Abstract
In the hippocampus, a CA3 pyramidal cell forms excitatory synapses with thousands of other pyramidal cells and inhibitory interneurons. By using sequential paired recordings from three connected cells, we show that the presynaptic properties of CA3 pyramidal cell terminals, belonging to the same axon, differ according to the type of target cell. Activation of presynaptic group III metabotropic glutamate receptors decreases transmitter release only at terminals contacting CA1 interneurons but not CA1 pyramidal cells. Furthermore, terminals contacting distinct target cells show different frequency facilitation. On the basis of these results, we conclude that the pharmacological and physiological properties of presynaptic terminals are determined, at least in part, by the target cells.
Neurons in the mammalian central nervous system exhibit various degrees of divergence toward targets belonging to distinct functional categories. A striking example is the weak divergence of Purkinje cells projecting to few deep cerebellar nuclei cells (1) as compared with the tens of thousand of contacts made by a hippocampal CA3 pyramidal cell with other pyramidal cells and interneurons (2, 3). Divergent connectivity represents a potent means to coordinate the behavior of neuronal populations in space and in time. Examples include the neuronal circuits underlying spinal reflexes or generating synchronous neuronal oscillations (4–6).
Several observations in invertebrates indicate that presynaptic terminals belonging to the same axon can exhibit different properties of transmitter release (7–11) and that correlations exist between the properties of the presynaptic terminal and the type of target it impinges on (12–14) suggesting that presynaptic properties may be controlled by the target cell via a retrograde signal (15–17). The match between the properties of the presynaptic terminal and the function of the target cell is an essential parameter for the understanding of the coordination of cell ensembles by divergent circuits.
Some evidence indicates that target cell specificity of transmitter release also occurs in the mammalian central nervous system. Physiological and anatomical studies in the spinal cord and in the hippocampus, have shown that the properties of release and the expression of presynaptic metabotropic glutamate receptors vary between terminals originating from the same axon (18, 19). Recent experiments indicating that differential short- and long-term facilitation of transmitter release depend on the target further support target cell specificity, although release properties between terminals belonging to the same axon were not compared (20–22).
Here we use sequential paired recordings, to compare modulation of transmitter release between presynaptic terminals from a single CA3 pyramidal cell axon (Schaffer collateral) but impinging on different target cells.
METHODS
Acute Hippocampal Slices.
Standard procedures were used to prepare 400-μm thick hippocampal slices from 20- to 30-day-old Wistar rats. After dissection, the slices were maintained at room temperature for at least 1 h in a submerged chamber containing artificial cerebrospinal fluid equilibrated with 95% O2 and 5% CO2 and then transferred to a superfusion chamber for recording. The artificial cerebrospinal fluid contained 119 mM NaCl, 2.5 mM KCl, 1 mM NaHPO4, 1.3 mM MgCl2, 2.5 mM CaCl2, 26 mM NaHCO3, and 11 mM glucose. Electrophysiological recordings were made at room temperature and the concentration of MgCl2 and of CaCl2 in the artificial cerebrospinal fluid was raised to 4 mM. Picrotoxin (100 μM) was always present in the perfusate and a cut between CA1 and CA3 was made to avoid spread of epileptiform activity into CA1, except when GABAergic inhibition was to be monitored or synaptic activity was to be evoked by puffing potassium on CA3 pyramidal cells. Whole-cell recording electrodes were filled with a solution containing 122.5 mM Cs gluconate, 10 mM CsCl, 10 mM Hepes, 10 mM 1,2-bis(2-aminophenoxy)ethane-N,N,N,N, -tetraacetic acid (BAPTA), and 8 mM NaCl. The pH was adapted to 7.2. For current-clamp recordings, Cs gluconate was substituted with K gluconate and CsCl was omitted. The resistance of the pipettes ranged from 2 to 4 MOhm. Field recordings were made with glass electrodes containing 3 M NaCl. Monopolar stimulation electrodes were made with a similar glass electrode containing artificial cerebrospinal fluid. Interneurons located in the strata radiatum or oriens were visually identified by using infrared differential interference contrast videomicroscopy and had either bipolar or multipolar shape, clearly distinguishable from displaced pyramidal cells. For the activation of Schaffer collaterals, one or two potassium puffs lasting 75–100 ms (two puffs were separated by 50 ms) were applied to the surface of the CA3 pyramidal cell layer every 30–45s with a patch pipette, containing 1 M KCl. We measured the charge transfer of the responses because the superimposition of N-methyl-d-aspartic acid receptor (NMDAR)-mediated excitatory postsynaptic currents (EPSCs) did not allow an accurate analysis of the frequency and amplitude of unitary events. The l(+)-amino-4-phosphonobutyric acid (L-AP4)-induced decrease in charge transfer was not due to a change in excitability of CA3 pyramidal cells because L-AP4 (50 μM) had no effect on the membrane potential of those cells recorded with intracellular microelectrodes (n = 5).
Organotypic Cultures.
