Abstract
This study examined the influence of experimenter position on hand use in gestural communication in a sample of 127 captive chimpanzees. Hand use in gestural communication was recorded while an experimenter was positioned either left, right, or directly in front of the subject. The type of gesture was also recorded as either a food beg or whole hand point. Both hand use and gesture were consistent across experimenter positions. Overall, a significant population-level right hand bias was found, particularly for food beg gestures. These results are consistent with previous findings in chimpanzees and suggest that lateralisation in gestural communication is left hemisphere dominant.
One of the more pronounced lateralised human behaviours is a left hemisphere dominance in the perception and processing of symbolic information, particularly among right-handed individuals (Rasmussen & Milner, 1977). Specifically, both speech perception and production have been reported to be primarily performed within distinct regions of the left hemisphere (Ojemann, 1991). Furthermore, clinical studies indicate that lesions to specific areas of the left hemisphere can result in certain forms of aphasia, whereas lesions in the homologous regions of the right hemisphere do not produce such deficits in linguistic processing (Damasio, 1991). Finally, there are neuroanatomical asymmetries in the left hemisphere, such as the planum temporale and pars triangularis, that are thought to underlie the observed specialisations in linguistic functions (Foundas et al., 1996; Geschwind & Levitsky, 1968; Habib et al., 1995).
The initial studies examining lateralisation in linguistic functions primary focused on the processing of auditory linguistic cues (i.e., speech) but recent studies have examined whether similar functional asymmetries are observed in the processing of non-speech linguistic systems, notably sign language (Grossi, Semenza, Corazza, & Volterra, 1996; Poizner, Klima, & Bellugi, 1987). For example, there are some reports of aphasia, as indicative of the production of meaningful signs, in right-handed but not left-handed signers (Corina et al., 1993; Poizner et al., 1987). More recently, functional imaging studies have found that the production of certain linguistic signs involves the left hemisphere (Corina, Vaid, & Bellugi, 1992). Taken together, these data suggest that the left hemisphere dominance in linguistic functioning is not modality-specific.
The relationship between sign language, speech, and hemispheric specialisation is not limited to independent investigations of linguistic processing within each modality. There have been a number of studies that have examined laterality in hand gestures in the context of speech and non-speech production (Dalby, Gibson, Grossi, & Schneider, 1980; Foundas et al., 1995; Kimura, 1973). For example, Kimura (1973) recorded hand use in gesture production by subjects when engaged and not engaged in concomitant speech acts. Kimura (1973) reported that subjects gesture with their right hand more while talking than with their left hand. Evidence of increased right hand movements in conjunction with speech acts has been suggested to be due to concurrent activation of adjacent cortical areas involved in speech and manual movements (Kimura, 1993).
Whether non-human primates exhibit lateralisation in communicative behaviours that are comparable to those observed in humans remains a topic of considerable debate, particularly in the context of evolutionary theories on the origin of language (Bradshaw & Rogers, 1993; Corballis, 1992; Hauser, 1996; Hopkins & Carriba, in press). There has been a resurgence of interest in the topic of functional laterality in non-human primates and there is evidence that at least some manifestations of laterality in communicative behaviours are evident in non-human primates and humans (see Hopkins & Carriba, in press). For example, both Old and New World monkeys have been reported to exhibit left-sided lateral asymmetries (i.e., right hemisphere) in the movements of the mouth in the production of communicative facial expressions, particularly for calls with negative affective valence (Hauser, 1993; Hook-Costigan & Rogers, 1998). There is also evidence for left hemisphere asymmetries in the perception of species-specific vocalisations by macaques (Hauser & Andersson, 1994; Petersen et al., 1978). In contrast to studies in monkeys, data on laterality in communicative behaviours in apes have focused on gestural communication and these data are suggestive of a left hemisphere asymmetry. This is most likely due to the fact that manual gestures are rarely reported in monkeys, whereas there appears to be more widespread evidence of manual gestural communication in great apes (see Leavens & Hopkins, 1999, for review). With specific reference to laterality and gestures, Shafer (1993) reported that 22 gorillas preferred to use their right hand for gestures while 10 preferred the left hand. More recently, and of specific interest to this study, Hopkins and Leavens (1998) reported a right hand population bias in gestural communication in a sample of 63 captive chimpanzees. In the report by Hopkins and Leavens (1998), a banana was placed outside the subjects' home cage and the experimenter recorded the hand use and gesture type. The position of the banana was randomly placed either on the left or right side of the subjects' home cage. A hand use by gesture interaction was found with a greater proportion of right hand use observed for food begs compared to pointing (also see Krause & Fouts, 1997; Leavens, Hopkins, & Bard, 1996).
