Abstract
Electrophysiological, microdialysis and behavioral studies support a modulatory role for corticotropin-releasing factor (CRF) in regulating the dorsal raphe nucleus (DRN)-serotonin (5-HT) system. CRF and 5-HT are implicated in the pathophysiology of depression, thus neuroanatomical substrates of CRF-DRN-5-HT interactions are of interest. Identification of co-transmitters within DRN CRF axon terminals is important for elucidating the complex effects underlying CRF afferent regulation of DRN neurons. This study investigated whether CRF-labeled axon terminals within the DRN contain immunoreactivity for vesicular glutamate transporters (isoforms vGlut1 and vGlut2) indicative of the excitatory neurotransmitter glutamate. Dual immunohistochemistry for CRF and either vGlut1 or vGlut2 was conducted within the same tissue section and immunofluorescence results indicated patterns of immunoreactivity consistent with previous reports. Abundant vGlut1- and vGlut2-immunoreactivity was found in puncta exhibiting a largely uniform distribution, whereas CRF-immunoreactivity was localized to topographically distributed varicose processes within the DRN. Profiles containing both CRF- and either vGlut1- or vGlut2-immunoreactivity were apparent in the DRN. Electron microscopy confirmed that immunoreactivity for CRF and vGlut1 was localized primarily to separate axon terminals in the DRN, with a subset co-localizing CRF and vGlut1. Examination of CRF and vGlut2 immunoreactivities in the DRN indicated that CRF and vGlut2 were found within the same axon terminal more frequently than CRF and vGlut1. Overall, these anatomical findings suggest that CRF may function, in part, with the excitatory neurotransmitter glutamate in the modulation of neuronal activity in the DRN.
Keywords: immunohistochemistry, dual immunoelectron microscopy, vGlut isoforms, CRF, dorsolateral DRN, ventromedial DRN
1. Introduction
Both the corticotropin-releasing factor (CRF) and serotonergic (5-HT) systems have been reported to be dysregulated in affective disorders such as depression [4,9,12,18,19,26,48,53,60,61,83]. CRF is a 41-amino acid peptide that regulates the stress response [79], functioning as both a neurohormone modulating activity of the hypothalamic-pituitary-adrenal (HPA) axis and also as a neurotransmitter exerting its effects in monoaminergic brain nuclei. CRF cell bodies are enriched in various brain regions including the paraventricular nucleus of the hypothalamus, the central nucleus of the amygdala, and the bed nucleus of the stria terminalis that project throughout the central nervous system, in particular to the brainstem [52,72].
The serotonergic dorsal raphe nucleus (DRN) is a heterogeneous nucleus that is organized into multiple subdivisions [11,43,69] which contain both 5-HT and non-5-HT neurons [28,43]. Serotonergic neurons contained within these subdivisions have distinct afferent and efferent connections [35,51,56] with forebrain regions involved in stress circuitry [38]. The DRN is topographically innervated by afferent fibers containing CRF [33,72,80]. Examination of the DRN revealed that CRF fibers have a caudal-rostral topographic organization, enriched in the caudal dorsolateral (DL) subregion and the more rostral ventromedial (VM) subregion [33,80]. Thus, the organization of CRF fibers within this nucleus is such that they are poised to regulate specific forebrain regions in accordance with their topographical distribution [80].
Previous work from our laboratory and others has described in detail the types of profiles contacted by CRF-containing fibers in the DRN [33,80,87]. The interaction of CRF immunolabeled axon terminals with postsynaptic targets varied in accordance with the specific DRN subregion examined. First, CRF labeled axon terminals were most frequently found associated with somatodendritic processes in the DL DRN, whereas axon terminals containing CRF in the VM DRN were most often associated with other axon terminals. Further, synaptic specializations between these CRF-immunoreactive terminals and dendrites varied by DRN subregion, with ultrastructural studies revealing mostly asymmetric synapses (Gray’s Type I; excitatory) in the DL DRN, and symmetric synapses (Gray’s Type II; inhibitory) in the VM subregion [80]. Finally, the neurochemical phenotype of dendrites targeted by CRF afferents in these subregions was examined. Dual immunoelectron microscopy studies showed that in both the DL and VM subregions of the DRN, CRF-containing axon terminals were more frequently associated with GABAergic profiles when compared with analysis of these same subregions in sections dual-labeled for CRF and 5-HT [87]. These ultrastructural studies support a complex anatomical organization of the DRN which underlies the complexity of interactions between the CRF and DRN-5-HT systems.
Glutamate is a potential candidate neurotransmitter that is likely to be localized within CRF axon terminals of the DRN. Glutamatergic innervation is supplied to the DRN by cortical as well as subcortical structures [30,35], and many of these regions are also rich in CRF neurons [56,72]. Also, CRF axon terminals in other monoaminergic nuclei such as the locus coeruleus (LC) co-localize glutamate, suggesting co-transport of excitatory amino acid transmitters [81]. Pre-embedding immunohistochemical detection of glutamate, however, is not an ideal marker for excitatory profiles, as glutamate is a precursor to the generation of GABA as well as a general metabolic substrate [31].
Two members of the vesicular glutamate transporter family, vGlut1 and vGlut2, have been shown using immunohistochemical methods to be selectively localized within glutamatergic axon terminals [6,15,16,23,64] where they are sufficient for the accumulation of glutamate into synaptic vesicles [7,49,75]. Immunodetection showed that vGlut1 and vGlut2 exhibit a complementary distribution in the CNS, and together delineate all axon terminals conventionally thought of as glutamatergic [7,15,75,85]. Furthermore, the distribution of CNS neurons containing vGlut1 and vGlut2 is largely complementary, with vGlut1 containing neurons located in the cerebral cortex, hippocampus and cerebellar cortex, and vGlut2 neurons predominant in subcortical regions, cerebellar nuclei, and brainstem regions [15,23,32,64,85]. Examination of CRF in conjunction with vGlut1 and vGlut2 in DRN axon terminals would provide information about the extent to which CRF and glutamate may be poised to modulate postsynaptic targets within the DRN.
To determine whether CRF-containing axon terminals are positioned to co-release glutamate, this study determined the extent to which immunoreactivity for CRF and vGlut are co-localized within axon terminals of the dorsolateral (DL) and ventromedial (VM) subregions of the rat DRN. Pre-embedding dual-immunolabeling was carried out in coronal tissue sections through the DRN using immunohistochemistry to visualize CRF and either vGlut1 or vGlut2 in the same section, and subsequently examined using either confocal or immunoelectron microscopy.
2. Results
CRF and vGlut1 immunofluorescence in the dorsal raphe nucleus
Using dual immunofluorescence, both the dorsolateral (DL) and ventromedial (VM) subregions of the dorsal raphe nucleus were examined. The DRN contained numerous dense, topographically distributed fibers immunoreactive for CRF and these CRF-labeled fibers displayed a pattern of distribution within the DRN consistent with previous immunohistochemical reports [33,80]. CRF immunoreactive fibers were distinct throughout the various DRN subregions, and their beaded, varicose appearance in the DL DRN is illustrated (Fig. 1A, C; arrows). Immunolabeling for vGlut1, as shown in the DL DRN (Fig. 1B, C; arrowheads), was observed in punctate profiles distributed throughout the DRN, appearing only to be lacking in regions presumed to be cell soma, in accordance with prior studies of vGlut1 in this nucleus [10]. It has been reported that antibodies directed against vGlut1 protein do not result in the immunolabeling of somatodendritic profiles [6,15]; rather only resulting in the immunodetection of excitatory synapses. Thus, our light microscopy studies are consistent with a synaptic localization of vGlut1 immunoreactivity within glutamatergic axon terminals. The combined visualization in the DL DRN of both CRF and vGlut1 immunolabeling (Fig. 1C, D) illustrated the distinct labeling patterns of both CRF and vGlut1, in fibers and puncta, respectively. Individual profiles exhibiting labeling for both CRF and vGlut1 (Fig. 1D; double arrows) were better discerned using high magnification (Fig. 1D).
Fig. 1. Photomicrographs of coronal sections through the dorsolateral (DL) DRN showing immunofluorescence for CRF and vGlut1.
