Abstract
Capsid-displayed adenoviral peptide libraries have been a significant, yet unfeasible goal in biotechnology. Three barriers have made this difficult: the large size of the viral genome, the low efficiency of converting plasmid-based genomes into packaged adenovirus and the fact that library amplification is hampered by the ability of two (or more) virus to co-infect one cell. Here, we present a novel vector system, pFex, which is capable of overcoming all three barriers. With pFex, modified fiber genes are recombined into the natural genetic locus of adenovirus through unidirectional Cre–lox recombination. Modified-fiber genes can be directly shuttled into replicating viral genomes in mammalian cells. The ‘acceptor’ vector does not contain the fiber gene, and therefore does not propagate until it has received a ‘donor’ fiber gene. Therefore, This methodology overcomes the low efficiency of transfecting large viral genomes and bypasses the need for transition to functional virus. Thus, with a fiber-shuttle library, one can generate and evaluate large numbers of fiber-modified adenovirus simultaneously. Finally, successful fiber genes can be rescued from virus and recombined back into shuttle plasmids, avoiding the need to propagate mixed viral pools. For proof of principal, we use this new system to screen a capsid-displayed peptide library for retargeted viral infection.
INTRODUCTION
The translation of adenoviral vectors as therapeutic agents has been hampered, in part, by broad viral tropism and diminished expression of the Coxsackie and adenoviral receptor (CAR) in many cancers. To overcome these limitations, genetic strategies have been developed to detarget the virus away from natural infection pathways and to retarget viral infection to alternative receptors through modifications to capsid genes. Unfortunately, the large viral genome and complex major late gene organization have made capsid gene alterations challenging. Moreover, capsid gene alterations can often interfere with protein structure, viral packaging and infection. Thus, with current technology, adenoviral retargeting by genetic alterations can be a risky endeavor.
Nevertheless, there are several examples where adenoviral tropism has been successfully directed to refractory cells and tissues through capsid-displayed peptides and proteins on viral fiber (1), penton base (2), hexon (3) and protein IX (4). Of these methodologies, fiber-displayed peptides have been the most successful and most widely applied (5). Moreover, it was recently discovered that the fiber protein was responsible for non-CAR mediated liver infection and sequestration through interactions with certain blood factors (6). The mutation of fiber regions that interact with these blood factors significantly reduces both liver clearance and immune-induced hepatic toxicity. Therefore, alterations to the fiber gene alone can result in novel cell targeting, improved in vivo biodistribution and reduced toxicity. In addition, the fiber gene region provides a new site for incorporating transgenes, where, if desired, gene expression can be temporally restricted to viral replication through linkage to major late transcriptional unit (MLTU) expression (7–10). Thus, the fiber gene region has been a major focus of the next generation of adenoviral vectors.
Here, we present a new vector system for generating fiber-modified adenoviruses. The system is robust, allowing one to shuttle modified fiber genes into adenoviral plasmids in Escherichia coli or into replicating adenovirus genomes in mammalian cells. The efficiency and sensitivity are such that fiber-gene libraries can be generated and screened.
MATERIALS AND METHODS
Cells and culture
The 293 cells (Quantum Biotech), FBJ cells and 293cre57 cells were maintained in Dulbecco's MEM (DMEM) with 10% FBS. The 911–S11 cells were maintained in DMEM with 5% FBS and 200 μg/ml G418. The 911–S11 cells express a previously characterized membrane-bound anti-fiber single chain antibody as a pseudo-receptor (11). The DPL-37 cells were maintained in DMEM with 10% FBS and 10 mM MgCl2. The PC-3 cells were obtained from ATCC (Manassas, VA, USA) and maintained in RPMI medium supplemented with 10% FBS. PC3-CAR was provided by Dr Hsieh (12). All medias were supplemented with Ciprofloxacin Hydrochloride 5 μg/ml (US Biological, Swampscott, MA, USA) and Gentamicin 50 μg/ml (Quality Biological Inc., Gaithersburg, MD, USA). All cells were maintained at 37°C in an atmosphere containing 5% CO2.
Generation of plasmid vectors
The pFex plasmid was assembled through several steps. First, ‘distal to fiber AgeI’ was created by PCR amplification and cloning with primers AdE-Dist 5′ and AdE-Dist 3′ (Supplementary Table S2) into pCR-2.1 to produce the vector ‘Step 1 pFex’. Second, ‘proximal to fiber’ was created by PCR amplification with primers loxmve1 and loxmve2 and subcloning into ‘Step 1 pFex’ using the SpeI and AgeI. The resulting vector is ‘Step 2 pFex’. The SacB gene was isolated from pAJ200 using the BglII and PvuI restriction sites. Next, the two half mutant lox sites, lox m2/66 and lox 71, were added by ligation with self annealed linkers 5′ lox m2/66 and 3′ lox m2/66, and 5′ lox 71 and 3′ lox 71, respectively. The resulting floxed SacB gene was then subcloned into Step 2 pFex to create ‘Step 3 pFex’. Finally, the modified segment was subcloned into pAdEasy-1 with SpeI and PacI, replacing the pre-existing region. The final vector construct is called pFEX and was verified by sequencing using primers pFEXfor01-11 and pFEXrev01-11 (Supplementary Table S2).
Fiber shuttle vectors were also constructed in a stepwise manner. The pBK-CMV-fiber (a generous gift from Drew Pardoll and Sara Pai, Johns Hopkins) contained three mutations, A32665T, A32667C (which created a unique BspEI site in the fiber-HI loop) and A32651G (N541S ablative mutation). Adenovirus containing the fiber N541S mutation are unable to infect or propagate [Figure 3, far left column 0:1 (WT:N541S) produces no viral bursts or plaques]. The pBK-CMV-fiber was digested with the SpeI and XhoI and the linkers S-lox m2/71-X5 and S-lox m2/71-X3 (Supplementary Table S3) were self-annealed and ligated into the vector creating ‘Step 1 fiber Shuttle Lox m2/71’. The linkers N-Lox 66-A-5 and N-Lox 66-A-3 were then subcloned into this vector by Acc65I and NotI, making ‘RP-Fib1’. The tripartite leader (TPL) splice acceptor site was subcloned downstream of the lox m2/71 site with linkers splce1 and splce 2 creating RP-Fib1R1. For CAR-ablated vectors, pBK-CMV-TAYT-fiber (ΔAd5 nucleotides 32506–32518, amino acids T489AYT492) was subcloned into shuttle through XhoI/NotI, creating ‘RP-Fib2’. The TPL splice acceptor was cloned as above creating ‘RP-Fib2R1’. Finally, plasmids RP-FBR1 and RP-FBR2 were generated by reverting the A32651G (N541S) mutation to wild-type with primers 5FBR-537REP and 3FBR-537REP and BglII and BspEI subcloning. To generate Ampicillin resistant versions, the floxed cassettes were subcloned into Puc19 through KpnI and SpeI, creating RPuc-Fib1, RPuc-Fib2, RPuc-Fib1R1, RPuc-Fib2R1, RPuc-FBr1 and RPuc-FBR2. RPuc-RGD4C-2 was created in two steps. First, annealed primers 5N-Dir and 3N-Dir were subcloned into the HI-loop BspeI site. Linkers 5′RGD and 3′RGD were then annealed and subcloned into the vector. RPuc-WTFib and RP-WTFib, were generated with primers WTFibFix-1 and WTFibFix-2 and subcloning through NcoI and NotI. RPuc-Rescue was generated by PCR amplification of SacB with primers Not-SacB (AATTGCGGCCGCCACTATTATTTAGTGAAATGAGATATTA) and Xho-SacB (ATCTCGAGAGAAGTGATGCACTTTGATATCGACCCAAG) and subsequent cloning into RPuc-FBR1, replacing fiber with SacB.