The purpose of the current study was threefold. First, in the Hopkins and Leavens (1998) study, the position of the food item was not controlled for across all subjects and was not presented as a within-subject variable. In short, it was not clear whether hand use or gesture type was influenced by the position of the food item when considered as a between-group variable. Moreover, the biased testing environment may have inadvertently masked more robust or pronounced laterality effects under non-biased conditions. Thus, one purpose of this study was to examine the relationship between hand use and gesture type under biased and unbiased test conditions. If right hand use is associated with higher incidences of food begs and this association is stable, then it can be hypothesised that right hand use should be more pronounced for food begs compared to other gesture types in both biased and unbiased circumstances. The second purpose of this study was to examine consistency and strength in hand use as a function of the position of the food item. In our previous studies (Hopkins & Leavens, 1998), subjects were tested on only one trial and the goal of this project was to examine consistency in hand use across three trials that were presented in different positions relative to the subject. If hand use in the production of intentional, referential gestures is reliable, then significant correlations in hand use should be found across various biased and unbiased testing circumstances. The third aim of this study was to increase the sample size from the original paper by Hopkins and Leavens (1998). In the original paper by Hopkins and Leavens (1998) 115 chimpanzees were tested but only 63 produced a manual gesture within the paradigm employed in that study. To increase our sample size and thus the number of individuals that reliably gestured, in this study we tested for hand use by having the experimenter hold the food rather than having the food displaced from the experimenter. In addition, the period of observation was increased so that the subjects would have a longer period of time in which to produce a gesture.
METHOD
Subjects
There were 127 subjects ranging in age from 7 to 57 years (Mean = 18.33 s.d. = 10.21) all of whom were housed at the Yerkes Regional Primate Research Main Center (YRPRC). Chimpanzees under the age of 7 were excluded in this study because previous studies have indicated that the frequency of gesturing is relatively low in subjects below this age (Leavens & Hopkins, 1998). Of the 127 subjects, there were 70 females and 57 males, respectively. Of the 70 females, 36 were mother-reared and 34 were nursery-reared. Of the 57 males, 22 were mother-reared and 35 were nursery-reared. Mother-reared chimpanzees were those reared by their biological, conspecific mother for more than 30 days of life. Nursery-reared subjects were those that were brought to the YRPRC nursery before 31 days of life. The standard protocol for hand rearing chimpanzees have been described in detail elsewhere (Bard, 1996).
Procedure
The procedure for this study was relatively straightforward. Each subject received three test trials that corresponded to the position of the experimenter. All subjects were tested in their home cages. The experimenter was positioned either to the left or the right, or directly centred in front of the chimpanzees. For each position, the experimenter was approximately 1 m from the chimpanzee. During each trial, the experimenter approached the subjects' home cage with his hands behind his back and a banana hidden in the laboratory coat. The side from which the experimenter approached the subject's home cage was counterbalanced across subjects. Once in position, the experimenter revealed the banana and offered it to the chimpanzees. The hand used by the experimenter to offer the banana was consistent across positions but varied randomly between subjects. The banana was held at the experimenter's midline and approximately .5m above the ground. The chimpanzee could then gesture for the food and a 30-second time limit was placed on the response. As soon as a gesture was solicited from the subject, they were given the banana. If no gesture was solicited during the 30-second time limit, the chimpanzee was still given the banana. Each subject was tested on all three trials during one test session with the position of the experimenter counterbalanced across trials and subjects. Every effort was made to ensure that the subjects were in the same posture in each of the three positions. On each trial, the experimenter made note of the hand use and gesture type. Hand use was recorded as either left or right. During the course of the study, only one subject exhibited a bilateral gesture on a single trial and this subject was re-tested to obtain a full set of unilateral responses for all individuals. Gesture type was characterised as either a food beg or point, following the definitions outlined by Leavens and Hopkins (1998, 1999). Briefly, pointing was defined as single-digit or whole-hand extensions directed towards the experimenter positioned outside the cage. In contrast, food begs were defined as an extended hand towards the experimenter with the hand assuming a cupped position. These operational definitions are consistent with those used in previous reports in captive and wild chimpanzees (Leavens & Hopkins, 1998; Plooij, 1978).
