Abstract
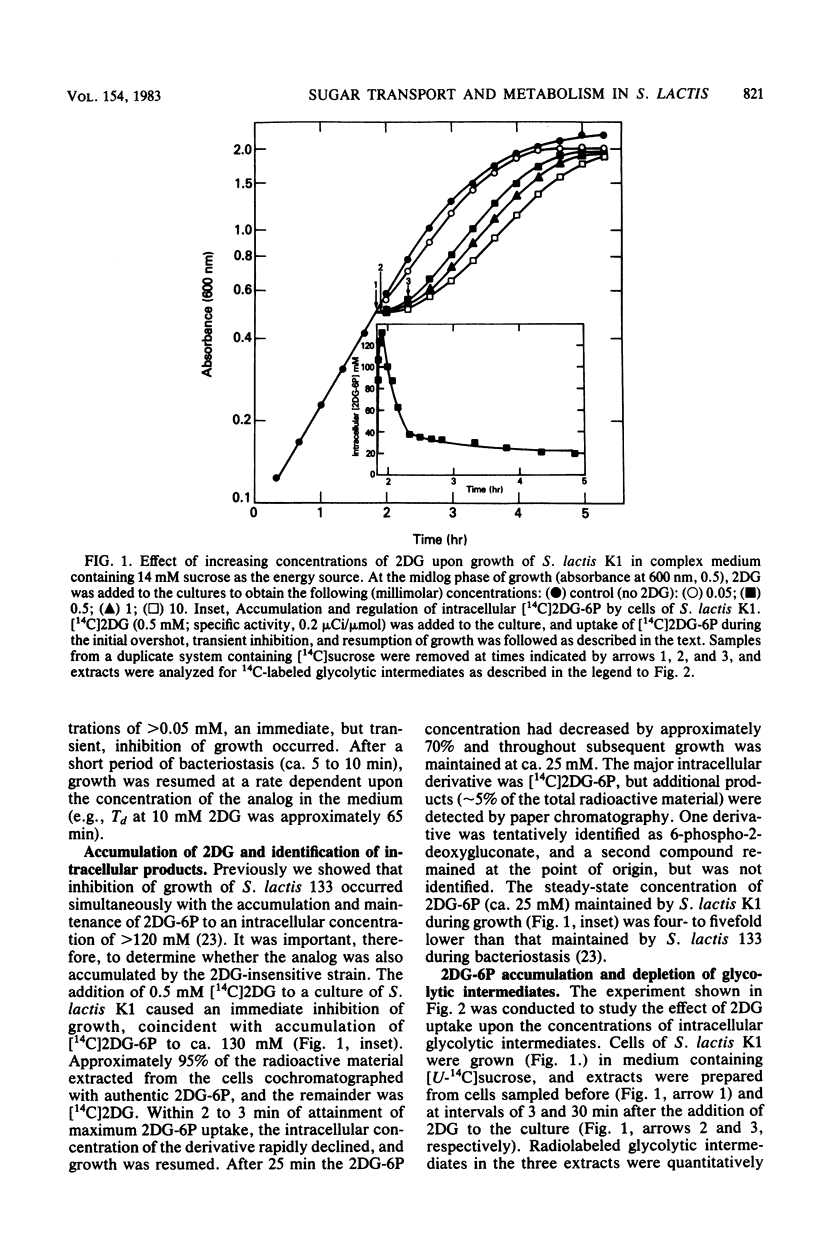
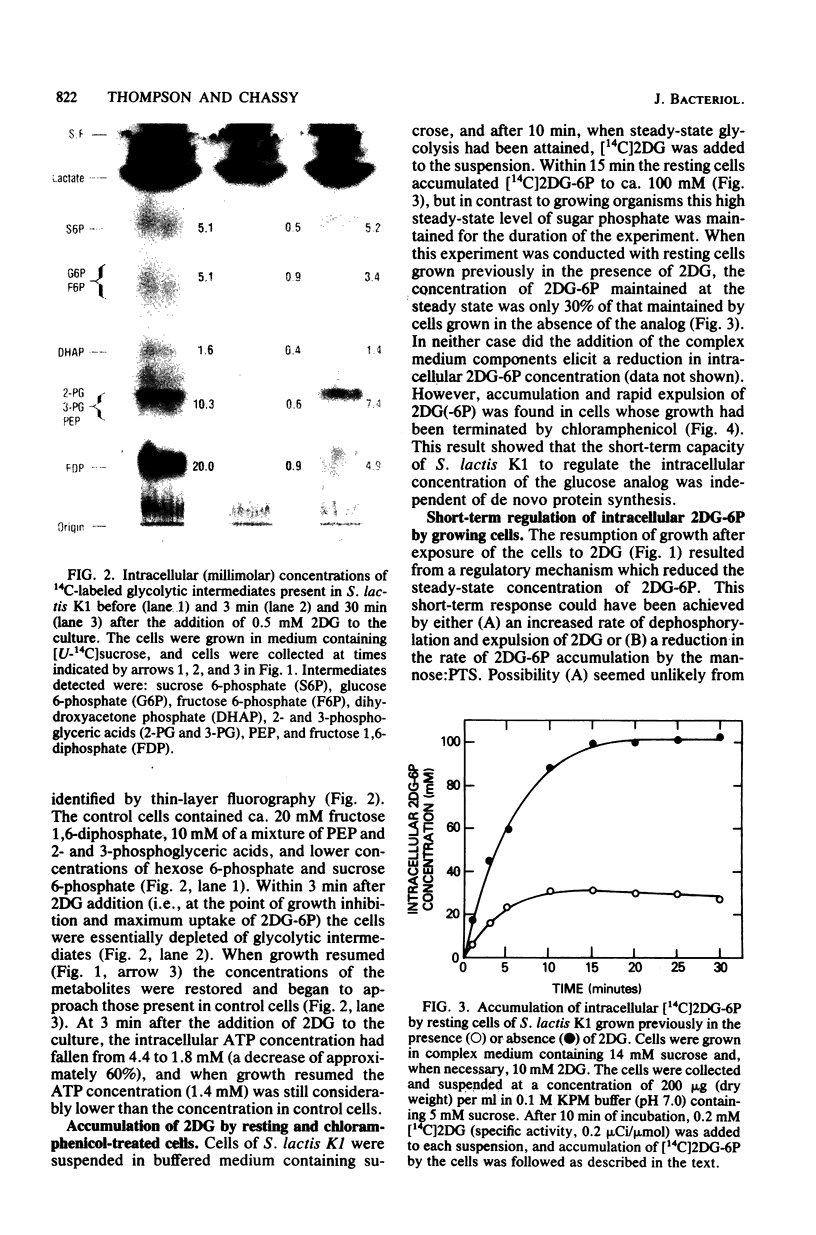
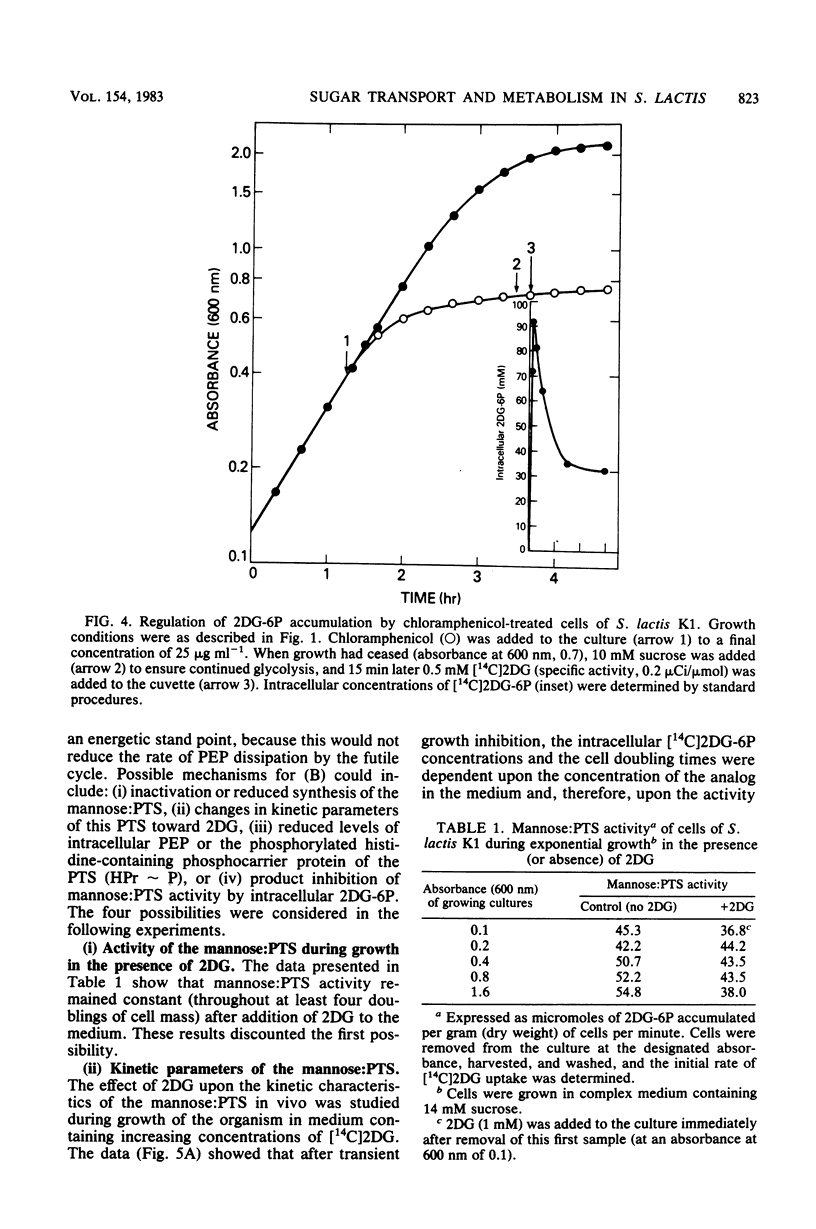
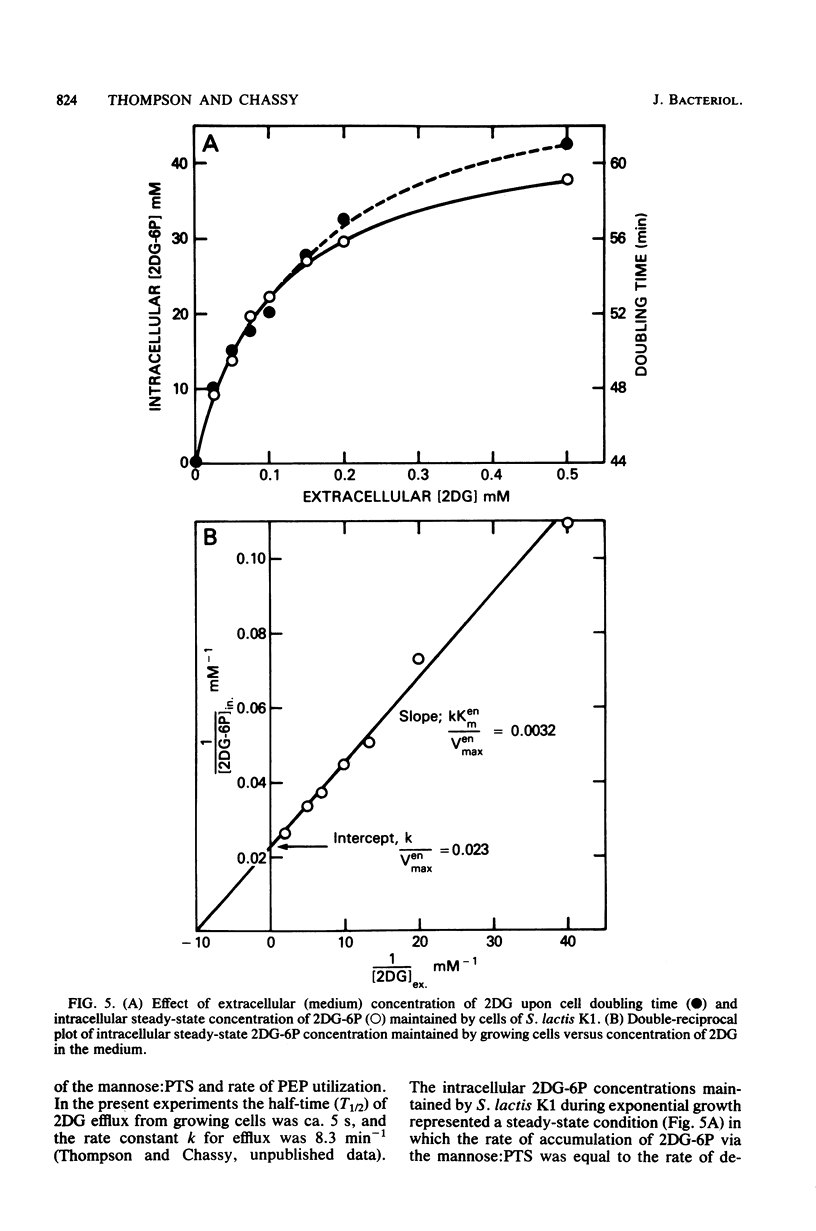
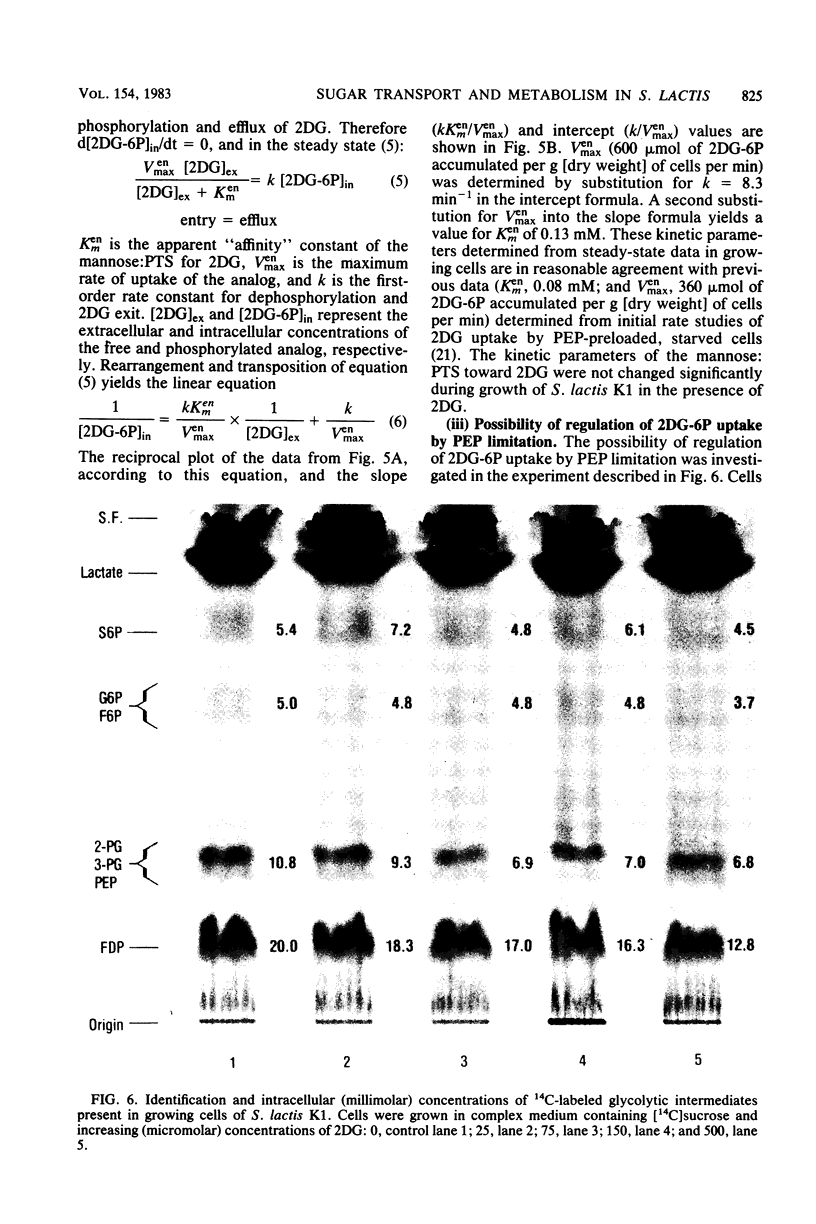
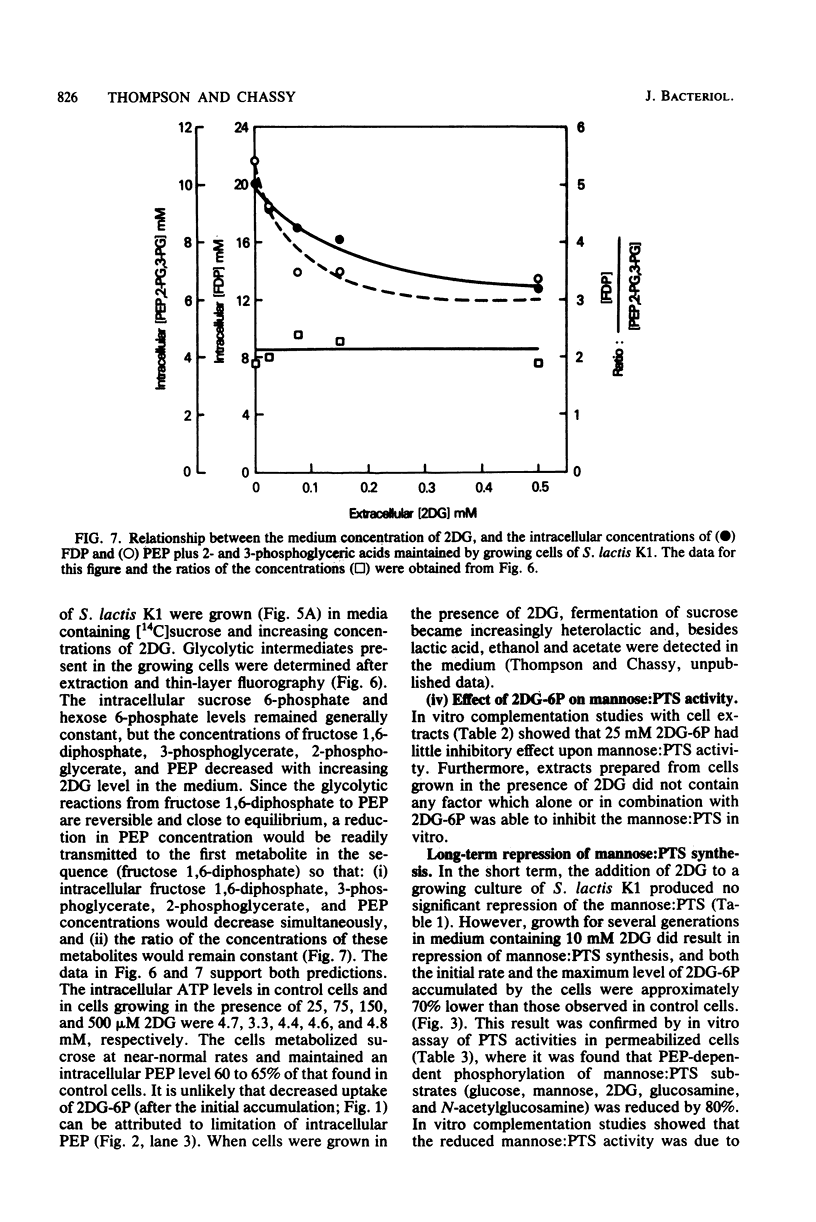
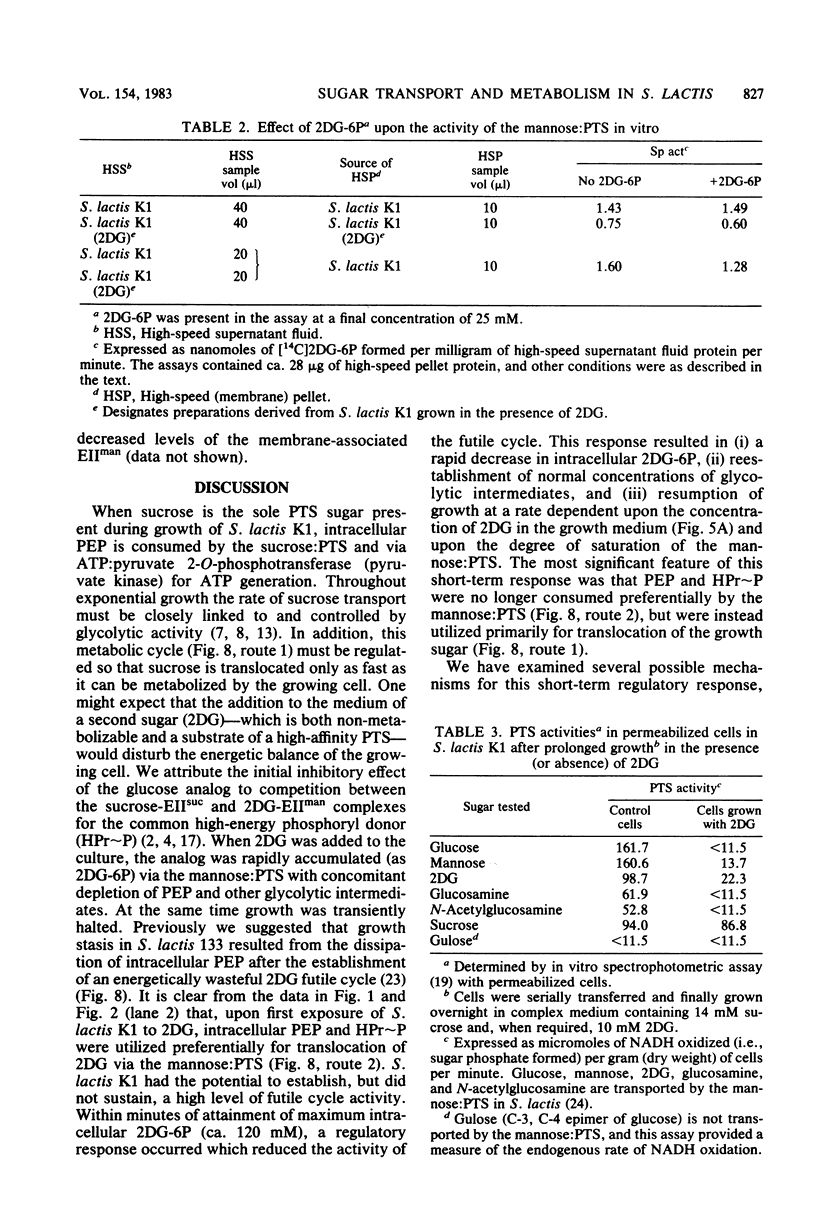
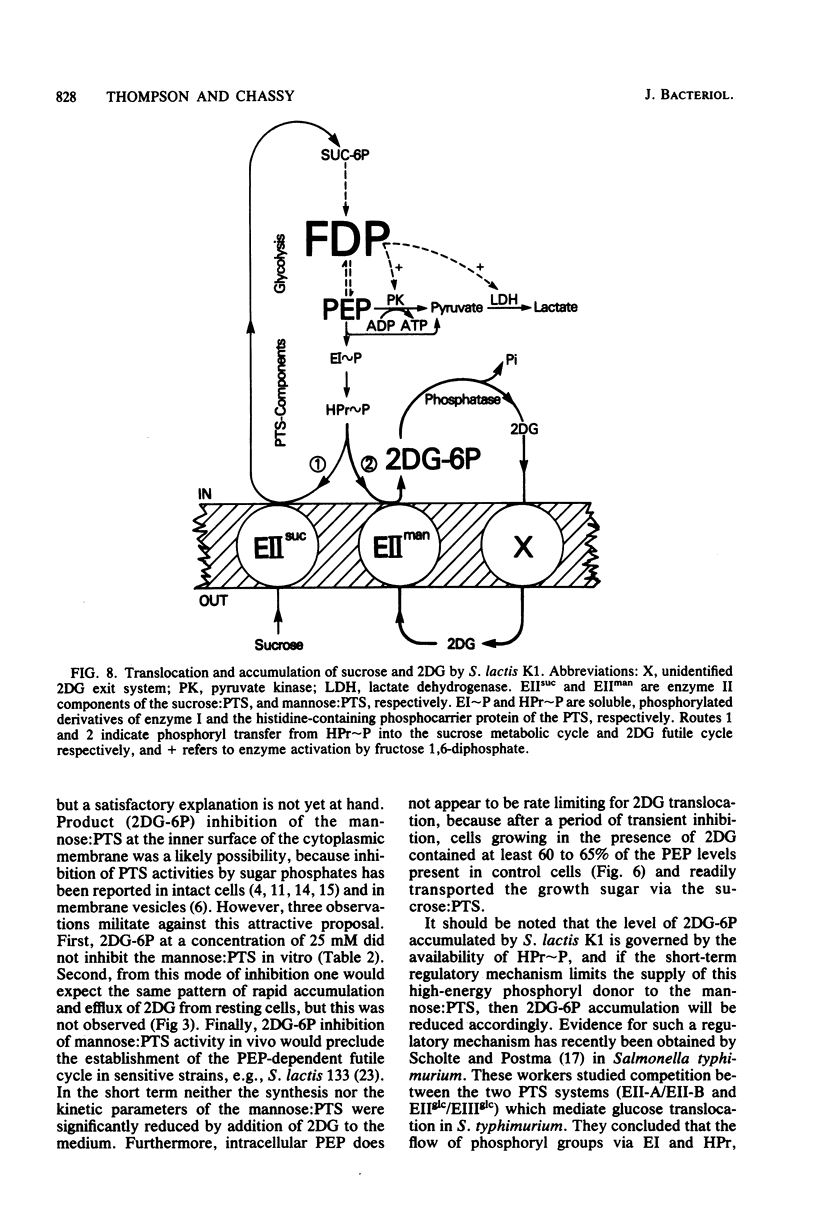
Streptococcus lactis K1 has the capacity to grow on many sugars, including sucrose and lactose, in the presence of high levels (greater than 500 mM) of 2-deoxy-D-glucose. Initially, growth of the organism was transiently halted by the addition of comparatively low concentrations (less than 0.5 mM) of the glucose analog to the culture. Inhibition was coincident with (i) rapid accumulation of 2-deoxy-D-glucose 6-phosphate (ca. 120 mM) and preferential utilization of phosphoenolpyruvate via the mannose:phosphotransferase system, (ii) depletion of phosphorylated