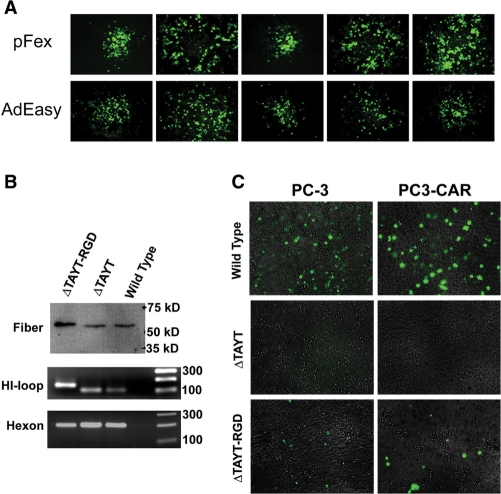
Figure 3.
Evaluation of adenovirus generated by the pFex system. (A) Viral burst comparison of pFex derived and AdEasy derived virus, both containing wild-type fiber, demonstrates equal replication and spreading rate as indicated by GFP-positive viral burst size. (B) Equal PFU of purified adenovirus containing wild-type fiber (AdTrack-WTFib), CAR-ablated fiber (ΔTAYT) or CAR ablated and RGD4C retargeted fiber (ΔTAYT-RGD) were boiled and evaluated by western blotting (top gel). Similar fiber quantities and appropriate size are demonstrated. Viral DNA of equal PFU of purified adenovirus was also subjected to PCR to evaluate content, size and homogeneity of the fiber-HI loop region (lower two gels). The larger fiber-PCR band of AdTrack-RGD4C-2 (ΔTAYT-RGD) is caused by the peptide encoding region. AdTrack-FBR2 (ΔTAYT) and AdTrack-WTFib (wild-type) do not contain recombinant HI loop peptides and therefore have identical size HI loop products. Hexon PCR was included as a reference control. (C) PC-3 and PC3-CAR prostate cancer cells were infected with 100 MOI of wild-type, CAR-ablated (ΔTAYT) and CAR-ablated and integrin retargeted (ΔTAYT-RGD) GFP-expressing adenovirus. Infected cells were identified by GFP-positive fluorescent microscopy. As predicted, ΔTAYT mutation completely detargeted virus and inclusion of the integrin targeting peptide, RGD4C, restored viral infection in a CAR independent manner.
All fiber shuttle vectors were sequenced with primers M13 forward, M13 Reverse, fiber-S2 and fiber-S3.
Generation and evaluation of adenovirus through plasmid-based recombination
The pAdTrack was recombined with the pFex plasmid vector either before or after fiber-region recombination in BJ5183 E. coli either by co-electroporation or by first generating a stable pFex-based BJ5183 coli followed by electroporation of the AdTrack shuttle vector (13,14). Recombinant vectors were propagated in DH5α. For fiber-region recombination, 10 ng of pFex was transformed with the 10 ng fiber shuttle vector into 40 μl of electrocompetent 294cre coli (Gene Bridges GmbH, Dresden, Germany) in a 0.2 cm cuvette at 2.5 KV, 200 ohms and 25 μFd (BioRad Gene Pulser). Following electroporation, samples were resuspended in 1.0 ml SOC broth, incubated at 42°C for 20 min and then shook vertically (225 rpm) at 37°C for 1 h. Recombinant plasmids were selected on appropriate antibiotic LB agar plates containing 5–7% sucrose. Clones were amplified, mini-prepped and the resulting plasmid electroporated into DH5α coli. In some cases, PCR was applied prior to DH5α transformation to detect the presence of recombinant clones (see ‘Recombination-specific PCR’ section subsequently and Figure 2B). Recombinant plasmids were screened by XhoI restriction mapping and confirmed by DNA sequencing. Viral plasmid products were linearized by PacI digestion and transfected into the desired packaging lines (293 or 911–S11). Viruses were step amplified to 5 × 150 mm2 flasks and then purified by commercial adenovirus purification kit (Adenopure™, Puresyn, Inc., Malvern, PA, USA). Benzonase was used in the purification process. Resulting virus were titered by Adeno-X™ Rapid Titer Kit (BD Biosciences). The correct splicing pattern upstream of the recombinant fiber gene was confirmed by reverse transcription of total RNA harvested from infected cells (Trizol, Invitrogen), followed by PCR amplification with primers Fib-RT1 (ACAAACUCUUCGCGGUCUUU) and Fib-RT2 (UAUCUUCAGACGGUCUUGCG), TOPO-TA cloning (pCR-21-TOPO, InVitrogen) and sequencing. These results confirmed natural linkage of the TPL to the fiber coding region.
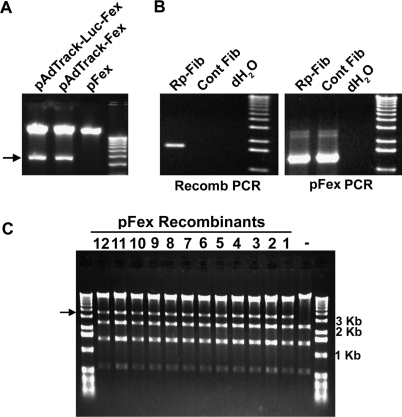
Figure 2.
E1 and fiber cassette exchange in plasmid vectors. (A) PacI restriction digestion of parental vector, pFex, and resulting recombinant vectors pAdTrack-Fex and pAdTrack-Luc-Fex following E1 cassette exchange in BJ5183 E. coli. The corresponding E1 Cassettes contain an additional PacI site, which results in the diagnostic 4.6 Kb band (arrow). (B) PCR across a single lox site of 294cre clones demonstrates the presence of fiber containing pFex (5′-primer within fiber gene and 3′-primer within pFex). The shuttle control does not contain lox sites and therefore does not recombine with pFex. On the other hand, PCR within the pFex vector demonstrates presence of the ‘acceptor’ pFex in both RP-Fib and shuttle-control samples. (C) XhoI restriction digest of 12 mini-preps randomly screened for the presence of the desired fiber-containing clone following fiber cassette exchange into pFex. The fiber gene contains an additional XhoI site, which generates the diagnostic 3.6 Kb product (arrow). All of the screened clones contained the desired plasmid product.