RESULTS
Consistency in hand use and gesture type
In the initial analysis, the associations between position and both hand use and gesture type were assessed using a phi-coefficient. For this analysis, both hand use and gesture type were separately correlated across the three positions using a phi-coefficient. The summary statistics can be seen in Table 1. For both gesture type and hand use, significant positive associations were found for all comparisons, indicating that subjects exhibited consistency in hand use and gesture type across all three positions.
TABLE 1.
Intercorrelations (phi coefficients) in hand use and gesture type across experimenter positions
| Left | Gesture type Centre | Right | Left | Hand use Centre | Right | |
|---|---|---|---|---|---|---|
| Left | 1.00 | 1.00 | ||||
| Centre | .394* | 1.00 | .294* | 1.00 | ||
| Right | .447* | .485* | 1.00 | .250* | .430* | 1.00 |
indicates p < .01. N = 127 for each analysis.
Interaction between hand use and gesture type
In the next analysis, the association between gesture type and hand use was compared within each of the positions. This analysis involved three separate 2 × 2 chi-square tests of independence with one analysis corresponding to each of the three positions. For the centre χ2(1, N = 127) = 31.63, p < .001, left χ2(1, N = 127) = 4.52, p < .04, and right χ2(1, N = 127) = 5.83, p < .02 positions, all three associations were significant. Depicted in Table 2 are the frequencies of left and right hand use as a function of gesture type and position. As can be seen, for all three positions, the relative proportion of right hand use was significantly higher for food begs compared to pointing.
TABLE 2.
Frequency of left and right hand use as a function of experimenter position and gesture type
| Left | Food beg Right | %Right | Left | Pointing Right | %Right | |
|---|---|---|---|---|---|---|
| Left | 18 | 20 | 53 | 60 | 29 | 36 |
| Centre | 6 | 56 | 90 | 37 | 28 | 43 |
| Right | 2 | 49 | 96 | 14 | 62 | 82 |
Sex and rearing effects on cumulative hand use
In the next analysis, hand use was characterised across all three positions in order to assess the effects of sex and rearing history on overall handedness. Each subject was characterised as being either strongly left-handed (left hand use on all three trials), mildly left-handed (left hand use on two of three trials), mildly right-handed (right hand use on two of three trials), or strongly right-handed (right hand use on all three trials). These distributions were then compared as function of rearing history and sex. The number of individuals classified into each handedness group can be seen in Figure 1. No significant interactions were found for these analyses; however, the overall distribution did differ significantly from chance as revealed by a chi-square goodness-of-fi t test χ2(3, N = 127) = 24.34, p < .001.
Figure 1.
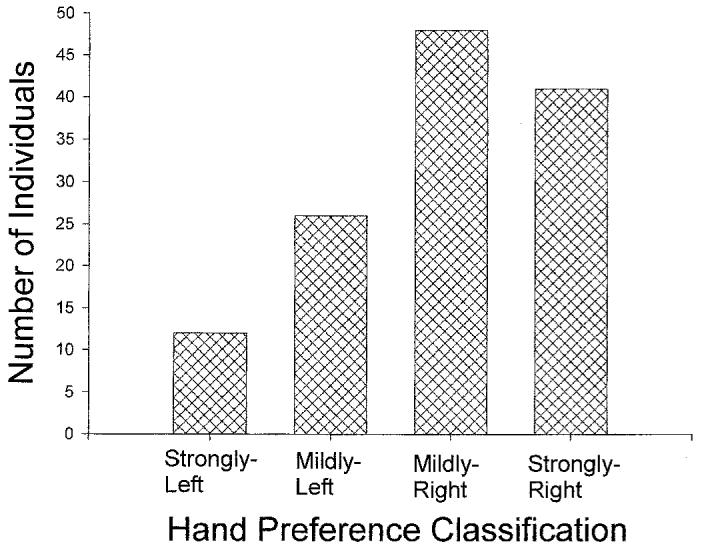
Number of individuals classified into each hand preference group based on the three trials presented to each subject.
