Abstract
The von Hippel-Lindau tumor suppressor protein (pVHL) is inactivated in the hereditary cancer syndrome von Hippel-Lindau disease and in the majority of sporadic renal carcinomas. pVHL is the substrate-binding subunit of the CBCVHL ubiquitin ligase complex that negatively regulates cell growth by promoting the degradation of hypoxia-inducible transcription factor subunits (HIF1/2α). Proteomics-based identification of novel pVHL substrates is hampered by their short half-life and low abundancy in mammalian cells. The usefulness of yeast two-hybrid (Y2H) approaches, on the other hand, has been limited by the failure of pVHL to adopt its native structure and by the absence of prolylhydroxylase activity critical for pVHL substrate recognition. Therefore, we modified the Y2H system to faithfully reconstitute the physical interaction between pVHL and its substrates. Our approach relies on the coexpression of pVHL with the cofactors Elongin B and Elongin C and with HIF1/2α prolylhydroxylases. In a proof-of-principle Y2H screen, we identified the known substrates HIF1/2α and new candidate substrates including diacylglycerol kinase iota, demonstrating that our strategy allows detection of stable interactions between pVHL and otherwise elusive cellular targets. Additional future applications may include structure/function analyses of pVHL-HIF1/2α binding and screens for therapeutically relevant compounds that either stabilize or disrupt this interaction.
INTRODUCTION
The yeast two-hybrid (Y2H) system has proved an invaluable tool for the identification of protein–protein interactions, both in standard laboratory screens and in high-throughput automated formats (1,2). However, despite the successful identification of thousands of binding partners from various organisms, the Y2H approach is subject to certain limitations. For example, interactions that critically depend on post-translational modifications by enzymatic activities absent from yeast cannot be detected. Furthermore, subunits of heterooligomeric complexes are likely to adopt non-native conformations in the absence of their natural binding partners, giving rise to biologically irrelevant Y2H interactions. Both potential problems are illustrated by the human CBCVHL E3 ubiquitin ligase complex (3) with its substrate recognition subunit, the von Hippel-Lindau tumor suppressor protein (pVHL).
CBCVHL is a heterooligomeric complex comprising the core subunits Cullin-2, Rbx1/Roc1, Elongin B (ELB) and Elongin C (ELC), and the pVHL substrate recognition subunit (3). Key cellular targets of the CBCVHL E3 ubiquitin ligase activity include the alpha subunit of hypoxia-inducible transcription factor 1, HIF1α, and its close homolog, HIF2α, which are important regulators of angiogenesis, glucose uptake and metabolism, cell growth and the cell cycle (4–6). Recognition of HIF1/2α by pVHL requires the post-translational hydroxylation of two conserved prolyl residues (P564 and P402 in human HIF-1α) by a new family of prolylhydroxylases (PHDs) (7–10). Under normoxic conditions, HIF1/2α is hydroxylated by PHDs, recruited to CBCVHL by pVHL, ubiquitylated and rapidly degraded by the 26S proteasome. Conversely, in a hypoxic cellular environment lacking the PHD substrate, molecular oxygen, HIF1/2α is not recognized by pVHL and thus stable, leading to the expression of HIF target genes (4–6). Mutational inactivation of pVHL causes the constitutive expression of HIF target genes even under normoxic conditions, which has been shown to be a key tumorigenic event in the hereditary cancer syndrome von Hippel-Lindau disease (11–13).
The identification of CBCVHL targets other than HIF1/2α has proven difficult in the past. Proteomics strategies relying on affinity purification schemes and subsequent mass spectrometric identification are complicated by the potential low abundance and short half-life of candidate proteins in mammalian cells. Y2H screens, in contrast, typically depend much less on the physiological abundance and half-life of interactors. In particular, Saccharomyces cerevisiae does not possess orthologs of Cullin-2, ELB and ELC, so that pVHL substrates are not subject to ubiquitylation by the CBCVHL ubiquitin ligase complex and subsequent proteasomal degradation. Consequently, it should, in principle, be possible to detect stable Y2H interactions of pVHL with substrates such as HIF1/2α, which are extremely short-lived and of low abundance in mammalian cells. However, Y2H screens using pVHL as bait are impeded by two major limitations: first, pVHL is unable to adopt its native conformation in the absence of the CBCVHL subunits ELB and ELC (14,15). In contrast, pVHL in the context of the ternary pVHL–ELB–ELC (VCB) subcomplex of CBCVHL possesses its native, stable fold and is amenable to structural and biochemical studies (14,16). In particular, the pVHL-binding site for HIF1/2α, and presumably other cellular substrates, is only formed in presence of ELB and ELC (17,18). Second, yeast does not possess PHD homologs, precluding the detection of hydroxyprolyl-mediated Y2H interactions.
Here, we describe a modified Y2H system for the detection of pVHL–substrate interactions that relies on the coexpression of pVHL with the cofactors ELB and ELC and with PHDs. We show that this system is able to faithfully recapitulate HIF1α binding to pVHL in yeast. Moreover, we report the identification of the known substrates HIF1/2α, along with new candidate substrates, in a proof-of-principle library screen, thus demonstrating the strength of the system in detecting even rare pVHL–substrate interactions.
MATERIALS AND METHODS
Yeast strains and media
The S. cerevisiae reporter strains CG1945 (MATa, ura3-52, his3-200, lys2-801, ade2-101, trp1-901, leu2-3,112, gal4-542, gal80-538, cyhr2, LYS2::GAL1UAS-GAL1TATA-HIS3, URA3::GAL417mers(X3)-CYC1TATA-lacZ) and CG1945BC (CG1945, ADE2::YIpDCE1-ElonginB-ElonginC) were described previously (19). Cells were grown in YPD medium (1% yeast extract, 2% peptone and 2% glucose), or in SC medium (0.67% yeast nitrogen base without amino acids, 2% glucose) supplemented with an amino acid mix lacking the indicated amino acids for selection and induction of the PMET25 promoter.
Plasmids
Plasmids used in this study are listed in Table 1. Bait plasmids were constructed using standard procedures by cloning the coding sequences for the short isoform of human pVHL (codons 54–213) and for human full-length PHD1, a PHD2 variant lacking residues 76–177 (10), or full-length PHD3 into multiple cloning sites 1 and 2, respectively, of pBridge (Clontech). Prey plasmids were constructed by cloning the coding sequences of full-length human HIF1α or full-length and truncated human DGKι into pACT2 (Clontech). Mammalian pVHL expression plasmids were constructed by cloning the coding sequences for the long (codons 1–213) and short (codons 54–213) isoforms, respectively, of human pVHL into pCMV-Tag2B (Stratagene). Construction details are available from the authors upon request. The mammalian expression plasmid pCMV-HA-DGKι (20) was a gift of S. Prescott. pACT2-HIF1αPPAG, pACT2-DGKι1-1065PPAA and pACT2-DGKι879-1065PA were generated by site-directed mutagenesis of the corresponding wild-type plasmids using the QuikChangeXL kit (Stratagene) according to the manufacturer's instructions. All inserts were fully sequenced.
