Abstract
OBJECTIVES
To determine whether cross-talk occurs between ER and NF-κB, to assess the functional consequences of such an ER/NF-κB interaction, and to identify other unknown regulatory proteins that may participate in the NF-κB transcriptional complex.
STUDY DESIGN
Electromobility gel shifts, reporter gene assays, and mass spectrometry were used to identify proteins interacting with the NF-κB DNA response element.
RESULTS
ER and the p65 subunit of NF-κB co-localized on DNA. This interaction was inhibitory for ER transcriptional activity. Sequencing of proteins bound to the NF-κB/DNA complex identified DNA modifying enzymes, scaffolding proteins, chaperones, and elements of the nuclear matrix.
CONCLUSION
These studies have identified an inhibitory interaction between estrogen receptors and the p65 subunit of NF-κB with implications for estrogen action in pregnancy and in cancer. New accessory proteins have also been identified that bind to protein complexes on the NF-κB DNA response element.
Keywords: placenta, trophoblast, HTR8/SVneo cells, Ishikawa cells, endometrial cancer, preterm labor, infection, inflammation, apoptosis
Introduction
Nuclear Factor-kappaB (NF-κB) is the principal transcription factor that regulates immune and inflammatory responses in tissues as diverse as the brain 1 and the reproductive tract. Central to the study of NF-κB in reproduction is its role in inducing pro-inflammatory cytokines in parturition, particularly when associated with intra-amniotic infection 2, and in cancers of reproductive tissues such as the endometrium. In such tumors, a pro-inflammatory environment is associated with tumorigenesis, inhibition of apoptosis, progression, and metastasis 3. In most untransformed cells, NF-κB complexes are largely cytoplasmic and remain transcriptionally inactive until the cell is stimulated 4. Once this occurs, the inhibitory protein bound to NF-κB, IκB, becomes phosphorylated on two serine residues and is subsequently proteolyzed by the 26S proteosome. NF-κB is then liberated and accumulates in the nucleus where it activates the expression of specific genes involved in immunity, inflammation, and proliferation5–8. NF-κB binds to its DNA response element as a dimer between the p65 (Rel A) subunit and the p50 or p52 subunit. An alternative conformation is a dimer between two p50 subunits.
In addition to NF-κB itself, additional proteins are recruited to the DNA. These form a protein complex including factors that recognize and bind to NF-κB itself. Such proteins enhance NF-κB binding to DNA and allow signals to be amplified and passed to the basic transcriptional unit, otherwise known as the preinitiation complex (PIC) 9. Other proteins within the complex activate RNA polymerases to initiate transcription10. Identification of the proteins that participate in this complex is an area of intense study.
Interestingly, proteins that interact with NF-κB include steroid hormone receptors such as glucocorticoid receptors (GR) 11–15. GR and NF-κB communicate directly by protein/protein interactions in a mutually inhibitory way, and this underlies many of the anti-inflammatory functions of glucocorticoids through GR. Similarly, we have previously shown that progesterone receptors (PR) inhibit NF-κB binding to its DNA consensus promoter sequence and induce the expression of NF-κB inhibitors A20 and ABIN-2 16. In turn, we have shown that progesterone through PR has anti-inflammatory effects that are likely linked to NF-κB inhibition. Thus, there is substantial evidence that cross-talk occurs between NF-κB and steroid hormone receptors with significant implications for disease states including infection and cancer, where NF-κB is highly active.
The purpose of these studies was to determine whether a similar interaction occurs between NF-κB and the estrogen receptor (ER). We first determined whether NF-κB co-localizes with ER on DNA. We also explored the consequences of such an interaction with respect to estrogen-mediated gene transcription using reporter gene assays. Finally, we employed mass spectrometry to identify other accessory proteins which interact with NF-κB and/or its DNA response element. We hypothesized that identifying these factors would provide new insight into how NF-κB-induced gene expression proceeds and what accessory proteins are needed to achieve transcriptional competency.
Materials and Methods
Cell culture and treatment
HTR-8/SVneo (HTR8) cells, immortalized cytotrophoblasts derived from first trimester placental tissue provided by Dr. Charles Graham, Toronto Canada, and Ishikawa endometrial cancer cells originally provided by Dr. Erlio Gurpide, New York, NY, were grown to 90% confluence in DMEM with 10% fetal bovine serum. In some experiments, interleukin 1-α, (IL-1) 0.2 ng/ml, was added to liberate NF-κB from its inactive complex with IκB in the cytoplasm and drive it into the nucleus. The treated cells were allowed to incubate 30 min at 37° C before harvesting.
Nuclear protein isolation
Harvested cells were washed, centrifuged at 1000 rpm for 5 min, and mixed with a low-salt buffer (10 mM HEPES, pH 7.9, 10 mM KCl, 0.1mM EDTA, 0.1mM EGTA, DTT and protease inhibitors added just before use). The mixture was incubated on ice 15 min allowing cells to swell, then spun down 3 min at 3000 rpm to dissolve the cytosolic proteins, which were then discarded. Five mg of nuclear protein extract was prepared by adding a high-salt buffer (20 mM HEPES, 400 mM NaCl, 1 mM EDTA, 1 mM EGTA, DTT and protease inhibitors) to the remaining precipitate which was stored at −80 ° C until use. To minimize non-specific binding in future operations, salmon sperm DNA (50 ng/μl) was added.
NF-kB consensus oligonucleotide
The sequence of the NF-κB oligonucleotide17 is shown below:
5′-AGTTGAGGGGACTTCCCAGGC-3′
3′-TCAACTCCCCTGAAGGGTCCG-5′
For electromobility shift assays (EMSAs), the double stranded oligonucleotide was used unmodified as shown above. To assess the peptide components bound to NF-κB on the response element oligonucleotide, sense and antisense strands of DNA were prepared. The sense strand had 4 additional cytosines attached to the 5’ end, and this strand was then biotinylated. An excess of this recombined biotinylated probe was added to the nuclear extract, and the mixture was rocked overnight at 10° C to bind the oligonucleotide to NF-κB and its transcriptional protein complex.
ERE consensus oligonucleotide
The estrogen response element (ERE) from the Xenopus vitellogenin A2 gene (bases -308 through -342 of the promoter) was also used in these studies:
5′-GTCCAAAGTCAGGTCACAGTGACCTGATCAAAGTT-3′
3′-CAGGTTTCAGTCCAGTGTCACTGGACTAGTTTCAA-5′
Electromobility gel shift assays (EMSAs)
EMSAs were performed as described below; all reactions were carried out at 4° C. Cells were grown to near confluence in dishes or flasks as described and treated with (or without) 0.2ng IL-1/ml to induce cytoplasmic to nuclear shuttling of active NF-κB. After a 15 min incubation, cells were washed with PBS, spun at 1000 rpm for 5 min, and the supernatant was aspirated to leave the cell pellet. Nuclear extract was then prepared as previously described 18. Briefly, the cell pellet was incubated in hypotonic buffer containing 10mM HEPES pH 7.9, 10mM KCl, 0.1mM each of EDTA and EGTA to which a protease inhibitor cocktail (Boehringer Mannheim, Indianapolis, IN) was added, followed by lysis with 0.625% Triton X-100. After centrifugation, the cytoplasmic components were removed. The pellet containing the nuclear extract was then suspended in a high-salt buffer containing 20mM HEPES and 400mM NaCl plus EDTA, EGTA and protease inhibitors. This suspension was rocked for 15 min and spun at 10,000 rpm for 5 min. The supernatant containing the nuclear extract was collected and stored at −80° C. Protein concentrations for each extract were determined, and equal amounts assayed for binding proteins on a 5% acrylamide gel. The probe was prepared by reacting the NF-κB or the ERE consensus oligo with T4 kinase and 32P-γ-ATP. One μl of probe containing approximately 200,000 cpm was incubated with 5 μg of cell extract derived from HTR8 or Ishikawa cells in binding buffer [10mM Tris pH 7.5, 50mM NaCl, 0.5mM DTT, 1mM MgCl2 0.5mM EDTA, 0.05mg/ml poly (di-dC) 4% (v/v) glycerol]. Supershifting with specific antibodies to the subunits p50 and p65 (Santa Cruz Biotechnologies, Oakland, CA) and with ER (DAKO, Chicago, IL) was also performed. The positive control cell line, RAW 264.7, was derived from activated macrophages. All assays were performed in duplicate.