In the previous analyses, hand use was considered on all three trials without regard to the type of gesture. The original analyses clearly demonstrated that gesture type does influence hand use. Therefore, in the next analysis, we compared the overall handedness scores as a function of the type of gesture produced in all three trials. Four groups of subjects were formed including (a) those that produced three food begs (n = 26, G1), (b) those that produced two food begs and one point (n = 20, G2), (c) those that produced two points and one food beg (n = 33, G3), and (d) those that produced three points (n = 48, G4). The four groups were compared on their overall handedness score (all right hand = 1.0, two right and 1 left = .33, two left and 1 right = −.33, and all left = −1.0) using a Kruskal-Wallis test. A significant main effect for group was found in this analysis χ2(3, N = 127) = 28.77, p < .001. The mean handedness score for each group can be seen in Figure 2. As a post-hoc analysis, a series of Mann-Whitney U-tests were performed comparing the handedness scores of each group to each other. Alpha was adjusted using Bonferoni's correction procedure to guard against Type I error due to the number of post-hoc tests performed (n = 7). The mean handedness score for group 1 (all food begs) differed significantly from group 3 (z = 3.34, p < .001) and group 4 (z = 4.84, p < .000). Group 2 differed significantly from group 4 (z = 2.88, p < .004). None of the remaining group comparisons was significantly different using the adjusted alpha level.
Figure 2.
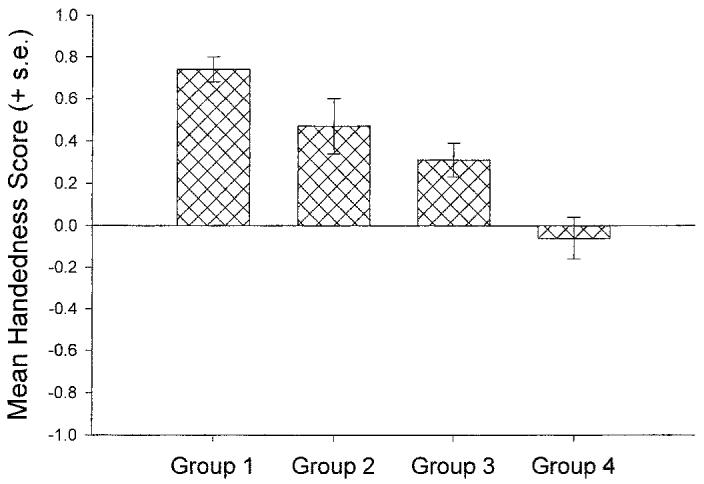
Mean handedness score for chimpanzees as a function of consistency in gesture type. Group 1 = use of food begs on all three trials. Group 2 = use of a food beg on two trials and a point on one trial. Group 3 = use of a food beg on one trials and a point on two trials. Group 4 = use of a point on all three trials. A mean handedness score of 1.0 reflects exclusive right hand use and a score of −1.0 reflects exclusive left hand use. Values between −1.0 and 1.0 reflect mixed hand use.
DISCUSSION
Three significant findings emerged in this study. First, chimpanzees exhibited a population-level right hand bias for food beg gestures but not for pointing. Second, the gesture-specific right hand bias in hand use was consistent across situational factors. Third, the hand used and type of gesture produced were significantly and positively correlated across situational factors.
