly depending on the composition of the extracellular matrix to which they are exposed, but the molecular basis for these differences has not been clarified. Now, two papers in this issue of The Journal of Cell Biology (Adams and Schwartz 2000; Wenk et al. 2000) show that these variations in cell morphology reflect differential activation of specific Rho GTPases.
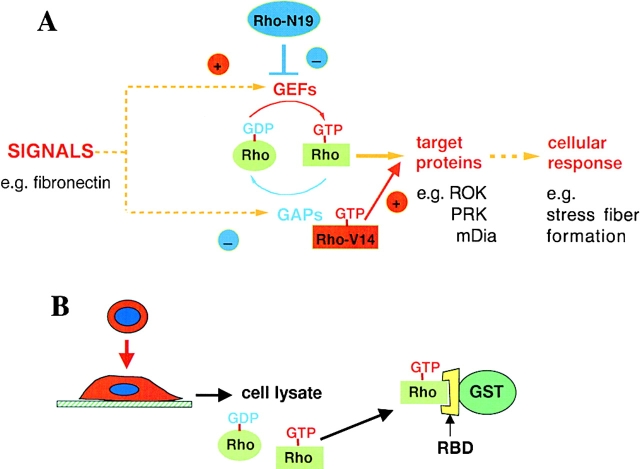
Cell morphology and migration are known to be regulated by members of the Rho family of GTPases, including Rho, Rac and Cdc42 (Hall 1998; Evers et al. 2000). These new papers use two established approaches to implicate Rho GTPases in responses to extracellular matrix proteins (Fig. 1). The first approach is to introduce dominant-negative or constitutively active mutants of a specific family member into cells. The second approach is to measure changes in the level of active Rho protein in cells, and is a recent addition to the battery of tools available for analyzing intracellular signaling. Rho GTPases cycle between an active, GTP-bound, conformation and an inactive, GDP-bound conformation. In the GTP-bound conformation the proteins are able to interact with their downstream targets and transmit signals to the cell. Cycling between the two conformations is regulated by guanine nucleotide exchange factors (GEFs), which promote release of GDP and allow GTP to bind, and GTPase-activating proteins (GAPs), which stimulate hydrolysis of GTP to yield GDP and free phosphate (Fig. 1 A; Scita et al. 2000). Dominant-negative mutants reduce the affinity of the protein for guanine nucleotides, and are presumed to bind to exchange factors but not be released because of their low affinity for GTP. The mutants thereby effectively mop up exchange factors and prevent them activating the endogenous counterpart (Feig 1999). Conversely, constitutively active mutants of Rho GTPases have mutations in critical amino acids required for GTP hydrolysis, and are locked in the GTP conformation because they cannot hydrolyze GTP (Scita et al. 2000). They therefore continuously signal to their downstream targets, and cannot be inactivated (Fig. 1 A).
Figure 1.
Methods for analyzing Rho, Rac and Cdc42 involvement in cellular responses. (A) Rho GTPases cycle between an inactive, GDP-bound conformation and an active, GTP-bound conformation. Incoming signals stimulate an increase in GTP-bound Rho, by activating exchange factors (GEFs) and/or by inactivating GTPase activating proteins (GAPs). Dominant-negative mutants of Rho proteins (e.g., Rho-N19) bind to GEFs, preventing them from activating endogenous Rho. Constitutively active mutants (e.g., Rho-V14) are unable to hydrolyze GTP, and signal continuously to downstream targets. (B) Active Rho-GTP can be isolated from cell lysates by incubation with a hybrid protein consisting of glutathione-S-transferase (GST) fused to the Rho-binding domain (RBD) of a downstream target, such as Rhoteckin.
The first indication that Rho GTPases were involved in signaling from extracellular matrix proteins came from studies of fibroblasts adhering to fibronectin. Here, incubation of cells with C3 transferase was found to inhibit fibronectin-stimulated activation of phosphatidylinositide 4-phosphate 5-kinase, to generate phosphatidylinositol (4,5) bisphosphate (PIP2) (Chong et al. 1994). C3 transferase also prevents fibronectin-induced tyrosine phosphorylation of proteins such as focal adhesion kinase (FAK) and paxillin (Barry et al. 1997), whereas constitutively active Rho can mimic the effect of fibronectin adhesion in nonadherent fibroblasts (Flinn and Ridley 1996). Subsequently, introduction of dominant-negative mutants of Rac and Cdc42 into fibroblasts indicated that they were both required for spreading of cells on fibronectin (Price et al. 1998). In addition, adhesion and spreading of T-lymphocytes to fibronectin is enhanced by activated Rac (D'Souza-Schorey et al. 1998), suggesting that Rac is generally involved in adhesion responses.