Chloramphenicol acetyl transferase reporter gene assay
The vit-tk-CAT reporter vector was constructed by placing the Xenopus vitellogenin A2 (vit) upstream sequence (-331 to −87) and the herpes simplex virus thymidine kinase (tk) promoter (−150 to +51) upstream from the bacterial gene for chloramphenicol acetyltransferase (CAT) 19. CAT assays were performed as previously described 18. These studies were performed using Ishikawa endometrial cancer cells because they express modest amounts of endogenous ER and are responsive to estrogen at the level of transcription. Briefly, subconfluent cells in 90 mm culture plates were cotransfected in triplicate by lipofection with 1μg of the estrogen-responsive reporter vit-tk-CAT and 3 μg of the β-galactosidase vector pCH110 (Clontech, Mountain View, CA). The total amount of DNA transfected was normalized to 20 μg per plate. The cells were cultured for 48 h in vehicle alone, 0.2ng/ml IL-1, 10−8 M estradiol, or both IL-1 and estradiol. Cell extracts were prepared by four cycles of freeze-thawing followed by centrifugation at 10,000g for 15 min. The efficiency of transcription was determined by assaying each culture plate for β-galactosidase activity with spectrophotometric analysis. The CAT analysis was performed with cell extract amounts equal to 50 units of β-galactosidase activity for 1 h at 37° C. The acetylated and nonacetylated forms of carbon 14-chloramphenicol were separated by thin-layer chromatography and quantitated from an autoradiograph of the thin-layer chromatography plate by densitometry. Substrate conversion was further quantitated by assessing the radioactivity present on the thin-layer chromatography plate in a scintillation counter. The results are reported as the fold induction of CAT compared to control. Statistical analysis was performed using the Student t test with p<0.05 as significant.
Streptavidin-biotin affinity chromatography
Streptavidin-agarose beads (Sigma) were washed 3 times with buffer (20 mM HEPES pH-7.0, 2 mM KCl, 0.2mM MgCl2, 0.1 mM DTT, 4% glycerol) and hybridized to the oligonucleotide/extract mixture by rocking overnight at 10°C. Unbound proteins and DNA were removed by washing three times with the same buffer.
Elution and purification
Bound proteins of lower molecular weight were removed from the beads by mixing the slurry with buffer containing 150 mM NaCl, followed by rocking 10 min. The slurry was spun at 3000 rpm, and the eluted proteins were saved for PAGE. Higher molecular weight proteins were eluted by adding 1.0 M NaCl buffer to the slurry, rocking, spinning down and drawing off supernatant as before. Both eluants were desalted by passing them through G25 columns and centrifuging at 1000 rpm for 3 min.
Electrophoresis
A polyacrylamide gel was prepared in the usual manner, except that 0.05% SDS was added according to the manufacturer’s instructions for staining. Eluants were loaded into two separate lanes, and a third lane loaded with a high molecular weight ladder size marker (GibCo). Gels were soaked in SYPRO RED stain in 7.5% acetic acid. Protein bands were visualized by UV, cut out, placed in separate tubes, and stored for peptide sequencing. Six individual bands were visualized and recovered from the gel, termed gel fragments (GF)1-6 (see Table I).
Table I. Identification of proteins interacting with the NF-κB/DNA binding complex.
The proteins are grouped by the gel fragment (GF) from which they were eluted. Peptides from six GF were eluted and identified. The number of matched peptides within each fragment is indicated in the second column. The third column indicates the expect number, which is the probability that the identified peptide is not the protein assigned. The lower the expect number, the higher the probability that the correct protein has been identified. The gi# refers to the assigned sequence in the database. Assigned protein names are indicated in the last column.
| sample | matched peptides # | expect | kDa | gi # | assigned protein |
|---|---|---|---|---|---|
| GF1 | >2 | 1.2×10−44 | 113 | 22902366 | ADP-ribosyltransferase (NAD+; poly (ADP-ribose) polymerase) |
| 2 | 3.3×10−4 | 109.3 | 4759280 | U5 snRNP-specific protein, 116 kD | |
| 1 | 4.2×10−3 | 58.3 | 22028256 | Similar to heterogeneous nuclear ribonucleoprotein U (scaffold attachment factor A) | |
| >2 | 1.4×10−2 | 135.5 | 19863446 | Splicing factor 3B subunit 3 (Spliceosome associated protein 130) (SAP 130) (SF3b130) (Pre- mRNA splicing factor SF3b 130 kDa subunit) | |
| 1 | 1.5×10−2 | 142 | 1082769 | RNA helicase A | |
| GF2 | >2 | 2.0×10−17 | 103 | 4501891 | actinin, alpha 1 |
| >2 | 2.7×10−16 | 74.3 | 21750187 | unnamed protein product | |
| >2 | 5.6×10−12 | 113 | 22902366 | ADP-ribosyltransferase (NAD+; poly (ADP-ribose) polymerase) | |
| >2 | 1.1×10−11 | 76.1 | 4826998 | splicing factor proline/glutamine rich (polypyrimidine tract binding protein associated) PTB- associated splicing factor | |
| 1 | 3.4×10−6 | 104.8 | 12025678 | actinin, alpha 4 | |
| 2 | 3.3×10−4 | 66.6 | 23200355 | Human DNA Topoisomerase I (70 Kda) In Non-Covalent Complex With A 22 Base Pair DNA Duplex | |
| 1 | 1.1×10−3 | 95.1 | 20521049 | KIAA0432 | |
| GF3 | >2 | 4.3×10−14 | 69.8 | 4503841 | thyroid autoantigen 70kDa (Ku antigen); thyroid autoantigen 70kD (Ku antigen) |
| >2 | 3.7×10−9 | 82.6 | 10863945 | ATP-dependant DNA helicase II; X-ray repair complementing defective repair in Chinese hamster cells 5 (double-strand- break rejoining; Ku autoantigen, 80kD); X-ray repair, complementing defective, repair in Chinese hamster; DNA repair protei | |
| 1 | 6.9×10−8 | 113 | 130781 | Poly [ADP-ribose] polymerase-1 (PARP-1) (ADPRT) (NAD(+) ADP- ribosyltransferase-1) (Poly[ADP- ribose] synthetase-1) | |
| 2 | 4.6×10−7 | 76.6 | 13644124 | similar to Nucleolin (Protein C23) | |
| 1 | 1.7×10−6 | 63.2 | 3287489 | Hsp89-alpha-delta-N | |
| 1 | 1.8×10−4 | 72.8 | 9082289 | chaperone protein HSP90 beta | |
| 2 | 6.6×10−4 | 72.3 | 5453840 | DEAD/H (Asp-Glu-Ala-Asp/His) box polypeptide 17 isoform 1; DEAD/H (Asp-Glu-Ala-Asp/His) box polypeptide 17 (72kD); probable RNA-dependent helicase p72 | |
| 1 | 7.4×10−4 | 81 | 4507241 | structure specific recognition protein 1 | |