As a whole, the results of this study support the previous findings by Hopkins and Leavens (1998) indicating preferential use of the right hand for food begs, and suggest that the previous findings were not due to the confounding of food position across subjects. Clearly, the position of the experimenter does influence hand use per se but the proportion of subjects using the right hand for food begs contrasted with points is relatively consistent across positions. The results of this study also extend the previous findings by Hopkins and Leavens (1998) because the sample size in this study (n = 127) was twice that of the previous report (n = 63).
The design of this study allowed for an assessment of consistency in both hand use and gesture type in this sample of chimpanzees. The results from these analyses revealed significant positive correlations across test conditions for both hand use and gesture type. Thus, subjects tend to use both the same hand and the same type of gesture across situational factors. That gesture type is consistent across situational factors may explain why the relative proportion of subjects who use the right hand for food begs is higher across positional factors. If we assume that right hand use is strongly associated with the production of food begs then it is not too surprising that a higher proportion of these responses were found in all three positions.
When considering overall hand use, there were significantly more right-compared to left-handed subjects in the population. There was no evidence that either sex or rearing history influenced the distribution of hand use. However, two observations are worth noting. First, the relative proportion of subjects exhibiting exclusive left or right hand use was 42% (53/127). This value is substantially higher than any other hand preference data we have reported in the YRPRC chimpanzees (see Hopkins, 1999; Hopkins & Pearson, 2000, for reviews) and suggests that gestures are a highly lateralised behaviour in the chimpanzee. Second, the total proportion of food begs was 40% (147/339), a value that is somewhat higher than the proportion reported by Leavens and Hopkins (1998). This finding may, in part, be due to differences in methodologies between the two studies. In this study, gestures were elicited by an experimenter holding the food outside the cage. In contrast, Leavens and Hopkins (1998) recorded gestures to food placed on the floor. It is possible that having a human hold food elicits more food begs because this is the social context in which this gesture is produced by wild chimpanzees (Goodall, 1986).
Finally, some have suggested that pointing towards foods outside their cages by chimpanzees reflects nothing more than attempted reaches to the food. To some extent these data have been refuted by the fact that chimpanzees, and other great apes, exhibit the “audience effect” (see Leavens & Hopkins, 1999 for review). The audience effect refers to the observation that apes do not gesture towards objects or foods without a social agent present to interpret their communicative act. The data from this study are relevant to the issue of whether gestures are frustrated reaching attempts, because previous studies in monkeys, apes, and humans have examined the effect of situational factors on hand preference (Carlson, 1985; Cronholm, Grodsky, & Behar, 1963; Fagard, 1998; Hopkins & Carriba, 2000; Welles, 1976). In these studies, hand preferences are recorded as a function of the position of the food items relative to the subject's midline. The most typical findings from these studies is that reaching is strongly influenced by situational factors such that monkeys and apes will show a bimodal distribution in hand use for foods positioned either to their left or right. In other words, if the food is positioned to the right, the non-human primates will strongly prefer their right hand and vice versa for foods positioned to their left. Hand use for midline presentations are typically bimodally distributed with equal numbers of left- and right-handed subjects. Based on these findings, if gestures were simply frustrated attempted reaches, then one would predict a high degree of left- or right-handedness based on the position of the experimenter (left or right) relative to the subject. Although this pattern was somewhat observed in this study, clear shifts to simple unimanual hand use for ipsilateral gestures were not found and are quite distinct from the data reported for simple reaching. Thus, it suggest that the mechanism that controls hand use for gestures is likely different from that for simple reaching.