All of these experiments showed that Rho, Rac and Cdc42 are required for responses induced by extracellular matrix proteins, but did not show that the proteins are actually activated by integrin signaling. Recently, however, a method to compare levels of activated, GTP-bound Rho, Rac or Cdc42 under different conditions has been developed. This involves incubating cell lysates with domains of target proteins that selectively pull down only the GTP-bound protein (Fig. 1 B; Sander et al. 1998; Ren et al. 1999). This approach has been used to show that adhesion of fibroblasts to fibronectin promotes transient Rac activation and more prolonged Rho activation (Ren et al. 1999; del Pozo et al. 2000). This is consistent with the actin cytoskeletal organization of fibroblasts spreading on fibronectin, where first Rac-regulated lamellipodium extension and subsequently Rho-regulated stress fibre formation is observed (Barry et al. 1997; Price et al. 1998). Interestingly, Rac can be weakly activated in nonadherent fibroblasts by growth factors such as PDGF, although activation is much stronger in adherent cells. However, downstream coupling of Rac to one of its targets, the serine-threonine kinase PAK, is completely prevented in nonadherent cells (del Pozo et al. 2000). This suggests that integrin engagement by ligand can act in two ways: first it can activate Rho GTPases, presumably through activation of an exchange factor, and it can regulate coupling to downstream targets, possibly by regulating formation of a complex between exchange factor, GTPase and target.
So far, all the experiments investigating Rho protein involvement in ECM-induced responses have been carried out with fibroblasts adhering to fibronectin. But cells in vivo never see one matrix protein in isolation, and are exposed to different combinations of extracellular matrix constituents both during development and during pathological responses such as inflammation and wound healing. In vitro the effects of different extracellular matrix constituents on cell behavior have been primarily studied by comparing the responses of cells on single matrix components. Now, Wenk et al. 2000 report that adding tenascin-C to a composite matrix of fibronectin/fibrin completely alters the morphology of cells.
In comparison to fibronectin, cells adhere weakly to tenascin-C, but can be highly motile. Tenascin-C is expressed selectively during development, wound healing and metastasis, at times when reduced adhesion to the matrix may be important (Mackie and Tucker 1999). Whereas cells adherent to fibronectin/fibrin spread and assemble stress fibres, when tenascin-C is added cells do not develop stress fibres, and instead extend multiple filopodia (Fig. 2). As expected from the morphology, adhesion of fibroblasts to fibronectin/fibrin activates RhoA, as previously observed for fibronectin alone (Ren et al. 1999). However, inclusion of tenascin-C in the matrix prevents activation of RhoA, consistent with the lack of stress fibers. As filopodium extension induced by cytokines is known to be regulated by Cdc42 (Kozma et al. 1995; Nobes et al., 1995), it would be expected that Cdc42 would be activated in cells adherent to tenascin-C/fibronectin/fibrin. It would also be interesting to know what the effect of tenascin-C is on the Rac activation observed during adhesion to fibronectin (del Pozo et al. 2000). This has not been specifically investigated, as Rac activity was not measured. Although the authors did not observe any difference in the level of active Cdc42 at one hour after adhesion to the different matrices, it is probable that global Cdc42 activity increases very transiently, and that by one hour this is over. Only transient activation of Rac is detected in response to fibronectin or growth factors, despite the fact that Rac-dependent lamellipodia continue to be observed well after overall Rac activity has returned to background levels (Sander et al. 1998, Sander et al. 1999; del Pozo et al. 2000). The acute increase in Rac/Cdc42-GTP following cell stimulation is likely to be rapidly downregulated, probably through GTPase activating proteins. Subsequently, very localized Rac/Cdc42 activity is probably sufficient to maintain the production of lamellipodia and filopodia. This active protein will represent only a small fraction of the total protein and will not be detectable as an overall increase in GTP-bound protein in pull-down assays. It will only be possible to detect this localized activity by microscopy-based approaches designed to identify where Rho proteins are active in single cells, and no doubt such methods will soon be developed.