| 1 | 1.7×10−2 | 81.9 | 22531287 | interferon-inducible IFI 16 | |
| 2 | 0.75 | 68.9 | 13528666 | Similar to splicing factor proline/glutamine rich (polypyrimidine tract-binding protein-associated) | |
| GF4 | 1 | 1.4×10−6 | 64.2 | 178152 | poly(ADP-ribose) polymerase |
| 1 | 4.0×10−3 | 50.7 | 3098601 | Ras-GAP SH3 binding protein | |
| 1 | 0.73 | 38.2 | 17939440 | Unknown (protein for IMAGE:3503708) | |
| 2 | 1.7×10−3 | 53.2 | 7656991 | coronin, actin binding protein, 1C; coronin, actin-binding protein, 1C | |
| GF5 | 1 | 1.3×10−2 | 43.8 | 5453842 | proliferation-associated 2G4, 38kDa; proliferation-associated 2G4, 38kD |
| 2 | 1.5×10−2 | 41.4 | 4758158 | neural precursor cell expressed, developmentally down-regulated 5 | |
| 1 | 0.08 | 40.8 | 21755396 | unnamed protein product | |
| 1(?) | 0.36 | 42.7 | 18606060 | Unknown (protein for IMAGE:3538792) | |
| >2 | 5.5×10−12 | 41.7 | 4501885 | beta actin; beta cytoskeletal actin | |
| GF6 | 2 | 2.6×10−2 | 30.9 | 825671 | B23 nucleophosmin (280 AA) replication factor C (activator 1) |
| 1 | 0.73 | 39.6 | 4506491 | 4, 37kDa; replication factor C (activator 1) 4 (37kD) | |
| 1 | 1.2×10−3 | 34.2 | 12654583 | ribosomal protein, large, P0 |
Protein in-gel digestion and nano-HPLC/MS/MS analysis
These studies were undertaken at the FDA, Washington, D.C. Protein in-gel digestion with trypsin was performed as previously described 20. HPLC/MS/MS analyses were performed in an LCQ DECA XP Plus ion trap mass spectrometer (ThermoFinnigan, San Jose, CA) coupled on-line to a nano-HPLC system (1100 Nano Pump, Agilent Technologies, San Jose, CA) and nanospray source. Two μl of the peptide solution in buffer A [5% acetronitrile/94.9% water/0.1% acetic acid (v/v/v)] was manually injected and separated in a nano-HPLC column (50 mm length x 75 μm inner diameter, 5-μm particle size, 100- pore diameter) packed in-house with Luna C18 resin (Phenornenex, St. Torrance, CA). The peptides were eluted from the column with a linear gradient of 25–80% buffer B [90% acetonitrile/9.9% water/0.1% acetic acid (v/v/v)] in buffer A over 30 min. The eluted peptides were electrosprayed directly into the LCQ mass spectrometer. The MS/MS spectra were acquired in a data-dependent mode. The four strongest ions in each MS spectrum were automatically selected for fragmentation. The resulting spectra were used to identify protein candidates in the NCBI nonredundant protein sequence database with the MASCOT search engine (Matrix Science Ltd., London, U.K.).
Results
Electromobility gel shift assays (EMSAs) were performed to determine the specific NF-κB subunits bound to the consensus DNA response element in HTR8 first trimester trophoblast cells and in Ishikawa endometrial cancer cells (Figure 1). Bands were compared to those obtained using LPS treated RAW264.7 macrophages, a positive control (lane 1). In both HTR8 (lane 3) and Ishikawa (lane 7) cells, the p65/p50 heterodimer and the p50/p50 homodimer bands were identified. Super-shifting experiments with antibodies specific to p65 and p50 confirmed the identities of these bands (data not shown). A middle band (identified as the “middle complex” on Figure 1), between the p50/p65 heterodimer and the p50/p50 homodimer bands was present and was more prominent under baseline conditions in Ishikawa cells (lane 7) compared to HTR8 cells (lane 3). With IL-1 stimulation, this center complex was enhanced in HTR8 cells (lane 4), and the p50/p50 homodimer band disappeared (lane 4), indicating that cytokine activation in HTR8 cells resulted in enhanced recruitment of proteins within the middle complex to the NF-κB DNA response element, and the p50 was driven into a more active heterodimeric complex with p65 (lane 5).
Figure 1. NF-κB isoforms in HTR8 cytotrophoblasts and Ishikawa endometrial cancer cells.
Three complexes are identified in HTR8 and Ishikawa H cells when protein extracts are hybridized with the radio-labeled NF-κB DNA response element: the p50/RelA(p65) band, the middle complex, and the p50/p50 band. IL-1 was used as an inducer of NF-κB nuclear activity. Excess cold (unlabeled) NF-κB DNA response element oligonucleotide was used to compete specific complexes, and cold estrogen response element was used to compete less specific bands. The unbound probe is seen at the base of the gel.
Ishikawa cells appear to exist in a constitutively activated pro-inflammatory state, consistent with their malignant phenotype, where IL-1 did not further enhance binding of proteins from the middle complex to the DNA, and the p50 dimeric state was not substantially altered (lane 8 compared to lane 7). Competition using an excess of unlabeled NF-κB response element oligonucleotide (in the presence of IL-1) competed away the p50/p65 heterodimeric upper band and reduced the intensity of the middle complex band (lane 5). A similar result was obtained under the same conditions in Ishikawa cells (lane 9). These findings indicate that the p50/p65 band is specific to the interaction between NF-κB and its response element, but the middle band contains some proteins that have relatively less affinity for the NF-κB response element (lane 9); however, as salmon sperm DNA was used in each reaction to compete away proteins that are non-specific, the proteins in the middle complex do recognize the NF-κB DNA response element with some specificity. Interestingly, and somewhat unexpectedly, competing with the estrogen response element (ERE) oligonucleotide strongly inhibited the middle complex, but did not inhibit the p50/p65 complex in HTR8 cells (lane 6); similarly, the middle band was also competed in Ishikawa cells, but not the p50/p65 or the p50/p50 complexes (lane 10). These data indicate that proteins within the middle complex bind to both the NF-κB response element and the ERE. The middle complex could also be competed by excess cold progesterone response element (PRE) DNA (data not shown). Note that this middle complex was not present in RAW macrophage control cells (Figure 1, lane 1), so the proteins binding to this middle complex are either absent from RAW cells, or the condition for their binding is altered in RAW cells compared to Ishikawa and HTR8 cells.