Recombination-specific PCR
Colony PCR was applied to confirm recombination of the fiber gene into pFex. Primers fiber-S2 and pFexrev07 produce a 1.6 Kb product that is specific for fiber containing clones (Figure 2B). Note that the PCR product size depends on the content of the fiber gene cassette. PCR primers pFexfor08 and pFex rev02 were used for positive controls. Clones positive by these assays were then mini-prepped and transformed into the more stable DH5α coli.
Efficiency of plasmid-based recombination
The 294cre E. coli were electroporated with 50 ng of pFex plasmid and 10-fold molar excess fiber shuttle. Cells were heat shocked for 20 min and grown for 2 h at 37° C, 225 r.p.m. Clones were quantified on LB + 50 μg/ml Kan for pFex containing colonies, LB + 100 μg/ml Amp for fiber shuttle colonies and LB + 7% sucrose + 50 μg/ml Kan as colonies per milliliter of growth medium.
Generation and evaluation of adenovirus through mammalian cell-based recombination
The fiber-less pAdTrack-Fex plasmid was pseudotyped by PacI digestion and transfection into the fiber gene expressing FBJ cell line (a generous gift of Dr David Johns, Johns Hopkins). Pseudotyped AdTrack-Fex virus was amplified, purified and titered as described earlier. For fiber gene recombination, approximately 106 293cre57 cells (15) (a generous gift of Dr Stephen Langer, University of Colorado) were transfected with 2 μg of fiber shuttle plasmid through magnet-assisted transfection (MATra-A, IBA, St Louis, MO, USA) in a 6-well plate, immediately followed by infection with pseudotyped AdTrack-Fex [multiplicity of infection (MOI = 1)]. After 3–5 days of transfection and infection, cells were harvested by scraping and virus eluted by 3–4 freeze per thaw cycles. The resulting supernatant was then used to infect 293 or 911–S11 cells.
Evaluation of purified adenoviral particles
The 106 plaque forming units (PFU) of each purified adenovirus were boiled in Laemmli buffer for western blot analysis. Blots were probed with 4D2 anti-fiber antibody (Abcam, Cambridge, MA, USA), anti-mouse IgG-HRP (Sigma, St Louis, MO, USA) and developed by ECL-Plus HRP detection assay (GE Healthcare). For PCR detection, 105 PFU was boiled and DNA subjected to Hexon (primers Ad5Hexon sense: ATGGCTACCCCTTCGATGAT and Ad5Hexon antisense: GATGAACCGCAGCGTCAAAC; 207 bp product) and fiber HI-loop [primers HIFlnk: TTCATTAATGTAGTTGTGGC and knlFIH: ACCATTACACTAAACGGTAC; 101 bp for wild-type and CAR-ablated (ΔTAYT) vectors and 140 bp for RGD4C vectors] PCR. PCR products were separated on agarose gel and stained with ethidium bromide.
Serial dilution experiments
RPuc-WTFib was serially diluted from 1 : 10 to 1 : 1 000 000 and mixed into a total of 3 μg of Rpuc-Fib1R1 (N541S). A negative control of N541S alone was also included. Shuttle dilutions were transfected into 293cre57 cells and immediately infected with pseudotyped AdTrack-Fex (MOI = 1). Five days later, recombinant virus was harvested and used to infect 293 cells in 100 mm plates (2—5 h). Cells were overlayed with noble agar and viral bursts allowed to form for 3–7 days. Only RPuc-WTFib recombinant virus form GFP-positive viral bursts (as in Figure 3, box arrow). Some pFex or N541S virus are infectious by complementation; however, these virus fail to spread and only appear to occupy a single cell (Figure 3, small arrows). The number of viral bursts was counted for each dilution to determine the sensitivity of detecting a single working fiber shuttle plasmid in the background of extensive mutant fiber shuttles.
Tropism studies
Four separate tropism studies were performed. First, 5 × 105 PC-3 and PC3-CAR cells were infected with AdTrack-WTFib, AdTrack-FBR2 or AdTrack-RGD4C-FBR2 at MOIs of 100 for 2 h in 6-well dishes. Infected cells were visualized 48—72 h post-infection by fluorescent and light microscopy. Collected images were overlayed to demonstrate number of cells infected and non-infected. In a second study, clones from the library selection, and CAR-ablated and wild-type controls were used to infect 293 cells (24 h after plating) with an MOI of 0.1. Ten fields of GFP-positive infected cells were counted 24 h after infection and results plotted as percent infected cells relative to wild-type fiber control. Finally, experiments investigating CAR-independent infection were performed in 96-well plate format. For anti-CAR siRNA experiments, 1 × 104 293 cells were plated and transfected with 5 nM of siRNA [Hs_CXADR_10 HP siRNA, Hs_CXADR_11 HP siRNA, All-Stars negative control siRNA or untransfected using HiPerfect Transfection Reagent (Qiagen, Valencia, CA, USA)]. Cells were infected with adenovirus (MOI = 1 and 10) for 2 h as triplicate samples, 72 h after transfection. For CAR overexpression experiments, 1 × 104 PC-3 or PC3-CAR cells were plated in 96-well plates. Cells were infected the next day with each adenovirus (MOI = 10 and 100) for 2 h in triplicate samples. For both studies, infection was quantified at 24—48 h post-infection by fluorescent microscopy (6×, equal exposure rates and processing) and fluorescent intensity (FLUOROstar Optima BMG fluorometer). Statistical significance was determined by Student's t-test.
Adenoviral peptide library
Primers for the random hexapeptide library, Bsp-Lib and Rnd-ext (BSP-Lib: TGGAGTTGTGTCTCCGGANNNNNNNNNNNNNNNNNNTCCGGATTCCTGTGTACCGCT; Rnd-ext: 5AGCGGTACACAGGAATCCGGA) were annealed and incubated with Klenow polymerase. Biotinylated primers Lib001 (Biotin-AGCGGTACACA-GGAATCC) and Lib002 (Biotin-TGGAGTTGTGTCTCCGGA) were then used for PCR amplification, and the resulting product was digested with BspEI. Flanking digestion products and uncut product were removed with streptavidin magnetic beads (New England Biolabs). The resulting library fragment was subcloned into RPuc-FBR2. Ligation conditions were optimized and large-scale electroporation to DH5α was used to generate ∼16 000 clones of the RPuc-FBR2-6X library. To generate library virus, 106 293cre57 cells were transfected with 3 μg of RPuc-FBR2-6X and infected with pseudotyped pAdTrack-Fex (MOI = 1). Five days later, cells and media were harvested, freeze-thawed and lysate was used to infect 293 cells on 100 mm plates. Infections were overlayed with agar and viral infection visualized by fluorescent microscopy. Both positive (RPuc-WTFib) and negative (N541S) shuttles were included for reference. Viral bursts were counted 48 h after infection and plaques isolated at later time points in 293 cells. Aliquots of viral lysate were treated with proteinase K, boiled and used to PCR amplify the floxed fiber region (FF01: TGTTCCTGTCCATCCGCACCCACTATCTTCATGTTG and FF02: AGGACTGTGTACTCTGTGTGTTGGGAGGGAGGTGGCA). The resulting PCR product was recombined with RPuc-Rescue in 294cre E. coli as described in the ‘Plasmid-based recombination’ section. Selected fiber clones were confirmed by restriction mapping and sequencing.