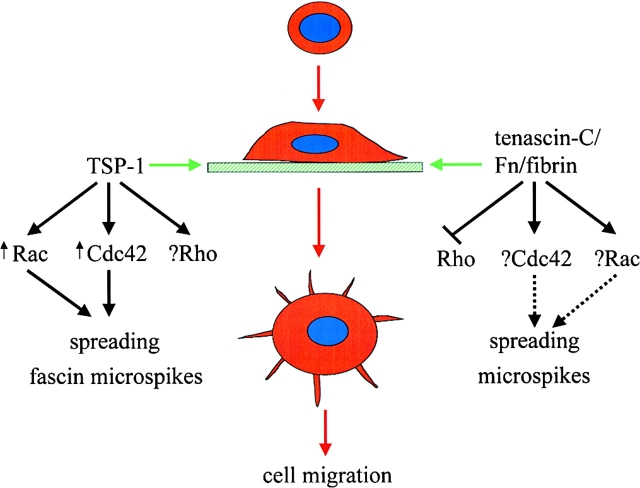
Figure 2.
Involvement of Rho, Rac and Cdc42 in responses to ECM. Adhesion of cells to thrombospondin-1 (TSP-1) leads to activation of Rac and Cdc42, which are required for extension of fascin-containing microspikes and cell migration. TSP-1 effects on Rho are not known. Adhesion of cells to a composite matrix of tenascin-C/fibronectin (FN)/fibrin prevents Rho activation. Effects on Rac and Cdc42 have not been determined.
Thrombospondin-1 (TSP-1) is another matrix protein that is selectively expressed during conditions of inflammation, wound healing, angiogenesis and tumorigenesis (Adams 1997a). Adhesion of several cell types to TSP-1 leads to a distinctive spreading response where microspikes containing F-actin and the actin-bundling protein fascin are extended, and no stress fibres are observed (Adams 1995). Adhesion to TSP-1 is now shown to induce activation of both Rac and Cdc42, as well as their downstream target PAK, and to be inhibited by dominant-negative mutants of Rac and Cdc42 (Adams and Schwartz 2000). Compared with fibronectin, activation of Rac and Cdc42 was more prolonged on TSP-1. Given the lack of stress fibres and lack of effect of C3 transferase on spreading of cells on TSP-1, it would be expected that Rho is not activated (although this has not been tested), and in fact the prolonged activation of Rac may inhibit Rho activation (Sander et al. 1999). As addition of fibronectin to TSP-1 progressively reduces extension of fascin-containing microspikes, and increases stress fiber formation (Adams 1997b), it would be interesting to know how this is reflected in the relative activation states of Rho, Rac and Cdc42. Interestingly, fascin is not only found in microspikes in TSP-adherent cells, but also localized in lamellipodia and filopodia induced by activated Rac and Cdc42 in the absence of TSP-1 (Adams and Schwartz 2000). This suggests that fascin plays a role in the formation or organization of these structures, and indeed introduction of anti-fascin antibodies inhibits cell adhesion, spreading and migration on either fibronectin or TSP-1. Whether Rac and Cdc42 directly modulate fascin actin-bundling activity, for example through phosphorylation, remains to be determined.
These two studies indicate the complexities of signaling to Rho GTPases by different ECM proteins, and the next step will be determine which exchange factors for Rho, Rac and Cdc42 are involved in ECM signaling, and how their activity is regulated depending on matrix composition. There is an ever-growing family of exchange factors, so this may not be a straight-forward task (Stam and Collard 1999). At present, most studies on Rho GTPases concentrate on Rho, Rac and Cdc42, simply because reagents for these are most readily available. In the future, however, it will be important to develop more global strategies to determine the involvement of all Rho family proteins (and indeed other related Ras superfamily members) in specific cellular responses. This will include measuring their activation status biochemically through pull-down assays and also determining where they and their targets are localized and active in cells through single-cell measurements. Critical to this will be the identification of targets for other Rho family members apart from Rho, Rac and Cdc42, as for example has been carried out for TC10 (Joberty et al. 1999). The future should hold many exciting developments in the investigation of Rho family proteins and ECM signaling.