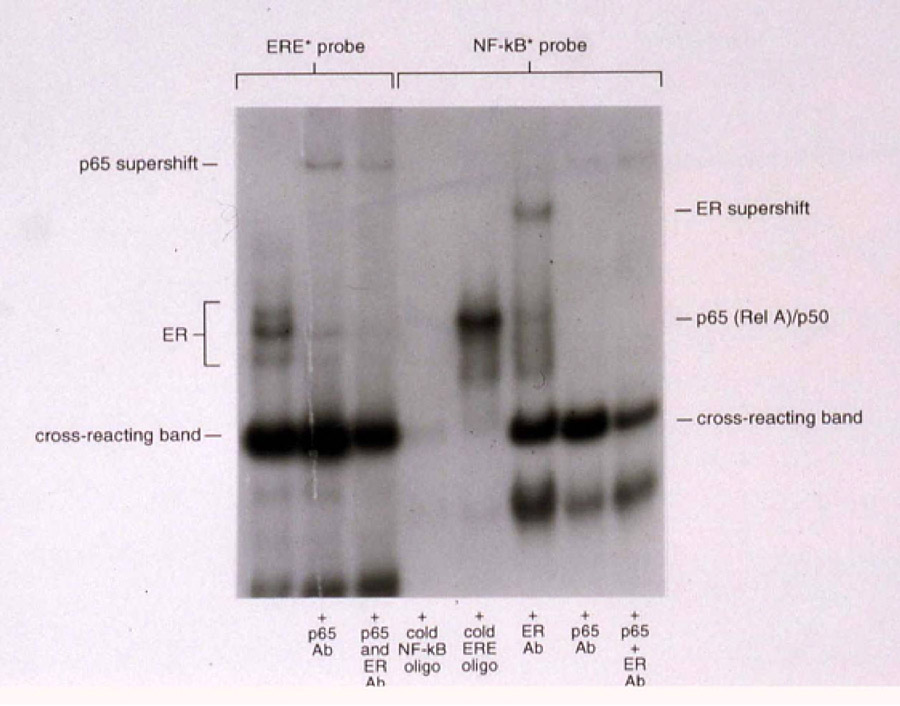
Ishikawa cells express ER as well as the NF-κB subunits p50 and p65. Figure 2 demonstrates that p65 as well as ER bind as a complex to the ERE and to the NF-κB response element, indicating the probable protein-protein interaction between ER and NF-κB in cells that express ER. In the first lane of Figure 2, the bands indicating ER binding to the ERE were identified. Interestingly, a strong band was seen below the ER-ERE complex that is identical to that previously identified as the “middle complex” in Figure 1. This complex was present in lanes 1-3 bound to the ERE and in lanes 4–6 bound to the NF-κB response element. We propose that proteins identified within this complex will have a role in both ER and NF-κB interaction with DNA. Strikingly, Figure 2 also demonstrates that p65 can be supershifted from the ER/ERE complex (lanes 2 and 3) and ER can be supershifted from the p50/65 complex (lane 5) using appropriate antibodies, indicating that when ER is expressed, it is contained within the p50/p65 complex and is possibly tethered to this complex by protein-protein interaction with p65, as has been previously reported for other steroid hormone receptors such as GR 10, 12–15 and PR 16.
Figure 2. Binding of NF-κB and ER to response elements.
In the first three lanes, the estrogen DNA response element (ERE) was labeled and used as a probe. In the last five lanes, the NF-κB DNA response element was used as a probe. Specific binding to the probe was assured by supershifting bands with antibodies to the NF-κB p65 subunit and ER. Excess cold oligonucleotides were used to assure specificity of bands. Note that in the second and third lanes, p65 supershifts from the ERE probe. Conversely, in the sixth lane, ER supershifts from the NF-κB response element.
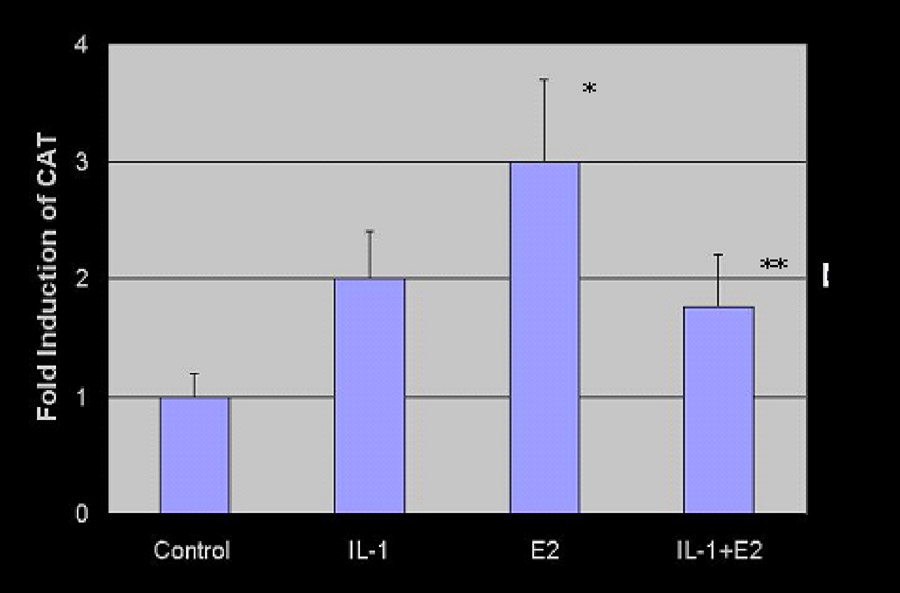
Reporter gene assays were performed to determine the possible implications of the findings from Figure 2 demonstrating a probable NF-κB/ER protein/protein interaction. Using IL-1 treatment to enhance nuclear NF-κB activation, the ability of estrogen to activate the vit-tk-CAT reporter gene was tested in Ishikawa cells that express modest levels of endogenous ER. As shown in Figure 3, cells were treated with vehicle alone (lane 1), IL-1 (lane 2), estradiol (lane 3), and estradiol + IL-1 (lane 4). IL-1, while inducing a modest increase in CAT alone (lane 2), limited estradiol-mediated transcription (lane 4), indicating that the interaction between p65 and ER is inhibitory, at least on the vit-tk promoter and within this cellular context (Figure 4 B, right panel). The fold induction of CAT was determined by the proportion of conversion of chloramphenicol substrate to acetylated products. These data were subjected to statistical analysis using the Student t test. Estradiol-induced CAT activity (lane 3) was significantly greater than control (lane 1), p = 0.01, while the addition of IL-1 to estradiol (lane 4) resulted in a significantly decreased level of CAT activity compared to estradiol alone (lane 3), p = 0.039. These data indicate that the addition of an inflammatory cytokine, IL-1, blunts the estrogen-dependent induction of CAT. Of note, the estrogen independent transcription of CAT (lane 2) was increased 2-fold in the presence of IL-1 compared to control (lane 1); however, this increase did not reach statistical significance. IL-1 is a principal factor that induces NF-κB activation and was used for that purpose in these experiments; however, these data do not directly prove that the effect requires NF-κB. Therefore, the mechanism underlying the inhibition of estradiol/ER transcription by IL-1 requires further study.
Figure 3. Chloramphenicol acetyl-transferase (CAT) reporter gene assay.
An estrogen-driven promoter was placed upstream of the CAT gene, as described in Materials and Methods. Cells were treated with vehicle alone (lane 1), IL-1 (lane 2), estradiol (lane 3), and estradiol + IL-1 (lane 4). The fold induction of the CAT gene was measured by densitometry. Error bars = SEM, computed from three independent experiments.
