Abstract
Mammalian genomes feature multiple genes encoding highly related keratin 6 (K6) isoforms. These type II keratins show a complex regulation with constitutive and inducible components in several stratified epithelia, including the oral mucosa and skin. Two functional genes, K6α and K6β, exist in a head-to-tail tandem array in mouse genomes. We inactivated these two genes simultaneously via targeting and homologous recombination. K6 null mice are viable and initially indistinguishable from their littermates. Starting at two to three days after birth, they show a growth delay associated with reduced milk intake and the presence of white plaques in the posterior region of dorsal tongue and upper palate. These regions are subjected to greater mechanical stress during suckling. Morphological analyses implicate the filiform papillae as being particularly sensitive to trauma in K6α/K6β null mice, and establish the complete absence of keratin filaments in their anterior compartment. All null mice die about a week after birth. These studies demonstrate an essential structural role for K6 isoforms in the oral mucosa, and implicate filiform papillae as being the major stress bearing structures in dorsal tongue epithelium.
Keywords: keratin, bullous diseases, skin, transgenic mouse, pachyonychia congenita
Introduction
Keratin intermediate filaments (IF) are heteropolymers of type I and type II sequences that occur in the cytoplasm of all epithelial cells. Consistent with this biochemical requirement, epithelial cells must coordinate the expression of at least one type I and one type II gene to produce a keratin IF network in their cytoplasm (Fuchs and Weber 1994). The keratin genes expressed in soft epithelia include the type II K1–K8 and the type I K9–K20. The expression of many type I and type II keratin genes is regulated in a pairwise and differentiation-specific fashion (O'Guin et al. 1990). Given their properties, abundance, and organization in the cytoplasm, keratin IF are poised to play an important role of mechanical support in epithelial cells and tissues. Such a role has been shown by transgenic mouse studies and through the discovery of mutations affecting keratin proteins in dominantly inherited epithelial fragility syndromes (Fuchs and Cleveland 1998; Irvine and McLean 1999; Takahashi et al. 1999).
Among type II keratins, keratin 6 (K6) is unique in three ways. First, it exists as multiple, highly related isoforms encoded by separate genes within the cluster of functional type II keratin genes. There are six functional K6 genes in human (Takahashi et al. 1995), two functional K6 genes and two K6 pseudogenes in mouse (Takahashi et al. 1998; Rothnagel et al. 1999), and potentially, three functional K6 genes in bovine (Ramirez et al. 1995). The significance of this diversity is unclear, as there is no common pattern of sequence diversification among K6 isoforms (Takahashi et al. 1998). Second, the K6 genes feature a complex regulation, with distinct constitutive and inducible components in various types of complex epithelia. Individual K6 genes are differentially regulated in human and mouse skin epithelia (Tyner and Fuchs 1986; Ramirez et al. 1995; Takahashi et al. 1995, Takahashi et al. 1998; Rothnagel et al. 1999). In addition, K6 is ectopically expressed after injury and in diseases involving altered proliferation or differentiation (McGowan and Coulombe 1998a). There is no obvious correlation between K6 isoform expression and the features of the epithelia expressing it. Third, inherited mutations affecting the K6a and K6b coding sequences in humans underlie type I (Bowden et al. 1995) and type II (Smith et al. 1998) pachyonychia congenita disease, respectively. These ectodermal dysplasias are characterized by severe dystrophy of the nail plate and alterations in a number of internal stratified epithelia (Irvine and McLean 1999). They differ principally in terms of the involvement of stratified epithelia other than nail. Again, the function of K6 isoforms in these epithelia is not readily apparent from the pathology associated with pachyonychia congenita diseases.
To address the issue of K6 isoform function(s), we inactivated the K6α/K6β locus in mouse via gene targeting and homologous recombination. The phenotype of K6 null mice points to the essential scaffolding function of K6α and K6β isoform proteins in the oral mucosa.
Materials and Methods
Construction of Targeting Vector and Generation of Transgenic Mice
Plasmid NeoTKXho, with functional PGK-Neo (Neo) and MC1TK (TK [thymidine kinase]) cassettes for selection, was obtained from Dr. Jeremy Nathans (Johns Hopkins University, Baltimore, MD) and used to generate the targeting vector. The 5′ arm of homology is a 6.0-kb XbaI fragment derived from the upstream regulatory region of the cloned mouse K6α (mK6α) gene. The 3′ arm of homology is a 3.8-kb SacI fragment that begins in the 3′ end of exon 1 extending through the 3′ noncoding region of the cloned mouse K6β (mK6β) gene. Both fragments were blunt end cloned in NeoTKXho, effectively destroying the original XbaI and SacI sites. The targeting construct was linearized at the unique NotI site before transfection. DNA (20 μg) was transfected by electroporation into 2 × 107 exponentially growing 129/Sv CK35 embryonic stem (ES) cells (Kress et al. 1998) using a BioRad Gene Pulser apparatus as described (Colucci-Guyon et al. 1994). After electroporation, ES cells were screened for G418 (GIBCO BRL) and gancyclovir (Syntex Research) resistance for 10 d and genotyped. Targeted ES cells were injected into C57Bl/6 blastocysts and transferred into (C57Bl/6xCBA)F1 pseudopregnant females. Chimeric males, identified by their agouti coat color, were mated with (C57Bl/6xDBA2)F1 females, and the resulting mice heterozygous for the disrupted K6 allele were interbred to obtain the K6 deficient mice. For genotyping, genomic DNA was extracted from mouse tails, digested with XbaI, electrophoresed, and transferred onto nylon membrane (Gene Screen Plus; NEN Life Science Products) for Southern blotting. Random labeled probe was generated using a 1.3-kb fragment within the 5′ arm of homology.