Hippocampal slice cultures were prepared from 5- to 6-day-old rats as described (23). After 2–4 weeks in vitro, cultures were placed in a superfusion chamber for recording. Recordings were done in the absence of picrotoxin. Interneurons located in the stratum oriens with proximal processes extending horizontally within the stratum were visually identified by using infrared differential interference contrast videomicroscopy and filled with Lucifer yellow (1 mg/ml intracellular solution) for anatomical confirmation at the end of the recordings. Only cells showing typical nonpyramidal morphology were considered interneurons (Fig. 4C). Sharp microelectrodes for intracellular recordings were filled with a 3 M KCl solution.
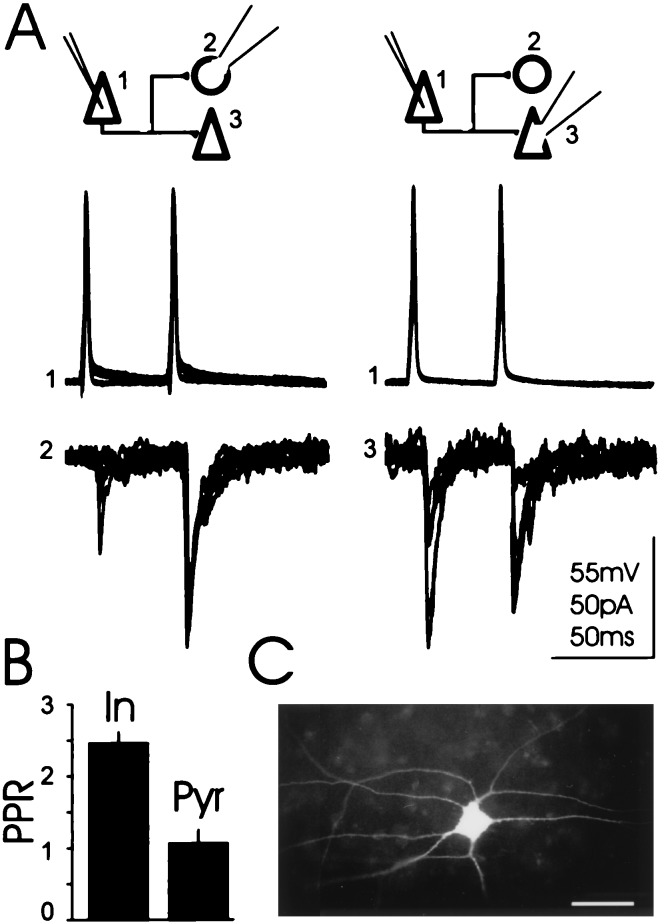
Figure 4.
Terminals originating from an individual axon but contacting different cell types display different PPR. (A) Voltage and current traces: action potentials triggered by intracellular current pulses in a presynaptic CA3 pyramidal cell (1) evoked unitary EPSCs in a monosynaptically connected alveus-oriens interneuron (2) and a subsequently recorded pyramidal cell (3). The traces are composed of seven superimposed sweeps. (B) Histogram: Summary graph (n = 3). PPR is p2/p1. (In, interneurons; Pyr, pyramidal cells) (C) Micrograph of a typical Lucifer yellow filled oriens/alveus interneuron in culture. The horizontally extending dendrites run parallel to the stratum pyramidale (Upper) and to the alveus (Lower). (Bar = 60 μm.)
Data were digitized on-line via a DigiData 1200 interface (Axon Instruments). The Student’s t test was used for statistical comparison. Values for coefficient of variation (CV) were calculated as √(σ(measured)2 − σ(noise)2)/M were M is the mean of the amplitude of the EPSC, σ(measured)2 is the variance of the recorded signal and σ(noise)2 is the variance of the noise. Average values are expressed as the mean ± SEM. Drugs used were picrotoxin, 1,2-bis(2-aminophenoxy)ethane-N,N,N,N,-tetraacetic acid (Sigma); RS-α-cyclopropyl-4-phosphonophenylglycine CPPG, d(−)-2-amino-5-phosphonopentanoic acid, L-AP4, α-methyl-4-carboxyphenylglycine, and 6-nitro-7-sulfamoylbenzo(f)quinoxaline-2,3-dione (NBQX; Tocris Cookson, Bristol).
RESULTS
Differential Action of L-AP4 on Excitatory Terminals Impinging on Interneurons vs. Pyramidal Cells.
Evoked excitatory synaptic responses were recorded simultaneously from the CA1 pyramidal cell dendritic field with an extracellular recording electrode placed in the stratum radiatum and, from whole cell-voltage clamped interneurons located in stratum radiatum of CA1 in rat hippocampal slices. The stimulation electrode was placed in the stratum radiatum of CA1. Interneurons were voltage clamped between −60 and −70 mV. The evoked EPSCs were mediated by α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor activation as they were abolished by NBQX (10 μM) perfused at the end of each experiment. Bath perfusion of 10 μM of the specific group III metabotropic glutamate receptor (mGLUR) agonist L-AP4 (24) reversibly depressed EPSCs recorded from interneurons (52 ± 4% depression; n = 6) leaving the field excitatory postsynaptic potential (fEPSP) unaffected (103 ± 2% of baseline).