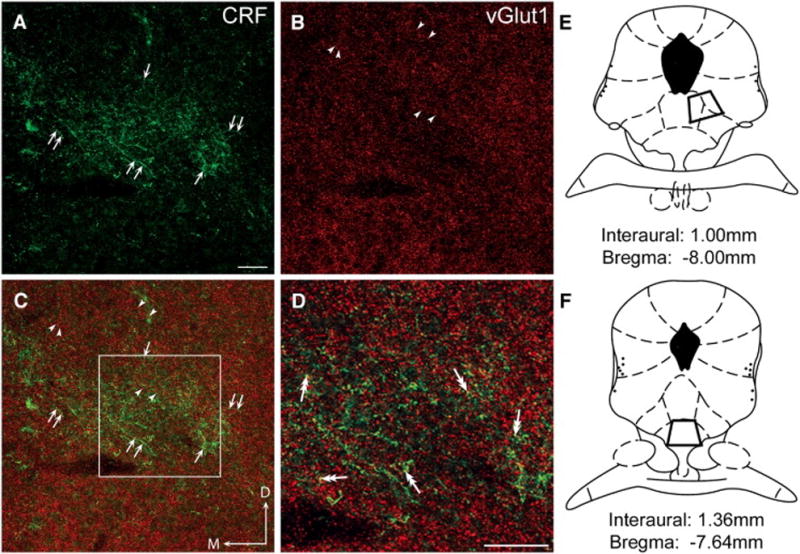
All images in these photomicrographs were obtained in the region of the DL DRN, ventral and to the right of the cerebral aqueduct, and dorsal to the medial longitudinal fasciculus. (A) CRF immunoreactivity was visualized using a FITC-conjugated secondary antibody (green) and the immunolabeled varicose fibers are indicated by arrows. The topographically organized fibers are consistent with previous reports of CRF-immunolabeled fiber distribution in this region and are also represented in panels C and D. (B) Vesicular glutamate transporter isoform1 (vGlut1) immunolabeling was detected using a TRITC-conjugated secondary antibody (red). Arrowheads indicate the punctate appearance of vGlut1 throughout the DL DRN in the same field as panel A. (C) The merged image illustrates the different patterns of distribution for both CRF (green; arrows) and vGlut1 (red; arrowheads) immunoreactivity in the DL DRN as depicted separately in panels A and B. Note that the CRF immunoreactivity is seen predominantly in varicose fibers, whereas vGlut1 is located in small puncta. (D) Dual-headed arrows indicate puncta that appear yellow in this higher magnification image of the region delineated by the square box in panel C, suggesting the presence of both CRF and vGlut1 in the same processes. (E and F) Regions sampled for both immunofluorescence and immunoelectron microscopic analysis of the DL DRN (E) and VM DRN (F) are illustrated in the schematic diagrams adapted from the Rat Brain Atlas [54] at level −8.00mm relative to bregma (DL DRN) and at level −7.64mm relative to bregma (VM DRN). Scale bar in A = 50 μm (also for B, C); scale bar in D = 50 μm. D:dorsal, M:medial
A similar situation was true of the ventromedial DRN (not shown). CRF fibers were distributed bilateral to the midline in the ventral portion of the nucleus and were continuous rostrally with CRF fibers that extended into the interfasicular region of the DRN [33,80]. Puncta containing vGlut1 were distributed throughout the VM DRN region, and high magnification of this portion of the nucleus, like the DL DRN, suggested the presence of both CRF and vGlut1 occasionally localized to the same profile.
Ultrastructural analysis of CRF and vGlut1 in the dorsolateral (DL) dorsal raphe nucleus
Dual labeling for CRF (immunogold-silver) and vGlut1 (immunoperoxidase) was carried out in coronal tissue sections through the DRN. The dorsolateral region of the DRN was delineated as described (see Methods) at the approximate rostro-caudal level of bregma −8.00mm (Fig. 1E; [54]). For all studies, tissue sections selected for analysis contained dense fibers in the DL DRN immunoreactive for CRF and prominent punctate profiles immunolabeled for vGlut1.
A total of 641 immunolabeled axon terminals were included in the analysis of the dorsolateral (DL) subregion of the DRN. All fields containing immunoreactivity for both CRF and vGlut1 were captured for analysis; however, only those axon terminals in which the synaptic vesicles could be distinguished were included in the quantification. Examination of the electron micrographs revealed that axon terminals immunoreactive for CRF and vGlut1 could be found in the same region of the neuropil (Fig. 2) as well as localized to the same axon terminal (Fig. 3).
Fig. 2. Axon terminals immunolabeled for CRF and vGlut1 are present in similar regions of the neuropil.
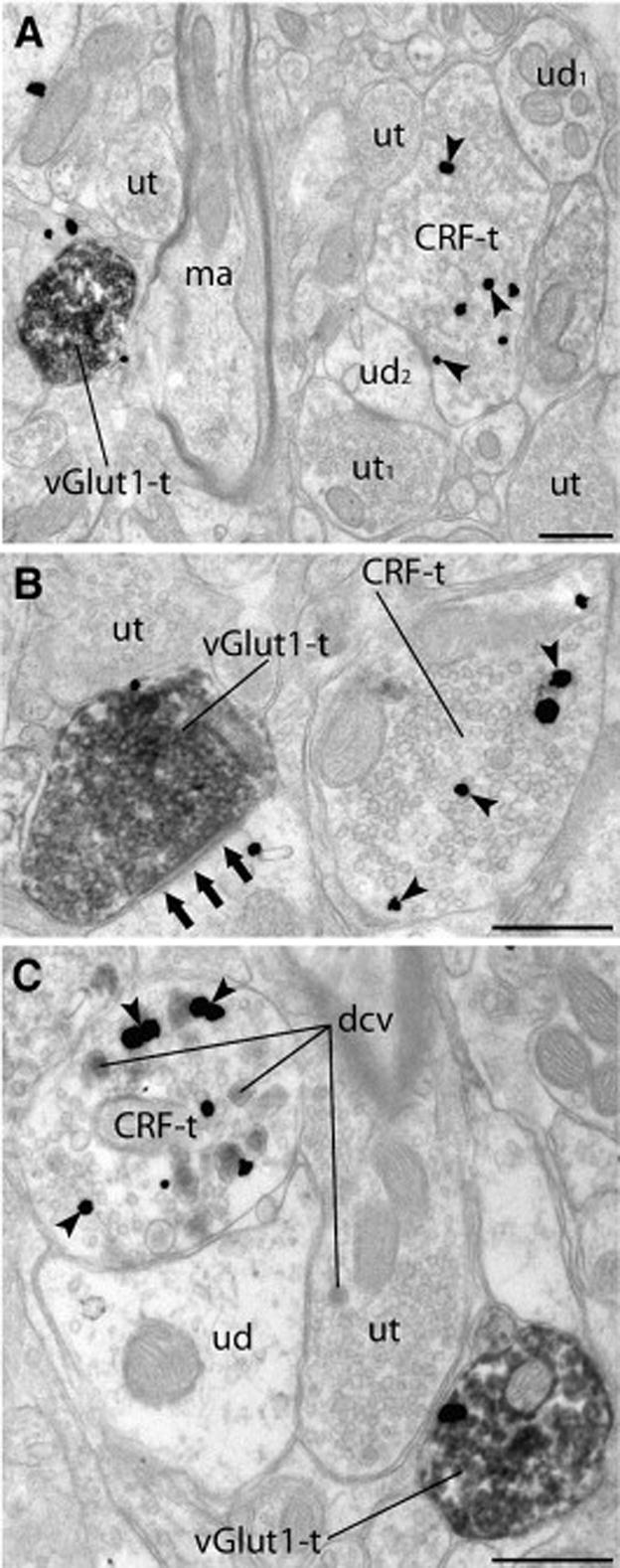
Ultrastructural analysis indicated that axon terminals immunoreactive for either CRF (CRF-t; silver enhanced 1nm gold particles) or vGlut1 (vGlut1-t; peroxidase) were often located in close proximity to each other (A–C) in the DL DRN. In this case (A), a CRF-t (immunoreactivity indicated by arrowheads), forms synaptic contacts onto two dendrites (ud1 and ud2) and is also apposed to an unlabeled terminal (ut). One of the unlabeled dendrites (ud2), in addition to receiving a synapse from the CRF-t, also receives a synapse from an unlabeled terminal (ut1) illustrating the complexity of synaptic contacts in the DRN. A vGlut-1 positive axon terminal (vGlut1) is in the same region of the neuropil, but not associated with the CRF-t. Other profiles including an unlabeled terminal (ut) and myelinated axon (ma) are visible nearby. In this image (B), separate axon terminals containing vGlut1 (vGlut1-t) and CRF (CRF-t) are found in closer proximity to each other. The CRF-t is discerned from a nearby unlabeled axon terminal (ut) by the presence of immunogold particles (arrowheads). This vGlut1-t is forming an asymmetric synapse (three arrows) onto a dendrite, consistent with its presence in glutamatergic axon terminals. The immunoperoxidase labeling indicating vGlut1 (vGlut1-t) within an axon terminal can be seen in stark contrast to a neighboring terminal (ut) considered to be unlabeled due to the lack of immunostaining. (C) A CRF-t containing prominent dense core vesicles (dcv) is apposed to an unlabeled dendrite (ud) which is also apposed by a ut. As shown, immunolabeling for CRF is often observed in association with the dcv. A vGlut1-t is visible nearby. Arrowheads: silver enhanced 1nm gold indicating CRF immunoreactivity; Scale bars=500nm.
Fig. 3. Ultrastructural evidence for the co-localization of CRF and vGlut1 within axon terminals of the DL DRN.