RNA Analyses
RNA was isolated using Trizol Reagent (Life Technologies) and quantitated. For reverse transcriptase (RT)-PCR, cDNA was generated from 1 μg of RNA (Advantage RT-PCR; CLONTECH Laboratories, Inc.) and PCR was performed using primers specific for mK6α or mK6β (Takahashi et al. 1998). β-Tubulin primers were used as internal controls. PCR conditions were: 4 min at 94°C, 30 cycles of 94°C for 45 s, 60°C for 45 s; 70°C for 2 min, and 10 min at 72°C. The expected PCR products for mK6α, mK6β, and β-tubulin are 1.8 kb, 1.8 kb, and 400 bp, respectively. For Northern blot analyses, 10 μg of RNA was resolved by formaldehyde-agarose gel electrophoresis, transferred to GeneScreen nylon membrane, and probed with a 400-bp fragment specific for the 3′ noncoding region of the mK6β transcript as described (Takahashi et al. 1998). After radioautographic exposure, the membrane was stripped and reprobed using a 692-bp fragment corresponding to the neomycin resistant (Neor) coding sequence.
Western Blot Analysis
Newborn mice were killed by decapitation and excised tongues were snap-frozen in liquid nitrogen. Total protein extracts were prepared as described (Paladini and Coulombe 1999). Protein concentrations were determined using a Bradford Assay Kit (BioRad). Equal amounts of protein were electrophoresed in 8.5% SDS-PAGE gels and electroblotted onto Protran nitrocellulose (Schleicher and Schuell). After rinsing, they were incubated with primary antibody, followed by goat anti–mouse HRP or goat anti–rabbit HRP (1:2,000) following the manufacturer's instruction. Primary antibodies used include rabbit polyclonal antisera directed against K6 and K17 (McGowan and Coulombe 1998b), K5 (Lersch et al. 1989) and K16 (Porter et al. 1998), and mouse mAbs to K4 (Sigma-Aldrich), K14 (Purkis et al. 1990), and plakoglobin (Transduction Laboratories).
Morphological Studies
For histology, tissues were fixed in Bouin's overnight at 4°C before paraffin embedding. 5-μm sections were stained with hematoxylin and eosin. Alternatively, they were immunostained using the antibodies described above and a peroxidase-based detection method (Kirkegaard and Perry Labs). For transmission EM, tongues from newborn mice were fixed in 2% glutaraldehyde, 1% paraformaldehyde in 0.1 M cacodylate buffer, pH 7.2, postfixed in aqueous osmium tetroxide, and embedded in epoxy resin as described (Paladini and Coulombe 1999). Thin sections were cut, counterstained with uranyl acetate and lead citrate, and examined using a Philips CM120 electron microscope.
Results
Generation of Mice with a K6α/β Null Mutation
The linearized K6 targeting vector (Fig. 1 A) was introduced in the 129/Sv CK35 ES line (Kress et al. 1998). Out of 450 G418/gancyclovir double resistant ES clones, four showed correct targeting by homologous recombination upon Southern blot analysis (5′ side of locus) and PCR (3′ side of locus). One correctly targeted line gave rise to germline transmission. To generate mice that are homozygous for the targeted allele, heterozygous males and females were mated. The targeted allele segregated in a Mendelian fashion. Out of 143 mice, 35 were homozygous (24.5%), 73 heterozygous (51%), and 37 wild-type (25.8%) as determined by Southern blot analysis (Fig. 1 B). These results indicate that K6α/β null mice are viable.
Figure 1.
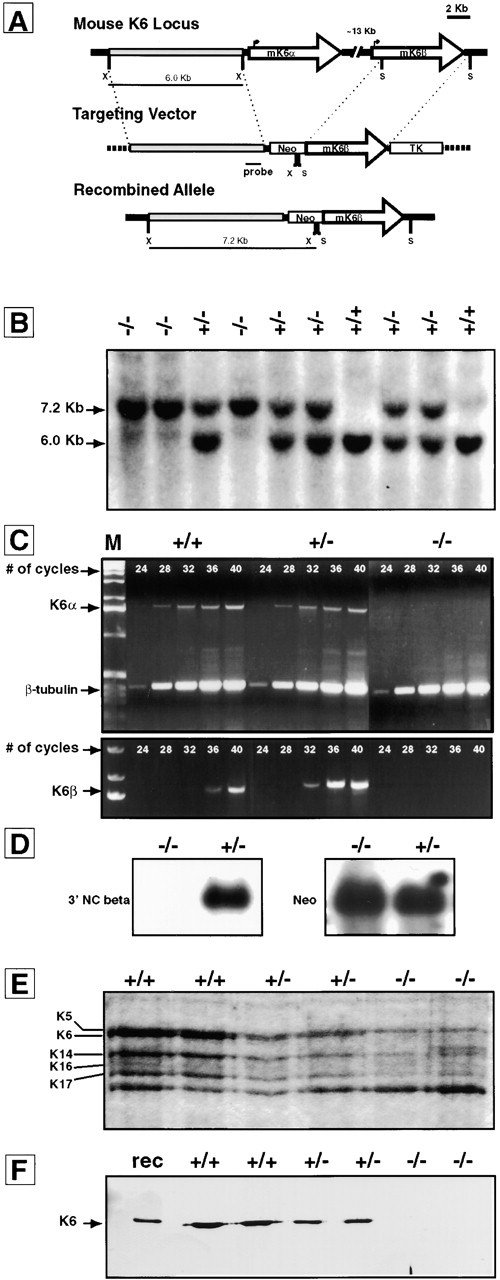
Generating K6 null mice. A, Targeting strategy used to inactivate the K6α and K6β isoforms genes. A 6.0-kb XbaI fragment (5′ side) and a 3.8-kb SacI fragment (3′ side) were blunt-end cloned on either side of a Neor cassette in plasmid NeoTKXho. A proper recombination event removes the entire coding sequences of mK6α, the intergenic sequences (∼13 kb), and the first half of exon 1 (including the start of translation) in mK6β, and regenerates the XbaI site at the 5′ end of the 5′ arm of homology in the recombined allele. X, XbaI; S, SacI. B, Southern blot analysis on XbaI digested genomic DNA reveals a 6.0- and a 7.2-kb band corresponding to wild-type and recombined alleles, respectively. C, RT-PCR was performed on total RNA isolated from primary keratinocytes cultured from newborn pups using K6α-specific or K6β-specific primers. Samples were removed after various number of cycles to visualize product accumulation. β-Tubulin primers were included as an internal control. D, Northern blot analysis was performed on total RNA (10 μg) extracted from primary keratinocytes using probes specific for either the 3′ end of mK6β or Neor. E, Proteins were isolated from primary keratinocyte cultures, resolved via SDS-PAGE, and visualized by Coomassie staining. F, Western blot analysis of the samples shown in E using an antiserum directed against a peptide sequence that is conserved between both K6α and K6β. Recombinant K6α is loaded in lane 1.