Table 1.
Plasmids used in this study
| Plasmid name | Vector | Insert(s) | Reference |
|---|---|---|---|
| pACT2 | pACT2 | None | Clontech |
| pACT2-HIF1α | pACT2 | Human full-length HIF1α | This study |
| pACT2-HIF1αPPAG | pACT2 | Human full-length HIF1α P402A/P564G | This study |
| pACT2-DGKι1-1065 | pACT2 | Human full-length DGKι | This study |
| pACT2-DGKι1-878 | pACT2 | Human DGKι aa 1–878 | This study |
| pACT2-DGKι1-667 | pACT2 | Human DGKι aa 1–667 | This study |
| pACT2-DGKι879-1065 | pACT2 | Human DGKι aa 879–1065 | This study |
| pACT2-DGKι668-1065 | pACT2 | Human DGKι aa 668–1065 | This study |
| pACT2-DGKι1-1065PPAA | pACT2 | Human full-length DGKι P147A/P903A | This study |
| pACT2-DGKι879-1065PA | pACT2 | Human DGKι aa 879–1065 P147A/P903A | This study |
| pBridge-pVHL/empty | pBridge | Human pVHL aa 54–213 (MCS 1) | This study |
| pBridge-pVHL/PHD1 | pBridge | Human pVHL aa 54–213 (MCS 1) | This study |
| Human full-length PHD1 (MCS 2) | |||
| pBridge-pVHL/PHD2 | pBridge | Human pVHL aa 54–213 (MCS 1) | This study |
| Human PHD2Δ76–177 (MCS 2) | |||
| pBridge-pVHL/PHD3 | pBridge | Human pVHL aa 54–213 (MCS 1) | This study |
| Human full-length PHD3 (MCS 2) | |||
| pBridge-empty/PHD1 | pBridge | None (MCS 1) | This study |
| Human full-length PHD1 (MCS 2) | |||
| pBridge-empty/PHD2 | pBridge | None (MCS 1) | This study |
| Human PHD2Δ76–177 (MCS 2) | |||
| pBridge-empty/PHD3 | pBridge | None (MCS 1) | This study |
| Human full-length PHD3 (MCS 2) | |||
| pCMV2B-pVHL19 | pCMV2B | Human pVHL aa 54–213 | This study |
| pCMV2B-pVHL30 | pCMV2B | Human pVHL aa 1–213 | This study |
| pcDNA-HA-DGKι | pCMV-HA | Human full length DGKι | Ref. 20 |
MCS, multiple cloning site; pVHL, von Hippel-Lindau protein; PHD, prolyl hydroxylase; DGK, diacylglycerol kinase.
Directed two-hybrid interactions
For the initial characterization of the two-hybrid system and for the verification of screening results, yeast cells were transformed simultaneously with bait and prey plasmids according to Knop et al. (21) and plated onto plates lacking leucine, tryptophane and methionine (SC-LWM). After 3 days, several transformants were picked with a sterile toothpick and resuspended in sterile water. Cell numbers were adjusted to an OD600 nm of 0.2, and 5 μl of the cell suspension were spotted onto selective plates as indicated. Interactions were evaluated after 3 days.
Y2H screen
CG1945BC yeast cells carrying the bait plasmid pBridge-pVHL/PHD3 were transformed with a human testis cDNA library in pACT2 (Clontech) according to the protocol for high transformation efficiency by Gietz and Woods (22). A total of 1.5 × 107 transformants were plated onto plates lacking leucine, tryptophane, methionine and histidine (SC-LWMH) and containing 5 mM 3-aminotriazol (3-AT). Colonies appearing after 3 days were replica plated onto SC-LWMH plates containing 5 or 25 mM 3-AT and incubated for 5 days. Subsequently, 5-bromo-4-chloro-3-indoyl β-d-galactoside (XGal) colony-lift filter assays were performed according to the Clontech Yeast Protocols Handbook. Prey plasmids from colonies growing in the presence of 5 and 25 mM 3-AT and scoring positive in XGal colony-lift filter assays were isolated and re-transformed together with pBridge-pVHL/PHD3 to verify the interaction, or with pBridge-empty/PHD3 to identify auto-activating library plasmids.
Western blots
Yeast patches growing on SC-LWM control plates were picked and dissolved in sterile water, diluted to identical OD600 nm values and further processed for western blot analysis according to Knop et al. (21). Expression levels were detected with antibodies against pVHL (Ig32; Pharmingen), HIF1α, PHD1, PHD2, PHD3 (all Novus Biologicals) and Gal4 transactivation domain (sc-1663; Santa Cruz).
Immunoprecipitation
HEK293T cells grown in DMEM, 10% FCS, 100 U/ml penicillin, 100 µg/ml streptomycin in 10 cm cell culture dishes were co-transfected with mammalian expression plasmids pCMV-2B-VHL and pCMV-HA-DGKι using the calcium phosphate method. Forty-eight hours after transfection, cells were harvested, washed with buffer ECB (50 mM Tris–HCl, 150 mM NaCl, 1 mM EDTA, pH 7.9), and resuspended in lysis buffer (ECB supplemented with 0.25% NP-40, 1 mM DTT, 25 μM MG132, 6 μg/ml antipain, 4.3 μg/ml leupeptin, 4.5 μg/ml aprotinin, 5 μg/ml trypsin inhibitor, 5 μg/ml pepstatin, 6 μg/ml chymostatin, 10 mM NEM). After 15 min incubation on ice, lysates were cleared by centrifugation (15 min, 20 000 g), and the protein concentrations of the supernatants were adjusted with lysis buffer. FLAG-tagged pVHL was immunoprecipitated by overnight incubation with 35 μl of FLAG-M2 beads (Sigma), followed by three wash steps with ECB containing 0.25% NP-40 and 1 mM DTT. Bound proteins were analyzed by western blots using anti-FLAG antibody (M2, Sigma) and a polyclonal rabbit antibody raised in-house against a recombinantly expressed, C-terminal fragment (amino acid residues 748–1065) of DGKι.