EPSCs recorded from interneurons located within the stratum oriens of CA1 and evoked by local stimulation were similarly depressed by L-AP4 (48 ± 8% depression, n = 5, not significantly different from the depression observed in the stratum radiatum interneurons, P > 0.5). The data were therefore pooled (Fig. 1). Higher concentrations of L-AP4 (50 μM) did not significantly alter the amount of depression (36 ± 7%, n = 12, P > 0.7). L-AP4-mediated depression of the EPSC varied over a wide range, from <10% to a maximum of 80%.
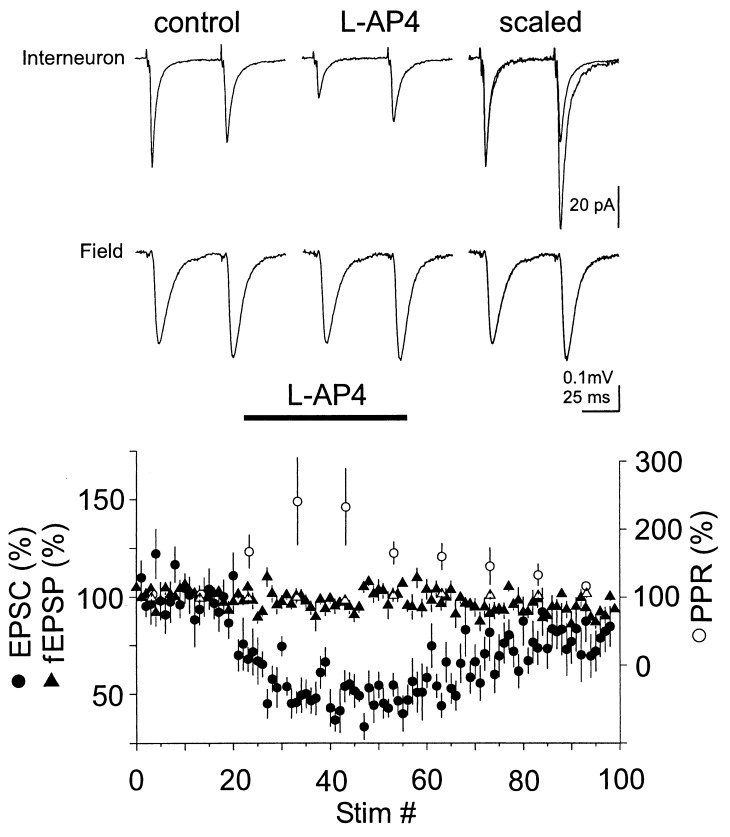
Figure 1.
L-AP4 reduces EPSCs recorded from interneurons in CA1 but not field EPSPs. Current and voltage traces: Data from a representative experiment in which field and whole-cell recordings were performed simultaneously. Each trace represents the average of 10–20 sweeps. (Lower) Summary graph of the action of L-AP4 (10 μM) on the amplitude of evoked AMPA receptor-mediated EPSCs recorded from CA1 interneurons voltage clamped at −70 mV (n = 11, •). Note the increase of the normalized PPR of the EPSCs recorded from interneurons upon perfusion of L-AP4 (○, bin size: 10 stimuli; the PPR was defined as (p2/p1) where p1 and p2 are the amplitude of the first and second EPSC, respectively). fEPSPs were monitored simultaneously by placing an extracellular-recording pipette in the stratum radiatum in six experiments (▴). No change in PPR could be detected in the field recordings (▵).
Application of (S)-α-methyl-4-carboxyphenylglycine, a group I and II mGLUR antagonist (2 mM, n = 3) or of (RS)-α-cyclopropyl-4-phosphonophenylglycine, a group II and III mGLUR antagonist (10 μM, n = 5) did not change the size of EPSCs recorded from interneurons and did not reduce the response to 50 μM L-AP4 (data not shown).
Several lines of evidence indicate that L-AP4 acted presynaptically to decrease the probability of transmitter release. (i) It strongly increased the paired pulse ratio (PPR, Fig. 1). (ii) L-AP4 similarly depressed NMDAR-mediated EPSCs recorded at +40 mV in the presence of 10 μM NBQX (42 ± 11% depression, n = 5). (iii) L-AP4 was effective in the presence of the Ca2+ buffer 1,2-bis(2-aminophenoxy)ethane-N,N,N,N,-tetraacetic acid (10 mM) and absence of nucleotides in the recording pipette. Furthermore, there was no “washout” of the effect even after prolonged (60 min) dialysis of the interneuron.