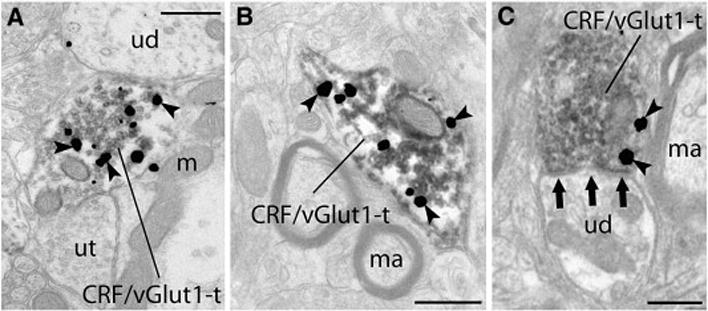
Silver enhanced gold particles (arrowheads) indicating CRF immunoreactivity were observed within axon terminals that contained immunoperoxidase associated with abundant clear synaptic vesicles indicating the presence of vGlut1 (A–D). (A) Although both unlabeled terminals (ut) and unlabeled dendrites (ud) could be identified throughout regions of the neuropil, individual terminals exhibited immunoreactivity for both CRF and vGlut1 (CRF/vGlut1-t). (B) As in all regions selected for analysis, immunoreactivity indicating the presence of CRF and vGlut1 was clearly localized to axon terminals, and here, myelinated axons (ma) lacking immunoreactivity can be seen in close proximity to the CRF/vGlut1-t. (C) Unlabeled dendrites (ud) were identified as one target of CRF/vGlut1-t, and here this CRF/vGlut1-t forms an asymmetric (excitatory) synapse (three grouped arrows) onto the unlabeled dendrite (ud). Scale bars=500nm.
When axon terminals immunoreactive for CRF and vGlut1 were located in the same field, they could be found associated with unlabeled profiles including dendrites and other axon terminals that lacked any detectable immunoreactivity (Fig. 2A). CRF and vGlut1 axon terminals were also found in close proximity to each other (Fig. 2B). Consistent with excitatory neurotransmission, vGlut1 axon terminals formed asymmetric synapses (Fig. 2B; three arrows) as evidenced by the electron-dense thickness of the postsynaptic membrane. As observed in previous studies [41,80,87], CRF immunolabeled axon terminals often contained large dense core vesicles (dcv) not associated with the zone of apposition, in addition to the numerous small clear synaptic vesicles containing classical neurotransmitters (Fig. 2C). Of the immunolabeled axon terminals in the DL DRN, 41.34% (n=265; 95% CI= 37.59–45.19%) of these contained only immunoreactivity for CRF whereas 45.87% (n=294; 95% CI= 42.05–49.74%) were singly labeled for vGlut1. Significantly fewer immunolabeled axon terminals, 12.79% (n=82; 95% CI= 10.42–15.60%), in the DL DRN demonstrated immunoreactivity for both CRF and vGlut1.
When axon terminals containing immunoreactivity for both CRF and vGlut1 were observed, numerous gold particles indicating CRF immunoreactivity could be seen contained within the axon terminal (Fig. 3). These dual labeled terminals could be found throughout the neuropil associated with unlabeled axon terminals or dendrites (Fig. 3A) or in the vicinity of myelinated axons (Fig. 3B). Often, dually immunolabeled CRF/vGlut1 terminals could be found synapsing onto unlabeled dendrites (Fig. 3C). Numerous axons and axon terminals lacking immunoreactivity were apparent in the same region of the neuropil.
Co-localization of CRF and vGlut1 in the ventromedial (VM) dorsal raphe nucleus
Analysis of the ventromedial DRN was carried out in tissue sections processed in parallel with those containing the dorsolateral DRN subregion. The approximate region selected for analysis of the VM DRN using electron microscopy is outlined by the trapezoid (Fig. 1F) centered immediately dorsal to the medial longitudinal fasciculus (mlf), where CRF fibers were organized bilateral to the midline. Dense fibers containing CRF-immunoreactivity, occasionally extending slightly into the interfasicular space, were present along with interspersed vGlut1-immunolabeled profiles in this subregion (not shown).
In the VM DRN, 544 immunolabeled axon terminals were randomly sampled for analysis. Consistent with the pattern of labeling in the DL DRN, axon terminals could be found that contained either CRF or vGlut1 immunoreactivity in the same region of the neuropil, associated with unlabeled profiles including dendrites, myelinated axons and other axon terminals. Some axon terminals in this subregion were observed to contain both CRF and vGlut1. Of the total number of immunolabeled axon terminals, those containing only CRF accounted for 36.03% (n=196; 95% CI= 32.11–40.15%) whereas vGlut1 immunopositive terminals comprised a larger percentage of immunolabeled axon terminals in this subregion at 59.01% (n=321; 95%CI= 54.83–63.07%). The remaining 5% (n=27; 95% CI= 3.43–7.12%) of immunolabeled axon terminals in the VM DRN were immunopositive for both CRF and vGlut1.
Comparison of CRF/vGlut1 distribution in the DL and VM DRN
The percentages of immunolabeled axon terminals containing only CRF, only vGlut1, or both CRF and vGlut1 was compared in the two DRN subregions examined. No difference was observed in the percentage of axon terminals containing only CRF between the DL and VM DRN (5.31%; 95% CI= −0.026–10.81%; p=0.0616). A significantly higher percentage of axon terminals within the VM DRN (as opposed to the DL DRN) contained only vGlut1 (13.14%; 95% CI= 7.44–18.71%; p=<0.0002). Finally, co-localization of CRF and vGlut1 within the same axon terminal was more frequently noted within the DL DRN (7.83%; 95% CI= 4.62–11.03%; p=<0.0002).
CRF and vGlut2 immunofluorescence in the dorsal raphe nucleus
Immunofluorescent labeling observed in sections processed for the detection of CRF and vGlut2 was similar to the pattern of labeling seen in sections analyzed for CRF and vGlut1 immunoreactivity. Using confocal microscopy, CRF-labeled processes and vGlut2 containing puncta were visible throughout the neuropil (Fig. 4A–B). Although separate profiles immunoreactive for either CRF or vGlut2 were most prevalent, immunolabeling that appeared yellow (Fig. 4A, B; double arrowheads) indicated the co-localization of CRF and vGlut2 within the same profile. These observations were characteristic of both the DL (Fig. 4A, B) and VM DRN (not shown).
Fig. 4. Evidence for the co-localization of CRF and vGlut2 in the DRN.
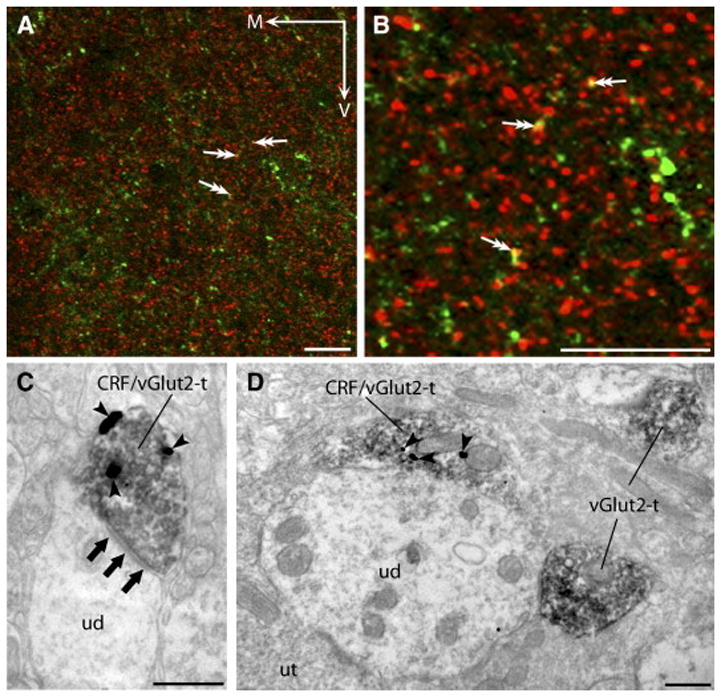
Confocal photomicrographs (A,B) revealed processes immunolabeled for CRF (green) and puncta immunopositive for vGlut2 (red) throughout both the DL (shown) and VM (not shown) DRN subregions. Puncta that appeared to contain both CRF and vGlut2 immunoreactivity (double arrows A, B) appeared yellow in color. The co-localization of CRF and vGlut2 within the indicated profiles was better discerned at higher magnification (B). Ultrastructural analysis revealed that CRF and vGlut2 were often co-localized within the same axon terminals in both the DL (C) and VM (D) DRN. Consistent with the excitatory role of glutamate, an axon terminal containing both CRF (arrowheads; 1nm gold particles) and vGlut2 (peroxidase) can be seen forming an asymmetric synapse (three grouped arrows) onto an unlabeled dendrite (ud). Here (D), an unlabeled dendrite (ud) is associated with an axon terminal (CRF/vGlut2-t) immunoreactive for both CRF (arrowheads) and vGlut2 (peroxidase). This ud also received contacts from an axon terminal containing only vGlut2 (vGlut2-t) and a terminal lacking detectable immunoreactivity (unlabeled terminal; ut). Scale bars A, B = 20 μm. M: medial, V: ventral. Scale bars C, D = 500 nm.
Ultrastructural analysis of CRF and vGlut2 in the dorsolateral (DL) and ventromedial (VM) dorsal raphe nucleus
Dual immunolabeling for CRF (immunogold-silver) and vGlut2 (immunoperoxidase) was carried out in the same coronal tissue section through the DRN. Sections containing the dorsolateral and ventromedial subregions were processed in parallel, and these subregions were demarcated as described (see Methods and Fig. 1E and F; [54]), with immunoreactivity for both CRF and vGlut2 apparent in all sections sampled.