RESULTS
Design of the vector system
We have generated a novel vector system for modifying the fiber gene region of the widely applied Adenovirus vector, pAdEasy-1 (13). The vector system, outlined in Figure 1, applies an ‘acceptor’ vector, pFex, and fiber ‘donor’ vectors, RP-FBR or RPuc-FBR (depending on required antibiotic). The pFex acceptor vector is a modified version of AdEasy-1, where the fiber gene and upstream TPL splice-acceptor-site (Ad5 nucleotides 31 023–32 768) have been replaced with the Bacillus subtilis (SacB) gene. SacB imparts negative selectivity to bacteria grown in 5% sucrose and thus offers a means to negatively select against non-recombined acceptor vectors (16). Correspondingly, the donor vectors contain the modified fiber gene, TPL splice acceptor site and regions for additional transgene incorporation. Both donor and acceptor gene cassettes are flanked by non-compatible half-mutant lox sites. As delineated by Langer et al. (15), the use of these specialized lox sites results in a unidirectional gene exchange with maintained orientation and lack of alternative recombination events. In the presence of Cre Recombinase, the fiber gene cassette replaces SacB, placing fiber in the natural location of the adenovirus genome. By including the natural TPL splice-acceptor sequence in the fiber shuttles, upstream lox sites are not included in the final spliced fiber transcript.
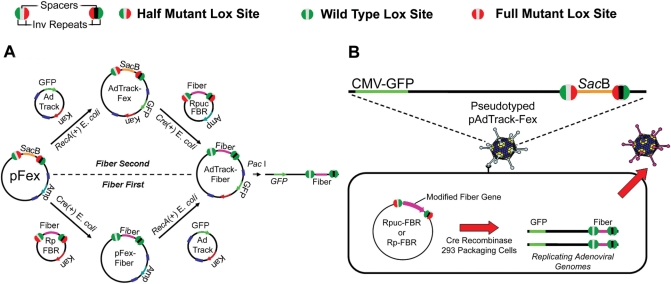
Figure 1.
Methods for site specifically transferring modified fiber gene cassettes into adenoviral plasmid vectors or replicating viral genomes in mammalian cells through unidirectional Cre–lox-mediated recombination. (A) The large viral plasmid vector pFex has two regions for accepting transgene cassettes, the Amp-resistant-E1-region cassette and the SacB-fiber-region cassette. The fiber-region-cassette can be recombined before (bottom) or after E1-region-cassette recombination (top). E1 cassettes are recombined through homologous recombination of adenovirus left-hand and right-hand homology regions in RecA positive BJ5183 coli. Recombinant plasmids are selected according to the newly acquired resistance cassette. On the other hand, fiber cassettes are recombined through half-mutant lox site (see legend: Green, wild-type half site; Red, mutant half site; Black and Gray represent non-compatible spacers) recombination in Cre recombinase expressing coli. Non-compatible spacers (gray center versus black center) prevent intragenic recombination. The resulting recombinant plasmids are selected by growth on sucrose containing plates. Following recombination, the donor plasmid product contains fully mutant lox sites (red circles), where the resulting recombinant vector contains fully wild-type lox sites (green circles); therefore, preventing any further recombination between the shuttles and viral vectors. The final resulting vectors are linearized and transfected into mammalian packaging cells to create viral particles (B) Fiber gene cassettes can be directly shuttled into adenoviral genomes in mammalian cells. E1-cassette-containing pFex vectors, such as AdTrack-pFex, can be pseudotyped (packaged in cells that express wild-type fiber) and used to infect packaging cell lines that express Cre recombinase. Transfection of infected cells with a fiber-gene shuttle results in site specific incorporation of the fiber cassette into replicative adenovirus. Only following recombination into a viral genome can the modified fiber gene be expressed. The resulting recombinant virus will be packaged in the newly synthesized modified-fiber capsids and amplified in other cell lines, such as 911–S11.
Fiber gene exchange in E. coli and mammalian cells
The vector system is flexible, allowing one to shuttle fiber cassettes into adenoviral plasmids in E. coli or replicating adenovirus genomes in mammalian cells (Figure 1A and B, respectively). When generating recombinant adenoviral plasmids, the modified-fiber cassette can be recombined from the smaller fiber shuttle vectors (RP-FBR or RPuc-FBR) into the larger viral genomic plasmid vector (pFex) either before or after incorporation of a modified E1 gene cassette. For example, if a single E1 cassette is to be tested with multiple fiber modifications, one can first generate E1-pFex (Figure 1A, top), followed by recombination with different fiber shuttles. Alternatively, if a single fiber modification is to be studied in the background of multiple E1 cassettes, one can generate a pFex-fiber (Figure 1A, bottom), followed by recombination with different E1 cassettes. The pFex vector is compatible with all AdEasy-based E1 shuttle vectors.
The E1 region shuttles are recombined into pFex through traditional RecA-mediated homologous recombination in E. coli, such as BJ5183 (Figure 2A). Efficiency of this step can be enhanced by generating stable pFex containing BJ5183 coli, as has been shown with the AdEasier method (14). Fiber cassette insertion, on the other hand, is completed in 294cre E. coli by temperature-induced expression of Cre recombinase (17). The desired modified-fiber clones can be selected through appropriate antibiotic resistance and growth on plates containing 5% sucrose, which negatively selects against SacB-containing parent vectors. If desired, the recombinant colonies can be confirmed in 294cre E. coli by colony PCR (Figure 2B). Plasmid-based efficiency of fiber gene exchange with this system is considerably better than previously reported RecA-mediated fiber gene recombinations (18). With pFex, often 100% of screened clones contain the fiber of interest (Figure 2C). Further, the efficiency is such that a few thousand clones can be generated in a small volume reaction (Supplementary Table 1).
In addition to plasmid-gene exchange, the pFex system allows modified-fiber cassettes to be transferred directly and site specifically into mature pFex-based viral genomes. To achieve this, pFex vectors are pseudotyped (packaged in cells that express wild-type fiber) and used to infect a Cre recombinase expressing mammalian cell that has been transfected with a fiber shuttle plasmid (Figure 1B). The modified-fiber gene is only expressed following recombination into the genome of a replicating pFex virus. Thus, only the desired recombinant virus will propagate (see Figure 3, viral burst). In the following sections, we use this methodology to generate numerous infective adenoviruses (Tables 1 and 2), including a library of adenoviruses displaying various peptides in the fiber HI-loop.
Table 1.
AdEasy and pFex derived vectors
| Name | Parent vector | E1 cassette | Fiber cassette | PFU/ml | VP/ml |
|---|---|---|---|---|---|
| AdTrack-AdEasya | pAdEasy-1 | CMV-GFP | – | 2.2 × 109 | 4 × 1011 |
| AdTrack-WTFiba | pFex | CMV-GFP | Wild Type Fiber | 1.7 × 109 | 1.9 × 1012 |
| AdTrack-FBR2b | pFex | CMV-GFP | ΔTAYT | 1.8 × 10 | 2.4 × 1011 |
| AdTrack-RGD4C-2b | pFex | CMV-GFP | ΔTAYT +RGD4C | 3.0 × 107 | 4.9 × 1011 |
aVirus amplified, purified and titered simultaneously (equal time and volumes at all steps).
bTiter determined on 911-S11 cells.