The lack of effect of L-AP4 on fEPSPs recorded in the dendritic field of CA1 pyramidal cells was not due to the extracellular-recording conditions because similar results were obtained with whole-cell recordings from CA1 pyramidal cells (10–50 μM L-AP4, 108 ± 8% of baseline, n = 5). If L-AP4 were to increase the sensitivity of pyramidal cells to synaptically released glutamate, this would compensate for a possible L-AP4-mediated decrease in release probability. This hypothesis is, however, unlikely because L-AP4 failed to change PPR of excitatory transmission onto pyramidal cells (Fig. 1) and 1,2-bis(2-aminophenoxy)ethane-N,N,N,N,-tetraacetic acid was present in the pipette during CA1 pyramidal whole-cell recordings.
Concentrations of L-AP4 of over one order of magnitude higher were necessary to reduce the slope of the fEPSPs to a similar extent as the EPSCs recorded from interneurons. A dose-response curve of the depression of fEPSPs was obtained with four different concentrations of L-AP4 (10, 200, 500, and 1,000 μM; n = 3–6). At 10 and 200 μM, no significant depression of fEPSPs was observed and the sigmoidal fit yielded a value for EC50 of 621 μM, similar to what has been described (25, 26) [the lower concentrations of L-AP4 needed by Baskys and Malenka (27) in hippocampal slices from rats up to 30 days of age may be attributed to developmental differences between rat strains: Sprague–Dawley (R. C. Malenka, personal communication) vs. Wistar (see Materials and Methods)].
Direct Activation of CA3 Pyramidal Cells.
The dendrites of CA1 interneurons extending into stratum radiatum and/or oriens receive excitatory inputs from both Schaffer collaterals and CA1 pyramidal cell axons whereas CA1 pyramidal cells rarely receive inputs from other CA1 pyramidal cells (28–30). The differential action of L-AP4 on EPSCs recorded from interneurons vs. pyramidal cells could thus be caused by the stimulation of different sets of afferents. To test whether the terminals of the Schaffer collaterals innervating interneurons were sensitive to L-AP4, we evoked EPSCs in an interneuron by exciting CA3 pyramidal cells, which give rise to Schaffer collaterals. Brief puffs of a potassium solution were applied via a patch pipette to the surface of the slice while moving the tip of the pipette along the CA3 pyramidal cell layer, until an increase in the frequency of the EPSCs could be recorded from a CA1 interneuron. NBQX (10 μM) was then bath-applied to block polysynaptic excitation of the interneuron via CA1 pyramidal cells, and the interneuron was voltage-clamped at +40 mV to record NMDAR-mediated EPSCs (31). The increase in EPSC frequency lasted between 0.5 and 4 s and the puffs were repeated every 30–45 s. Bath perfusion of the NMDAR antagonist d(−)-2-amino-5-phosphonopentanoic acid (50 μM) at the end of each experiment, abolished the response to the puffs. Fig. 2A shows that application of L-AP4 (10–20 μM) reversibly decreased by 55 ± 10% (n = 4) the charge transfer through NMDAR-activated channels when measured during the first 1–4 s after the puff. These results demonstrate that transmitter release at synapses between Schaffer collaterals and CA1 interneurons is decreased by activation of group III mGLURs.
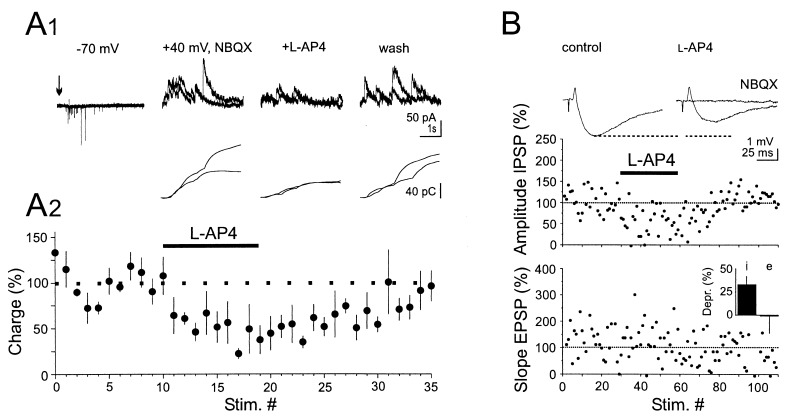
Figure 2.
(A) Synaptic transmission at Schaffer collateral/interneuron synapses is decreased by L-AP4. (A1) Potassium puffs (2 × 75 ms, 50 ms interval, arrow) applied every 30 s to the surface of the CA3 stratum pyramidale evoked a burst of α-amino-3-hydroxy-5-methyl-4-isoxalozolepropionic acid receptor-mediated EPSCs in a CA1 interneuron voltage- clamped at −70 mV. After bath-application of NBQX (10 μM) the neuron was voltage clamped at +40 mV to record NMDAR-mediated EPSCs. Application of L-AP4 (15 μM) reversibly reduced NMDAR-mediated EPSCs. The current traces are composed of two superimposed sweeps. Lower traces: charge transfer of the current traces illustrated above. (A2). Summary graph of four similar experiments. (B) L-AP4 reduces feed-forward inhibition. Threshold extracellular stimulation in CA3 evoked an EPSP-IPSP sequence in a CA1 pyramidal cell recorded in current-clamp mode (membrane potential manually clamped at −55 mV). The voltage traces represent the average of 20 sweeps. L-AP4 (50 μM) reduced the amplitude of IPSPs but not the slope of EPSPs. Note that NBQX (10 μM) abolished the EPSP and the IPSP. (Inset) Comparison of the action of L-AP4 on IPSPs (i) and EPSPs (e) for the four experiments where a depression of the IPSP was observed.