In the DL DRN, 862 immunolabeled axon terminals were randomly obtained for analysis to determine the extent of CRF and vGlut2 co-localization. Axon terminals containing CRF or vGlut2 immunoreactivity were often observed in close proximity throughout the neuropil, with labeling for CRF and vGlut2 found in separate axon terminals as well as within the same axonal profile. The majority of immunolabeled axon terminals in the DL DRN were immunopositive for either vGlut2 alone or dually-labeled for both CRF and vGlut2 (Fig. 4C). Of the immunolabeled axon terminals, 42.34% (n=365; 95% CI= 39.08–45.67%) of these contained only immunoreactivity for vGlut2 whereas 36.78% (n=317; 95% CI= 33.62–40.04%) exhibited immunolabeling for both CRF and vGlut2. The remaining 20.88% (n=180; 95% CI= 18.30–23.72%) of immunolabeled axon terminals in the DL DRN demonstrated immunoreactivity for only CRF. Axon terminals containing both CRF and vGlut2 were often found in synaptic contact with unlabeled dendrites (Fig. 4C).
In the VM DRN, 736 randomly captured immunoreactive profiles were examined. The majority of axon terminals that were immunolabeled, 49.59%, contained only immunoreactivity for vGlut2 (n=365; 95% CI= 45.99–53.19%), whereas significantly fewer axon terminals, 18.34%, were immunoreactive exclusively for CRF (n=135; 95% CI= 15.71–21.30%). The remaining immunolabeled axon terminals, 32.07% (n=236; 95% CI= 28.80–35.53%), contained both CRF and vGlut2 immunoreactivity (Fig. 4D). Unlabeled dendrites were frequently found in association with numerous vGlut2-containing axon terminals, with one or more of these vGlut2 axon terminals also containing CRF immunoreactivity (Fig. 4D).
Comparison of CRF/vGlut2 distribution in the DL and VM DRN
The percentage of immunolabeled axon terminals containing only CRF, only vGlut2, or both CRF and vGlut2 was compared between the two DRN subregions examined. The percentage of axon terminals containing only CRF did not differ between the DL and VM DRN (2.54%; 95% CI= −1.39%–6.41%; p=0.2034). Similar to sections assessed for CRF/vGlut1 immunoreactivity, more axon terminals in the VM DRN were found to contain glutamate as indicated by the presence of vGlut2 (7.25%; 95% CI=2.35–12.11%; p=0.0037). In the DL DRN, co-localization of CRF and vGlut2 within the same axon terminal was greater than in the VM DRN (4.71%; 95% CI=0.03–9.34%; p=0.0485).
3. Discussion
This study provides an anatomical correlate for the co-existence of CRF and glutamate within a subset of axon terminals in the midbrain serotonergic dorsal raphe nucleus. The immunohistochemical co-localization of CRF with either vGlut1 or vGlut2 suggests that, as in other monoaminergic nuclei [81], the co-release of CRF and glutamate may function to regulate postsynaptic targets in the DRN. Although CRF and vGlut1 were present in primarily separate axon terminals, CRF and vGlut2 were frequently found within the same axon terminal. The finding that significantly more CRF axon terminals in the DL subregion co-localize vGlut1 and vGlut2 compared to the VM subregion further supports the heterogeneous organization of this nucleus. These results also support previous findings that CRF axon terminal associations with dendrites are predominantly excitatory in the DL DRN as opposed to the VM DRN [80].
Methodological considerations
The specificity of the antisera used in this analysis has been confirmed by previous studies in our laboratory and the laboratories of others [10,13,65,82]. The primary antisera for CRF and vGlut1 or vGlut2 were generated in different species, thus the potential for false positive immunolabeling is considered unlikely. A small number of axon terminals did exhibit dual-immunolabeling for CRF and vGlut1 (Fig. 3), however, a significant number of axon terminals immunoreactive for either CRF or vGlut1 alone (Fig. 2) were observed in both DRN subregions. A larger proportion of axon terminals within both DRN subregions were found to co-localize CRF and vGlut2 (Fig. 4) even though numerous singly labeled axon terminals were also observed throughout the neuropil. Together, these observations along with previous control experiments and antibody characterization support the validity of these findings.
Caveats associated with the experimental approach used include antibody penetration, as immunolabeling of the desired antigens is carried out in thick tissue sections. Thus, only the most superficial ultra-thin sections at the tissue-aclar interface were used in the analysis to minimize any bias of the immunolabeling associated with antibody penetration [36]. Axonal profiles that lacked CRF, vGlut1 or vGlut2 were considered to be unlabeled in this study. The possibility exists, however, that these axon terminals did contain immunoreactivity for CRF, vGlut1 or vGlut2 but that the protein levels were too low to be detected with the existing methods. This also is considered unlikely, as numerous axon terminals either exhibiting or lacking both CRF and/or either vGlut1 or vGlut2 could be identified in all fields of tissue that were evaluated.
In some instances the zone of synaptic contact between axon terminals and dendrites could be determined, and asymmetric synapses could be found supporting the excitatory nature of the vGlut1 and vGlut2 axon terminals. However, this study aimed only to examine the extent of CRF and vGlut1 or vGlut2 co-localization within DRN axon terminals, thus the inability to discern the types of synaptic contacts did not detract from these findings. Further, previous data examining the ultrastructural characteristics of vGlut1 and vGlut2 immunolabeled axon terminals in the DRN provided evidence that asymmetric synapses were associated with the majority of vGlut1 and vGlut2 axon terminals when profiles were examined in serial ultra-thin sections [10].
The vGlut family of transporters is structurally and functionally similar, and are essential machinery for the proton-driven increase of glutamate in synaptic vesicles prior to synaptic release [7,77]. The vGlut family currently consists of three known isoforms: vGlut1, vGlut2, and vGlut3 [1,14,15,20,23,74,76]. Together, vGlut1 and vGlut2 delineate all accepted glutamatergic neuronal populations in the brain [15,85] and have distinct, complementary distributions throughout the CNS [15,23,25,49,50].
The third isoform in the vGlut family, vGlut3, is for the most part structurally and functionally similar to both vGlut1 and vGlut2 [14,20,62,66,74], however numerous differences in its distribution suggest non-traditional roles for vGlut3 in glutamate signaling. First, the distribution of vGlut3 in neuronal populations is sparse compared to that of both vGlut1 and vGlut2 [20,24]. Further, vGlut3 is co-localized within neurons containing classical neurotransmitters such as GABA, serotonin, and acetylcholine [14,24,66] and vGlut3 immunoreactivity is not only detectable in presynaptic terminals (like vGlut1 and vGlut2), but also found in astrocytes and the somato-dendritic regions of neurons [73]. Furthermore, the serotonergic DRN is one region where vGlut3 is robustly present in both symmetric and asymmetric synapses of serotonergic as well as non-serotonergic neurons [20,66], whereas vGlut1 and vGlut2 immunoreactivities are found only in axon terminals that form asymmetrical synapses [6,15,16,23,64]. For these reasons, this study investigated only the co-localization of vGlut1 or vGlut2 with CRF to determine the extent of co-localization of CRF and glutamate.
Potential sources of CRF/vGlut1 and CRF/vGlut2 afferents
The DRN receives glutamatergic input from cortical as well as subcortical structures and many of these regions are rich in CRF neurons [30,35,56,72]. The specific origins of CRF afferent projections to the DRN have not been elucidated; however, forebrain regions projecting to the DRN enriched in CRF cell bodies include the central nucleus of the amygdala, the bed nucleus of the stria terminalis, and the paraventricular nucleus of the hypothalamus [39,56,72].
Brain regions including the medial prefrontal cortex, amygdala, various hypothalamic regions, the parabrachial nuclei, and laterodorsal tegmental nuclei among others [35] send glutamatergic afferents to the DRN. In addition to providing glutamatergic input to the DRN, a separate study using immunohistochemistry indicated that all of these regions contain some number of CRF neurons [56,72] suggesting the possibility of co-release of glutamate with CRF in the DRN. These glutamatergic inputs may be involved in the regulation of affective behaviors as well as biological rhythms, arousal, and sensory and motor functions.
Despite their numerous similarities, vGlut1 and vGlut2 have distinct distributions within glutamatergic neurons of the rat CNS [15,23,25,49,50]. For example, vGlut1 containing neurons are predominant in locations including the cerebral and cerebellar cortex, hippocampus, and thalamus. The medial prefrontal cortex is one area rich in vGlut1 neurons [32] and this region has also been shown to project substantially to the entire DRN [56,67]. vGlut2 neurons, in contrast, are predominantly localized to subcortical regions including the diencephalon, brainstem, and portions of the spinal cord [15,23,25,49,50] and numerous subcortical regions sending glutamatergic projections to the DRN are enriched in vGlut2 neurons [30,35]. This suggests that brain regions enriched in neurons containing vGlut1 or vGlut2 are positioned to modulate the DRN.
Although the vGlut1 and vGlut2 isoforms are differentially distributed throughout the brain, both subtypes can be found within the same nucleus, although their localization is restricted to different neuronal populations within this nucleus. The paraventricular nucleus of the hypothalamus contains mRNA for both vGlut1 and vGlut2, although the hybridization signal for vGlut2 is stronger than that of vGlut1 [89]. The localization of vGlut mRNA within parvocellular neurons of the paraventricular nucleus of the hypothalamus [37] where neurons containing CRF are also located [72] suggests that axon terminals in the DRN immunoreactive for CRF/vGlut2 and to a lesser extent CRF/vGlut1 may originate at least to some extent in this region. Both CRF [52,72] and vGlut2 [27] are present in neurons of the bed nucleus of the stria terminalis as well, suggestive of another brain region from which CRF/vGlut2 containing axon terminals within the DRN may arise. Future experiments consisting of anterograde tract tracing studies from these regions combined with immunohistochemistry for CRF and vGlut would need to be executed for confirmation of such circuitry.