Table 2.
Peptide library and selected recombinant viruses
| Name | E1 cassette | CAR binding | Fiber cassette | PFU/ml |
|---|---|---|---|---|
| AdTrack-FBR2-6X | CMV-GFP | ΔTAYT | XXXXXX | – |
| AdTrack-FBR2-A2 | CMV-GFP | ΔTAYT | TGEKGG | 1.6 × 105 |
| AdTrack-FBR2-A3 | CMV-GFP | ΔTAYT | GGAAGA | 1.4 × 106 |
| AdTrack-FBR2-A4 | CMV-GFP | ΔTAYT | GGGDRG | 1.6 × 106 |
Titer determined on 911-S11 cells, X–refers to randomized peptide sequence.
pFex derived adenovirus
The modifications of the pFex vector do not impart any negative effects on viral propagation. To confirm this, we used pFex vectors to generate a virus containing wild-type adenovirus serotype 5 (Ad5) fiber using the cellular recombination method outlined in Figure 1B. The resulting virus, AdTrack-WTFib, is identical to the traditionally generated AdTrack-AdEasy virus, except for the incorporated lox sites. We then simultaneously amplified, purified and titered both viruses to observe any differences. Both viruses produced identical infectious titers (PFU) (Table 1). We further compared viral packaging and spread through viral burst assays. Specifically, 293 cells were infected with each virus at MOI less than 1 and evaluated for viral replication and spread by burst size. Viral bursts were visualized at early stages by GFP fluorescent microscopy and five randomly selected bursts from each sample were measured (longest point diameter). AdTrack-AdEasy and AdTrack-WTFib had identical burst size, indicating little to no difference between AdEasy versus pFex derived virus (Figure 3A). We conclude that the incorporated lox sites in pFex have no significant inhibitory impact on viral lifecycle or production.
We then applied the pFex system to generate previously characterized tropism-modified adenovirus for proof of principal. First, the natural CAR-mediated infection pathway was ablated through FG loop mutation (ΔT489AYT492) (19). These CAR detargeted viruses (referred to as ΔTAYT) were then redirected to bind and infect cells via integrins by inserting the cysteine-constrained, integrin-binding peptide RGD4C (CDCRGDCFC) into the fiber HI-loop (20). Purified particles of these two viruses, AdTrack-FBR2 (ΔTAYT) and AdTrack-RGD4C-2 (ΔTAYT-RGD) contain only the desired recombinant fiber DNA and recombinant fiber protein at expected size and proportions (Figure 3B). The 911–S11 cells, which contain an anti-fiber pseudoreceptor (11), were used to propagate and titer these CAR-ablated vectors. In cell infection assays, fiber FG loop mutation predictably ablates CAR mediated infection of PC-3 and PC3-CAR prostate cancer cell lines (Figure 3C). The incorporation of the integrin targeting peptide expectedly provides an alternative infection pathway for both cell lines. Notably, the CAR overexpressing cell line, PC3-CAR, was infected by wild-type fiber adenovirus (AdTrack-WTFib) at a higher rate than the parental PC-3 cell line, which is known to express lower levels of CAR (12). On the other hand, the CAR detargeted and integrin retargeted virus (ΔTAYT-RGD) infected both PC-3 and PC3-CAR cell lines with equal efficiency. Though integrin retargeted infection is low when compared to wild-type fiber in this cell line (∼5%), such vectors are valuable for infecting cancer cells, which often lack CAR expression (2). These results confirm previous reports of FG loop detargeting and the subsequent retargeted infection through capsid-displayed integrin-binding peptides (21,22).
Efficiency and sensitivity of generating modified adenovirus with pFex
Cre–lox recombination offers a means to site specifically recombine gene cassettes into large vectors, including cellular genomes. However, in traditional Cre–lox recombination, the symmetric lox site remains unchanged following recombination. Thus, the reactant and product are identical and the reverse reaction occurs with equal efficiency. Langer et al. (15) have overcome this limitation by designing half-mutant lox sites, which, after recombination, produce a unique and non-functional product in the donor vector, rendering the reaction unidirectional. The application of this unidirectional cassette exchange theoretically provides added efficacy by preventing any reverse recombination over time.
In vitro evaluation of gene exchange efficiency was evaluated with four separate fiber shuttle vectors. In this plasmid-based gene exchange, numerous fiber gene cassettes were recombined into the 34 Kb pFex plasmid, producing ∼1800 recombinant clones per milliliter of transformation culture in the absence of detectable background (Supplementary Table S1). While this efficiency could be easily scaled to generate a large diversity plasmid library, direct translation to active adenoviral particles is unrealistic. Therefore, the true value of the system is best evaluated by the ability to generate recombinant infectious virus.
To evaluate the efficiency of exchanging modified fiber genes into viral genomes in mammalian cells (Figure 1B), we performed serial dilution mixing experiments using a wild-type fiber shuttle (Rpuc-WTFib) and a non-infective fiber mutant (N541S) shuttle. Recombinant AdTrack-WTFib virus produces GFP-positive viral bursts visible by fluorescent microscopy in agar overlayed 293 cells (Figure 4, large arrow), where AdTrack-N541S is non-infective. The wild-type fiber shuttle was serially diluted (1 : 10 to 1 : 1 000 000) and mixed into a constant amount of mutant fiber shuttle, and the resulting plasmid mixes were transfected into 293cre57 cells, which were simultaneously infected with pseudotyped AdTrack-Fex virus (MOI = 1). Five days after transfection and infection, recombinant viral pools were harvested and applied to 293 cells with agar overlay, in order to determine the sensitivity of detecting spreading AdTrack-WTFib virus (as indicated by a GFP-positive viral burst). This is the most stringent assay possible as adenovirus with fiber N541S mutation do not produce infective virus particles. Therefore, this mutant fiber protein will potentially hybridize and compete with the wild-type fiber in a co-infected or co-transfected cell. A sensitivity of 0.01–0.001% was determined by finding at least one viral burst in ratios of 1 WTFib shuttle to 10 000 or 100 000 fiber-N541S mutant shuttles (Figure 4). This success is unprecedented for fiber gene exchange and suggests that fiber peptide adenovirus libraries can be simultaneously generated and screened for the ability to create infective and spreading virus.
Figure 4.
Efficiency and sensitivity of fiber gene exchange. Serial dilution mixing experiments demonstrate fiber cassette exchange sensitivity. Fiber shuttle RPuc-WTFib was serially diluted from 1:10 to 1:1 000 000 and mixed into a constant amount of mutant Rpuc-Fib1R1 (N541S) plasmid. This plasmid mix was recombined into AdTrack-pFex genomes by transfection into 293cre57 cells infected with pseudotyped AdTrack-Fex at an MOI of 1. Cell lysates were used to infect 293 cells, which were then overlayed by agar. Recombinant AdTrack-WTFib viruses were identified by viral burst (block arrow). At least a single viral burst was detectable in dilutions of 1:10 000 to 1:100 000 wild-type fiber shuttle, indicating a sensitivity of 0.01–0.001%. Some non-spreading viral infections were present (small arrows), indicating infection by AdTrack-pFex or AdTrack-N541S, which was pseudotyped in cells co-inhabited by AdTrack-WTFib viral genomes. Fiber N541S alone control (0:1) was unable to create any viral bursts.