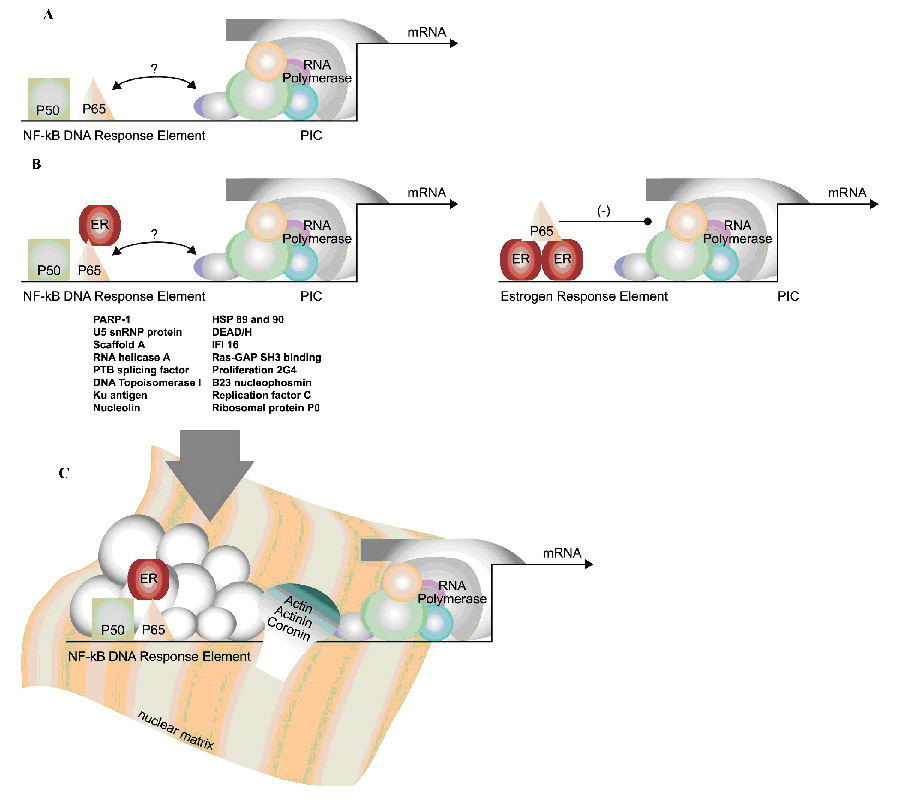
Figure 4. Identification of NF-κB/DNA binding proteins.
A. It was known that NF-κB, as a transcription factor, binds to consensus DNA response elements in the promoters of responsive genes. However, how NF-κB signals to the preinitiation complex (PIC) composed of RNA polymerase and other members was not known. B. These studies identified ER as one protein capable of binding to the p65 subunit of NF-κB when bound to DNA (left panel). This was also confirmed when ER was bound to the estrogen response element (right panel). By reporter gene assays, this interaction was inhibitory for ER-mediated transcription of the CAT gene. C. Mass spectrometry identified a large cohort of proteins that were eluted from the NF-κB/DNA complex. These include the proteins listed above the arrow as well as actin, actinin, and coronin, shown contacting the nuclear matrix and RNA polymerase.
We next turned our attention to the unknown proteins bound to the NF-κB DNA response element; we were particularly interested in those proteins that comprise the prominent “middle complex”, the binding of which are enhanced with the addition of IL-1 (Figure 1). From Figure 1, it is clear that some of these proteins also bind to an ERE (and a PRE, data not shown) because the band can be competed by adding excess cold ERE or PRE instead of excess cold NF-κB response element DNA. However, the addition of non-specific salmon sperm DNA to the mixture did not compete for these proteins, indicating that they are specific to the response elements. To identify these factors, the double-stranded NF-κB DNA response element was bound to agarose beads, and protein extracts from HTR8 cells were allowed to hybridize with the beads. Proteins interacting with the DNA on the beads were eluted and gel purified. The protein extracts were submitted to the FDA for peptide sequencing as described above. The six bands from the original gel were designated as gel fragments (GF), and the proteins are reported for each GF in Table I. The identities of the interacting proteins, linked to peptide sequence number (the gi#) are also shown in Table I, along with the statistical probability that they are accurately identified (the number in the expect column). The lower the expect number, the higher the probability that the proteins have been accurately identified. Also, a number of peptides were identified more than once, providing further reassurance that the identities were correct.
The proteins can be divided into four general categories based upon function (Table II). They include DNA binding proteins and modifying enzymes, RNA binding proteins and modifying enzymes, components of the nuclear matrix, and heat shock chaperone proteins. The diverse interacting proteins identified have the functional capacity to link the NF-κB complex with both the basal transcriptional machinery and the nuclear matrix, as well as with previously described transcriptional activators. We identified factors previously reported to interact with NF-κB, including PARP-1 21, interferon-inducible (IFI) 16 22, α-actinin 4 23, and RNA helicase A 24. Interestingly, we also found members of a multi-protein complex, including PARP-1, Ku antigen, RNA helicase A, beta actin, scaffold attachment factor A, and PTB associated splicing factor, that controls transcription in the context of steroid hormone signaling 25, 26. Scaffold attachment factor A was an intriguing discovery because a closely related protein, scaffold attachment factor B1, is known to bind to ER and to inhibit estrogen-mediated transcription. These findings indicate other avenues of cross-talk between NF-κB and hormone receptors and provide a more complete picture of the NF-κB initiation complex than has been previously reported.
Table II.
Proteins that bind to NF-κB and/or the NF-κB DNA response element by functional category.
| DNA Binding Factors and Enzymes | RNA Binding Factors and Enzymes | Nuclear Matrix and Cytoskeletal Components | Chaperone Proteins |
|---|---|---|---|
| PARP-1 | U5 snRNP | Actinin-α1 | Heat shock protein 89 |
| PTB-associated factor | RNA helicase A | Actinin-α4 | Heat shock protein 90 |
| Ku antigen | PO ribosomal protein | Coronin | |
| Scaffold attachment A | RNA helicase p72 | β-actin | |
| SAP 130- TFTC | Ras-GAP SH3 binding | ||
| Nucleolin protein 23 | |||
| Proliferation 2G4 factor | |||
| B23 nucleophosmin | |||
| Replication factor C | |||
| DNA topoisomerase | |||
| Interferon-inducible 16 |
Comments
NF-κB is activated in important processes including infection-induced parturition2 and in gynecologic malignancies 27. However, the molecular mechanism controlling NF-κB transcriptional activation of important genes, including pro-inflammatory cytokines, is incompletely understood. It is known that NF-κB binds to a consensus DNA response element in the promoter of responsive genes, yet how NF-κB, as a distal controlling factor, signals to the preinitiation complex to induce RNA polymerase is unknown (Figure 4 A). In these studies, we used two methods to identify proteins that comprise the NF-κB/DNA complex, electromobility gel supershifting with antibodies against suspected interacting proteins (NF-κB subunits p50 and p65 as well as ER) and direct peptide sequencing of proteins of high abundance that were eluted from the NF-κB-bound consensus DNA response element. Each method allowed the identification of different factors which provides new and evocative data relative to the composition of the NF-κB transcriptional unit.
Previous studies from our laboratory and others describe multiple points of crosstalk between NF-κB and steroid hormone signaling. We have shown that progesterone inhibits NF-κB function on multiple levels, including preventing its binding to DNA. Cytoplasmic NF-κB is also bound by inactivating proteins A20 and ABIN-2, induced at the level of transcription by ligand activated PR 16. The p65 subunit of NF-κB interacts with other transcription factors, particularly the steroid hormone receptors such as GR 10, 11 , and our previous studies indicate a likely protein-protein interaction between NF-κB and PR 16. In general, both GR and PR inhibit NF-κB transcriptional activity; however, how NF-κB modulates steroid hormone signaling is less understood.