K6α/β expression was assessed at the RNA and protein levels in keratinocyte cultures established from newborn pups derived from heterozygous matings. The two isoforms are strongly induced upon placing newborn mouse keratinocytes in primary culture, thus providing a stringent test of whether the K6 locus has been inactivated. Internally controlled RT-PCR was first performed to examine expression at the mRNA level at four days after cell plating. Primers specific for K6α amplify the expected 1.8-kb product in wild-type and heterozygous samples, but not in homozygous samples (Fig. 1 C). Identical results are obtained when using primers specific for K6β mRNA (Fig. 1 C). A similar PCR-based test for the possibility of a neo-K6β read-through transcript tested negative (data not shown). Northern analyses were also performed on the same type of RNA samples to confirm these results (Fig. 1 D). When probing for the 3′ noncoding region of K6β, a strong hybridization band with expected mobility (∼2 kb; Takahashi et al. 1998) is readily detected in heterozygous mouse samples, but not in homozygous samples (Fig. 1 D). Reprobing the stripped membrane with a probe specific for the Neor sequence yielded strong hybridization to a single band of ∼1.3 kb only in heterozygous and homozygous samples (Fig. 1 D).
Equal amounts of total proteins extracted from primary keratinocytes were subjected to SDS-PAGE and visualized by Coomassie staining. In wild-type and heterozygous cells, bands corresponding to K5, K6, K14, K16, and K17 are present (Fig. 1 E). In cells derived from homozygous mice, the band corresponding to K6 has disappeared. Western blot analysis was performed on these samples using a rabbit antiserum directed against a peptide that is conserved at the COOH terminus of K6α/β (McGowan and Coulombe 1998b). Strong immunoreactivity for K6 is present in wild-type and heterozygous mouse samples, but not in homozygous mouse samples (Fig. 1 F). Both the Coomassie-stained gel and K6 immunoblot suggest that K6 levels are reduced by half in heterozygous mice. Likewise, the levels of type I keratins, especially K17, appear to be reduced in null mouse samples. Additional studies are required to establish these as consistent findings. Together, these analyses establish that mice homozygous for the targeted allele do not express the K6α and K6β mRNA, and are K6 protein null.
Homozygous K6α/β Null Mice Suffer from Severe Blistering in the Oral Mucosa
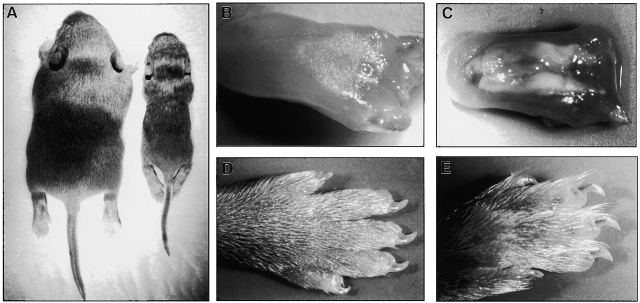
Pups born from K6α/β null heterozygous mice appear normal at birth. All newborns initially feed and display a full stomach of milk as determined by visual inspection. Within a few days, a subset of mice (∼25%) become smaller, weak, and have noticeably less, and eventually no milk in their stomachs (Fig. 2 A). These mice generally die between five to ten days postpartum, weighing about half of their littermate controls. Genotype analysis reveals that these are the K6α/β null mice. At the time of death, these mice do not show any obvious developmental anomaly other than reduced size and frail appearance. The fur and nails appear normal (Fig. 2D and Fig. E).
Figure 2.
Appearance of wild-type and K6 null mice. A, Dorsal view of 10-d-old wild-type and K6 null mice. Note the size difference between the littermates. B, Wild-type tongue. The surface is smooth and uniform. C, Tongue from K6 null mice. White plaques cover the dorsal surface, extending from the posterior region (right) near the pharynx towards, but not reaching, the tip of the tongue (left). D, Paws from wild-type mice are similar to null mice. E, The paw of the null mouse appears smaller, probably due to poor nutrition and slow growth. Development of fur and nails do not appear altered in null mice.