Activation of Group III mGLURs Inhibits Feed-Forward Inhibition.
A selective depression of EPSPs recorded from interneurons is expected to decrease GABAergic feed-forward inhibition in CA1 pyramidal cells evoked by electrical stimulation of the stratum radiatum. This possibility was tested by setting the stimulation intensity slightly above threshold to elicit an EPSP followed by an inhibitory postsynaptic potential (IPSP) in current-clamped CA1 pyramidal cells. At the end of each experiment total blockade of both EPSPs and IPSPs by NBQX (10 μM) ensured that no direct inhibitory afferents to the recorded CA1 pyramidal cells were stimulated. In four of seven experiments we found a clear and reversible reduction in the amplitude of the disynaptic IPSP whereas the slope of the EPSP remained unaffected (Fig. 2B). The action of L-AP4 was not due to a direct effect on GABAergic terminals because evoked monosynaptic IPSCs recorded from CA1 pyramidal cells in the presence of NBQX (10 μM) were unaffected by L-AP4 (50 μM, n = 2), as previously reported by others (26). The facts that the stimulation intensity was set just above threshold and that the IPSP occurred within 3–4 ms from the onset of the EPSP strongly suggest that we evoked disynaptically mediated feed-forward inhibition rather than trisynaptic feed-back inhibition.
Target Cell-Specific Depression of Transmitter Release by L-AP4 at Excitatory Terminals Originating from the Same Axon.
Two different explanations could account for the results described above: (i) a subpopulation of CA3 pyramidal cells innervates only CA1 interneurons, and their terminals are sensitive to L-AP4 or (ii) CA3 pyramidal cells innervate both CA1 pyramidal cells and interneurons but only synaptic transmission to interneurons exhibits L-AP4-mediated presynaptic inhibition.
To address these possibilities we obtained sequential paired recordings from interneurons and pyramidal cells receiving synaptic contacts from the same identified presynaptic CA3 pyramidal cell. For this purpose we took advantage of the high probability of finding monosynaptically connected pairs of neurons in hippocampal organotypic slice cultures (32). We recorded from interneurons located in the stratum oriens, because they are easier to visualize. We first confirmed the results obtained in acute slices, using paired recordings: in pyramidal cell/alveus-oriens interneuron pairs L-AP4 (50 μM) depressed unitary EPSCs by 37 ± 5% (n = 12) but did not affect unitary EPSCs recorded between pairs of pyramidal cells (108 ± 6% of baseline, n = 6). Connections between cell pairs were considered monosynaptic if the distribution of the latencies between the peak of the action potential and the peak of the first derivative of the EPSC varied by less than ± 1 ms. A typical experiment involving sequential paired recordings is illustrated in Fig. 3A. Action potentials triggered in a CA3 pyramidal cell by intrasomatic current injection elicited EPSCs in a monosynaptically connected alveus-oriens interneuron voltage-clamped at −70 mV. Perfusion of L-AP4 (50 μM) reversibly depressed the unitary EPSCs. After wash of L-AP4, the patch pipette was retracted from the interneuron, and a new whole-cell recording was made from a pyramidal cell monosynaptically excited by the same presynaptic CA3 pyramidal cell. Application of L-AP4 had no effect on the amplitude of these EPSCs. Fig. 3B illustrates a summary graph of three similar experiments. These results provide direct evidence that activation of group III mGLURs decreases glutamate release only at presynaptic terminals contacting interneurons.
Figure 3.
L-AP4 differentially affects transmitter release from terminals arising from an individual axon that contacts different cell types. (A) Voltage and current traces: action potentials triggered by intracellular current pulses in a presynaptic CA3 pyramidal cell (1) evoked unitary EPSCs in a monosynaptically connected alveus-oriens interneuron (2). L-AP4 (50 μM) reversibly depressed the response. The monosynaptic response between the same presynaptic pyramidal cell and a subsequently recorded postsynaptic pyramidal cell (3) was not affected by L-AP4. The traces are composed of five superimposed sweeps. Plots: time course of this experiment for the amplitudes of the EPSCs; (Insets) EPSC latency histograms. (B) Summary graphs (n = 3; bin size: 3 stimuli). In one experiment, the postsynaptic cells were recorded in the reverse order.
Target Cell-Specific Facilatory Properties of Transmitter Release at Excitatory Terminals Originating from the Same Axon.