References
- Adams J.C. Formation of stable microspikes containing actin and the 55 kDa actin bundling protein, fascin, is a consequence of cell adhesion to thrombospondin-1implications for the anti-adhesive activities of thrombospondin-1. J. Cell Sci. 1995;108:1977–1990. doi: 10.1242/jcs.108.5.1977. [DOI] [PubMed] [Google Scholar]
- Adams J.C. Thrombospondin-1 Int. J. Biochem. Cell Biol. 29 1997. 861 865a [DOI] [PubMed] [Google Scholar]
- Adams J.C. Characterization of cell-matrix adhesion requirements for the formation of fascin microspikes Mol. Biol. Cell. 8 1997. 2345 2363b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J.C., Schwartz M.A. Stimulation of fascin microspikes by thrombospondin-1 is mediated by the GTPases Rac and Cdc42. J. Cell Biol. 2000;150:807–822. doi: 10.1083/jcb.150.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry S.T., Flinn H.M., Humphries M., Critchley D.R., Ridley A.J. Requirement for Rho in integrin signalling. Cell Adhes. Commun. 1997;4:387–398. doi: 10.3109/15419069709004456. [DOI] [PubMed] [Google Scholar]
- Chong L.D., Traynor-Kaplan A., Bokoch G.M., Schwartz M.A. The small GTP-binding protein Rho regulates a phosphatidylinositol 4-phosphate 5-kinase in mammalian cells. Cell. 1994;79:507–513. doi: 10.1016/0092-8674(94)90259-3. [DOI] [PubMed] [Google Scholar]
- del Pozo M.A., Price L.S., Alderson N.B., Ren X.D., Schwartz M.A. Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. EMBO (Eur. Mol. Biol. Organ.) J. 2000;19:2008–2014. doi: 10.1093/emboj/19.9.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza-Schorey C., Boettner B., Van Aelst L. Rac regulates integrin-mediated spreading and increased adhesion of T lymphocytes. Mol. Cell Biol. 1998;18:3936–3946. doi: 10.1128/mcb.18.7.3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers E.E., Zondag G.C., Malliri A., Price L.S., ten Klooster J., van der Kammen R.A., Collard J.G. Rho family proteins in cell adhesion and cell migration. Eur. J. Cancer. 2000;36:1269–1274. doi: 10.1016/s0959-8049(00)00091-5. [DOI] [PubMed] [Google Scholar]
- Feig L.A. Tools of the tradeuse of dominant-inhibitory mutants of Ras-family GTPases. Nat. Cell Biol. 1999;1:E25–E27. doi: 10.1038/10018. [DOI] [PubMed] [Google Scholar]
- Flinn H.M., Ridley A.J. The small GTP-binding protein Rho stimulates tyrosine phosphorylation of focal adhesion kinase, p130 and paxillin. J. Cell Sci. 1996;109:1133–1141. doi: 10.1242/jcs.109.5.1133. [DOI] [PubMed] [Google Scholar]
- Giancotti F.G., Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Joberty G., Perlungher R.R., Macara I.G. The Borgs, a new family of Cdc42 and TC10 GTPase-interacting proteins. Mol. Cell. Biol. 1999;19:6585–6597. doi: 10.1128/mcb.19.10.6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma R., Ahmed S., Best A., Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol. Cell. Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie E.J., Tucker R.P. The tenascin-C knockout revisited. J. Cell Sci. 1999;112:3847–3853. doi: 10.1242/jcs.112.22.3847. [DOI] [PubMed] [Google Scholar]
- Nobes C.D., Hall A. Rho, Rac and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Price L.S., Leng J., Schwartz M.A., Bokoch G.M. Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol. Biol. Cell. 1998;9:1863–1871. doi: 10.1091/mbc.9.7.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X.D., Kiosses W.B., Schwartz M.A. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander E.E., van Delft S., ten Klooster J.P., Reid T., van der Kammen R.A., Michiels F., Collard J.G. Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell-cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J. Cell Biol. 1998;143:1385–1398. doi: 10.1083/jcb.143.5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander E.E., ten Klooster J.P., van Delft S., van der Kammen R.A., Collard J.G. Rac downregulates Rho activityreciprocal balance between both GTPases determines cellular morphology and migratory behavior. J. Cell Biol. 1999;147:1009–1022. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scita G., Tenca P., Frittoli E., Tocchetti A., Innocenti M., Giardina G., Di Fiore P.P. Signaling from Ras to Rac and beyondnot just a matter of GEFs. EMBO (Eur. Mol. Biol. Organ.) J. 2000;11:2393–2398. doi: 10.1093/emboj/19.11.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam J.C., Collard J.G. The DH protein family, exchange factors for Rho-like GTPases. Prog. Mol. Subcell. Biol. 1999;22:51–83. doi: 10.1007/978-3-642-58591-3_4. [DOI] [PubMed] [Google Scholar]
- Wenk M.B., Midwood K.S., Schwarzbauer J.E. Tenascin-C suppresses Rho activation. J. Cell Biol. 2000;150:913–919. doi: 10.1083/jcb.150.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]