K6 is constitutively expressed in several of the epithelia making up the oral mucosa. With the K6α/β null mice dying possibly due to poor feeding, we examined the gross anatomy of the oral mucosa in dissected preparations. The dorsal surface of the tongue (Fig. 2 C) and the ventral surface of the upper palate (data not shown) are covered with white plaques located towards the posterior end of the mouth in K6α/β null mice. Wild-type (Fig. 2 B) and heterozygous littermates (not shown) showed no signs of these lesions.
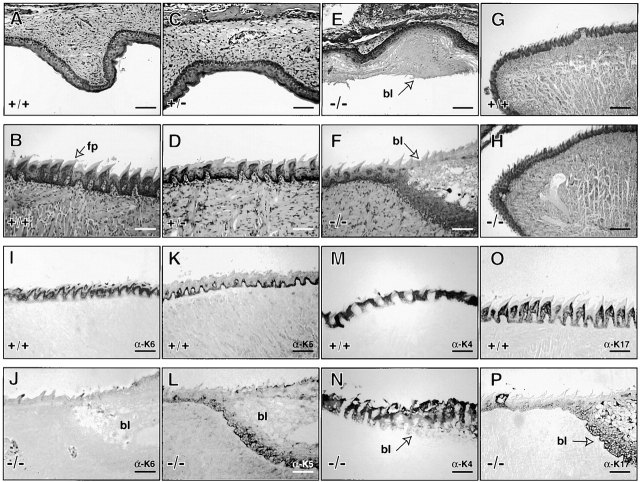
Histological studies were conducted to investigate the oral lesions in homozygous null mice. Examination of hematoxylin and eosin-stained sections confirmed the occurrence of severe blistering in the posterior region of the tongue and upper palate in homozygous mice, as compared with wild-type controls and heterozygous mice (Fig. 3, A–F). The blisters extend across the dorsal surface of the tongue, with the plane of cleavage located within the suprabasal layer of dorsal tongue and upper palate epithelia (Fig. 3E and Fig. F). Within such blisters, the epithelium is thickened and shows signs of intraepithelial edema. Beneath the plane of cleavage, several rows of cells remain attached to the extracellular matrix, whereas above, the epithelial architecture is destroyed, giving way to cell debris and inflammatory cells. The anterior region of the tongue and upper palate displays a normal morphology at this level of resolution (Fig. 3G and Fig. H). Mild cytolysis can occasionally be seen in the esophagus, but not in the forestomach of null mice (data not shown).
Figure 3.
Histological analysis of the oral mucosa in K6 null mice. A–H, Hematoxylin and eosin staining of wild-type, heterozygous, and null mice tongues. In wild-type mice, the upper palate exhibits normal epithelial differentiation (A) and the dorsal tongue shows well-developed filiform papillae (B). Similar results are found in heterozygous mice (C and D). In K6 null mice, the upper palate contains large amounts of cellular debris facing the oral cavity (E). F, Shows the transition zone between unaffected and affected dorsal tongue epithelium. Blisters (bl) form as a result of cleavage within the suprabasal layers of the epithelium. The tips of both wild-type and null mice tongues are similar at low magnification (G and H). I–P, Immunohistochemical staining of wild-type and K6 null tongue. Antibodies to K6 shows reactivity in wild-type, but not in null mouse tongue sections (I and J). The immunostaining reaction in these samples was overdeveloped in an attempt to detect any residual K6 expression in K6 null mice tongues. Antibodies to K5 stain the basal layer of wild-type tongues (K) while staining the basal and adjacent suprabasal cells in blistered dorsal epithelium (L). K4 antibody staining detects the interpapillary region in both types of samples. The regular staining pattern is severely disturbed in blistered epithelium (M and N). Wild-type staining for K17 shows strong reactivity in the papillae (O), whereas reactivity adjacent to the blister is basal and restricted to the lower most portion of blistered epithelium (P). Bars: (A–F, I–P) 200 μm; (G and H) 100 μm.
Immunostaining of similar sections confirmed the lack of K6α/β protein in oral mucosa of K6 null mice (Fig. 3 J). In wild-type tongue sections, K6 staining occurs in the suprabasal layer of the ventral tongue, in the upper and lower palate, and buccal mucosa. In dorsal tongue epithelium, a strong signal occurs in fungiform (data not shown) and filiform papillae (Fig. 3 I). Based on antibody staining, K5 is expressed in the basal layer throughout the oral mucosa of wild-type mice (Fig. 3 K). In K6α/β null mice, a normal pattern of K5 immunoreactivity is seen in intact epithelia, while the signal extends into the suprabasal layer in the blister floor (Fig. 3 L). Strong immunoreactivity for K4, a major type II keratin in the oral mucosa (O'Guin et al. 1990), occurs in the interpapillary regions of the dorsal tongue for both control and null mice (Fig. 3M and Fig. N). Interestingly, antibodies to K4 faintly stain the disorganized suprabasal layers in the blister roof, where the papillae are difficult to outline. Antibodies to K17, a potential type I partner for K6, display strong reactivity with the epithelium of the filiform papillae and fungiform papillae, and weaker reactivity with basal cells throughout the dorsal tongue mouse (Fig. 3 O). In null mice, K17 antibodies stain the basal layer and like K5, the signal extends into the suprabasal layers in blistered epithelium (Fig. 3 P). Follow-up studies are needed to carefully assess the fate of K17 protein in the absence of K6 isoforms in complex tissues such as filiform papillae. The available data indicate that K6α/β proteins are essential to maintain the mechanical integrity of several types of epithelia in the oral mucosa. They also show that in the absence of trauma, there are no obvious anomalies in the morphogenesis and differentiation of the various types of epithelia in the oral mucosa.