Adenoviral peptide library screen for retargeted infection
For proof of principal, we generated and screened a low diversity fiber-displayed hexapeptide adenovirus library (Table 2). A random peptide cassette was cloned into the HI-loop of the CAR-ablated (ΔTAYT) fiber shuttle, RPuc-FBR2. The resulting plasmid library (RPuc-FBR2-6X) produced ∼16 000 plasmid clones, reflecting the maximum diversity. One million 293cre57 cells were transfected with 3 μg of RPuc-FBR2-6X and infected with pseudotyped pAdTrack-Fex (MOI of 1). Five days later, the recombinant virus pool was harvested. The 293 cells were then infected to identify CAR-independent viral plaques. Both positive (RPuc-WTFib) and negative (N541S) control samples were included in this experiment for reference purposes.
Viral burst from each sample were quantified 48 h post-infection. In comparison to the wild-type control, the FBR2-6X library produced ∼0.075% of the number of bursts. There were no infected cells bursts in the negative (N541S) control. Individual plaques were randomly isolated and amplified in 24-well plates. Aliquots were taken for PCR amplification of the floxed fiber cassette. The resulting PCR product was then recombined with RPuc-Rescue, an acceptor plasmid that contains half-mutant lox sites flanking a SacB gene for sucrose selection, in 294cre E. coli (Figure 5). Once recombined into RPuc-Rescue, the cloned fiber gene is available for sequencing, further modification, or recombination with pFex viral genomes to create a subsequent virus.
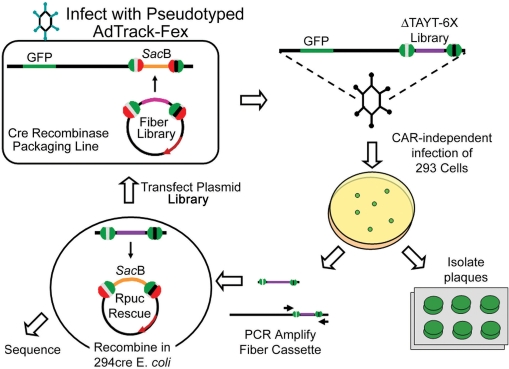
Figure 5.
Schematic for generating, selecting and rescuing adenoviral peptide libraries. Clockwise from top left: CAR-ablated fiber-peptide library cassettes are unidirectionally shuttled into fiber-less pFex viral genomes in 293cre57 cells. Some of the resulting CAR detargeted and peptide retargeted viruses infect the target cells. Resulting viral plaques can be isolated and amplified. The floxed fiber cassette can be amplified by PCR and recombined with RPuc-Rescue in 294cre E. coli. Growth in appropriate antibiotics and 5% sucrose selects new fiber shuttles that are directly applicable to sequencing, further modification or recombination to generate new virus in plasmid or viral form.
Three plaques from the hexapeptide library were cloned and sequenced to identify three unique peptides (Table 2). These clones were amplified, purified, titered and evaluated for retargeting ability in comparison to CAR-ablated virus. Adenovirus AdTrack-FBR2-A2 (ΔTAYT-A2) was poorly propagated in 293 cells, possibly due to peptide inhibition during packaging. The two remaining viruses, FBR2-A3 and FBR-2A4, do propagate and are capable of retargeting adenoviral infection to 293 cells (Supplementary Figure S1). To evaluate if this infection was CAR-independent, we knocked down CAR protein levels by transfecting 293 cells with two separate anti-CAR siRNAs. CAR-specific knock-down by both siRNAs reduced wild-type fiber infection by over 50%, but did not reduce the number of infected A3, A4 or RGD peptide retargeted viruses (Figure 6A). This infection rate was also quantified by fluorometer (Supplementary Figure 2A). Further, we evaluated the infection rates of these viruses in PC-3 and the CAR overexpressing subline, PC3-CAR. Wild-type adenovirus infected PC3-CAR cells with 26-fold better efficiency than the low CAR expressing PC-3 parental cell line. However, CAR overexpression did not enhance the infection rate of the A3, A4 or RGD peptide retargeted virus (Figure 6B and Supplementary Figure 2B). Notably, the infection rate of the A3 retargeted virus was superior to the RGD retargeted virus in both cell lines. These experiments indicate that the ligand for FBR2-A3 and FBR2-A4 viruses is expressed in both 293 cells and PC-3 prostate cancer cells.
Figure 6.
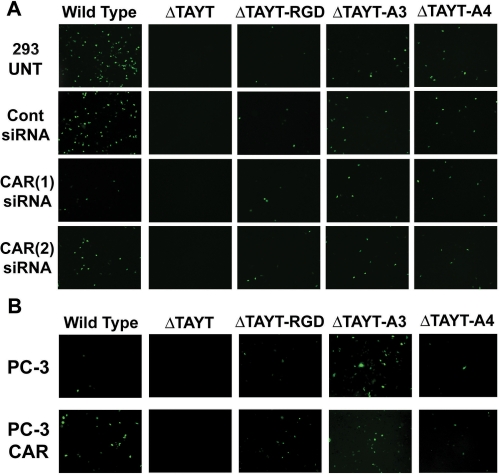
CAR-independent infection by selected retargeted adenovirus. (A) The 293 cells were transfected with two separate anti-CAR siRNAs, a negative control siRNA or non-transfected (UNT). Cells were infected with each virus at an MOI of 1, 72 h after transfection. Infected GFP-positive cells were visible the following day by fluorescent microscopy (6×) at equal exposure times. The infection rate of adenoviruses with wild-type fiber was reduced by over 50% with both anti-CAR siRNAs. CAR knock-down had no effect on infected cell numbers in CAR-detargeted and peptide retargeted viruses (B) PC-3 and the CAR overexpressing subline, PC3-CAR, were infected with each virus at an MOI of 10. Infected GFP-positive cells were visible the following day by fluorescent microscopy (6×) at equal exposure times. The infection rate of adenoviruses with wild-type fiber increased several fold in the CAR overexpressing line. However, there was no visible increase in infected cell number in CAR-detargeted and peptide retargeted viruses.