glycolytic intermediates, and (iii) a 60% reduction in intracellular ATP concentration. During the 5- to 10-min period of bacteriostasis the intracellular concentration of 2-deoxy-D-glucose 6-phosphate rapidly declined, and the concentrations of glycolytic intermediates were restored to near-normal levels. When growth resumed, the cell doubling time (Td) and the steady-state levels of 2-deoxy-D-glucose 6-phosphate maintained by the cells were dependent upon the medium concentration of 2-deoxy-D-glucose. Resistance of S. lactis K1 to the potentially toxic analog was a consequence of negative regulation of the mannose:phosphotransferase system by two independent mechanisms. The first, short-term response occurred immediately after the initial "overshoot" accumulation of 2-deoxy-D-glucose 6-phosphate, and this mechanism reduced the activity (fine control) of the mannose:phosphotransferase system. The second, long-term mechanism resulted in repression of synthesis (coarse control) of enzyme IImannose. The two regulatory mechanisms reduced the rate of 2-deoxy-D-glucose translocation via the mannose:phosphotransferase system and minimized the activity of the phosphoenolpyruvate-dependent futile cycle of the glucose analog (J. Thompson and B. M. Chassy, J. Bacteriol. 151:1454-1465, 1982). Phosphoenolpyruvate was thus conserved for transport of the growth sugar and for generation of ATP required for biosynthetic and work functions of the growing cell.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dills S. S., Apperson A., Schmidt M. R., Saier M. H., Jr Carbohydrate transport in bacteria. Microbiol Rev. 1980 Sep;44(3):385–418. doi: 10.1128/mr.44.3.385-418.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald R. J., Fitzgerald D. B. Inhibition of caries in hamsters by 2-deoxy-D-glucose. J Dent Res. 1977 Nov;56(11):1431–1431. doi: 10.1177/00220345770560113501. [DOI] [PubMed] [Google Scholar]