Absence of Keratin IF in the Anterior Compartment of the Filiform Papillae
The dorsal tongue epithelium is covered in four different types of lingual papillae: fungiform, circumvallate, foliate, and filiform, the latter being the most abundant (Hume and Potten 1976). Three distinct programs of epithelial differentiation are executed in filiform papillae, giving rise to the anterior (AC), posterior (PC), and buttress (BC) columns (Fig. 4 A). In situ hybridization using probes that are specific for K6α (Fig. 4 B) or K6β (Fig. 4 C) mRNAs in wild-type samples establishes that both isoforms are expressed in the anterior and buttress columns. We next assessed the ultrastructure of filiform papillae using samples harvested from mice born that day (P1) to avoid any extensive damage caused by suckling.
Figure 4.
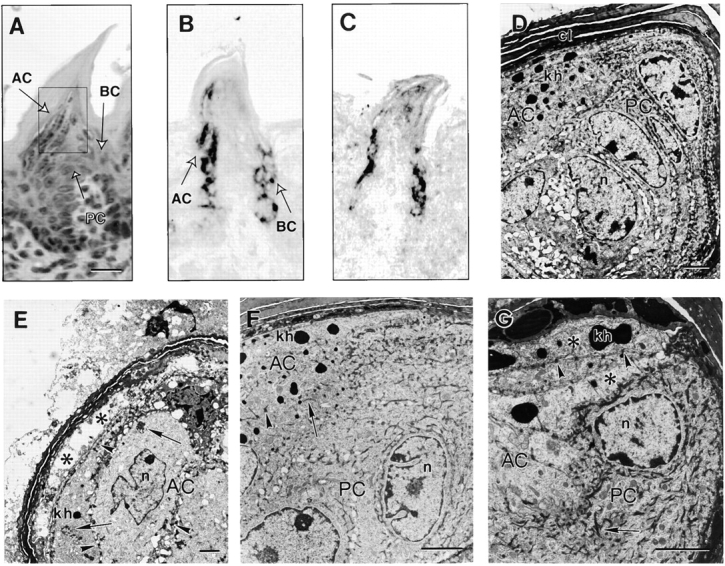
Ultrastructure of newborn filiform papillae. A, Hematoxylin and eosin stained wild-type papillae. The three programs of differentiation are labeled AC (anterior column), PC (posterior column), and BC (buttress column). The electron micrographs shown in frames D–G originate from the region defined in the box. In situ hybridization using K6α- (B) or K6β- (C) specific probes shows expression of both isoforms in the anterior and buttress columns. D, In wild-type posterior filiform papillae, AC cells can be readily recognized by keratohyalin granules (kh). PC cells feature prominent keratin bundles, but no kh. cl, Cornified layers; n, nucleus. E, In K6 null mouse posterior filiform papillae, AC cells feature early signs of cytolysis (asterisks). Keratohyalin granules and desmosomes (arrowheads) are present. Arrows show aggregated keratin protein. F, In anterior filiform papillae of wild-type mice, AC cells exhibit the normal complement of keratin IF bundles, keratohyalin granules, and desmosomes. G, In contrast, filaments in null mice are completely devoid in AC cells (asterisks) while still featuring numerous keratohyalin granules and desmosomes. PC cells exhibit numerous IF bundles (arrows). Bars: (A) 12.5 μm; (D–G) 2 μm.
At the ultrastructural level in wild-type mice, differentiating keratinocytes in the anterior column feature electron dense granules (Fig. 4 D) similar to those found in the granular layer of epidermis (Manabe and O'Guin 1994). Posterior column keratinocytes have a rounder shape and feature large bundles of densely packed keratin IF, but no keratohyalin granules (Fig. 4 D). These cells express the hard keratins that are characteristic of hair and nails (Manabe and O'Guin 1994). No obvious alterations are present in the filiform papillae of heterozygous mice. In contrast, samples taken from the same region in K6α/β null mice show dramatic alterations in anterior column keratinocytes (Fig. 4 E) readily visible through their cytolytic appearance and unusually clear cytoplasm. These cells appear to have a normal complement of keratohyalin granules and desmosomes, but seem to completely lack keratin IF (Fig. 4 E). To confirm the latter, we examined samples taken from the anterior region of newborn (P1) tongues, since it is subjected to less trauma (Fig. 2 C). In comparison to wild-type (Fig. 4 F), the anterior column cells in null mouse samples have no visible keratin IF, whereas differentiating cells in the posterior column feature a well-developed array of keratin IF bundles (Fig. 4 G). These findings suggest that anterior column cells depend upon K6 isoform proteins for the elaboration of a keratin IF array, and that their absence account for the early stages of blister formation in dorsal tongue epithelium.