Implications for DRN circuitry
Numerous studies have implicated both stress and CRF in the modulation of DRN activity. Electrophysiological data demonstrated that high doses of CRF have primarily excitatory effects on neuronal discharge [33]. CRF afferents to the DRN are engaged in responses to swim stress, a potent physiological stressor [58,63]. Following an initial exposure to swim stress, GABAergic neurons located in the DL DRN are activated, as evidenced by c-fos expression [63]. The activated GABA neurons also express the CRF type 1 receptor (CRF1) and activation of DL DRN GABA neurons was attenuated by a CRF1 antagonist [63]. These studies and others support an excitatory role for CRF on DRN neurons and are supported by the current finding that numerous CRF axon terminals in the DRN contain glutamate.
The potential exists for GABAergic interneurons located in the DRN to modulate serotonergic output to forebrain regions. Prior studies have revealed that GABA neurons synapse in the DRN [21,86], the DRN contains GABA receptors [8], DRN-5-HT neurons express GABA receptors [17,84,88] and in vitro extracellular electrophysiological studies showed that 5-HT neuronal activity is modulated by both GABAA and GABAB receptor subtypes [29]. Taken together these studies all support a role for GABA in the mediation of CRF’s excitatory effects on the DRN-5-HT system. Thus, the co-localization of glutamate within a subset of CRF axon terminals in the DRN provides us with an extended understanding of the anatomical circuitry in this nucleus that is engaged in complex responses to stress. These findings are in support of our working hypothesis which postulates that CRF axon terminals exert excitatory effects on GABAergic interneurons to subsequently inhibit DRN-5-HT neurons that project to forebrain regions involved in stress mediation.
Glutamate and CRF in affective disorders
Numerous reports indicate that CRF is involved in the pathophysiology of affective disorders [3,5,22,34,46]. For instance, elevated CRF levels in cerebrospinal fluid (CSF) have been reported in a number of depressed patients [48]. Studies of postmortem brain tissue from suicide victims also indicated that CSF levels of CRF were elevated related to controls [2] and CRF receptor binding sites were also reduced in the frontal cortex consistent with the elevated CRF levels [47]. Rodent studies examining the CRF neurotransmitter system using anatomical and behavioral studies have shown that CRF is involved in the regulation of the dorsal raphe nucleus [33,57,59,63,80,87] which contains the majority of forebrain-projecting serotonergic neurons [68,70,71], suggesting that CRF may be a key intermediary in stress-related affective disorders such as depression.
The glutamatergic system has also been implicated in affective disorders, specifically, depression [40,42]. Repeated antidepressant administration effectively increased vGlut1 mRNA and protein in the cortex and hippocampus, without consistently increasing the expression of other isoforms in the vesicular glutamate transporter family [78]. It is likely that the other vesicular glutamate transporter isoforms (vGlut2 and vGlut3) are differentially regulated by pharmacological substrates, just as their topographical distribution is different within the rat CNS [78]. The finding that cortical vGlut1 expression is modified by medications with antidepressant efficacy suggests that vGlut1 may offer some therapeutic target for depression treatment [44,78], although it appears that this mechanism involving increased vGlut1 expression may well be independent of the CRF system.
In summary, this study provides anatomical evidence that a number of CRF-containing axon terminals within the midbrain serotonergic DRN co-localize glutamate. Although a higher percentage of CRF axon terminals contained vGlut2, CRF axon terminals also contained vGlut1 but to a lesser extent. This suggests that these axon terminals have the potential to co-release CRF along with glutamate and thus may be poised to modulate excitatory regulation of post-synaptic targets within the DRN.
4. Experimental Procedures
Subjects
Adult male Sprague-Dawley rats weighing 250–300 g were housed two or three per cage on a 12-hour light schedule in a temperature controlled (20°C) colony room with ad libitum access to standard rat chow and water. The care and use of animals were approved by the Institutional Animal Care and Use Committee (IACUC) of Thomas Jefferson University and were conducted in accordance with the NIH Guide for the care and use of laboratory animals. Only the minimum number of animals necessary was used, and all efforts were made to minimize animal suffering.
Tissue Preparation
Rats were deeply anesthetized with sodium pentobarbital (60 mg/kg) and transcardially perfused through the ascending aorta with 50 ml of 3.8% acrolein (Electron Microscopy Sciences, Hatfield, PA) and 200 ml of 2% paraformaldehyde (Electron Microscopy Sciences) in 0.1 M phosphate buffer (PB; pH 7.4). Immediately following perfusion/fixation, brains were removed, and blocks containing the DRN were cut and placed in 2% paraformaldehyde for an additional 30 minutes, and then placed into 0.1M PB. Sections through the DRN were cut at a thickness of 40 μm with a Vibratome (Technical Products Incorporated, St. Louis, MO) and collected in chilled 0.1 M PB. Alternate sections from twelve rats were processed for immunofluorescence and immunoelectron microscopy.
Immunofluorescence labeling for CRF and vGlut1 or vGlut2
A detailed description for the processing of tissue sections containing the DRN for immunofluorescent visualization was given previously [87]. Briefly, sections were incubated overnight with rotary agitation in a primary antibody made in rabbit directed against corticotropin-releasing factor (CRF; Dr. W. Vale, Salk Institute; 1:2,000) and subsequently in a solution containing guinea pig anti-vesicular glutamate transporter 1 (vGlut1; 1:2,000; Chemicon, Temecula, CA) or guinea pig anti-vesicular glutamate transporter 2 (vGlut2; 1:2,000; Chemicon, Temecula, CA) in 0.1% BSA/0.25% Triton X-100 in 0.1 M TS. Tissue sections were rinsed extensively and incubated in a secondary antibody cocktail containing tetramethyl rhodamine isothiocyanate (TRITC) donkey anti-guinea pig (1:100; Jackson ImmunoResearch Laboratories, West Grove, PA) and fluorescein isothiocyanate (FITC) donkey anti-rabbit (1:100; Jackson ImmunoResearch Laboratories). Following incubation, sections were washed, mounted onto gelatinized slides, dried, dehydrated and coverslipped using DPX (Aldrich, Milwaukee, WI). Images of immunohistochemically labeled profiles in the DRN were captured using a Zeiss LSM 510 Meta (Carl Zeiss; Inc., Thornwood, NY) confocal microscope and imported using the LSM 5 image browser. Files were subsequently exported as BMP files and opened in Adobe Photoshop 7.0 where text and labels were added and images were minimally adjusted for brightness and contrast.
Electron microscopy processing for CRF and vGlut1 or vGlut2
The dual-immunolabeling protocol for electron microscopic analysis of DRN sections has also been previously described in detail [87]. Briefly, DRN tissue sections were incubated overnight in rabbit anti-CRF (1:8,000) antibody, rinsed, and incubated overnight in either guinea pig anti-vGlut1 (1:2,000) or guinea pig anti-vGlut2 (1:2,000) antibody. Sections were rinsed, incubated in biotinylated goat anti-guinea pig (Jackson ImmunoResearch Laboratories, West Grove, PA; 1:400) and then incubated in avidin-biotin complex (ABC; Vector Laboratories, Burlingame, CA). vGlut1 or vGlut2 was subsequently visualized by an empirically determined 5–6-minute reaction in 3,3′-diaminobenzidine tetrahydrochloride dihydrate (DAB; Sigma, St. Louis, MO) activated with 30% H2O2. For the gold-silver localization of CRF, sections were rinsed and incubated in goat anti-rabbit IgG conjugated to 1-nm gold particles (Amersham Biosciences, Piscataway, NJ). Sections were rinsed, exposed to 2% glutaraldehyde (Electron Microscopy Sciences), and a silver enhancement kit (Amersham) was used for silver intensification of the 1nm gold particles. The optimal silver enhancement times were determined empirically for each experiment and ranged from 13–15 minutes. After intensification, tissues were rinsed, incubated in 2% osmium tetroxide, rinsed again, dehydrated, and flat embedded in Epon 812 (Electron Microscopy Sciences; [36]). Thin sections approximately 70–80 nm in thickness were cut with a diamond knife (Diatome-U.S., Fort Washington, PA) using an LKB ultramicrotome and then collected on copper mesh grids (Electron Microscopy Sciences) and visualized using a Morgagni™ 268(D) transmission electron microscope (FEI Company, Hillsboro, OR).
Controls
Immunohistochemical processing of control sections for both immunofluorescence and dual immunoelectron microscopy studies was carried out using an identical protocol as above, however the primary antiserum was omitted. Analysis of the immunofluorescence and both immunoperoxidase and immunogold labeling at the light and ultrastructural level revealed that omission of the primary antibody abolished immunostaining.
The specificity of the antiserum against the rat/human CRF antibody raised in rabbit has been meticulously characterized in previous experiments [13,65,82]. Specifically, an immunodot blot assay revealed that this CRF antiserum showed a lack of cross reactivity [82] with either melanin-concentrating hormone (MCH) or a closely related peptide, α-melanocyte-stimulating hormone (α-MSH), to which antiserum directed against CRF has previously been reported to cross-react [45]. Likewise, omission of the primary CRF antiserum as well as preadsorption [82] of the primary antisera with CRF peptide resulted in tissue sections that exhibited no immunolabeling for CRF.