RESULTS
Reconstitution of pVHL substrate interactions in a Y2H system
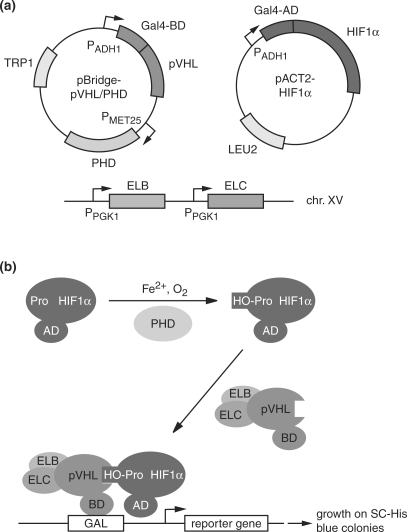
Due to serious doubts about the usefulness of free, presumably non-native pVHL as bait in standard Y2H screens, our aim was to establish a system that was able to identify selectively, or at least preferentially, interactors and bona fide substrates of native pVHL in the context of the stable, ternary VCB complex. To that end, we relied on a previously established reporter yeast strain constitutively expressing ELB and ELC, CG1945BC (19). This strain was transformed with a plasmid derived from the pBridge vector that allows simultaneous expression of the bait pVHL fused to the Gal4 DNA-binding domain (BD) with one of the three human PHDs (Figure 1a). To test the functionality of this system, we used a prey plasmid expressing HIF1α fused to the Gal4 activation domain (AD) (Figure 1a). According to the rationale of the system (Figure 1b), proline residues 402 and/or 564 of HIF1α should be hydroxylated by the PHDs. The prolyl hydroxylation enables pVHL to bind HIF1α, provided that pVHL is stabilized in its native conformation by ELB and ELC. This hydroxyprolyl-dependent interaction between bait and prey should direct the quartenary BD-pVHL/ELB/ELC/AD-HIF1α complex to Gal4 binding sites upstream of the HIS3 and lacZ reporter genes via the BD fused to pVHL, thus inducing the activation of reporter gene expression by the AD fused to HIF1α. The two-hybrid interaction is monitored by the ability of the reporter yeast strain to grow on media lacking histidine, and by the blue staining of colonies in XGal filter assays.
Figure 1.
Yeast two-hybrid system for the detection of pVHL–substrate interactions. (a) Essential elements of the system. The BD-pVHL bait and AD-HIF1α prey fusions are expressed from the pBridge and pACT2 vectors under the control of the constitutive PADH1 promoter. The pBridge vector in addition allows for the expression of PHD1, 2 or 3 under the control of the PMET25 promoter, which is induced in the absence of methionine. The yeast reporter strain CG1945BC is a derivative of CG1945 expressing ELB and ELC under the control of the constitutive PPGK1 promoter from chromosome XV next to the ADE2 locus (19). (b) Schematic representation of reporter gene activation by the PHD-, ELB-, ELC-dependent interaction between BD-pVHL and AD-HIF1α. Pro, HO-Pro; unmodified and hydroxylated form, respectively, of PHD target prolyl residues.
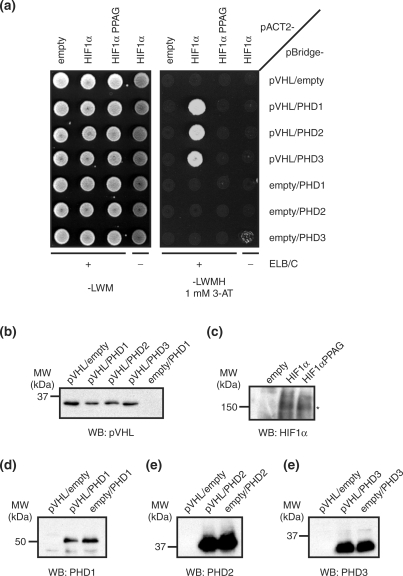
Using this system, we observed a robust interaction between pVHL and HIF1α that was strictly dependent on the presence of one of the three PHDs (Figure 2a). Consistent with this requirement for a PHD, no interaction was detected between pVHL and a HIF1α variant lacking the critical PHD target residues Pro402 and Pro564, HIF1αPPAG. Furthermore, no interaction could be detected in a control reporter strain lacking ELB and ELC, indicating that the two-hybrid interaction required the native conformation of pVHL in the context of the VCB complex. Importantly, western blot analysis demonstrated that the lack of the two-hybrid interaction in the negative controls was not the consequence of reduced expression levels of pVHL, the PHDs or HIF1α PPAG (Figure 2b–f). Together, these results indicate that the observed two-hybrid interaction between pVHL and HIF1α faithfully recapitulates the biologically relevant interaction found in human cells.
Figure 2.
Yeast two-hybrid interaction between pVHL and HIF1α. (a) CG1945BC (+ELB/C) or CG1945 (–ELB/C) yeast cells transformed with the indicated combinations of bait (pBridge-) and prey (pACT2-) plasmids were diluted to an OD600 nm of 0.2 and spotted on SC-Leu-Trp-Met (—LWM) control plates or SC-Leu-Trp-Met-His (—LWMH) reporter plates containing 1 mM 3-aminotriazole (3-AT). (b)–(f) Expression levels of components of the yeast two-hybrid system. Yeast cells were directly taken from the SC-Leu-Trp-Met control plates shown in (a), dissolved in sterile water and diluted to identical OD600 nm values. Protein expression levels were determined by western blots (WB) using antibodies against pVHL (b), HIF1α (c), PHD1 (d), PHD2 (e) and PHD3 (f). Shown are representative sets of samples taken from CG1945BC cells expressing the indicated pBridge (b, d–f) and pACT2 (c) constructs. Expression levels were independent of the presence of ELB/ELC and of specific combinations of bait and prey plasmids, as tested in random samples obtained from several different yeast colonies on the SC-Leu-Trp-Met control plates (data not shown). In (c), an asterisk indicates the HIF1α band.
Screening for novel pVHL interactors
While the role of pVHL in the regulation of HIF1/2α is well understood (23–25) and a number of novel cellular functions for pVHL have emerged recently (26–28), the search for additional substrates of the CBCVHL ubiquitin ligase complex remains a key objective in elucidating the pathology of von Hippel-Lindau disease. The existence of three differently regulated PHD isoforms exhibiting differential activities towards HIF1/2α (7,8,29) and the apparently intact regulation of HIF1/2α in some subtypes of von Hippel-Lindau disease (30,31) suggests that additional cellular targets of CBCVHL exist. Our Y2H system should principally be able to identify such targets.