Expression Levels of Other Keratin Proteins in K6α/β Null Tongue Epithelia
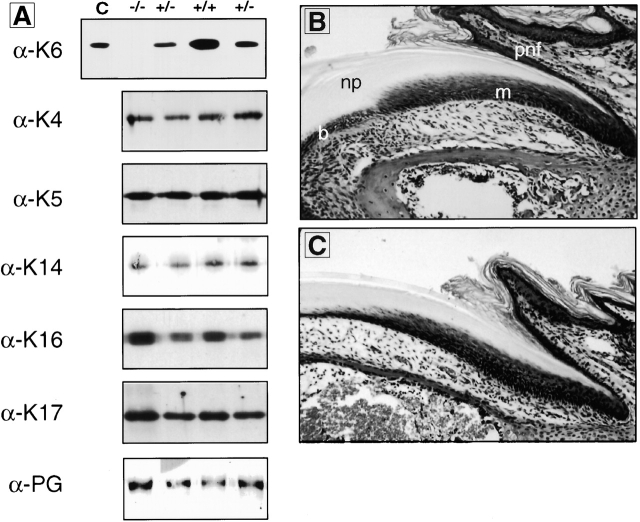
Western blot analysis was next performed on protein extracts prepared from whole tongues (Fig. 5 A). An mAb to plakoglobin, a component of desmosomes and adherens junction, was used to control for protein loading. The K6 antiserum detected the expected ∼60 kD antigen(s) in wild-type and heterozygous, but not in homozygous null samples. As compared with controls, K5 and K4 protein levels do not appear to be altered in null samples. The levels of K16 and K17, the presumed pairing partners for K6, do not appear to be significantly altered either, once protein loading differences are taken into account (see plakoglobin blot). However, it is conceivable that the likely induction of K16 and K17 gene expression in blistered epithelium (e.g., see Fig. 3 P) could mask a decrease in the steady state levels of these proteins in intact tissue.
Figure 5.
Additional analyses of K6 null mice. A, Examination of keratin protein levels in tongue epithelium. Western blot analysis was performed on 2 μg of total protein extracts from wild-type, heterozygous, and homozygous samples with various keratin-specific antibodies as indicated. An additional blot was reacted with antiplakoglobin (PG) to provide an internal reference for protein loadings. The K6 antibody does not detect any K6 protein the tongue extracts. No consistent change is observed in the steady state levels of other keratins. B and C, Histology of nail tissue. Hematoxylin and eosin staining of nail cross-sections from 10-d-old wild-type (B) and K6α/β null (C) mice. At this age, the proximal nail fold (pnf), matrix (m), nail bed (b), and nail plate (np) all appear normal.
Lack of Detectable Alterations in Nail Epithelia
The dominant clinical trait of all pachyonychia congenita patients is a severe thickening and dystrophy of the nail owing to hyperproliferation and aberrant differentiation in the nail bed epithelium (see McGowan and Coulombe 2000). Examination of histological cross-sections of the nail tissue shows that at the time of death (less than five to ten days after birth), no major differences in morphology can be observed in null mice as compared with wild-type (Fig. 5B and Fig. C).
Discussion
Targeted inactivation of the K6α and K6β isoform genes in the mouse genome results in the complete absence of detectable K6 protein in relevant epithelia and the appearance of severe blisters in the oral mucosa. These blisters develop early after birth, coinciding with the onset of suckling. They occur in the posterior region of the dorsal tongue epithelium and the upper palate directly above it, where suckling produces the most mechanical rubbing (Koch et al. 1997). Correlating with the onset of blistering, the mice fail to adequately feed and gain weight. All homozygous K6 null mice become very weak and die about seven days after birth. In addition to the original mixed genetic background, the same phenotype was observed in the C57/Bl6 and 129 SvJ inbred stains (data not shown). These findings suggest that K6 isoform proteins play an essential structural role in many epithelia within the oral mucosa. This includes the filiform papillae, which are poised to play a major role in handling mechanical forces in the oral mucosa given their shape, properties, number, and localization. A similar structural scaffolding function has been described for several other keratins (see Fuchs and Cleveland 1998; Irvine and McLean 1999; Takahashi et al. 1999). Our studies so far do not address the impact of secondary alterations in the steady state levels of other keratin proteins in K6α/β null epithelial cells. This represents an important, but complex issue that can be best addressed in follow up studies at the cellular level.
The severity of the oral blisters seen in the absence of K6 proteins contrasts with those resulting from an absence of K4, another prominent type II keratin found in the oral mucosa. The K4 null mice feature subclinical blistering associated with mild cytolytic changes in the oral mucosa, tongue, and esophagus (Ness et al. 1998). In dorsal tongue, the K4-K13 pair is strongly expressed in the postmitotic layers of the interpapillary epithelium (O'Guin et al. 1990). The differences noted between K4 null and K6 null mice suggest that the integrity of filiform papillae represents a more critical determinant of the mechanical properties of dorsal tongue epithelium than is the interpapillary epithelium. However, when analyzed for its keratin protein profile the K4 null tongue epithelium show an upregulation of a ∼60-kD band, likely a type II keratin (Ness et al. 1998). There is no evidence for an increase in the levels of a known type II keratin in K6α/β null mice, providing yet another explanation for the mild nature of the K4 null phenotype as compared with K6α/β null mice.
At the time of death, K6 null mice do not show any obvious alteration in nail morphology, a finding that contrasts sharply with the clinical finding in pachyonychia congenita patients. It is possible that K6 null mice die too young to develop nail changes. In support of this, visible nail lesions develop over months after birth in pachyonychia congenita patients (Joseph 1964). Alternatively, this discrepancy could reflect the existence of differences in the functional anatomy of mouse and human nail epithelia. Consistent with this, the nail bed epithelium is significantly shorter in mouse compared with human. Finally, it is not clear whether the nail bed alterations that result from inherited point mutations or small deletions in K6 coding sequences correspond to a loss- or gain-of-function phenotype (see McGowan and Coulombe 1998a, and Irvine and McLean 1999, for discussions). Experiments in which the lifespan of K6 null mice is extended may shed some light on this issue.