This vGlut1 and vGlut2 antisera have also been well described regarding the specificity of the immunolabel. Previous reports using similar experimental conditions report that the immunizing peptide abolishes immunostaining [10], and also established that this antibody resulted in a similar pattern of staining to that previously seen for vGlut1 and vGlut2 using other well described antisera [16,76,77].
Data Analysis
Both dorsolateral (DL) and ventromedial (VM) DRN subregions were selected for ultrastructural analysis and the approximate locations of each subregion are illustrated on the respective schematic illustration (Fig. 1E, F) obtained from the rat brain atlas [54]. The dorsolateral region was selected in coronal sections through the rat brain where both immunolabels were robust at a level 8.00 mm posterior to bregma, with the mid-point of the region sampled located at approximately 0.2 mm lateral to the midline and 6.00 mm ventral to the surface of the skull (Fig. 1E). The aqueduct served as a dorsal boundary. Likewise, the ventromedial region was sampled on the basis of the CRF distribution and presence of vGlut1 or vGlut2 in coronal sections through the rat brain at a level 7.64 mm caudal to bregma, with the midpoint of the region sampled located on the midline with a ventral coordinate of 6.6 mm (Fig. 1F). The medial longitudinal fasciculus served as a ventral border for this region.
A total of twelve male Sprague Dawley rats were used in this study. The photomicrographs depicted in Figure 1 (A–D) are representative of the immunolabeling pattern for both CRF and vGlut1 in the DL DRN, and the labeling pattern of vGlut2 was very similar to that of vGlut1. Semi-quantitative analysis of data from CRF and vGlut1-or vGlut2-labeled sections was performed only in areas of the tissue in which both labels were detectable. Superior preservation of the ultrastructural morphology was a key criterion when selecting tissue sections to be used for the analysis. Thirteen coronal tissue sections through the DRN from six rats were used for the ultrastructural analysis of CRF and vGlut1 interactions and nineteen sections from six rats were used for the examination of CRF and vGlut2 distributions in the DRN. All fields of the neuropil in which CRF immunopositive axons or axon terminals were present in conjunction with axons or axon terminals containing labeling for vGlut1 or vGlut2 were captured using an ORCA camera (Hamamatsu, Bridgewater, NJ) and AMT Image Capture Engine software (Advanced Microscopy Techniques Corp., Danvers, MA).
Examination of associations between CRF-and either vGlut1- or vGlut2-immunoreactive axon terminals were carried out on the most superficial portions of the tissue in direct contact with the aclar to minimize artificial differences resulting from reagent penetration [36]. Cellular elements were based on characterizations described by Peters et al. [55]. Axon terminals were distinguished from unmyelinated axons based on synaptic vesicle presence and a diameter greater than 0.1 μm. Synaptic terminals were characterized by a junctional complex and a restricted zone of parallel membrane apposition with slight enlargement of the intercellular space and/or associated postsynaptic thickening. When synapses were observed, they were categorized as follows [55]: asymmetric synapses (Gray’s Type I) were characterized by a presynaptic terminal interacting with a postsynaptic dendrite or axon with thick postsynaptic densities and a synaptic cleft of 20 nm wide between presynaptic and postsynaptic structures; symmetric synapses (Gray’s Type II) had thinner, less prominent post-synaptic densities with narrower synaptic clefts (approximately 12 nm) than that observed at asymmetric synapses. Appositions (non-synaptic contacts) were defined by closely spaced parallel plasma membranes of the axon of interest and the membranes of dendrites, other axon terminals, myelinated, and unmyelinated axons. A minimum of two-three gold particles within the axon terminal were required for inclusion in the semi-quantitative analysis. Text and labels were added to micrographs using Adobe Photoshop 7.0 and images were minimally adjusted for brightness and contrast.
Statistical Analysis
Regions of the neuropil containing immunolabeling for CRF and either vGlut1 or vGlut2 were photographed and the number of immunolabeled axon terminals was determined. Axon terminals included in the analysis were characterized as containing one of the following: only CRF, only vGlut1 or vGlut2, or both CRF and vGlut1 or vGlut2. The percentage of axon terminals containing each of the previously described immunolabels relative to the total number of immunolabeled axon terminals per subregion was calculated for each tissue set (CRF/vGlut1 and CRF/vGlut2) and the 95% confidence interval with no continuity correction expressed. Within tissues processed for CRF/vGlut1 or CRF/vGlut2, the DL and VM subregions were compared and the difference between the percentages along with the 95% confidence interval is reported, along with the corresponding p value (two-tailed) for the data set. The difference between subregions was determined to be significant if p < 0.05.
Acknowledgments
This work was supported by DA 15395 and MH 58250. M.W. was previously supported by the Foerderer Fellowship and is presently supported by the Mary Smith Fellowship, both from Thomas Jefferson University. The authors would like to gratefully acknowledge Mulan Li of Thomas Jefferson University for assistance with the immunohistochemistry and ultrathin sectioning and Dr. Rita J. Valentino for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aihara Y, Mashima H, Onda H, Hisano S, Kasuya H, Hori T, Yamada S, Tomura H, Yamada Y, Inoue I, Kojima I, Takeda J. Molecular cloning of a novel brain-type Na(+)-dependent inorganic phosphate cotransporter. J Neurochem. 2000;74:2622–5. doi: 10.1046/j.1471-4159.2000.0742622.x. [DOI] [PubMed] [Google Scholar]
- 2.Arato M, Banki CM, Bissette G, Nemeroff CB. Elevated CSF CRF in suicide victims. Biol Psychiatry. 1989;25:355–9. doi: 10.1016/0006-3223(89)90183-2. [DOI] [PubMed] [Google Scholar]
- 3.Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 4.Asberg M, Thoren P, Traskman L, Bertilsson L, Ringberger V. “Serotonin depression”--a biochemical subgroup within the affective disorders? Science. 1976;191:478–80. doi: 10.1126/science.1246632. [DOI] [PubMed] [Google Scholar]
- 5.Bale TL. Stress sensitivity and the development of affective disorders. Horm Behav. 2006 doi: 10.1016/j.yhbeh.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 6.Bellocchio EE, Hu H, Pohorille A, Chan J, Pickel VM, Edwards RH. The localization of the brain-specific inorganic phosphate transporter suggests a specific presynaptic role in glutamatergic transmission. J Neurosci. 1998;18:8648–59. doi: 10.1523/JNEUROSCI.18-21-08648.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellocchio EE, Reimer RJ, Fremeau RT, Jr, Edwards RH. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science. 2000;289:957–60. doi: 10.1126/science.289.5481.957. [DOI] [PubMed] [Google Scholar]
- 8.Bowery NG, Hudson AL, Price GW. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience. 1987;20:365–83. doi: 10.1016/0306-4522(87)90098-4. [DOI] [PubMed] [Google Scholar]
- 9.Chaouloff F. Physiopharmacological interactions between stress hormones and central serotonergic systems. Brain Res Brain Res Rev. 1993;18:1–32. doi: 10.1016/0165-0173(93)90005-k. [DOI] [PubMed] [Google Scholar]
- 10.Commons KG, Beck SG, Bey VW. Two populations of glutamatergic axons in the rat dorsal raphe nucleus defined by the vesicular glutamate transporters 1 and 2. Eur J Neurosci. 2005;21:1577–86. doi: 10.1111/j.1460-9568.2005.03991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Descarries L, Watkins KC, Garcia S, Beaudet A. The serotonin neurons in nucleus raphe dorsalis of adult rat: a light and electron microscope radioautographic study. J Comp Neurol. 1982;207:239–54. doi: 10.1002/cne.902070305. [DOI] [PubMed] [Google Scholar]
- 12.Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- 13.Foote SL, Cha CI. Distribution of corticotropin-releasing-factor-like immunoreactivity in brainstem of two monkey species (Saimiri sciureus and Macaca fascicularis): an immunohistochemical study. J Comp Neurol. 1988;276:239–64. doi: 10.1002/cne.902760208. [DOI] [PubMed] [Google Scholar]
- 14.Fremeau RT, Jr, Burman J, Qureshi T, Tran CH, Proctor J, Johnson J, Zhang H, Sulzer D, Copenhagen DR, Storm-Mathisen J, Reimer RJ, Chaudhry FA, Edwards RH. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc Natl Acad Sci U S A. 2002;99:14488–93. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fremeau RT, Jr, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–60. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- 16.Fujiyama F, Furuta T, Kaneko T. Immunocytochemical localization of candidates for vesicular glutamate transporters in the rat cerebral cortex. J Comp Neurol. 2001;435:379–87. doi: 10.1002/cne.1037. [DOI] [PubMed] [Google Scholar]