In these studies, we hypothesized that in addition to GR and PR, ER also interacts with p65. To test this hypothesis, EMSA supershifting using antibodies allowed us to confirm the likely interaction between ER and p65 (Figure 2). We concluded this to be the case because incubating with antibodies against p65 and ER caused the same EMSA band to supershift on the NF-κB as well as the estrogen DNA response elements, indicating that the ER was together with p65 on the DNA. An alternative explanation to a direct p65-ER protein interaction is the possibility that the interaction with ER is with other proteins of the transcriptional machinery, not with p65 itself. However, others have previously reported an ER-NF-κB interaction that represses interleukin-6 gene expression 28. These findings indicate that ER can potentially modulate NF-κB transcriptional activity. We next questioned whether NF-κB can inhibit ER activity.
Through reporter gene assays we confirmed that in ER-positive Ishikawa endometrial cancer cells, treating with IL-1 results in the repression of ER-mediated transcription on a reporter gene (Figure 3 and 4 B, right panel). As IL-1 induces NF-κB, we propose that these data are consistent with the hypothesis that NF-κB inhibits ER transcriptional activity within this cellular context. However, it is possible that IL-1 inhibits ER activity through another mechanism other than NF-κB, and further studies will be necessary to clarify the mechanism for IL-1 mediated ER repression. On the other hand, a number of studies have documented an inhibitory effect of estradiol on inflammation and on cytokines 29, 30; even the partial estrogen raloxifene may be effective in this regard, and the pathway is reported to involve inhibition of NF-κB 31, 32. These studies strongly suggest a mutual repressive interaction between ER and NF-κB, and our laboratory is pursuing studies to determine the effect of NF-κB activation and inhibition on the expression of endogenous estrogen-controlled genes.
To further define the composition of the NF-κB transcriptional unit and to identify unknown proteins, the NF-κB response element DNA oligonucleotide was linked to agarose beads, incubated with nuclear extract from HTR8 cytotrophoblast cells (which lack ER), and the bound proteins were identified by mass spectrometry. Under the conditions of elution employed, it is likely that these proteins comprise a set of factors that are bound to the NF-κB/DNA complex through protein/protein interactions and not through protein/DNA interactions, which are more difficult to interrupt. Nevertheless, these studies identified proteins that are likely to be part of the general transcription complex as well as factors previously reported to specifically interact or co-localize with other transcription factors, such as PR (PARP-1 and PTB-associated splicing factor), and/or with NF-κB itself (PARP-1, RNA helicase A, α-actinin 4, and IFI 16). Four general categories of proteins were identified from six gel fragments (GF) that were isolated (Tables I and II): DNA-modifying enzymes, RNA polymerase accessory proteins, elements of the nuclear matrix and cytoskeleton, and heat shock chaperones. From the proteins identified, several have previously been linked to steroid hormone signaling and to cancer. These are described below.
Full length (113 kDa) ADP-ribosyltransferase NAD+; poly(ADP-ribose) polymerase or PARP-1 was consistently identified in multiple gel fragments (GF1-3), and its cleaved product (64.2 kDa) was sequenced from GF4. PARP-1 is a nuclear enzyme that catalyzes the poly (ADP-ribosyl)ation of target proteins including nucleosomal core histones, histone H1, high mobility group proteins and topoisomerases. It plays a role in DNA repair, recombination, and chromatin remodeling and binds to transcription factors such as PR in combination with the Ku autoantigen 33, which was also identified in the NF-κB transcriptional unit (Table I). PARP-1 has been implicated in the control of other steroid hormone receptors such as retinoic acid and thyroid receptors, where the interaction is inhibitory 34. It is involved in regulation of cell cycle genes, cleaved as a result of apoptosis 35, and is lost or mutated in cancers, indicating its function as a tumor suppressor 36, 37. PARP-1 cooperates with p53 as an inhibitor of tumorigenesis, maintaining chromosome stability 38. In addition to directly interacting with PR, PARP-1 is co-expressed with PR and down-regulated in advanced endometrial tumors 39; it has also been reported to restore DNA integrity in association with progesterone after ovulation 40. Most interestingly, PARP-1 has been previously implicated in AP-1 and NF-κB mediated transcription of inflammatory factors 21. Our finding that it is part of the NF-κB/DNA protein complex is consistent with the hypothesis that PARP-1 is not only a ubiquitous protein and DNA interacting molecule, but also a specific co-factor that modulates NF-κB transcriptional activity.
Splicing factor proline/glutamine rich (polypyrimidine tract binding protein associated) PTB-associated splicing factor is a multi-function nuclear protein that complexes with RNA binding protein [p54(nrb)] to repair DNA and to participate in transcription 41. It regulates IGF-1 signaling by binding to and repressing the IGF response element of the P-450 cholesterol side-chain cleavage enzyme 42 and cooperates with RNA-binding protein [p54(nrb)] and Ku autoantigen (Table I) to repair DNA single strand breaks 43. PTB-associated splicing factor is part of the androgen receptor protein binding complex on DNA 44, and has been reported to be a negative co-regulator of PR, acting to functionally withdraw progesterone action at the time of parturition 45.
Interferon-inducible (IFI) 16 is a member of the interferon-inducible p200 family of proteins. Members of this family have been reported to bind to NF-κB p50 and p65 subunits as well as AP-1 factors to modulate (mainly inhibit) transcriptional activity 46. IFI 16 contains a DNA binding domain and a nuclear localization signal, and its expression is attenuated by glucocorticoids 47. IFI 16 has been implicated in bringing about irreversible cell senescence in prostate cancer cells 48. While generally considered a repressor of transcription 49, this factor binds directly to the C-terminal region of p53 and augments p53-mediated transcriptional response 50.
In conclusion, we have shown that ER and NF-κB co-localize on DNA with implications for ER-mediated gene transcription on an ERE (Figure 4B). Our data indicate that this is an inhibitory interaction with respect to estrogen-mediated transcription, and we hypothesize that estrogen signaling will be down-regulated with infection. Also, in view of the constitutive activation of NF-κB in cancer cells such as Ishikawa (Figure 1), it is possible that the inhibition of hormone receptor action by NF-κB may be linked with the progression towards hormone independence in such tumors.
These studies also provide unique insights into the complex process of NF-κB transcriptional initiation in HTR8 cytotrophoblast cells. They highlight the complexity of the NF-κB/DNA response element molecular machinery (Figure 4C), which includes factors that have been previously reported to bind directly to NF-κB itself (PARP-1, RNA helicase A, α-actinin 4, and IFI 16), proteins previously implicated in steroid hormone signaling (PARP-1, Ku Antigen, scaffold attachment factor A, and PTB-associated splicing factor), as well as structural proteins that likely function to link the entire complex to the RNA polymerase holoenzyme (actin).
The identification of cytoskeletal proteins such as actin, actinin, and coronin, known as interacting units of the nuclear matrix as well as proteins with the capacity to interact with the basal transcriptional unit, highlight the central role the nuclear matrix plays in ordering NF-κB transcription. As in Figure 4C, it is proposed that these cytoskeletal proteins comprise a molecular motor with the capability of moving RNA polymerase II along during transcription 51, and these studies support the hypothesis that transcription factors such as NF-κB recruit the molecular machine to DNA.
Acknowledgments
The authors thank Dr. Chip Petricoin and the members or his research group who performed the protein sequencing at the FDA.