We also compared short-term changes in synaptic transmission between CA3 pyramidal cell connections to pyramidal cells and oriens-alveus interneurons in organotypic cultures. Two action potentials were triggered in a presynaptic CA3 pyramidal cell by current injection at an interval of 50 ms. Postsynaptic cells were voltage-clamped at −60 mV. We consistently observed a greater PPR of unitary EPSCs at connections between pyramidal cells and alveus-oriens interneurons (1.96 ± 0.35, n = 10 pairs) than at connections between two pyramidal cells (1.1 ± 0.22, n = 8 pairs, significantly different P < 0.05). To establish whether the observed differences in PPR were target cell-specific we performed sequential paired recordings, as illustrated in Fig. 4. The results revealed significant differences in PPR between presynaptic terminals originating from a single axon but contacting different target cells (n = 3, paired Student’s t test, P < 0.04). Amplitude fluctuations of EPSCs also depended on the target cell. In sequential paired recordings, the mean CV of the amplitude of the first and second EPSC at a pyramidal to oriens-alveus interneuron synapse was 0.75 ± 0.06 and 0.51 ± 0.06, respectively, whereas the CV at a pyramidal to pyramidal synapse was 0.28 ± 0.5 and 0.28 ± 0.4 (n = 3). The CV of the first EPSC recorded in alveus-oriens interneurons was significantly different from the CV of the first EPSC recorded in pyramidal cells (paired Student’s t test, P < 0.02).
DISCUSSION
Do all terminals of an axon have the same properties? The present study clearly shows that for the axons of hippocampal CA3 pyramidal cells this is not the case. By using sequential paired recordings, we demonstrate that both pharmacological and physiological properties of Schaffer collateral terminals belonging to the same axon can be clearly different depending on the type of target cell they innervate. Thus, presynaptic properties modulating transmitter release are determined in part by the target cell and not exclusively by the presynaptic neuron.
Bath perfusion of L-AP4 depressed transmitter release from Schaffer collateral terminals impinging on interneurons but not on CA1 pyramidal cells. Which mGLUR(s) subtype is involved? The effectiveness of the agonist L-AP4 and the inability of α-methyl-4-carboxyphenylglycine (an antagonist with some specificity for group I and II mGLURs) to block its action, even at high concentrations, point to the involvement of group III mGLURs and exclude groups I and II. Recent morphological data indicate that the group III mGLUR subtype 7 is selectively expressed on Schaffer collateral terminals impinging on stratum oriens interneurons (19). The mGLUR7 could, thus, underlie the observed target cell-specific inhibition of transmitter release. Some experimental results are, however, inconsistent with this hypothesis. First, the concentration of L-AP4 (10–50 μM) used in our study is much lower compared with the EC50 (160–500 μM) of the recombinant mGLUR7 for L-AP4 (33–35) and CPPG did not antagonize its action. These discrepancies may result from a difference in the percentage of receptor occupancy necessary to induce an effect in native and expression systems as well as from the weak potency of CPPG on mGLUR7. Second, the depression of transmitter release from Schaffer collateral terminals by L-AP4 was observed on both oriens and stratum radiatum interneurons. This result may suggest that presynaptic mGLURs other than mGLUR7 are involved in target-specific presynaptic inhibition. Development of more specific antagonists is needed to identify the presynaptic mGLUR(s).
Paired pulse facilitation at synapses formed by CA3 pyramidal cell axons was two to three times greater when the target was an alveus-oriens interneuron compared with a pyramidal cell. Does the observed difference represent a difference in the sensitivity of the target cell to repetitively released glutamate or a difference in the presynaptic properties of release? The rate of facilitation observed in the postsynaptic neuron can be influenced by purely postsynaptic factors, such as the desensitization of AMPA receptors (36, 37) or by a postsynaptic Ca2+ signal pathway (38, 39). These factors are, however, unlikely to determine the observed differences in PPR because, first, reduction of AMPA receptor desensitization by aniracetam does not affect PPR at the pyramidal to pyramidal cell synapse (40), second, recovery from desensitization of AMPA receptors is too rapid do explain the low PPR observed in pyramidal cells as compared with alveus-oriens interneurons (41), and third, the presence of 1,2-bis(2-aminophenoxy)ethane-N,N,N,N,-tetraacetic acid in our recording pipettes excludes the involvement of Ca2+ signal pathways. The difference in PPR is thus likely to indicate target cell-specific differences in the properties of release.
Several possibilities could account for the target cell-specific depression of transmitter release by group III mGLUR activation, including differences in the transduction molecules downstream to the receptors, or changes in the spatial distribution between receptors and effectors at different presynaptic terminals, or alterations in the expression of mGLURs. This last possibility is supported by immunohistological evidence indicating that the density of mGLURs is higher at Schaffer collateral terminals impinging on interneurons than pyramidal cells (19).
How can target cell-specific differences in PPR be explained? Differences in PPR are generally interpreted as differences in release probability (40, 42) but this may not apply to functionally or anatomically different terminals. A difference in the depletability of the readily releasable vesicle pool, in the Ca2+-binding site responsible for facilitation, in the amount of presynaptic Ca2+ influx upon repetitive stimulation, or in the presynaptic Ca2+-buffering characteristics also may lead to a different PPR. Further experiments will be necessary to address this issue.