In addition, the association between hand use for gestures and simple reaching is non-significant. For 116 chimpanzees in this study, simple reaching data were available and these data were correlated with the gesture hand preference data. Simple reaching data were collected by throwing peanuts or raisins to random locations in the subject's home cage and recording the hand use to pick up the food item. For each subject 25 reaching responses were recorded and each observation of reaching was kept independent from the others by throwing only a single food item into the cage at a time and requiring that subjects reposition themselves between responses. For each subject, a handedness index was calculated by subtracting the left hand responses from the number of right hand responses and dividing by the total number of responses (R−L)/(R+L). Handedness indices were calculated for both the simple reaching and gesture data collected in this study. A Spearman rank order correlation failed to reveal significant association between the handedness indices for simple reaching and overall hand use in gestural communication (rho = .067, N = 116, n.s.). We further correlated the handedness index value for simple reaching with the dichotomous handedness score for the left, centre, and right positions and none of these associations reached statistical significance (rho values =−.069, .175), and −.004, respectively). Finally, we analysed the association between hand use for gestures and simple reaching as a function of gesture type and position. Within each position, we selected subjects on the basis of whether they gestured with a food beg or point, and then correlated the dichotomous hand use score with their simple reaching score. For food begs, there was no significant association between hand use and the simple reaching handedness score when the experimenter was positioned either to the left, centre, or right of the subject (rho values = −.009, .219, and −.240, respectively). Similarly, for points, no significant associations were found between hand use for gestures and simple reaching when the experimenter was positioned either to the left, centre or right of the subject (rho values = −.105, .160, and .204, respectively). Taken together, these findings suggest that hand use for gestural communication occurs relatively independent of hand use for simple reaching. Moreover, it is unlikely that the gestures reflect frustrated attempts to reach for the food, although we recognise that we did not record gaze alternation and other behaviours that would possibly have indicated the motivation and intention of the chimpanzees' gestures (but see Leavens & Hopkins, 1998).
Finally, it is not clear why the proportion of right hand use in gestures is specific to food begs. There is no evidence that pointing is used differently from food begs in terms of their communicative function (see Leavens & Hopkins, 1999). Thus, the difference does not appear to be associated with differences in function. It is possible that differences in the morphology of the gestures produces this effect, but the morphological differences between the two gestures are not that distinct and we are not of the opinion that this is a likely explanation. A more plausible explanation for this effect may be in the neurobiological origin of the expression of this bias. The food beg is a gesture that wild chimpanzees (Plooij, 1978) produce whereas the pointing described in this paper is more of an acquired or learned behaviour specific to captive chimpanzees. Both of these gestures are used referentially and intentionally by captive chimpanzees but the expression of each gesture differs because of different mechanisms controlling their development. Clearly this issue needs further investigation but we would emphasise that the differences appear to be very robust, and have now been replicated and reported in at least four different studies (see Krause & Fouts, 1997; Leavens et al., 1996; Hopkins & Leavens, 1998; this study).
In conclusion, the results of this study indicate that chimpanzees prefer to use their right hand for gestural communication, particularly food beg gestures. Situational factors have some influence on overall hand preference but preferential use of the right hand for food begs were consistent, irrespective of the position of the experimenter. Neither sex nor rearing history influenced hand preference. Whether gestures represent a unique or distinctly lateralised behaviour compared to other motor functions will warrant further investigation. Moreover, whether lateralisation in gestural communication is associated with asymmetries of the great ape brain, notably those associated with language functions in humans, warrants further investigation (see Gannon, Holloway, Broadfield, & Braun, 1998; Hopkins, Marino, Rilling & MacGregor, 1998). Further studies in chimpanzees and other great apes should shed important light on the evolution of manual gestures in relation to theories on the origin of language and speech in humans.
Acknowledgments
The research was supported by NIH grants NS-29574, NS-36605 and RR-00165 to the Yerkes Regional Primate Research Center. The Yerkes Center is fully accredited by the American Association for Accreditation of Laboratory Animal Care. APA guidelines for the ethical treatment of animals were adhered to during all aspects of this study. Michael Wesley was also supported in part by funds provided by the Summer Undergraduat e Research Experience at Emory University.