Grant support to KL: NIH R01CA99908-1, the Cory/Beach Foundation, Mrs. Shirley Leslie, Dean and Alice Irvin, the UNM Cancer Research and Treatment Center Proteomics Core Facility
Footnotes
Condensation: This work characterizes the proteins bound to the Nuclear Factor-kappa B (NF-κB) DNA response element which includes estrogen receptors (ER). The interaction between NF-κB and ER inhibits estrogen signaling in reporter gene assays, predicting for a blunted hormonal response in the setting of infection and cancer.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bacot SM, Lenz P, Frazier-Jessen MR, Feldman GM. Activation by prion peptide PrP106-126 induces a NF-kappaB-driven proinflammatory response in human monocyte-derived dendritic cells. J Leukoc Biol. 2003;74:118–25. doi: 10.1189/jlb.1102521. [DOI] [PubMed] [Google Scholar]
- 2.Leslie KK, Lee SL, Woodcock SM, et al. Acute intrauterine infection results in an imbalance between pro- and anti-inflammatory cytokines in the pregnant rabbit [In Process Citation] Am J Reprod Immunol. 2000;43:305–11. doi: 10.1111/j.8755-8920.2000.430510.x. [DOI] [PubMed] [Google Scholar]
- 3.Modugno F, Ness RB, Chen C, Weiss NS. Inflammation and endometrial cancer: a hypothesis. Cancer Epidemiol Biomarkers Prev. 2005;14:2840–7. doi: 10.1158/1055-9965.EPI-05-0493. [DOI] [PubMed] [Google Scholar]
- 4.Lin A, Karin M. NF-[kappa]B in cancer: a marked target. Seminars in Cancer Biology. 2003;13:107–114. doi: 10.1016/s1044-579x(02)00128-1. [DOI] [PubMed] [Google Scholar]
- 5.Ashburner BP, Westerheide SD, Baldwin AS., Jr The p65 (RelA) subunit of NF-kappaB interacts with the histone deacetylase (HDAC) corepressors HDAC1 and HDAC2 to negatively regulate gene expression. Mol Cell Biol. 2001;21:7065–77. doi: 10.1128/MCB.21.20.7065-7077.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madrid LV, Mayo MW, Reuther JY, Baldwin AS., Jr Akt stimulates the transactivation potential of the RelA/p65 Subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38. J Biol Chem. 2001;276:18934–40. doi: 10.1074/jbc.M101103200. [DOI] [PubMed] [Google Scholar]
- 7.Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J Clin Invest. 2001;107:241–6. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldwin AS., Jr Series introduction: the transcription factor NF-kappaB and human disease. J Clin Invest. 2001;107:3–6. doi: 10.1172/JCI11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roeder RG. Transcriptional regulation and the role of diverse coactivators in animal cells. FEBS Lett. 2005;579:909–15. doi: 10.1016/j.febslet.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Nissen RM, Yamamoto KR. The glucocorticoid receptor inhibits NFkappaB by interfering with serine-2 phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev. 2000;14:2314–29. doi: 10.1101/gad.827900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hermoso MA, Cidlowski JA. Putting the brake on inflammatory responses: the role of glucocorticoids. IUBMB Life. 2003;55:497–504. doi: 10.1080/15216540310001642072. [DOI] [PubMed] [Google Scholar]
- 12.Ray A, Prefontaine KE. Physical association and functional antagonism between the p65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor. Proc Natl Acad Sci U S A. 1994;91:752–6. doi: 10.1073/pnas.91.2.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caldenhoven E, Liden J, Wissink S, et al. Negative cross-talk between RelA and the glucocorticoid receptor: a possible mechanism for the antiinflammatory action of glucocorticoids. Mol Endocrinol. 1995;9:401–12. doi: 10.1210/mend.9.4.7659084. [DOI] [PubMed] [Google Scholar]
- 14.Scheinman RI, Gualberto A, Jewell CM, Cidlowski JA, Baldwin AS., Jr Characterization of mechanisms involved in transrepression of NF-kappa B by activated glucocorticoid receptors. Mol Cell Biol. 1995;15:943–53. doi: 10.1128/mcb.15.2.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wissink S, van de Stolpe A, Caldenhoven E, Koenderman L, van der Saag PT. NF-kappa B/Rel family members regulating the ICAM-1 promoter in monocytic THP-1 cells. Immunobiology. 1997;198:50–64. doi: 10.1016/s0171-2985(97)80026-5. [DOI] [PubMed] [Google Scholar]
- 16.Davies S, Dai D, Feldman I, Pickett G, Leslie KK. Identification of a novel mechanism of NF-kappaB inactivation by progesterone through progesterone receptors in Hec50co poorly differentiated endometrial cancer cells: induction of A20 and ABIN-2. Gynecol Oncol. 2004;94:463–70. doi: 10.1016/j.ygyno.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 17.Lenardo MJ, Baltimore D. NF-kappa B: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989;58:227–9. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- 18.Leslie KK, Tasset DM, Horwitz KB. Functional analysis of a mutant estrogen receptor isolated from T47Dco breast cancer cells. Am J Obstet Gynecol. 1992;166:1053–61. doi: 10.1016/s0002-9378(11)90590-0. [DOI] [PubMed] [Google Scholar]
- 19.Klein-Hitpass L, Schorpp M, Wagner U, Ryffel GU. An estrogen-responsive element derived from the 5' flanking region of the Xenopus vitellogenin A2 gene functions in transfected human cells. Cell. 1986;46:1053–61. doi: 10.1016/0092-8674(86)90705-1. [DOI] [PubMed] [Google Scholar]
- 20.Ahram M, Best CJ, Flaig MJ, et al. Proteomic analysis of human prostate cancer. Mol Carcinog. 2002;33:9–15. doi: 10.1002/mc.10019. [DOI] [PubMed] [Google Scholar]
- 21.Virag L. Poly(ADP-ribosyl)ation in asthma and other lung diseases. Pharmacol Res. 2005;52:83–92. doi: 10.1016/j.phrs.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Min W, Ghosh S, Lengyel P. The interferon-inducible p202 protein as a modulator of transcription: inhibition of NF-kappa B, c-Fos, and c-Jun activities. Mol Cell Biol. 1996;16:359–68. doi: 10.1128/mcb.16.1.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Babakov VN, Bobkov DE, Petukhova OA, et al. [alpha-Actinin-4 and p65/RelA subunit of NF-kappaB transcription factor are co-localized and migrate together into the nucleus in EGF-stimulated A431 cell] Tsitologiia. 2004;46:1064–72. [PubMed] [Google Scholar]
- 24.Tetsuka T, Uranishi H, Sanda T, et al. RNA helicase A interacts with nuclear factor kappaB p65 and functions as a transcriptional coactivator. Eur J Biochem. 2004;271:3741–51. doi: 10.1111/j.1432-1033.2004.04314.x. [DOI] [PubMed] [Google Scholar]
- 25.Eggert M, Michel J, Schneider S, et al. The glucocorticoid receptor is associated with the RNA-binding nuclear matrix protein hnRNP U. J Biol Chem. 1997;272:28471–8. doi: 10.1074/jbc.272.45.28471. [DOI] [PubMed] [Google Scholar]