After having successfully established the system in directed interaction assays, we thus tested its potential by performing a Y2H screen of a human testis cDNA library. We used the pBridge-pVHL/PHD3 bait plasmid because PHD3 appears to possess the least stringent substrate specificity of the three human PHDs (32) and is localized in both nucleus and cytoplasm in mammalian cells (33). We screened 1.5 × 107 transformants on SC-LWMH plates containing 5 mM 3-aminotriazol, replica plated onto plates containing 5 or 25 mM 3-aminotriazol, and performed XGal filter lift assays from these plates. Prey plasmids from colonies growing on 5 and 25 mM 3-aminotriazol and scoring positive in the XGal assay were isolated and re-transformed into the reporter strain either together with the bait plasmid pBridge-pVHL/PHD3 to verify the two-hybrid interaction, or with pBridge-empty/PHD3 to identify bait-independent, auto-activating library plasmids. About 100 true-positive interactors were further tested for two-hybrid interactions in the absence of PHD3 or ELB/ELC, respectively. Among the true-positive interactors, multiple full-length cDNAs of ELC and ELB were identified that mediated a PHD3-independent interaction with pVHL, as expected (Table 2). Importantly, we identified several cDNAs encoding fragments of HIF1α and HIF2α, thus providing convincing proof-of-principle for the usefulness of our system in Y2H screens. All HIF1/2α fragments spanned one of the two critical prolyl residues and mediated an interaction with pVHL that was entirely dependent on PHD3, ELB and ELC (Table 2 and data not shown), again illustrating the unique specificity of the system.
Table 2.
pVHL interactors identified in the yeast two-hybrid screen
| Prey | Longest fragment founda | Number of hits | PHD3 requirementb | LxxLAP motifc |
|---|---|---|---|---|
| Elongin C | aa 4–112 (CT) | 26 | No | No |
| Elongin B | aa 2–118 (CT) | 10 | No | No |
| HIF1α | aa 232–439, | 6 | Yes | Yes |
| aa 537–777 | ||||
| HIF2α | aa 331–537 | 1 | Yes | Yes |
| DGKι | aa 668–910 | 2 | Yes | Yes |
| KIF2C | aa 558–681 | 1 | Yes | Yes |
| THO1 | aa 578–657 (CT) | 1 | Yes | (Yes) |
| Calpain 7 | aa 536–679 | 8 | Yes | (Yes) |
| YY1AP1 | aa 604–739 (CT) | 20 | Yes | No |
| RBP-MS/type 3 | aa 23–201 | 1 | Yes | No |
| RB1CC1 | aa 1407–1594 (CT) | 1 | Yes | No |
| FKBP38 | aa 97–344 | 1 | Yes | No |
aAmino acid residues as determined by sequencing of the prey plasmid insert from the 5′ end; (CT), residue number denotes the authentic C-terminus of the protein; otherwise, the second residue number indicates the end of the sequenced region, not the 3′ end of the prey plasmid insert. Because an oligo(dT)-primed cDNA library was used, most inserts include the authentic 3′ end of the coding sequence and some 3′ untranslated region of the cDNA.
bAs tested by co-transformation of the prey plasmid with pBridge-pVHL/empty.
cBrackets indicate presence of divergent motifs that may still be recognized by PHDs (32).
Besides these known interactors, we identified eight new binding partners that interacted with pVHL in a PHD3- and ELB/ELC-dependent manner, some of them possessing variants of the LxxLAP target motif for PHDs found in HIF1/2α (Table 2 and data not shown). In summary, the Y2H screen led to the identification of both known and potential novel substrates of pVHL.
A novel, PHD-dependent two-hybrid interactor of pVHL
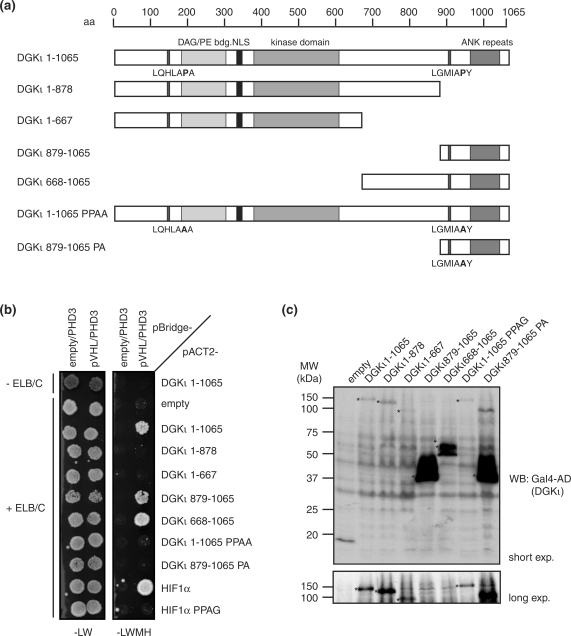
A particularly interesting new interactor is diacylglycerolkinase iota (DGKι), a member of an enzyme family involved in the regulation of intracellular diacylglycerol and phosphatidic acid levels (20,34). DGKι is predominantly expressed in brain and retina, two tissues that are most frequently affected in VHL disease. Intriguingly, DGKι contains two LxxLAP motifs at residues Pro147 and Pro903 that are potential targets for PHD-catalyzed prolyl hydroxylation (Figure 3), one of which was present in the original isolate from the two-hybrid screen (DGKι668–1065; Figure 4a and Table 2).
Figure 3.
DGKι possesses two LxxLAP motifs. Sequence alignments of the N-terminal (top) and C-terminal (bottom) PHD recognition motifs found in HIF1α, HIF2α and DGKι. The LxxLAP consensus motif is underlined, with the target prolyl residue in bold.
Figure 4.
Two-hybrid interaction between pVHL and the candidate substrate, DGKι. (a) Schematic representation of full-length DGKι and the constructs used in two-hybrid assays. Key domains and sequence motifs are indicated at the top, and the two LxxLAP motifs are indicated at the bottom of the bars. (b) ELB-, ELC- and LxxLAP motif-dependent interaction between pVHL and DGKι. Two-hybrid interactions between BD-pVHL and the indicated AD-DGKι and AD-HIF1α constructs were assayed as in Figure 2a. (c) Expression levels of AD-DGKι constructs. Protein samples were prepared as in Figure 2b–f. Shown are representative samples taken from CG1945BC cells expressing the indicated DGKι constructs. Expression levels were determined by western blot (WB) using Gal4-AD antibody. Asterisks indicate bands of the different DGKι constructs.