DISCUSSION
The fiber capsid protein is responsible for the initial interaction between the virus and the cell, through high-affinity binding with the cellular receptor, CAR (23,24), followed by internalization, which is triggered by viral penton base interaction with cellular integrins (25). The fiber gene is the fifth and last gene in the MLTU, an approximately ∼29 Kb, multiple open reading frame (ORF) transcript expressed late in the viral lifecycle (26). Alternative splicing of the MLTU links each ORF with a 5′-leader for preferential translation and a 3′-polyadenylation site (27–29). When designing fiber gene-modified virus, one must avoid interrupting splice donor and acceptor sites because they cannot only affect fiber, but also other ORF in the MLTU. Apart from splicing, modification of the fiber coding region can also negatively affect fiber protein folding, trimerization, assembly into the virus and therefore viral production. The HI-loop, a serotype variable region, is an accepted site for incorporating targeting motifs while still allowing successful viral propagation (30). However, each ligand is unique and may affect fiber differently (31). Additionally, incorporation of a targeting peptide into the fiber protein backbone may interfere with the ability of the peptide to bind its ligand. Therefore, rigorous evaluation of each retargeted virus is required to confirm that they have no deleterious effects on viral production or peptide–ligand binding. Because of these potential problems, and the time and effort required to make genetically retargeted virus, there is significant risk in pursuing these projects.
For this reason, adenoviral display peptide libraries have been a significant, yet difficult goal in adenoviral biotechnology. While high diversity libraries can be generated in plasmid form, transformation to a packaged adenovirus library is difficult due to inefficiencies in transfecting the large viral genome and low rates of conversion to a packaged and infective adenovirus. For example, most protocols recommend transfection of 2–5 μg of linearized adenoviral plasmid DNA (∼5 × 1010 − 1 × 1011 plasmid particles) into 2 × 106 293 cells to obtain full infection within 7–10 days. This corresponds to an MOI of 50 000. On the other hand, infection with an MOI of 1 will generally provide full infection in less than half the time. Therefore, the efficiency of viral production is several logs better with infection when compared to transfection. While it is notable that conjugation of terminal protein complex can improve the conversion rate of viral DNA into packaged adenoviral particles by a few logs (32), this efficiency is still far below that of natural viral infection. Here, we describe a methodology to produce fiber peptide libraries by applying the large ‘acceptor’ vector as a packaged and infective virus that genetically lacks the fiber gene. The fiber peptide library is then transferred from a small, more easily transfected shuttle, into infected adenoviral genomes in mammalian cells. The fiber protein is only expressed following successful gene transfer, thus only recombinant virus will properly package and propagate. This step overcomes the major limitation of converting the large linearized viral plasmid genome into a packageable and infectious adenovirus particle.
Through serial dilution mixing experiments, we demonstrate that, with this system, libraries of at least 105 can be generated and screened to identify successfully propagating virus (Figure 4). These pilot studies, completed in 6-well dishes, can be easily expanded to increase the applicable diversity. Moreover, the applicable diversity may be improved by transfecting smaller amounts of the shuttle plasmid library (to achieve a low plasmid copy number per cell) during library generation. This could potentially minimize the number of chimeric viruses formed during this first step. Later, during library selection and amplification, there is the possibility of co-infection by a neighboring virus. The resulting co-infection would create chimeric viruses, potentially inhibiting the propagation of a successful clone. With the pFex Rescue system (Figure 5), successful clones can be rescued from infected cells by PCR and recombined back into the RPuc-Rescue plasmid. This step, while applied to individual plaques in our screen, can also be applied to a population of cells infected with a viral pool. With this methodology, one can avoid the negative effects associated with amplifying a viral pool. The resulting rescue plasmids produce working fiber shuttles, which can be sequenced, applied to generate a new viral clone or applied as a pool to a second round of library selection (Figure 5).
Here, a single round of selection was applied to a moderately diverse library of 16 000 potential fiber-displayed hexapeptides to identify adenoviruses which infect cells in a CAR independent manner. With a projected three amino acid diversity of 8000 (203 = 8000), this library should contain at least one integrin targeting RGD motif, currently one of the best peptide motifs for retargeting adenoviruses to CAR-deficient cells. One of the selected peptides, A3, redirects infection of detargeted viruses to 293, PC-3 and PC3-CAR cells at higher levels than the widely applied integrin targeting peptide, RGD4C. The selected retargeting peptides, like RGD and other peptides, appear to reduce the overall output of infectious viruses (PFU). However, it is possible that additional rounds of biopanning may select for peptides which not only infect the target cell, but also minimize interference with fiber protein folding and capsid assembly (i.e. more favorable viral particle to PFU ratios). We anticipate that the pFex system can be used in this way to generate and screen higher diversity libraries through iterative selection rounds, as outlined in Figure 5, to identify CAR-independent adenovirus to most any cell type.
SUPPLEMENTARY DATA
Supplementary data are available at NAR Online.
ACKNOWLEDGEMENTS
We thank Victor W. van Beusechem (VU University Medical Center, Amsterdam) for providing the 911-S11 cell line, Stephen Langer (University of Colorado, Boulder) for providing the 293cre57 cell line and for technical advice, Drew Pardoll, Sara Pai and David Johns (Johns Hopkins University School of Medicine) for providing parent modified Fiber vectors and FBJ cell line, respectfully, and Bert Vogelstein for providing pAdEasy-1, pAdTrack, pAdTrack-CMV, BJ5183 and AdEasier cells. This article was funded by National Institutes of Health (2P50CA58236-09A1), (1R01CA121153-01A2 to R.R.); the Robert & Donna Tompkins Foundation, an award from David H. Koch through the Prostate Cancer Foundation, and the Department of Defense Prostate Cancer Research Program under Award Number (DAMD17-03-2-0033), which is managed by the U.S. Army Medical Research and Material Command. Funding to pay the Open Access publication charges for this article was provided by the Prostate Cancer Foundation.
Conflict of interest statement. None declared.