REFERENCES
- Bard KA. Responsive care: Behavioral intervention for nursery-reared chimpanzees. Jane Goodall Institute; Ridgefield, CT: 1996. Available from the. 06877. [Google Scholar]
- Bradshaw JL, Rogers L. The evolution of lateral asymmetries, language, tool-use and intellect. Academic Press; San Diego: 1993. [Google Scholar]
- Carlson DF. Development of the infants' hand preference for visually directed reaching: Preliminary report of a longitudinal study. Infant Mental Health Journal. 1985;6:158–174. [Google Scholar]
- Corballis MC. The lopsided brain: Evolution of the generative mind. Oxford University Press; New York: 1992. [Google Scholar]
- Corina DP, Pizner H, Bellugi U, Feinberg T, Dowd D, O'Grady-Batch IL. Dissociation between linguistic and non-linguistic gestural system: A case of compositionality. Brain and Language. 1993;43:414–447. doi: 10.1016/0093-934x(92)90110-z. [DOI] [PubMed] [Google Scholar]
- Corina DP, Vaid J, Bellugi U. The linguistic basis for left hemisphere specialization. Science. 1992;255:1258–1260. doi: 10.1126/science.1546327. [DOI] [PubMed] [Google Scholar]
- Cronholm JM, Grodsky M, Behar I. Situational factors in the lateral preference in rhesus monkeys. Journal of Genetic Psychology. 1963;103:167–174. doi: 10.1080/00221325.1963.10532510. [DOI] [PubMed] [Google Scholar]
- Dalby JT, Gibson D, Grossi V, Schneider RD. Lateralized hand gesture during speech. Journal of Motor Behavior. 1980;12:292–297. doi: 10.1080/00222895.1980.10735228. [DOI] [PubMed] [Google Scholar]
- Damasio HC. Neuroanatomical correlates of aphasia. In: Sarno MT, editor. Acquired aphasia. 2nd Edn Academic Press; New York: 1991. pp. 45–70. [Google Scholar]
- Fagard J. Changes in grasping skills and the emergence of bimanual coordination during the first year of life. In: Connolly KJ, editor. The psychobiology of the hand. Cambridge University Press; London: 1998. pp. 123–143. [Google Scholar]
- Foundas AL, Leonard CM, Gilmore RL, Fennell EB, Heilman KM. Pars triangularis asymmetry and language dominance. Proceeding of the National Academy of Sciences. 1996;93:719–722. doi: 10.1073/pnas.93.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foundas AL, Macauley BL, Raymer AM, Maher LM, Heilman KM, Rothi LJ. Gesture laterality in aphasic and apraxic stroke patients. Brain and Cognition. 1995;29:204–213. doi: 10.1006/brcg.1995.1277. [DOI] [PubMed] [Google Scholar]
- Gannon PJ, Holloway RL, Broadfield DC, Braun AR. Asymmetry of chimpanzee planum temporale: Humanlike pattern of Wernicke's brain language area homologue. Science. 1998;279:220–222. doi: 10.1126/science.279.5348.220. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Levitsky W. Human brain: Left–right asymmetries in the temporal speech region. Science. 1968;151:186–187. doi: 10.1126/science.161.3837.186. [DOI] [PubMed] [Google Scholar]
- Goodall J. The chimpanzees of Gombe: Patterns in adaptation. Harvard University Press; Cambridge, MA: 1986. [Google Scholar]
- Grossi G, Semenza C, Corazza S, Volterra V. Hemispheric specialization for sign language. Neuropsychologia. 1996;34:737–740. doi: 10.1016/0028-3932(96)00008-5. [DOI] [PubMed] [Google Scholar]
- Habib M, Robichon F, Levrier O, Khalil R, Salamon G. Diverging asymmetries of the temporo-parietal cortical areas: A reappraisal of Geschwind/Galaburda theory. Brain and Language. 1995;48:238–258. doi: 10.1006/brln.1995.1011. [DOI] [PubMed] [Google Scholar]
- Hauser M. The evolution of communication. MIT Press; Cambridge, MA: 1996. [Google Scholar]
- Hauser MC. Right hemisphere dominance in the production of facial expression in monkeys. Science. 1993;261:475–477. doi: 10.1126/science.8332914. [DOI] [PubMed] [Google Scholar]
- Hauser MD, Andersson K. Left hemisphere dominance for processing vocalizations in adult, but not infant, rhesus monkeys: Field experiments. Proceeding of the National Academy of Sciences. 1994;91:3946–3948. doi: 10.1073/pnas.91.9.3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook-Costigan MA, Rogers LJ. Lateralised use of the mouth in production of vocalizations by marmosets. Neuropsychologia. 1998;36:1265–1273. doi: 10.1016/s0028-3932(98)00037-2. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. On the other hand: Statistical issues in the assessment and interpretation of hand preference data in nonhuman primates. International Journal of Primatology. 1999;20:851–866. [Google Scholar]