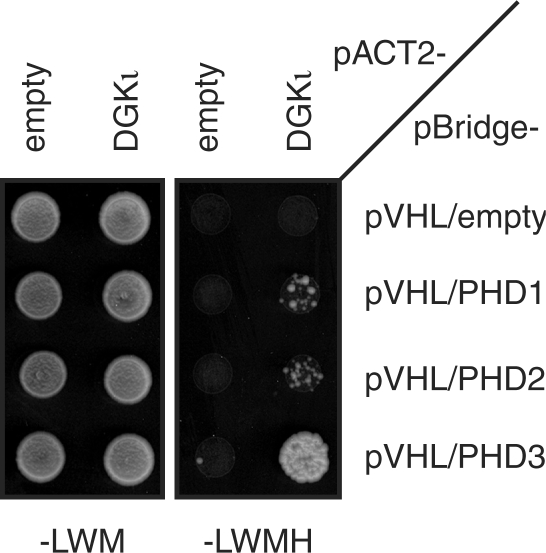
We thus further characterized the two-hybrid interaction between DGKι and pVHL. To map the region of pVHL binding more precisely, we generated different truncated versions of DGKι fused to the AD (Figure 4a). While full-length DGKι1-1065 interacted with pVHL in a PHD3- and ELB/ELC-dependent manner (Figure 4b), C-terminally truncated variants lacking the LxxLAP motif at Pro903 failed to interact, demonstrating that the C-terminal LxxLAP motif is required for the interaction with pVHL. Consistently, the C-terminal fragment DGKι879-1065 including the C-terminal LxxLAP motif was sufficient for the pVHL interaction. Next, we directly tested the importance of the LxxLAP motif for pVHL interaction by mutating the critical prolyl residue, either alone (DGKι879-1065PA) or in concert with the N-terminal LxxLAP motif (DGKι1-1065PPAA). Importantly, neither of the two DGKι variants interacted with pVHL (Figure 4b), strongly suggesting that the two-hybrid interaction depends on the PHD3-catalyzed prolyl hydroxylation of residue Pro903. The expression levels of these mutant prey constructs were found to be very similar to those of the respective wild-type constructs (Figure 4c). Furthermore, we analyzed the PHD specificity of the interaction and found that full-length DGKι interacted strongly with pVHL in the presence of PHD3, while only a very weak interaction was detectable in the presence of PHD1 or PHD2 (Figure 5). This result is consistent with the possibility that PHD3 has evolved to recognize a broader range of substrates than the PHD1 and PHD2 isoenzymes (32).
Figure 5.
PHD3-dependent two-hybrid interaction between pVHL and DGKι. Two-hybrid interactions between BD-pVHL and AD-DGKι in the absence and presence of the indicated PHD isoforms were assayed as in Figure 2a.
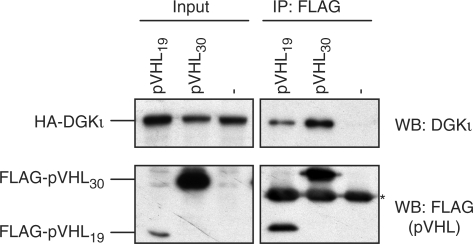
Finally, we wished to test whether pVHL and DGKι also interact in human cells. To this end, 293T cells were transiently transfected with expression plasmids for full-length DGKι and the long and short isoforms of pVHL, respectively. DGKι was efficiently co-immunoprecipitated with both pVHL isoforms, but not in the negative control lacking exogenous pVHL (Figure 6). Thus, the novel two-hybrid interactor DGKι physically associates with pVHL in human cells, further strengthening the validity of our two-hybrid approach.
Figure 6.
pVHL and DGKι interact in human cells. Lysates (Input) of human 293T cells overexpressing HA-tagged DGKι in the absence (–) or presence of the indicated FLAG-tagged pVHL isoform were subjected to immunoprecipitation using anti-FLAG antibody (IP: FLAG). Immunoprecipitated pVHL and DGKι were detected by western blot (WB) using anti-FLAG and anti-DGKι antibodies as indicated. The asterisk marks the light chain of the anti-FLAG antibody used for immunoprecipitation.
DISCUSSION
Mutations in the von Hippel-Lindau tumor suppressor gene (VHL) are the basis of the familial tumor syndrome von Hippel-Lindau disease and the majority of sporadic renal clear cell carcinomas (35–37). Mutational inactivation of pVHL, the substrate-binding subunit of the CBCVHL ubiquitin ligase complex, leads to the constitutive stabilization of the key cellular substrate, HIF1/2α, and presumably other cellular targets. However, systematic approaches for the identification of CBCVHL substrates have so far been largely unsuccessful, despite the great medical relevance.
We have designed and established a powerful adaptation of the Y2H system that allows to screen for novel targets of the pVHL tumor suppressor protein. Our strategy relies on the reconstitution of the VCB complex in yeast (19), which enables pVHL to adopt its native 3D conformation, and on the coexpression of the substrate modifying enzymes of the PHD family. In a novel combination, our system introduces both a new enzymatic activity and structural cofactors into the yeast background to screen for protein–protein interactions requiring a complex interplay of post-translational modification and physical interaction. This combination allows for the rigorous testing of positive clones by omitting either the enzymatic activity or the cofactors. Our strategy is not only useful in order to eliminate a large number of false-positive interactors resulting from interaction with non-native bait, but also, more importantly, enables for the first time to distinguish between potential substrates and regulatory or scaffolding proteins. This is illustrated by our proof-of-principle library screen, which identified the known pVHL substrates HIF1/2α as strictly PHD-dependent interactors. To our knowledge, this is the first identification of HIF1/2α in a Y2H screen and, in fact, in any approach aiming at the identification of pVHL substrates. Previous standard Y2H screens in the absence of ELB, ELC and PHDs had not only failed to identify HIF1/2α, but also often led to the isolation of molecular chaperones and enzymes of the protein degradation machinery, suggesting that most of these proteins bound to non-native pVHL (27,38–40). Of note, we did not identify any chaperone or notorious false-positive Y2H interactor in our screen (Table 2), underlining its superior design with respect to the integrity of the native 3D structure of pVHL. Together, our results demonstrate that our adaptation of the Y2H system can successfully be used to screen for pVHL substrates of even extremely low cellular abundance such as HIF1/2α, as long as their transcripts are represented in a cDNA library. This is a significant advantage over proteomics strategies based on affinity purification, which often fail to identify low abundance binding partners because of insufficient sensitivity of detection and/or high background from abundant proteins.