Oral mucosa lesions also develop in pachyonychia congenita patients, whom often feature oral leukoplakia on the lateral margins of the tongue and buccal mucosa along the interdental lines (Gorlin et al. 1990). It appears, however, that the severity of the oral lesions is significantly greater in K6 null mice than in pachyonychia congenita patients. Two arguments can account for this difference. First, it seems likely that induction of wild-type K6 isoform genes in response to tissue damage (see Takahashi et al. 1995) attenuates the dominance of the mutated allele in pachyonychia congenita patients. Second, differences exist in the morphology of filiform papillae in mouse and human tongue: a single spine projects from the base of the papilla in mouse, whereas multiple spines are seen in human (Kobayashi et al. 1994).
The phenotype of K6α/β null mice is strikingly different from that of transgenic mice expressing a dominant negative mutant K6α gene (Wojcik et al. 1999). The latter mice feature spectacular skin lesions early after birth, but their oral mucosa is reportedly normal. The reasons for this are unclear at the present time, but may include strain-related differences.
Acknowledgments
The authors are grateful to Ms. Sandrine Vandormael-Pournin, Mr. Bing Yan, and Mr. James C. Morrell for their kind assistance. We also thank Drs. Elaine Fuchs (University of Chicago, Chicago, IL), Rebecca Porter and Birgit Lane (University of Dundee, Scotland, UK), and Irene Leigh (ICRF, London, UK) for providing antibodies, and to members of the Coulombe laboratory for advice and support.
These studies were supported by grants F32-AR088553 (P. Wong) and R01-AR42047 (P.A. Coulombe) from the National Institutes of Health, from the Centre National de la Recherche Scientifique and Institut Pasteur (C. Babinet), and from the Ministry of Education, Science, Sports and Culture of Japan (K. Takahashi).
Footnotes
Abbreviations used in this paper: ES, embryonic stem cells; IF, intermediate filaments; K6, keratin 6; mK6α, mouse K6α; mK6β, mouse K6β; Neor, neomycin resistance; RT, reverse transcriptase; TK, thymidine kinase.
References
- Bowden P.E., Haley J.L., Kansky A., Rothnagel J.A., Jones D., Turner R.J. Mutation of a type II keratin gene (K6a) in pachyonychia congenita. Nat. Genet. 1995;10:363–365. doi: 10.1038/ng0795-363. [DOI] [PubMed] [Google Scholar]
- Colucci-Guyon E., Portier M.M., Dunia I., Paulin D., Pournin S., Babinet C. Mice lacking vimentin develop and reproduce without an obvious phenotype. Cell. 1994;79:679–694. doi: 10.1016/0092-8674(94)90553-3. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Weber K. Intermediate filamentsstructure, dynamics, function, and disease. Ann. Rev. Biochem. 1994;63:345–382. doi: 10.1146/annurev.bi.63.070194.002021. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Cleveland D.W. A structural scaffolding of intermediate filaments in health and disease. Science. 1998;279:514–519. doi: 10.1126/science.279.5350.514. [DOI] [PubMed] [Google Scholar]
- Gorlin R.J., Cohen M.M., Levin L.S. Pachyonychia congenita. In: Gorlin R.J., Cohen M.M., Levin L.S., editors. Oxford Monographs on Medical Genetics No. 19Syndromes of the Head and Neck. Oxford University Press; New York: 1990. pp. 445–448. [Google Scholar]
- Hume W.J., Potten C.S. The ordered columnar structure of mouse filiform papillae. J. Cell Sci. 1976;22:149–160. doi: 10.1242/jcs.22.1.149. [DOI] [PubMed] [Google Scholar]
- Irvine A.D., McLean W.H.I. Human keratin diseasesthe increasing spectrum of disease and subtlety of the phenotype-genotype correlation. Br. J. Dermatol. 1999;140:815–828. doi: 10.1046/j.1365-2133.1999.02810.x. [DOI] [PubMed] [Google Scholar]
- Joseph H.L. Pachyonychia congenita. Arch. Dermatol. 1964;90:594–603. doi: 10.1001/archderm.1964.01600060060011. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Kamakura M., Ishii K. Three-dimensional fine structure of the lingual papillae and their connective tissue cores in the human tongue. Acta Anat. Nippon. 1994;69:624–635. [PubMed] [Google Scholar]
- Koch P.J., Mahoney M.G., Ishikawa H., Pulkkinen L., Uitto J., Shultz L., Murphy G.F., Whitaker-Menezes D., Stanley J.R. Targeted disruption of the pemphigus vulgaris antigen (desmoglein 3) gene in mice causes loss of keratinocyte cell adhesion with a phenotype similar to pemphigus vulgaris. J. Cell Biol. 1997;137:1091–1102. doi: 10.1083/jcb.137.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress C., Vandormael-Pournin S., Baldacci P., Cohen-Tannoudji M., Babinet C. Nonpermissiveness for mouse embryonic stem (ES) cells derivation circumvented by a single backcross to 129/Sv strainestablishment of ES cell lines bearing the Omd conditional mutation. Mammal. Genome. 1998;9:998–1001. doi: 10.1007/s003359900914. [DOI] [PubMed] [Google Scholar]