- 17.Gao B, Fritschy JM, Benke D, Mohler H. Neuron-specific expression of GABAA-receptor subtypes: differential association of the alpha 1- and alpha 3-subunits with serotonergic and GABAergic neurons. Neuroscience. 1993;54:881–92. doi: 10.1016/0306-4522(93)90582-z. [DOI] [PubMed] [Google Scholar]
- 18.Gold PW, Goodwin FK, Chrousos GP. Clinical and biochemical manifestations of depression. Relation to the neurobiology of stress (1) N Engl J Med. 1988;319:348–53. doi: 10.1056/NEJM198808113190606. [DOI] [PubMed] [Google Scholar]
- 19.Gold PW, Goodwin FK, Chrousos GP. Clinical and biochemical manifestations of depression. Relation to the neurobiology of stress (2) N Engl J Med. 1988;319:413–20. doi: 10.1056/NEJM198808183190706. [DOI] [PubMed] [Google Scholar]
- 20.Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, Gasnier B, Giros B, El Mestikawy S. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci. 2002;22:5442–51. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harandi M, Aguera M, Gamrani H, Didier M, Maitre M, Calas A, Belin MF. gamma-Aminobutyric acid and 5-hydroxytryptamine interrelationship in the rat nucleus raphe dorsalis: combination of radioautographic and immunocytochemical techniques at light and electron microscopy levels. Neuroscience. 1987;21:237–51. doi: 10.1016/0306-4522(87)90336-8. [DOI] [PubMed] [Google Scholar]
- 22.Heim C, Nemeroff CB. The impact of early adverse experiences on brain systems involved in the pathophysiology of anxiety and affective disorders. Biol Psychiatry. 1999;46:1509–22. doi: 10.1016/s0006-3223(99)00224-3. [DOI] [PubMed] [Google Scholar]
- 23.Herzog E, Bellenchi GC, Gras C, Bernard V, Ravassard P, Bedet C, Gasnier B, Giros B, El Mestikawy S. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J Neurosci. 2001;21:RC181. doi: 10.1523/JNEUROSCI.21-22-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herzog E, Gilchrist J, Gras C, Muzerelle A, Ravassard P, Giros B, Gaspar P, El Mestikawy S. Localization of VGLUT3, the vesicular glutamate transporter type 3, in the rat brain. Neuroscience. 2004;123:983–1002. doi: 10.1016/j.neuroscience.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 25.Hisano S, Hoshi K, Ikeda Y, Maruyama D, Kanemoto M, Ichijo H, Kojima I, Takeda J, Nogami H. Regional expression of a gene encoding a neuron-specific Na(+)-dependent inorganic phosphate cotransporter (DNPI) in the rat forebrain. Brain Res Mol Brain Res. 2000;83:34–43. doi: 10.1016/s0169-328x(00)00194-7. [DOI] [PubMed] [Google Scholar]
- 26.Holsboer F. The rationale for corticotropin-releasing hormone receptor (CRH-R) antagonists to treat depression and anxiety. J Psychiatr Res. 1999;33:181–214. doi: 10.1016/s0022-3956(98)90056-5. [DOI] [PubMed] [Google Scholar]
- 27.Hur EE, Zaborszky L. Vglut2 afferents to the medial prefrontal and primary somatosensory cortices: a combined retrograde tracing in situ hybridization. J Comp Neurol. 2005;483:351–73. doi: 10.1002/cne.20444. [DOI] [PubMed] [Google Scholar]
- 28.Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 29.Judge SJ, Ingram CD, Gartside SE. GABA receptor modulation of 5-HT neuronal firing: characterization and effect of moderate in vivo variations in glucocorticoid levels. Neurochem Int. 2004;45:1057–65. doi: 10.1016/j.neuint.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Kalen P, Karlson M, Wiklund L. Possible excitatory amino acid afferents to nucleus raphe dorsalis of the rat investigated with retrograde wheat germ agglutinin and D-[3H]aspartate tracing. Brain Res. 1985;360:285–97. doi: 10.1016/0006-8993(85)91244-2. [DOI] [PubMed] [Google Scholar]
- 31.Kaneko T, Fujiyama F. Complementary distribution of vesicular glutamate transporters in the central nervous system. Neurosci Res. 2002;42:243–50. doi: 10.1016/s0168-0102(02)00009-3. [DOI] [PubMed] [Google Scholar]
- 32.Kaneko T, Fujiyama F, Hioki H. Immunohistochemical localization of candidates for vesicular glutamate transporters in the rat brain. J Comp Neurol. 2002;444:39–62. doi: 10.1002/cne.10129. [DOI] [PubMed] [Google Scholar]
- 33.Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology. 2000;22:148–62. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- 34.Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848:141–52. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- 35.Lee HS, Kim MA, Valentino RJ, Waterhouse BD. Glutamatergic afferent projections to the dorsal raphe nucleus of the rat. Brain Res. 2003;963:57–71. doi: 10.1016/s0006-8993(02)03841-6. [DOI] [PubMed] [Google Scholar]
- 36.Leranth C, Pickel VM. Electron Microscopic Preembedding Double-Immunostaining Methods. In: Heimer L, Zaborszky L, editors. Neuroanatomical tract-tracing methods, 2: recent progress. Plenum; New York: 1989. pp. 129–172. [Google Scholar]
- 37.Lin W, McKinney K, Liu L, Lakhlani S, Jennes L. Distribution of vesicular glutamate transporter-2 messenger ribonucleic Acid and protein in the septum-hypothalamus of the rat. Endocrinology. 2003;144:662–70. doi: 10.1210/en.2002-220908. [DOI] [PubMed] [Google Scholar]
- 38.Lowry CA. Functional subsets of serotonergic neurones: implications for control of the hypothalamic-pituitary-adrenal axis. J Neuroendocrinol. 2002;14:911–23. doi: 10.1046/j.1365-2826.2002.00861.x. [DOI] [PubMed] [Google Scholar]
- 39.Merchenthaler I. Corticotropin releasing factor (CRF)-like immunoreactivity in the rat central nervous system. Extrahypothalamic distribution. Peptides. 1984;5(Suppl 1):53–69. doi: 10.1016/0196-9781(84)90265-1. [DOI] [PubMed] [Google Scholar]
- 40.Michael N, Erfurth A, Ohrmann P, Arolt V, Heindel W, Pfleiderer B. Neurotrophic effects of electroconvulsive therapy: a proton magnetic resonance study of the left amygdalar region in patients with treatment-resistant depression. Neuropsychopharmacology. 2003;28:720–5. doi: 10.1038/sj.npp.1300085. [DOI] [PubMed] [Google Scholar]
- 41.Milner TA, Reis DJ, Pickel VM, Aicher SA, Giuliano R. Ultrastructural localization and afferent sources of corticotropin-releasing factor in the rat rostral ventrolateral medulla: implications for central cardiovascular regulation. J Comp Neurol. 1993;333:151–67. doi: 10.1002/cne.903330203. [DOI] [PubMed] [Google Scholar]
- 42.Mirza Y, Tang J, Russell A, Banerjee SP, Bhandari R, Ivey J, Rose M, Moore GJ, Rosenberg DR. Reduced anterior cingulate cortex glutamatergic concentrations in childhood major depression. J Am Acad Child Adolesc Psychiatry. 2004;43:341–8. doi: 10.1097/00004583-200403000-00017. [DOI] [PubMed] [Google Scholar]
- 43.Molliver ME. Serotonergic neuronal systems: what their anatomic organization tells us about function. J Clin Psychopharmacol. 1987;7:3S–23S. [PubMed] [Google Scholar]
- 44.Moutsimilli L, Farley S, Dumas S, El Mestikawy S, Giros B, Tzavara ET. Selective cortical VGLUT1 increase as a marker for antidepressant activity. Neuropharmacology. 2005;49:890–900. doi: 10.1016/j.neuropharm.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 45.Nahon JL, Presse F, Bittencourt JC, Sawchenko PE, Vale W. The rat melanin-concentrating hormone messenger ribonucleic acid encodes multiple putative neuropeptides coexpressed in the dorsolateral hypothalamus. Endocrinology. 1989;125:2056–65. doi: 10.1210/endo-125-4-2056. [DOI] [PubMed] [Google Scholar]
- 46.Nemeroff CB. Psychopharmacology of affective disorders in the 21st century. Biol Psychiatry. 1998;44:517–25. doi: 10.1016/s0006-3223(98)00068-7. [DOI] [PubMed] [Google Scholar]
- 47.Nemeroff CB, Owens MJ, Bissette G, Andorn AC, Stanley M. Reduced corticotropin releasing factor binding sites in the frontal cortex of suicide victims. Arch Gen Psychiatry. 1988;45:577–9. doi: 10.1001/archpsyc.1988.01800300075009. [DOI] [PubMed] [Google Scholar]
- 48.Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–4. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- 49.Ni B, Rosteck PR, Jr, Nadi NS, Paul SM. Cloning and expression of a cDNA encoding a brain-specific Na(+)-dependent inorganic phosphate cotransporter. Proc Natl Acad Sci U S A. 1994;91:5607–11. doi: 10.1073/pnas.91.12.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ni B, Wu X, Yan GM, Wang J, Paul SM. Regional expression and cellular localization of the Na(+)-dependent inorganic phosphate cotransporter of rat brain. J Neurosci. 1995;15:5789–99. doi: 10.1523/JNEUROSCI.15-08-05789.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Hearn E, Molliver ME. Organization of raphe-cortical projections in rat: a quantitative retrograde study. Brain Res Bull. 1984;13:709–26. doi: 10.1016/0361-9230(84)90232-6. [DOI] [PubMed] [Google Scholar]