Our system sets the precedence for a method that can easily be adapted to other heterooligomeric ubiquitin ligases, in particular cullin-based complexes with their diverse substrate recognition requirements (3). Prime examples of high medical importance are Cullin-5 complexes which, like CBCVHL, contain ELB and ELC as core subunits, but use members from the SOCS (suppressor of cytokine signaling) box superfamily as substrate-binding subunits (3,41,42). Some SOCS box proteins like CIS-1, SOCS-1, SOCS-2 and SOCS-3 recruit phosphotyrosine-modified substrates via SH2 domains and are important regulators of immune responses and other cytokine-regulated processes (43). Remarkably, these SOCS proteins exhibit high substrate specificity despite similarities in the composition of their SH2 domains (44), and regions outside the SH2 domains also contribute to substrate binding (45,46). Replacing the bait vector of our system with one that encodes SOCS proteins as baits together with the critical tyrosine kinases (which are absent from yeast) should be a straightforward adaptation to study the exact binding requirements of SOCS proteins and to screen for novel substrates. SOCS proteins with other functional domains are less well understood, but recent findings suggest that an ankyrin domain-containing family member, ASB4, is modified by an asparagine hydroxylase called FIH (factor inhibiting HIF) (47), an oxygen-converting enzyme related to the PHDs which was first identified as a negative regulator of HIF transcriptional activity (48). ASB4 plays an important role in vascular differentiation and has been proposed to recruit yet unknown substrates to a Cullin-5 ubiquitin ligase complex in an asparagine hydroxylation-dependent manner (47). Consequently, employing a bait vector encoding ASB4 as bait together with FIH in our Y2H system could proof a successful adaptation to screen for ASB4 substrates.
Our Y2H screen led to the identification of several potential novel CBCVHL substrates that showed a dependency on PHD and ELB/ELC, some containing an LxxLAP motif for recognition by PHDs similar to the one found in HIF1/2α (Table 2). One such candidate exhibiting interaction characteristics resembling those of HIF1/2α is DGKι. We showed that the Y2H interaction between pVHL and DGKι requires its C-terminal LxxLAP motif, which is not conserved among other DGK isoforms. Intriguingly, and in contrast to other DGK isoforms, which show a rather broad tissue distribution, expression of DGKι appears to be strongest in brain and retina (20), which are both frequently affected in pVHL disease. This correlation might hint to a medically relevant functional interaction between pVHL and DGKι, a possibility that is currently addressed in our laboratory.
In addition to the identification of further pVHL targets in Y2H screens using various cDNA libraries, our system is well suited to perform detailed analyses of the physical interaction between pVHL and full-length HIF1/2α. This should be a welcome addition to the currently available experimental approaches, which are limited to qualitative pull-down experiments and quantitative, but laborious biochemical measurements. The possibility to evaluate the Y2H interaction between pVHL and HIF1/2α in liquid culture using colorimetric β-galactosidase activity assays based on ortho-nitrophenyl-β-d-galactopyranoside (ONPG) even provides the opportunity for semi-quantitative analyses. Additionally, since the PHDs are active in yeast, their specificity and mechanism of HIF1/2α regulation could also be further characterized by mutational analysis, using the pVHL-HIF1/2α interaction as a functional read-out.
Further future applications of the system could include screens for therapeutically relevant chemical compounds that specifically alter the interaction between pVHL and HIF1/2α (4,49,50). For example, drugs that rescue or improve the binding of HIF1/2α to mutant pVHL could lead to improved down-regulation of HIF1/2α in patients carrying certain partial loss-of-function VHL alleles. Conversely, compounds interrupting the physiological interaction between pVHL and HIF1/2α that occurs under hypoxic conditions might be useful in the treatment of acute ischemia. Such compounds may conveniently be screened for in liquid culture high-throughput formats using ONPG β-galactosidase activity assays. In the past, Y2H-based approaches have successfully been used to identify small-molecule inhibitors of protein–protein interactions, for example a modulator of neuronal N-type calcium channels from a library of 156 000 small molecules (51), and an inhibitor of TGFβ receptor signaling from a library of 23 000 compounds (52). We found that Co2+, a known inhibitor of the interaction between pVHL and HIF1/2α in mammalian cells (53,54), is also effective in the context of our Y2H system (Supplementary Figure S1), which further underlines the feasibility of small molecule screens using this system.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
ACKNOWLEDGEMENTS
We thank Stefan Jentsch for continued support; Richard Klausner, Peter Ratcliffe, Steven McKnight and Steven Prescott for materials; Stefan Müller and Christian Schuberth for critical reading of the manuscript. This work was supported by Emmy Noether grant Bu 951/1-3 and/1-4 of the Deutsche Forschungsgemeinschaft to A.B. Funding to pay the Open Access publication charges for this article was provided by the Deutsche Forschungsgemeinschaft.
Conflict of interest statement. None declared.