- Lersch R., Stellmach V., Stocks C., Giudice G., Fuchs E. Isolation, sequence, and expression of a human keratin K5 genetranscriptional regulation of keratins and insights into pairwise control. Mol. Cell. Biol. 1989;9:3685–3697. doi: 10.1128/mcb.9.9.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe M., O'Guin W.M. Existence of trichohyalin-keratohyalin hybrid granulesco-localization of two major intermediate filament-associated proteins in non-follicular epithelia. Differentiation. 1994;58:65–75. doi: 10.1046/j.1432-0436.1994.5810065.x. [DOI] [PubMed] [Google Scholar]
- McGowan K.M., Coulombe P.A. The wound repair associated keratins 6, 16, and 17insights into the role of intermediate filaments in specifying cytoarchitecture Harris J.R., Herrmann H. Subcellular BiochemistryIntermediate Filaments 1998. 141 165 Plenum Publishing Co; London: a [PubMed] [Google Scholar]
- McGowan K.M., Coulombe P.A. Onset of keratin 17 expression coincides with the definition of major epithelial lineages during mouse skin development J. Cell Biol. 143 1998. 469 486b [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan K.M., Coulombe P.A. Keratin 17 expression in the hard epithelial context of the hair and nail, and its relevance to the pachyonychia congenita phenotype. J. Invest. Dermatol. 2000;114:1101–1107. doi: 10.1046/j.1523-1747.2000.00986.x. [DOI] [PubMed] [Google Scholar]
- Ness S.L., Edelmann W., Jenkins T.D., Liedtke W., Rustgi A.K., Kucherlapati R. Mouse keratin 4 is necessary for internal epithelial integrity. J. Biol. Chem. 1998;273:23904–23911. doi: 10.1074/jbc.273.37.23904. [DOI] [PubMed] [Google Scholar]
- O'Guin W.M., Schermer A., Lynch M., Sun T.-T. Differentiation-specific expression of keratin pairs. In: Goldman R.D., Steinert P.M., editors. Cellular and Molecular Biology of Intermediate Filaments. Plenum Publishing Co; London: 1990. pp. 301–334. [Google Scholar]
- Paladini R.D., Coulombe P.A. The functional diversity of epidermal keratins revealed by the partial rescue of the keratin 14 null phenotype by keratin 16. J. Cell Biol. 1999;146:1185–1201. doi: 10.1083/jcb.146.5.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R.M., Hutcheson A.M., Rugg E.L., Quinlan R.A., Lane E.B. cDNA cloning, expression, and assembly characteristics of mouse keratin 16. J. Biol. Chem. 1998;273:32265–32272. doi: 10.1074/jbc.273.48.32265. [DOI] [PubMed] [Google Scholar]
- Purkis P.E., Steel J.B., Mackenzie I.C., Nathrath W.B., Leigh I.M., Lane E.B. Antibody markers of basal cells in complex epithelia. J. Cell Sci. 1990;97:39–50. doi: 10.1242/jcs.97.1.39. [DOI] [PubMed] [Google Scholar]
- Ramirez A., Vidal M., Bravo A., Larcher F., Jorcano J.L. A 5′-upstream region of a bovine keratin 6 gene confers tissue-specific expression and hyperproliferation-related induction in transgenic mice. Proc. Natl. Acad. Sci. USA. 1995;92:4783–4787. doi: 10.1073/pnas.92.11.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothnagel J.A., Seki T., Ogo M., Longley M.A., Wojcik S.M., Rundman D., Bickenbach J.R., Roop D.R. The mouse keratin 6 isoforms are differentially expressed in the hair follicle, footpad, tongue, and activated epidermis. Differentiation. 1999;65:119–130. doi: 10.1046/j.1432-0436.1999.6520119.x. [DOI] [PubMed] [Google Scholar]
- Smith F.J., Jonkman M.F., van Goor H., Coleman C.M., Covello S.P., Uitto J., McLean W.H. A mutation in human keratin K6b produces a phenocopy of the K17 disorder pachyonychia congenita type 2. Hum. Mol. Genetics. 1998;7:1143–1148. doi: 10.1093/hmg/7.7.1143. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Paladini R.D., Coulombe P.A. Cloning and characterization of multiple human genes and cDNAs encoding highly related type II keratin 6 isoforms. J. Biol. Chem. 1995;270:18581–18592. doi: 10.1074/jbc.270.31.18581. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yan B., Yamanishi K., Imamura S., Coulombe P.A. The two functional type II keratin 6 genes of mouse show a differential regulation and evolved independently from their human orthologs. Genomics. 1998;53:170–183. doi: 10.1006/geno.1998.5476. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Coulombe P.A., Miyachi Y. Using transgenic models to study the pathogenesis of keratin-based inherited diseases. J. Dermatol. Sci. 1999;21:73–95. doi: 10.1016/s0923-1811(99)00023-7. [DOI] [PubMed] [Google Scholar]
- Tyner A.L., Fuchs E. Evidence for posttranscriptional regulation of the keratins expressed during hyperproliferation and malignant transformation in human epidermis. J. Cell Biol. 1986;103:1945–1955. doi: 10.1083/jcb.103.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik S.M., Imakado S., Seki T., Longley M.A., Petherbridge L., Bundman D.S., Bickenbach J.R., Rothnagel J.A., Roop D.R. Expression of MK6a dominant-negative and C-terminal mutant transgenes in mice has distinct phenotypic consequences in the epidermis and hair follicles. Differentiation. 1999;65:97–112. doi: 10.1046/j.1432-0436.1999.6520097.x. [DOI] [PubMed] [Google Scholar]