- 26.Eggert H, Schulz M, Fackelmayer FO, Renkawitz R, Eggert M. Effects of the heterogeneous nuclear ribonucleoprotein U (hnRNP U/SAF-A) on glucocorticoid-dependent transcription in vivo. J Steroid Biochem Mol Biol. 2001;78:59–65. doi: 10.1016/s0960-0760(01)00074-7. [DOI] [PubMed] [Google Scholar]
- 27.Pallares J, Martinez-Guitarte JL, Dolcet X, et al. Abnormalities in the NF-kappaB family and related proteins in endometrial carcinoma. J Pathol. 2004;204:569–77. doi: 10.1002/path.1666. [DOI] [PubMed] [Google Scholar]
- 28.Ray P, Ghosh SK, Zhang DH, Ray A. Repression of interleukin-6 gene expression by 17 beta-estradiol: inhibition of the DNA-binding activity of the transcription factors NF-IL6 and NF-kappa B by the estrogen receptor. FEBS Lett. 1997;409:79–85. doi: 10.1016/s0014-5793(97)00487-0. [DOI] [PubMed] [Google Scholar]
- 29.Steffan RJ, Matelan E, Ashwell MA, et al. Control of chronic inflammation with pathway selective estrogen receptor ligands. Curr Top Med Chem. 2006;6:103–11. doi: 10.2174/156802606775270279. [DOI] [PubMed] [Google Scholar]
- 30.Kanda N, Watanabe S. 17beta-estradiol inhibits the production of RANTES in human keratinocytes. J Invest Dermatol. 2003;120:420–7. doi: 10.1046/j.1523-1747.2003.12067.x. [DOI] [PubMed] [Google Scholar]
- 31.Olivier S, Close P, Castermans E, et al. Raloxifene-induced myeloma cell apoptosis: a study of NF-{kappa}B inhibition and gene expression signature. Mol Pharmacol. 2006 doi: 10.1124/mol.105.020479. [DOI] [PubMed] [Google Scholar]
- 32.Demyanets S, Pfaffenberger S, Kaun C, et al. The estrogen metabolite 17beta-dihydroequilenin counteracts interleukin-1alpha induced expression of inflammatory mediators in human endothelial cells in vitro via NF-kappaB pathway. Thromb Haemost. 2006;95:107–16. [PubMed] [Google Scholar]
- 33.Sartorius CA, Takimoto GS, Richer JK, Tung L, Horwitz KB. Association of the Ku autoantigen/DNA-dependent protein kinase holoenzyme and poly(ADP-ribose) polymerase with the DNA binding domain of progesterone receptors. J Mol Endocrinol. 2000;24:165–82. doi: 10.1677/jme.0.0240165. [DOI] [PubMed] [Google Scholar]
- 34.Miyamoto T, Kakizawa T, Hashizume K. Inhibition of nuclear receptor signalling by poly(ADP-ribose) polymerase. Mol Cell Biol. 1999;19:2644–9. doi: 10.1128/mcb.19.4.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Virag L. Structure and function of poly(ADP-ribose) polymerase-1: role in oxidative stress-related pathologies. Curr Vasc Pharmacol. 2005;3:209–14. doi: 10.2174/1570161054368625. [DOI] [PubMed] [Google Scholar]
- 36.Rajaee-Behbahani N, Schmezer P, Ramroth H, et al. Reduced poly(ADP-ribosyl)ation in lymphocytes of laryngeal cancer patients: results of a case-control study. Int J Cancer. 2002;98:780–4. doi: 10.1002/ijc.10234. [DOI] [PubMed] [Google Scholar]
- 37.Tong WM, Cortes U, Hande MP, et al. Synergistic role of Ku80 and poly(ADP-ribose) polymerase in suppressing chromosomal aberrations and liver cancer formation. Cancer Res. 2002;62:6990–6. [PubMed] [Google Scholar]
- 38.Tong WM, Hande MP, Lansdorp PM, Wang ZQ. DNA strand break-sensing molecule poly(ADP-Ribose) polymerase cooperates with p53 in telomere function, chromosome stability, and tumor suppression. Mol Cell Biol. 2001;21:4046–54. doi: 10.1128/MCB.21.12.4046-4054.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghabreau L, Roux JP, Frappart PO, et al. Poly(ADP-ribose) polymerase-1, a novel partner of progesterone receptors in endometrial cancer and its precursors. Int J Cancer. 2004;109:317–21. doi: 10.1002/ijc.11731. [DOI] [PubMed] [Google Scholar]
- 40.Murdoch WJ. Perturbation of sheep ovarian surface epithelial cells by ovulation: evidence for roles of progesterone and poly(ADP-ribose) polymerase in the restoration of DNA integrity. J Endocrinol. 1998;156:503–8. doi: 10.1677/joe.0.1560503. [DOI] [PubMed] [Google Scholar]
- 41.Shav-Tal Y, Zipori D. PSF and p54(nrb)/NonO--multi-functional nuclear proteins. FEBS Lett. 2002;531:109–14. doi: 10.1016/s0014-5793(02)03447-6. [DOI] [PubMed] [Google Scholar]
- 42.Urban RJ, Bodenburg Y. PTB-associated splicing factor regulates growth factor-stimulated gene expression in mammalian cells. Am J Physiol Endocrinol Metab. 2002;283:E794–8. doi: 10.1152/ajpendo.00174.2002. [DOI] [PubMed] [Google Scholar]
- 43.Bladen CL, Udayakumar D, Takeda Y, Dynan WS. Identification of the polypyrimidine tract binding protein-associated splicing factor. p54(nrb) complex as a candidate DNA double-strand break rejoining factor. J Biol Chem. 2005;280:5205–10. doi: 10.1074/jbc.M412758200. [DOI] [PubMed] [Google Scholar]
- 44.Ishitani K, Yoshida T, Kitagawa H, Ohta H, Nozawa S, Kato S. p54nrb acts as a transcriptional coactivator for activation function 1 of the human androgen receptor. Biochem Biophys Res Commun. 2003;306:660–5. doi: 10.1016/s0006-291x(03)01021-0. [DOI] [PubMed] [Google Scholar]
- 45.Dong X, Shylnova O, Challis JR, Lye SJ. Identification and characterization of the protein-associated splicing factor as a negative co-regulator of the progesterone receptor. J Biol Chem. 2005;280:13329–40. doi: 10.1074/jbc.M409187200. [DOI] [PubMed] [Google Scholar]
- 46.Min W, Ghosh S, Lengyel P. The interferon-inducible p202 protein as a modulator of transcription: inhibition of NF-kappa B, c-Fos, and c-Jun activities. Mol Cell Biol. 1996;16:359–68. doi: 10.1128/mcb.16.1.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith JB, Herschman HR. The glucocorticoid attenuated response genes GARG-16, GARG-39, and GARG-49/IRG2 encode inducible proteins containing multiple tetratricopeptide repeat domains. Arch Biochem Biophys. 1996;330:290–300. doi: 10.1006/abbi.1996.0256. [DOI] [PubMed] [Google Scholar]
- 48.Xin H, Curry J, Johnstone RW, Nickoloff BJ, Choubey D. Role of IFI 16, a member of the interferon-inducible p200-protein family, in prostate epithelial cellular senescence. Oncogene. 2003;22:4831–40. doi: 10.1038/sj.onc.1206754. [DOI] [PubMed] [Google Scholar]
- 49.Johnstone RW, Kerry JA, Trapani JA. The human interferon-inducible protein, IFI 16, is a repressor of transcription. J Biol Chem. 1998;273:17172–7. doi: 10.1074/jbc.273.27.17172. [DOI] [PubMed] [Google Scholar]
- 50.Johnstone RW, Wei W, Greenway A, Trapani JA. Functional interaction between p53 and the interferon-inducible nucleoprotein IFI 16. Oncogene. 2000;19:6033–42. doi: 10.1038/sj.onc.1204005. [DOI] [PubMed] [Google Scholar]
- 51.Grummt I. Actin and myosin as transcription factors. Curr Opin Genet Dev. 2006;16:191–6. doi: 10.1016/j.gde.2006.02.001. [DOI] [PubMed] [Google Scholar]