REFERENCES
- 1.Bartel PL, Fields S. Analyzing protein-protein interactions using two-hybrid system. Meth. Enzymol. 1995;254:241–263. doi: 10.1016/0076-6879(95)54018-0. [DOI] [PubMed] [Google Scholar]
- 2.Cagney G, Uetz P, Fields S. High-throughput screening for protein-protein interactions using two-hybrid assay. Meth. Enzymol. 2000;328:3–14. doi: 10.1016/s0076-6879(00)28386-9. [DOI] [PubMed] [Google Scholar]
- 3.Petroski MD, Deshaies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 2005;6:9–20. doi: 10.1038/nrm1547. [DOI] [PubMed] [Google Scholar]
- 4.Semenza GL. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 5.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat. Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 6.Mazure NM, Brahimi-Horn MC, Berta MA, Benizri E, Bilton RL, Dayan F, Ginouves A, Berra E, Pouyssegur J. HIF-1: master and commander of the hypoxic world. A pharmacological approach to its regulation by siRNAs. Biochem. Pharmacol. 2004;68:971–980. doi: 10.1016/j.bcp.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 7.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 8.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 9.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 10.Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 11.Kondo K, Klco J, Nakamura E, Lechpammer M, Kaelin WG., Jr Inhibition of HIF is necessary for tumor suppression by the von Hippel-Lindau protein. Cancer Cell. 2002;1:237–246. doi: 10.1016/s1535-6108(02)00043-0. [DOI] [PubMed] [Google Scholar]
- 12.Maranchie JK, Vasselli JR, Riss J, Bonifacino JS, Linehan WM, Klausner RD. The contribution of VHL substrate binding and HIF1-alpha to the phenotype of VHL loss in renal cell carcinoma. Cancer Cell. 2002;1:247–255. doi: 10.1016/s1535-6108(02)00044-2. [DOI] [PubMed] [Google Scholar]
- 13.Kim WY, Safran M, Buckley MR, Ebert BL, Glickman J, Bosenberg M, Regan M, Kaelin WG., Jr Failure to prolyl hydroxylate hypoxia-inducible factor alpha phenocopies VHL inactivation in vivo. EMBO J. 2006;25:4650–4662. doi: 10.1038/sj.emboj.7601300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stebbins CE, Kaelin WG, Jr, Pavletich NP. Structure of the VHL-ElonginC-ElonginB complex: implications for VHL tumor suppressor function. Science. 1999;284:455–461. doi: 10.1126/science.284.5413.455. [DOI] [PubMed] [Google Scholar]
- 15.Sutovsky H, Gazit E. The von Hippel-Lindau tumor suppressor protein is a molten globule under native conditions: implications for its physiological activities. J. Biol. Chem. 2004;279:17190–17196. doi: 10.1074/jbc.M311225200. [DOI] [PubMed] [Google Scholar]
- 16.Knauth K, Bex C, Jemth P, Buchberger A. Renal cell carcinoma risk in type 2 von Hippel-Lindau disease correlates with defects in pVHL stability and HIF-1alpha interactions. Oncogene. 2006;25:370–377. doi: 10.1038/sj.onc.1209062. [DOI] [PubMed] [Google Scholar]
- 17.Min JH, Yang H, Ivan M, Gertler F, Kaelin WG, Jr, Pavletich NP. Structure of an HIF-1alpha -pVHL complex: hydroxyproline recognition in signaling. Science. 2002;296:1886–1889. doi: 10.1126/science.1073440. [DOI] [PubMed] [Google Scholar]
- 18.Hon WC, Wilson MI, Harlos K, Claridge TD, Schofield CJ, Pugh CW, Maxwell PH, Ratcliffe PJ, Stuart DI, et al. Structural basis for the recognition of hydroxyproline in HIF-1 alpha by pVHL. Nature. 2002;417:975–978. doi: 10.1038/nature00767. [DOI] [PubMed] [Google Scholar]
- 19.Pause A, Peterson B, Schaffar G, Stearman R, Klausner RD. Studying interactions of four proteins in the yeast two-hybrid system: structural resemblance of the pVHL/elongin BC/hCUL-2 complex with the ubiquitin ligase complex SKP1/cullin/F-box protein. Proc. Natl Acad. Sci. USA. 1999;96:9533–9538. doi: 10.1073/pnas.96.17.9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding L, Traer E, McIntyre TM, Zimmerman GA, Prescott SM. The cloning and characterization of a novel human diacylglycerol kinase, DGKiota. J. Biol. Chem. 1998;273:32746–32752. doi: 10.1074/jbc.273.49.32746. [DOI] [PubMed] [Google Scholar]
- 21.Knop M, Siegers K, Pereira G, Zachariae W, Winsor B, Nasmyth K, Schiebel E. Epitope tagging of yeast genes using a PCR-based strategy: more tags and improved practical routines. Yeast. 1999;15:963–972. doi: 10.1002/(SICI)1097-0061(199907)15:10B<963::AID-YEA399>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 22.Gietz RD, Woods RA. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Meth. Enzymol. 2002;350:87–96. doi: 10.1016/s0076-6879(02)50957-5. [DOI] [PubMed] [Google Scholar]
- 23.Kim WY, Kaelin WG. Role of VHL gene mutation in human cancer. J. Clin. Oncol. 2004;22:4991–5004. doi: 10.1200/JCO.2004.05.061. [DOI] [PubMed] [Google Scholar]
- 24.Haase VH. The VHL tumor suppressor in development and disease: functional studies in mice by conditional gene targeting. Semin. Cell Dev. Biol. 2005;16:564–574. doi: 10.1016/j.semcdb.2005.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maynard MA, Ohh M. Von Hippel-Lindau tumor suppressor protein and hypoxia-inducible factor in kidney cancer. Am. J. Nephrol. 2004;24:1–13. doi: 10.1159/000075346. [DOI] [PubMed] [Google Scholar]
- 26.Hergovich A, Lisztwan J, Barry R, Ballschmieter P, Krek W. Regulation of microtubule stability by the von Hippel-Lindau tumour suppressor protein pVHL. Nat. Cell Biol. 2003;5:64–70. doi: 10.1038/ncb899. [DOI] [PubMed] [Google Scholar]
- 27.Li Z, Wang D, Na X, Schoen SR, Messing EM, Wu G. The VHL protein recruits a novel KRAB-A domain protein to repress HIF-1alpha transcriptional activity. EMBO J. 2003;22:1857–1867. doi: 10.1093/emboj/cdg173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thoma CR, Frew IJ, Hoerner CR, Montani M, Moch H, Krek W. pVHL and GSK3beta are components of a primary cilium-maintenance signalling network. Nat. Cell. Biol. 2007;9:588–595. doi: 10.1038/ncb1579. [DOI] [PubMed] [Google Scholar]