- Hopkins WD, Carriba SF. The effect of situational factors on hand preference in chimpanzees (Pan troglodytes) Neuropsychologia. 2000;38:403–409. doi: 10.1016/s0028-3932(99)00092-5. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Carriba SF. Laterality in communicative behaviors in nonhuman primates: A critical analysis. In: Rogers L, Andrews R, editors. Comparative vertebrate lateralization. Oxford University Press; Oxford: (in press) [Google Scholar]
- Hopkins WD, Leavens DA. Hand use and gestural communication in chimpanzees (Pan troglodytes) Journal of Comparative Psychology. 1998;112:95–99. doi: 10.1037/0735-7036.112.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Marino L, Rilling J, MacGregor L. Planum temporale asymmetries in great apes as revealed by magnetic resonance imaging (MRI) NeuroReport. 1998;9:2913–2918. doi: 10.1097/00001756-199808240-00043. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Pearson K. Chimpanzee (Pan troglodytes) handedness : Variability across multiple measures of hand use. Journal of Comparative Psychology. 2000;114:126–135. doi: 10.1037/0735-7036.114.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura D. Manual activity during speaking: I. Right-handers. Neuropsychologia. 1973;11:45–50. doi: 10.1016/0028-3932(73)90063-8. [DOI] [PubMed] [Google Scholar]
- Kimura D. Neuromotor mechanisms in human communication. Oxford University Press; Oxford: 1993. [Google Scholar]
- Krause MA, Fouts RS. Chimpanzee (Pan troglodytes) pointing: Hand shapes, accuracy, and the role of eye gaze. Journal of Comparative Psychology. 1997;111:330–336. doi: 10.1037/0735-7036.111.4.330. [DOI] [PubMed] [Google Scholar]
- Leavens DA, Hopkins WD. Intentional communication by chimpanzees (Pan troglodytes): A cross-sectional study of the use of referential gestures. Developmental Psychology. 1998;34:813–822. doi: 10.1037//0012-1649.34.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavens DA, Hopkins WD. The whole-hand point: The structure and function of pointing from a comparative perspective. Journal of Comparative Psychology. 1999;113:417–425. doi: 10.1037/0735-7036.113.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavens DA, Hopkins WD, Bard KA. Indexical and referential pointing in chimpanzees (Pan troglodytes) Journal of Comparative Psychology. 1996;110:346–353. doi: 10.1037/0735-7036.110.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojemann GA. Cortical organization for language. Journal of Neurosurgery. 1991;71:316–326. doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- Petersen MR, Beecher MD, Zoloth SR, Moody DB, Stebbins WC. Neural lateralization of species-specific vocalizations in Japanese macaques (Macaca fuscata) Science. 1978;202:324–327. doi: 10.1126/science.99817. [DOI] [PubMed] [Google Scholar]
- Plooij F. Some basic traits of language in wild chimpanzees. In: Lock A, editor. Action, gesture and symbol: The emergence of language. Academic Press; London: 1978. pp. 111–132. [Google Scholar]
- Poizner H, Klima ES, Bellugi U. What the hands reveal about the brain. Cambridge University Press; Cambridge, MA: 1987. [Google Scholar]
- Rasmussen T, Milner B. The role of early left-brain injury in determining lateralization of cerebral speech function. Annals of the New York Academy of Sciences. 1977;299:355–369. doi: 10.1111/j.1749-6632.1977.tb41921.x. [DOI] [PubMed] [Google Scholar]
- Shafer DD. Patterns of hand preference in gorillas and children. In: Ward JP, Hopkins WD, editors. Primate laterality: Current behavioral evidence of primate asymmetries. Springer-Verlag; New York: 1993. pp. 267–283. [Google Scholar]
- Welles JF. A comparative study of prehension in anthropoids. Saugetierkund. Mitteil. 1976;24:26–37. [Google Scholar]