Presynaptic metabotropic glutamate autoreceptors are known to be involved in activity-dependent inhibition of synaptic efficacy, which can last from hundreds of milliseconds to several tens of minutes (27, 43–46). Paired pulse facilitation, on the other hand, represents a change in release probabilities that decays within a few tens of milliseconds (40, 42, 47). Hence, depending on the frequency of synaptic activity, one or the other of these mechanisms will prevail. In the present study, we show a differential distribution of these mechanisms between presynaptic terminals impinging onto different target cells. By altering its firing frequency, a presynaptic neuron may thus differentially modulate the excitatory synaptic input to different target cells. To establish the conditions necessary for the endogenous activation of group III mGLURs on terminals contacting interneurons, the development of more specific antagonists is needed.
Acknowledgments
We would like to thank L. Rietschin and L. Heeb for technical assistance, U. Gerber, E. Audinat, S. M. Thompson, J. Mertz, and J. Rossier for helpful discussions and comments on the manuscript. Supported by the Swiss National Science Foundation and the Centre National de la Recherche Scientifique.
ABBREVIATIONS
- CV
coefficient of variation
- EPSC
excitatory postsynaptic current
- EPSP
excitatory postsynaptic potential
- fEPSP
field EPSP
- IPSP
inhibitory postsynaptic potential
- L-AP4
l(+)-2-amino-4-phosphonobutyric acid
- mGLUR
metabotropic glutamate receptor
- NBQX
6-nitro-7-sulfamoylbenzo(f)quinoxaline-2,3-dione
- PPR
paired pulse ratio
- NMDAR
N-methyl-d-aspartic acid receptor
- AMPA
[alpha]-amino-3-hydroxy-5-methyl-4-isoxalozolepropionic acid
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1. Wojtowicz J M, Marshall K C, Hendelman W J. Neuroscience. 1978;3:607–618. doi: 10.1016/0306-4522(78)90002-7. [DOI] [PubMed] [Google Scholar]
- 2.Andersen P, Trommald M, Jensen V. Adv Second Messenger Phosphoprotein Res. 1994;29:340–351. [PubMed] [Google Scholar]
- 3.Li X G, Somogyi P, Ylinen A, Buzsaki G. J Comp Neurol. 1994;339:181–208. doi: 10.1002/cne.903390204. [DOI] [PubMed] [Google Scholar]
- 4.Baldissera F, Hultborn H, Illert M. In: Handbook of Physiology, Section 1: The Nervous System. Brooks V B, editor. Vol. 2. Bethesda, MD: Am. Physiol. Soc.; 1981. pp. 509–595. [Google Scholar]
- 5.Eeckman F H, Freeman W J. Brain Res. 1990;528:238–244. doi: 10.1016/0006-8993(90)91663-2. [DOI] [PubMed] [Google Scholar]
- 6.Jefferys J G, Traub R D, Whittington M A. Trends Neurosci. 1996;19:202–208. doi: 10.1016/s0166-2236(96)10023-0. [DOI] [PubMed] [Google Scholar]
- 7.Atwood H L. Nature (London) 1967;215:57–58. doi: 10.1038/215057a0. [DOI] [PubMed] [Google Scholar]
- 8.Atwood H L, Bittner G D. J Neurophysiol. 1971;34:157–170. doi: 10.1152/jn.1971.34.1.157. [DOI] [PubMed] [Google Scholar]
- 9.Frank E. J Physiol (London) 1973;233:635–658. doi: 10.1113/jphysiol.1973.sp010327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laurent G, Sivaramakrishnan A. J Neurosci. 1992;12:2370–2380. doi: 10.1523/JNEUROSCI.12-06-02370.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardner D. J Neurophysiol. 1991;66:2150–2154. doi: 10.1152/jn.1991.66.6.2150. [DOI] [PubMed] [Google Scholar]
- 12.Muller K J, Nicholls J G. J Physiol (London) 1974;238:357–369. doi: 10.1113/jphysiol.1974.sp010529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis G W, Murphey R K. J Neurosci. 1993;13:3827–3838. doi: 10.1523/JNEUROSCI.13-09-03827.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katz P S, Kirk M D, Govind C K. J Neurosci. 1993;13:3075–3089. doi: 10.1523/JNEUROSCI.13-07-03075.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lnenicka G A, Mellon D. J Physiol (London) 1983;345:285–296. doi: 10.1113/jphysiol.1983.sp014978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis G W, DiAntonio A, Petersen S A, Goodman C S. Neuron. 1998;20:305–315. doi: 10.1016/s0896-6273(00)80458-4. [DOI] [PubMed] [Google Scholar]
- 17.Davis G W, Goodman C S. Nature (London) 1998;392:82–86. doi: 10.1038/32176. [DOI] [PubMed] [Google Scholar]