- 29.Berra E, Benizri E, Ginouves A, Volmat V, Roux D, Pouyssegur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J. 2003;22:4082–4090. doi: 10.1093/emboj/cdg392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clifford SC, Cockman ME, Smallwood AC, Mole DR, Woodward ER, Maxwell PH, Ratcliffe PJ, Maher ER. Contrasting effects on HIF-1alpha regulation by disease-causing pVHL mutations correlate with patterns of tumourigenesis in von Hippel-Lindau disease. Hum. Mol. Genet. 2001;10:1029–1038. doi: 10.1093/hmg/10.10.1029. [DOI] [PubMed] [Google Scholar]
- 31.Hoffman MA, Ohh M, Yang H, Klco JM, Ivan M, Kaelin WG., Jr von Hippel-Lindau protein mutants linked to type 2C VHL disease preserve the ability to downregulate HIF. Hum. Mol. Genet. 2001;10:1019–1027. doi: 10.1093/hmg/10.10.1019. [DOI] [PubMed] [Google Scholar]
- 32.Li D, Hirsila M, Koivunen P, Brenner MC, Xu L, Yang C, Kivirikko KI, Myllyharju J. Many amino acid substitutions in a hypoxia-inducible transcription factor (HIF)-1alpha-like peptide cause only minor changes in its hydroxylation by the HIF prolyl 4-hydroxylases: substitution of 3,4-dehydroproline or azetidine-2-carboxylic acid for the proline leads to a high rate of uncoupled 2-oxoglutarate decarboxylation. J. Biol. Chem. 2004;279:55051–55059. doi: 10.1074/jbc.M410287200. [DOI] [PubMed] [Google Scholar]
- 33.Metzen E, Berchner-Pfannschmidt U, Stengel P, Marxsen JH, Stolze I, Klinger M, Huang WQ, Wotzlaw C, Hellwig-Burgel T, et al. Intracellular localisation of human HIF-1 alpha hydroxylases: implications for oxygen sensing. J. Cell Sci. 2003;116:1319–1326. doi: 10.1242/jcs.00318. [DOI] [PubMed] [Google Scholar]
- 34.Topham MK. Signaling roles of diacylglycerol kinases. J. Cell. Biochem. 2006;97:474–484. doi: 10.1002/jcb.20704. [DOI] [PubMed] [Google Scholar]
- 35.Maher ER, Kaelin WG., Jr von Hippel-Lindau disease. Medicine. 1997;76:381–391. doi: 10.1097/00005792-199711000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Kaelin WG, Jr, Maher ER. The VHL tumour-suppressor gene paradigm. Trends Genet. 1998;14:423–426. doi: 10.1016/s0168-9525(98)01558-3. [DOI] [PubMed] [Google Scholar]
- 37.Lonser RR, Glenn GM, Walther M, Chew EY, Libutti SK, Linehan WM, Oldfield EH. von Hippel-Lindau disease. Lancet. 2003;361:2059–2067. doi: 10.1016/S0140-6736(03)13643-4. [DOI] [PubMed] [Google Scholar]
- 38.Tsuchiya H, Iseda T, Hino O. Identification of a novel protein (VBP-1) binding to the von Hippel-Lindau (VHL) tumor suppressor gene product. Cancer Res. 1996;56:2881–2885. [PubMed] [Google Scholar]
- 39.Li Z, Na X, Wang D, Schoen SR, Messing EM, Wu G. Ubiquitination of a novel deubiquitinating enzyme requires direct binding to von Hippel-Lindau tumor suppressor protein. J. Biol. Chem. 2002;277:4656–4662. doi: 10.1074/jbc.M108269200. [DOI] [PubMed] [Google Scholar]
- 40.Corn PG, McDonald ER, III, Herman JG, El-Deiry WS. Tat-binding protein-1, a component of the 26S proteasome, contributes to the E3 ubiquitin ligase function of the von Hippel-Lindau protein. Nat. Genet. 2003;35:229–237. doi: 10.1038/ng1254. [DOI] [PubMed] [Google Scholar]
- 41.Kamura T, Maenaka K, Kotoshiba S, Matsumoto M, Kohda D, Conaway RC, Conaway JW, Nakayama KI. VHL-box and SOCS-box domains determine binding specificity for Cul2-Rbx1 and Cul5-Rbx2 modules of ubiquitin ligases. Genes Dev. 2004;18:3055–3065. doi: 10.1101/gad.1252404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kile BT, Schulman BA, Alexander WS, Nicola NA, Martin HM, Hilton DJ. The SOCS box: a tale of destruction and degradation. Trends Biochem. Sci. 2002;27:235–241. doi: 10.1016/s0968-0004(02)02085-6. [DOI] [PubMed] [Google Scholar]
- 43.Alexander WS, Hilton DJ. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu. Rev. Immunol. 2004;22:503–529. doi: 10.1146/annurev.immunol.22.091003.090312. [DOI] [PubMed] [Google Scholar]
- 44.Machida K, Mayer BJ. The SH2 domain: versatile signaling module and pharmaceutical target. Biochim. Biophys. Acta. 2005;1747:1–25. doi: 10.1016/j.bbapap.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 45.Nicholson SE, Willson TA, Farley A, Starr R, Zhang JG, Baca M, Alexander WS, Metcalf D, Hilton DJ, et al. Mutational analyses of the SOCS proteins suggest a dual domain requirement but distinct mechanisms for inhibition of LIF and IL-6 signal transduction. EMBO J. 1999;18:375–385. doi: 10.1093/emboj/18.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Babon JJ, McManus EJ, Yao S, DeSouza DP, Mielke LA, Sprigg NS, Willson TA, Hilton DJ, Nicola NA, et al. The structure of SOCS3 reveals the basis of the extended SH2 domain function and identifies an unstructured insertion that regulates stability. Mol. Cell. 2006;22:205–216. doi: 10.1016/j.molcel.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 47.Ferguson JE, III, Wu Y, Smith K, Charles P, Powers K, Wang H, Patterson C. ASB4 is a hydroxylation substrate of FIH and promotes vascular differentiation via an oxygen-dependent mechanism. Mol. Cell. Biol. 2007;27:6407–6419. doi: 10.1128/MCB.00511-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science. 2002;295:858–861. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- 49.Giaccia A, Siim BG, Johnson RS. HIF-1 as a target for drug development. Nat. Rev. Drug Discov. 2003;2:803–811. doi: 10.1038/nrd1199. [DOI] [PubMed] [Google Scholar]
- 50.Hewitson KS, Schofield CJ. The HIF pathway as a therapeutic target. Drug Discov. Today. 2004;9:704–711. doi: 10.1016/S1359-6446(04)03202-7. [DOI] [PubMed] [Google Scholar]
- 51.Young K, Lin S, Sun L, Lee E, Modi M, Hellings S, Husbands M, Ozenberger B, Franco R. Identification of a calcium channel modulator using a high throughput yeast two-hybrid screen. Nat. Biotechnol. 1998;16:946–950. doi: 10.1038/nbt1098-946. [DOI] [PubMed] [Google Scholar]
- 52.Joshi PB, Hirst M, Malcolm T, Parent J, Mitchell D, Lund K, Sadowski I. Identification of protein interaction antagonists using the repressed transactivator two-hybrid system. Biotechniques. 2007;42:635–644. doi: 10.2144/000112434. [DOI] [PubMed] [Google Scholar]
- 53.Yuan Y, Hilliard G, Ferguson T, Millhorn DE. Cobalt inhibits the interaction between hypoxia-inducible factor-alpha and von Hippel-Lindau protein by direct binding to hypoxia-inducible factor-alpha. J. Biol. Chem. 2003;278:15911–15916. doi: 10.1074/jbc.M300463200. [DOI] [PubMed] [Google Scholar]
- 54.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.