REFERENCES
- 1.Wickham TJ, Roelvink PW, Brough DE, Kovesdi I. Adenovirus targeted to heparan-containing receptors increases its gene delivery efficiency to multiple cell types. Nat. Biotechnol. 1996;14:1570–1573. doi: 10.1038/nbt1196-1570. [DOI] [PubMed] [Google Scholar]
- 2.Wickham TJ, Carrion ME, Kovesdi I. Targeting of adenovirus penton base to new receptors through replacement of its RGD motif with other receptor-specific peptide motifs. Gene Ther. 1995;2:750–756. [PubMed] [Google Scholar]
- 3.Vigne E, Mahfouz I, Dedieu JF, Brie A, Perricaudet M, Yeh P. RGD inclusion in the hexon monomer provides adenovirus type 5-based vectors with a fiber knob-independent pathway for infection. J. Virol. 1999;73:5156–5161. doi: 10.1128/jvi.73.6.5156-5161.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dmitriev IP, Kashentseva EA, Curiel DT. Engineering of adenovirus vectors containing heterologous peptide sequences in the C terminus of capsid protein IX. J. Virol. 2002;76:6893–6899. doi: 10.1128/JVI.76.14.6893-6899.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurachi S, Koizumi N, Sakurai F, Kawabata K, Sakurai H, Nakagawa S, Hayakawa T, Mizuguchi H. Characterization of capsid-modified adenovirus vectors containing heterologous peptides in the fiber knob, protein IX, or hexon. Gene Ther. 2006 doi: 10.1038/sj.gt.3302859. [DOI] [PubMed] [Google Scholar]
- 6.Shayakhmetov DM, Gaggar A, Ni S, Li ZY, Lieber A. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J. Virol. 2005;79:7478–7491. doi: 10.1128/JVI.79.12.7478-7491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivera AA, Wang M, Suzuki K, Uil TG, Krasnykh V, Curiel DT, Nettelbeck DM. Mode of transgene expression after fusion to early or late viral genes of a conditionally replicating adenovirus via an optimized internal ribosome entry site in vitro and in vivo. Virology. 2004;320:121–134. doi: 10.1016/j.virol.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 8.Carette JE, Graat HC, Schagen FH, Abou El Hassan MA, Gerritsen WR, van Beusechem VW. Replication-dependent transgene expression from a conditionally replicating adenovirus via alternative splicing to a heterologous splice-acceptor site. J. Gene. Med. 2005;7:1053–1062. doi: 10.1002/jgm.754. [DOI] [PubMed] [Google Scholar]
- 9.Hoti N, Chowdhury W, Hsieh JT, Sachs MD, Lupold SE, Rodriguez R. Valproic acid, a histone deacetylase inhibitor, is an antagonist for oncolytic adenoviral gene therapy. Mol. Ther. 2006;14:768–778. doi: 10.1016/j.ymthe.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Hoti N, Li Y, Chen CL, Chowdhury WH, Johns DC, Xia Q, Kabul A, Hsieh JT, Berg M, et al. Androgen receptor attenuation of Ad5 replication: implications for the development of conditionally replication competent adenoviruses. Mol. Ther. 2007;15:1495–1503. doi: 10.1038/sj.mt.6300223. [DOI] [PubMed] [Google Scholar]
- 11.van Beusechem VW, Grill J, Mastenbroek DC, Wickham TJ, Roelvink PW, Haisma HJ, Lamfers ML, Dirven CM, Pinedo HM, et al. Efficient and selective gene transfer into primary human brain tumors by using single-chain antibody-targeted adenoviral vectors with native tropism abolished. J. Virol. 2002;76:2753–2762. doi: 10.1128/JVI.76.6.2753-2762.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okegawa T, Li Y, Pong RC, Bergelson JM, Zhou J, Hsieh JT. The dual impact of coxsackie and adenovirus receptor expression on human prostate cancer gene therapy. Cancer Res. 2000;60:5031–5036. [PubMed] [Google Scholar]
- 13.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc. Natl Acad. Sci. USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeng M, Smith SK, Siegel F, Shi Z, Van Kampen KR, Elmets CA, Tang DC. AdEasy system made easier by selecting the viral backbone plasmid preceding homologous recombination. Biotechniques. 2001;31:260–262. doi: 10.2144/01312bm04. [DOI] [PubMed] [Google Scholar]
- 15.Langer SJ, Ghafoori AP, Byrd M, Leinwand L. A genetic screen identifies novel non-compatible loxP sites. Nucleic Acids Res. 2002;30:3067–3077. doi: 10.1093/nar/gkf421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pierce JC, Sauer B, Sternberg N. A positive selection vector for cloning high molecular weight DNA by the bacteriophage P1 system: improved cloning efficacy. Proc. Natl Acad. Sci. USA. 1992;89:2056–2060. doi: 10.1073/pnas.89.6.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buchholz F, Angrand PO, Stewart AF. A simple assay to determine the functionality of Cre or FLP recombination targets in genomic manipulation constructs. Nucleic Acids Res. 1996;24:3118–3119. doi: 10.1093/nar/24.15.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lie ALM, Bakker CT, Wesseling JG, Bosma PJ. AdEasy-based cloning system to generate tropism expanded replicating adenoviruses expressing transgenes late in the viral life cycle. Gene Ther. 2005 doi: 10.1038/sj.gt.3302546. [DOI] [PubMed] [Google Scholar]
- 19.Roelvink PW, Mi Lee G, Einfeld DA, Kovesdi I, Wickham TJ. Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing adenoviridae. Science. 1999;286:1568–1571. doi: 10.1126/science.286.5444.1568. [DOI] [PubMed] [Google Scholar]
- 20.Koivunen E, Wang B, Ruoslahti E. Phage libraries displaying cyclic peptides with different ring sizes: ligand specificities of the RGD-directed integrins. Biotechnology. 1995;13:265–270. doi: 10.1038/nbt0395-265. [DOI] [PubMed] [Google Scholar]
- 21.Wickham TJ, Tzeng E, Shears LL, 2nd, Roelvink PW, Li Y, Lee GM, Brough DE, Lizonova A, Kovesdi I. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins. J. Virol. 1997;71:8221–8229. doi: 10.1128/jvi.71.11.8221-8229.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mizuguchi H, Koizumi N, Hosono T, Ishii-Watabe A, Uchida E, Utoguchi N, Watanabe Y, Hayakawa T. CAR- or alphav integrin-binding ablated adenovirus vectors, but not fiber-modified vectors containing RGD peptide, do not change the systemic gene transfer properties in mice. Gene Ther. 2002;9:769–776. doi: 10.1038/sj.gt.3301701. [DOI] [PubMed] [Google Scholar]
- 23.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 24.Tomko RP, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackie viruses. Proc. Natl Acad. Sci. USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 26.Nevins JR, Darnell JE. Groups of adenovirus type 2 mRNA's derived from a large primary transcript: probable nuclear origin and possible common 3′ ends. J. Virol. 1978;25:811–823. doi: 10.1128/jvi.25.3.811-823.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berget SM, Moore C, Sharp PA. Spliced segments at the 5′ terminus of adenovirus 2 late mRNA. Proc. Natl Acad. Sci. USA. 1977;74:3171–3175. doi: 10.1073/pnas.74.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chow LT, Gelinas RE, Broker TR, Roberts RJ. An amazing sequence arrangement at the 5′ ends of adenovirus 2 messenger RNA. Cell. 1977;12:1–8. doi: 10.1016/0092-8674(77)90180-5. [DOI] [PubMed] [Google Scholar]
- 29.Ziff EB, Evans RM. Coincidence of the promoter and capped 5′ terminus of RNA from the adenovirus 2 major late transcription unit. Cell. 1978;15:1463–1475. doi: 10.1016/0092-8674(78)90070-3. [DOI] [PubMed] [Google Scholar]
- 30.Krasnykh V, Dmitriev I, Mikheeva G, Miller CR, Belousova N, Curiel DT. Characterization of an adenovirus vector containing a heterologous peptide epitope in the HI loop of the fiber knob. J. Virol. 1998;72:1844–1852. doi: 10.1128/jvi.72.3.1844-1852.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belousova N, Krendelchtchikova V, Curiel DT, Krasnykh V. Modulation of adenovirus vector tropism via incorporation of polypeptide ligands into the fiber protein. J. Virol. 2002;76:8621–8631. doi: 10.1128/JVI.76.17.8621-8631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyake S, Makimura M, Kanegae Y, Harada S, Sato Y, Takamori K, Tokuda C, Saito I. Efficient generation of recombinant adenoviruses using adenovirus DNA-terminal protein complex and a cosmid bearing the full-length virus genome. Proc. Natl Acad. Sci. USA. 1996;93:1320–1324. doi: 10.1073/pnas.93.3.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.