- 18.Koerbe R H, Mendell L M. J Neurophysiol. 1991;65:590–597. doi: 10.1152/jn.1991.65.3.590. [DOI] [PubMed] [Google Scholar]
- 19.Shigemoto R, Kulik A, Roberts J D, Ohishi H, Nusser Z, Kaneko T, Somogyi P. Nature (London) 1996;381:523–525. doi: 10.1038/381523a0. [DOI] [PubMed] [Google Scholar]
- 20.Thomson A M. J Physiol (London) 1997;502:1. doi: 10.1111/j.1469-7793.1997.131bl.x. , 131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ali A B, Deuchars J, Pawelzik H, Thomson A M. J Physiol (London) 1998;507:1. doi: 10.1111/j.1469-7793.1998.201bu.x. , 201–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maccaferri G, Tóth K, McBain J C. Science. 1998;279:1368–1370. doi: 10.1126/science.279.5355.1368. [DOI] [PubMed] [Google Scholar]
- 23.Gähwiler B H. J Neurosci Methods. 1981;4:329–342. doi: 10.1016/0165-0270(81)90003-0. [DOI] [PubMed] [Google Scholar]
- 24.Conn P J, Pin J P. Toxicology. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 25.Koerner J F, Cotman C W. Brain Res. 1982;251:105–115. doi: 10.1016/0006-8993(82)91278-1. [DOI] [PubMed] [Google Scholar]
- 26.Gereau IV R W, Conn P J. J Neurosci. 1995;15:6879–6889. doi: 10.1523/JNEUROSCI.15-10-06879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baskys A, Malenka R C. J Physiol (London) 1991;444:687–701. doi: 10.1113/jphysiol.1991.sp018901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knowles W D, Schwartzkroin P A. J Neurosci. 1981;1:318–322. doi: 10.1523/JNEUROSCI.01-03-00318.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lacaille J C, Mueller A L, Kunkel D D, Schwartzkroin P A. J Neurosci. 1987;7:1979–1993. doi: 10.1523/JNEUROSCI.07-07-01979.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deuchars J, Thomson A M. Neuroscience. 1996;74:1009–1018. doi: 10.1016/0306-4522(96)00251-5. [DOI] [PubMed] [Google Scholar]
- 31.Weisskopf M G, Nicoll R A. Nature (London) 1995;376:256–259. doi: 10.1038/376256a0. [DOI] [PubMed] [Google Scholar]
- 32.Debanne D, Guérineau N C, Gähwiler B H, Thompson S M. J Neurophysiol. 1995;73:1282–1294. doi: 10.1152/jn.1995.73.3.1282. [DOI] [PubMed] [Google Scholar]
- 33.Okamoto N, Hori S, Akazawa C, Hayashi Y, Shigemoto R, Mizuno N, Nakanishi S. J Biol Chem. 1994;269:1231–1236. [PubMed] [Google Scholar]
- 34.Saugstad J A, Kinzie J M, Mulvihill E R, Segerson T P, Westbrook G L. Mol Pharmacol. 1994;45:367–372. [PubMed] [Google Scholar]
- 35.Gereau IV R W, Conn P J. J Neurophysiol. 1995;74:122–129. doi: 10.1152/jn.1995.74.1.122. [DOI] [PubMed] [Google Scholar]
- 36.Trussell L O, Zhang S, Raman I M. Neuron. 1993;10:1185–1196. doi: 10.1016/0896-6273(93)90066-z. [DOI] [PubMed] [Google Scholar]
- 37.Geiger J R, Lubke J, Roth A, Frotscher M, Jonas P. Neuron. 1997;18:1009–1023. doi: 10.1016/s0896-6273(00)80339-6. [DOI] [PubMed] [Google Scholar]
- 38.Wang J H, Kelly P T. J Neurophysiol. 1996;76:276–286. doi: 10.1152/jn.1996.76.1.276. [DOI] [PubMed] [Google Scholar]
- 39.Bao J X, Kandel E R, Hawkins R D. Science. 1997;275:969–973. doi: 10.1126/science.275.5302.969. [DOI] [PubMed] [Google Scholar]
- 40.Debanne D, Guérineau N C, Gähwiler B H, Thompson S M. J Physiol (London) 1996;491:163–176. doi: 10.1113/jphysiol.1996.sp021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colquhoun D, Jonas P, Sakmann B. J Physiol (London) 1992;458:261–287. doi: 10.1113/jphysiol.1992.sp019417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dobrunz L E, Stevens C F. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- 43.Forsythe I D, Clements J D. J Physiol (London) 1990;429:1–16. doi: 10.1113/jphysiol.1990.sp018240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobayashi K, Manabe T, Takahashi T. Science. 1996;273:648–650. doi: 10.1126/science.273.5275.648. [DOI] [PubMed] [Google Scholar]
- 45.Scanziani M, Salin P A, Vogt K E, Malenka R C, Nicoll R A. Nature (London) 1997;385:630–634. doi: 10.1038/385630a0. [DOI] [PubMed] [Google Scholar]
- 46.Forsythe I D, Barnes-Davies M. Curr Biol. 1997;7:R362–R365. doi: 10.1016/s0960-9822(06)00175-8. [DOI] [PubMed] [Google Scholar]
- 47.Zucker R S. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]