- 52.Olschowka JA, O’Donohue TL, Mueller GP, Jacobowitz DM. The distribution of corticotropin releasing factor-like immunoreactive neurons in rat brain. Peptides. 1982;3:995–1015. doi: 10.1016/0196-9781(82)90071-7. [DOI] [PubMed] [Google Scholar]
- 53.Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol Rev. 1991;43:425–73. [PubMed] [Google Scholar]
- 54.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. North Ryde; Academic Press: 1998. [Google Scholar]
- 55.Peters A, Palay SL, Webster Hd. The Fine Structure of the Nervous System: Neurons and Their Supporting Cells. 3. Oxford University Press; 1991. [Google Scholar]
- 56.Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–68. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- 57.Price ML, Curtis AL, Kirby LG, Valentino RJ, Lucki I. Effects of corticotropin-releasing factor on brain serotonergic activity. Neuropsychopharmacology. 1998;18:492–502. doi: 10.1016/S0893-133X(97)00197-8. [DOI] [PubMed] [Google Scholar]
- 58.Price ML, Kirby LG, Valentino RJ, Lucki I. Evidence for corticotropin-releasing factor regulation of serotonin in the lateral septum during acute swim stress: adaptation produced by repeated swimming. Psychopharmacology (Berl) 2002;162:406–14. doi: 10.1007/s00213-002-1114-2. [DOI] [PubMed] [Google Scholar]
- 59.Price ML, Lucki I. Regulation of serotonin release in the lateral septum and striatum by corticotropin-releasing factor. J Neurosci. 2001;21:2833–41. doi: 10.1523/JNEUROSCI.21-08-02833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raadsheer FC, Hoogendijk WJ, Stam FC, Tilders FJ, Swaab DF. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology. 1994;60:436–44. doi: 10.1159/000126778. [DOI] [PubMed] [Google Scholar]
- 61.Raadsheer FC, van Heerikhuize JJ, Lucassen PJ, Hoogendijk WJ, Tilders FJ, Swaab DF. Corticotropin-releasing hormone mRNA levels in the paraventricular nucleus of patients with Alzheimer’s disease and depression. Am J Psychiatry. 1995;152:1372–6. doi: 10.1176/ajp.152.9.1372. [DOI] [PubMed] [Google Scholar]
- 62.Reimer RJ, Fon EA, Edwards RH. Vesicular neurotransmitter transport and the presynaptic regulation of quantal size. Curr Opin Neurobiol. 1998;8:405–12. doi: 10.1016/s0959-4388(98)80068-8. [DOI] [PubMed] [Google Scholar]
- 63.Roche M, Commons KG, Peoples A, Valentino RJ. Circuitry underlying regulation of the serotonergic system by swim stress. J Neurosci. 2003;23:970–7. doi: 10.1523/JNEUROSCI.23-03-00970.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sakata-Haga H, Kanemoto M, Maruyama D, Hoshi K, Mogi K, Narita M, Okado N, Ikeda Y, Nogami H, Fukui Y, Kojima I, Takeda J, Hisano S. Differential localization and colocalization of two neuron-types of sodium-dependent inorganic phosphate cotransporters in rat forebrain. Brain Res. 2001;902:143–55. doi: 10.1016/s0006-8993(01)02290-9. [DOI] [PubMed] [Google Scholar]
- 65.Sawchenko PE, Swanson LW, Vale WW. Co-expression of corticotropin-releasing factor and vasopressin immunoreactivity in parvocellular neurosecretory neurons of the adrenalectomized rat. Proc Natl Acad Sci U S A. 1984;81:1883–7. doi: 10.1073/pnas.81.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schafer MK, Varoqui H, Defamie N, Weihe E, Erickson JD. Molecular cloning and functional identification of mouse vesicular glutamate transporter 3 and its expression in subsets of novel excitatory neurons. J Biol Chem. 2002;277:50734–48. doi: 10.1074/jbc.M206738200. [DOI] [PubMed] [Google Scholar]
- 67.Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–42. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- 68.Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- 69.Steinbusch HW, Nieuwenhuys R, Verhofstad AA, Van der Kooy D. The nucleus raphe dorsalis of the rat and its projection upon the caudatoputamen. A combined cytoarchitectonic, immunohistochemical and retrograde transport study. J Physiol (Paris) 1981;77:157–74. [PubMed] [Google Scholar]
- 70.Steinbusch HW, Verhofstad AA, Joosten HW. Localization of serotonin in the central nervous system by immunohistochemistry: description of a specific and sensitive technique and some applications. Neuroscience. 1978;3:811–9. doi: 10.1016/0306-4522(78)90033-7. [DOI] [PubMed] [Google Scholar]
- 71.Steinbusch HWM, Nieuwenhuys R. The Raphe Nuclei of the Rat Brainstem: A Cytoarchitectonic and Immunohistochemical Study. In: Emson PC, editor. Chemical Neuroanatomy. Raven Press; New York: 1983. pp. 131–207. [Google Scholar]
- 72.Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–86. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- 73.Takamori S. VGLUTs: ‘Exciting’ times for glutamatergic research? Neurosci Res. 2006;55:343–51. doi: 10.1016/j.neures.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 74.Takamori S, Malherbe P, Broger C, Jahn R. Molecular cloning and functional characterization of human vesicular glutamate transporter 3. EMBO Rep. 2002;3:798–803. doi: 10.1093/embo-reports/kvf159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. 2000;407:189–94. doi: 10.1038/35025070. [DOI] [PubMed] [Google Scholar]
- 76.Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of differentiation-associated brain-specific phosphate transporter as a second vesicular glutamate transporter (VGLUT2) J Neurosci. 2001;21:RC182. doi: 10.1523/JNEUROSCI.21-22-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Todd AJ, Hughes DI, Polgar E, Nagy GG, Mackie M, Ottersen OP, Maxwell DJ. The expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in neurochemically defined axonal populations in the rat spinal cord with emphasis on the dorsal horn. Eur J Neurosci. 2003;17:13–27. doi: 10.1046/j.1460-9568.2003.02406.x. [DOI] [PubMed] [Google Scholar]
- 78.Tordera RM, Pei Q, Sharp T. Evidence for increased expression of the vesicular glutamate transporter, VGLUT1, by a course of antidepressant treatment. J Neurochem. 2005 doi: 10.1111/j.1471-4159.2005.03192.x. [DOI] [PubMed] [Google Scholar]
- 79.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–7. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 80.Valentino RJ, Liouterman L, Van Bockstaele EJ. Evidence for regional heterogeneity in corticotropin-releasing factor interactions in the dorsal raphe nucleus. J Comp Neurol. 2001;435:450–63. doi: 10.1002/cne.1043. [DOI] [PubMed] [Google Scholar]
- 81.Valentino RJ, Rudoy C, Saunders A, Liu XB, Van Bockstaele EJ. Corticotropin-releasing factor is preferentially colocalized with excitatory rather than inhibitory amino acids in axon terminals in the peri-locus coeruleus region. Neuroscience. 2001;106:375–84. doi: 10.1016/s0306-4522(01)00279-2. [DOI] [PubMed] [Google Scholar]
- 82.Van Bockstaele EJ, Colago EE, Valentino RJ. Corticotropin-releasing factor-containing axon terminals synapse onto catecholamine dendrites and may presynaptically modulate other afferents in the rostral pole of the nucleus locus coeruleus in the rat brain. J Comp Neurol. 1996;364:523–534. doi: 10.1002/(SICI)1096-9861(19960115)364:3<523::AID-CNE10>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 83.van Praag H. Depression, suicide, and serotonin metabolism in the brain. In: RM P, JC B, editors. Neurobiology of Mood Disorders. Vol. 1. Williams and Wilkins; Baltimore: 1984. pp. 601–618. [Google Scholar]
- 84.Varga V, Sik A, Freund TF, Kocsis B. GABA(B) receptors in the median raphe nucleus: distribution and role in the serotonergic control of hippocampal activity. Neuroscience. 2002;109:119–32. doi: 10.1016/s0306-4522(01)00448-1. [DOI] [PubMed] [Google Scholar]
- 85.Varoqui H, Schafer MK, Zhu H, Weihe E, Erickson JD. Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J Neurosci. 2002;22:142–55. doi: 10.1523/JNEUROSCI.22-01-00142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang QP, Ochiai H, Nakai Y. GABAergic innervation of serotonergic neurons in the dorsal raphe nucleus of the rat studied by electron microscopy double immunostaining. Brain Res Bull. 1992;29:943–8. doi: 10.1016/0361-9230(92)90169-x. [DOI] [PubMed] [Google Scholar]
- 87.Waselus M, Valentino RJ, Van Bockstaele EJ. Ultrastructural evidence for a role of gamma-aminobutyric acid in mediating the effects of corticotropin-releasing factor on the rat dorsal raphe serotonin system. J Comp Neurol. 2005;482:155–65. doi: 10.1002/cne.20360. [DOI] [PubMed] [Google Scholar]
- 88.Wirtshafter D, Sheppard AC. Localization of GABA(B) receptors in midbrain monoamine containing neurons in the rat. Brain Res Bull. 2001;56:1–5. doi: 10.1016/s0361-9230(01)00487-7. [DOI] [PubMed] [Google Scholar]
- 89.Ziegler DR, Cullinan WE, Herman JP. Distribution of vesicular glutamate transporter mRNA in rat hypothalamus. J Comp Neurol. 2002;448:217–29. doi: 10.1002/cne.10257. [DOI] [PubMed] [Google Scholar]