- HOFFEE P., ENGLESBERG E., LAMY F. THE GLUCOSE PERMEASE SYSTEM IN BACTERIA. Biochim Biophys Acta. 1964 Mar 30;79:337–350. [PubMed] [Google Scholar]
- Kaback H. R. Regulation of sugar transport in isolated bacterial membrane preparations from Escherichia coli. Proc Natl Acad Sci U S A. 1969 Jul;63(3):724–731. doi: 10.1073/pnas.63.3.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg H. L. Formation and utilization of PEP in microbial carbohydrate transport. Curr Top Cell Regul. 1981;18:313–327. doi: 10.1016/b978-0-12-152818-8.50024-4. [DOI] [PubMed] [Google Scholar]
- London J., Hausman S. Xylitol-mediated transient inhibition of ribitol utilization by Lactobacillus casei. J Bacteriol. 1982 May;150(2):657–661. doi: 10.1128/jb.150.2.657-661.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason P. W., Carbone D. P., Cushman R. A., Waggoner A. S. The importance of inorganic phosphate in regulation of energy metabolism of Streptococcus lactis. J Biol Chem. 1981 Feb 25;256(4):1861–1866. [PubMed] [Google Scholar]
- Postma P. W., Roseman S. The bacterial phosphoenolpyruvate: sugar phosphotransferase system. Biochim Biophys Acta. 1976 Dec 14;457(3-4):213–257. doi: 10.1016/0304-4157(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Roberts K. R., Hayes M. L. Effects of 2-deoxy D-glucose and other sugar analogues on acid production from sugars by human dental plaque bacteria. Scand J Dent Res. 1980 Jun;88(3):201–209. doi: 10.1111/j.1600-0722.1980.tb01215.x. [DOI] [PubMed] [Google Scholar]
- SHERMAN J. R. Rapid enzyme assay technique utilizing radioactive substrate, ion-exchange paper, and liquid scintillation counting. Anal Biochem. 1963 Jun;5:548–554. doi: 10.1016/0003-2697(63)90075-7. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr Catalytic activities associated with the enzymes II of the bacterial phosphotransferase system. J Supramol Struct. 1980;14(3):281–294. doi: 10.1002/jss.400140303. [DOI] [PubMed] [Google Scholar]
- Schachtele D. F., Leung W. L. Effect of sugar analogues on growth, sugar utilization, and acid production by Streptococcus mutans. J Dent Res. 1975 May-Jun;54(3):433–440. doi: 10.1177/00220345750540030301. [DOI] [PubMed] [Google Scholar]
- Scholte B. J., Postma P. W. Competition between two pathways for sugar uptake by the phosphoenolpyruvate-dependent sugar phosphotransferase system in Salmonella typhimurium. Eur J Biochem. 1981;114(1):51–58. doi: 10.1111/j.1432-1033.1981.tb06171.x. [DOI] [PubMed] [Google Scholar]
- St Martin E. J., Wittenberger C. L. Characterization of a phosphoenolpyruvate-dependent sucrose phosphotransferase system in Streptococcus mutans. Infect Immun. 1979 Jun;24(3):865–868. doi: 10.1128/iai.24.3.865-868.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Fitzgerald R. J. Lack of caries inhibitory property of 2-deoxy-D-glucose in rats. J Dent Res. 1978 Mar;57(3):440–440. doi: 10.1177/00220345780570030301. [DOI] [PubMed] [Google Scholar]
- Thompson J., Chassy B. M. Novel phosphoenolpyruvate-dependent futile cycle in Streptococcus lactis: 2-deoxy-D-glucose uncouples energy production from growth. J Bacteriol. 1982 Sep;151(3):1454–1465. doi: 10.1128/jb.151.3.1454-1465.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Chassy B. M. Uptake and metabolism of sucrose by Streptococcus lactis. J Bacteriol. 1981 Aug;147(2):543–551. doi: 10.1128/jb.147.2.543-551.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. In vivo regulation of glycolysis and characterization of sugar: phosphotransferase systems in Streptococcus lactis. J Bacteriol. 1978 Nov;136(2):465–476. doi: 10.1128/jb.136.2.465-476.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Saier M. H., Jr Regulation of methyl-beta-d-thiogalactopyranoside-6-phosphate accumulation in Streptococcus lactis by exclusion and expulsion mechanisms. J Bacteriol. 1981 Jun;146(3):885–894. doi: 10.1128/jb.146.3.885-894.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Thomas T. D. Phosphoenolpyruvate and 2-phosphoglycerate: endogenous energy source(s) for sugar accumulation by starved cells of Streptococcus lactis. J Bacteriol. 1977 May;130(2):583–595. doi: 10.1128/jb.130.2.583-595.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadeboncoeur C., Mayrand D., Trahan L. A comparative study of enzymes involved in glucose phosphorylation in oral streptococci. J Dent Res. 1982 Jan;61(1):60–65. doi: 10.1177/00220345820610011401. [DOI] [PubMed] [Google Scholar]