Abstract
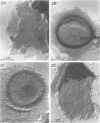
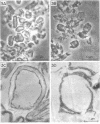
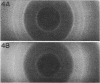
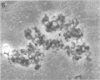
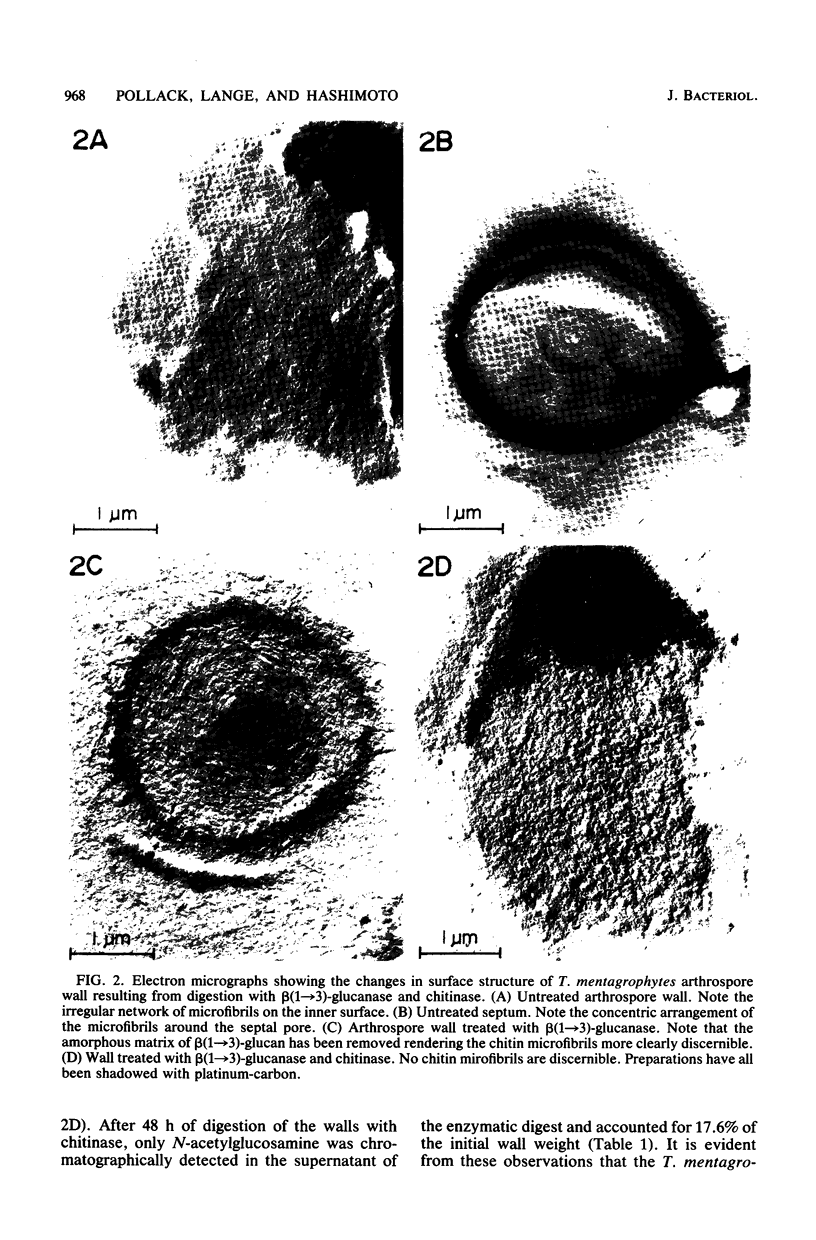
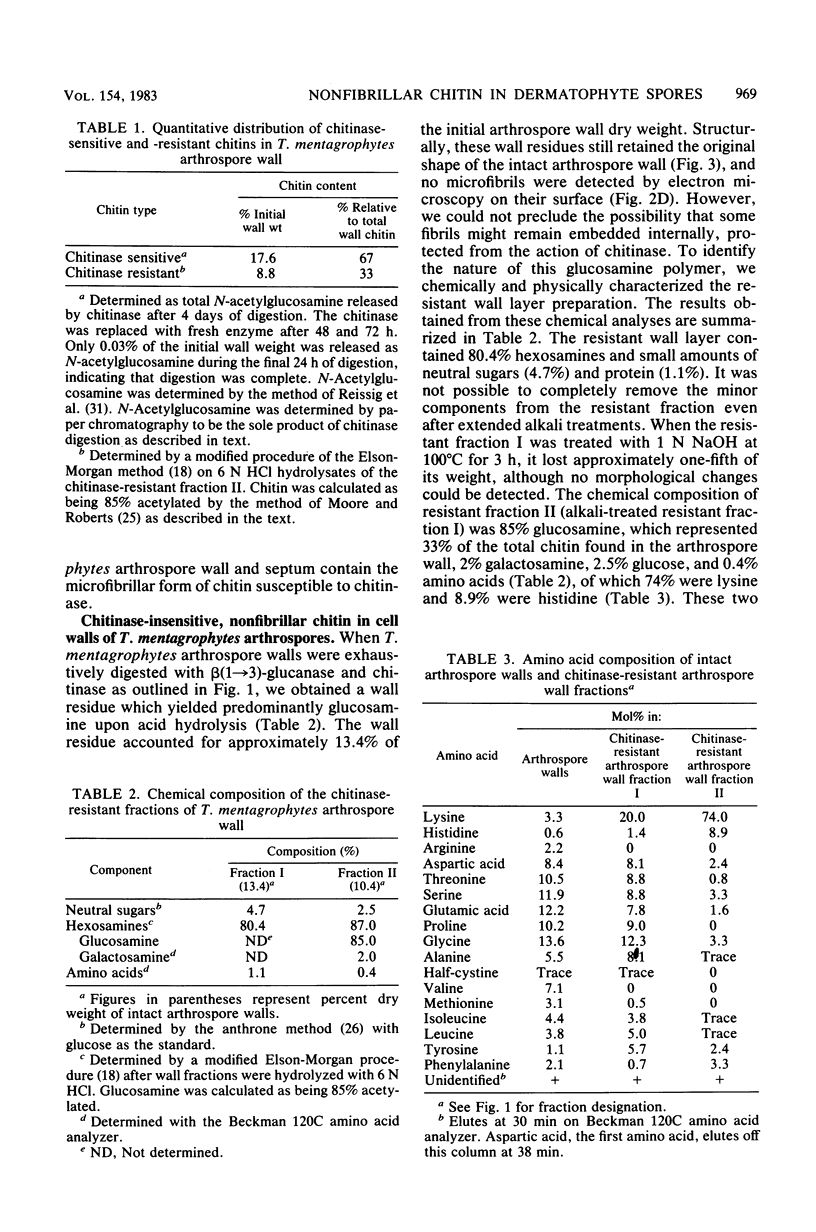
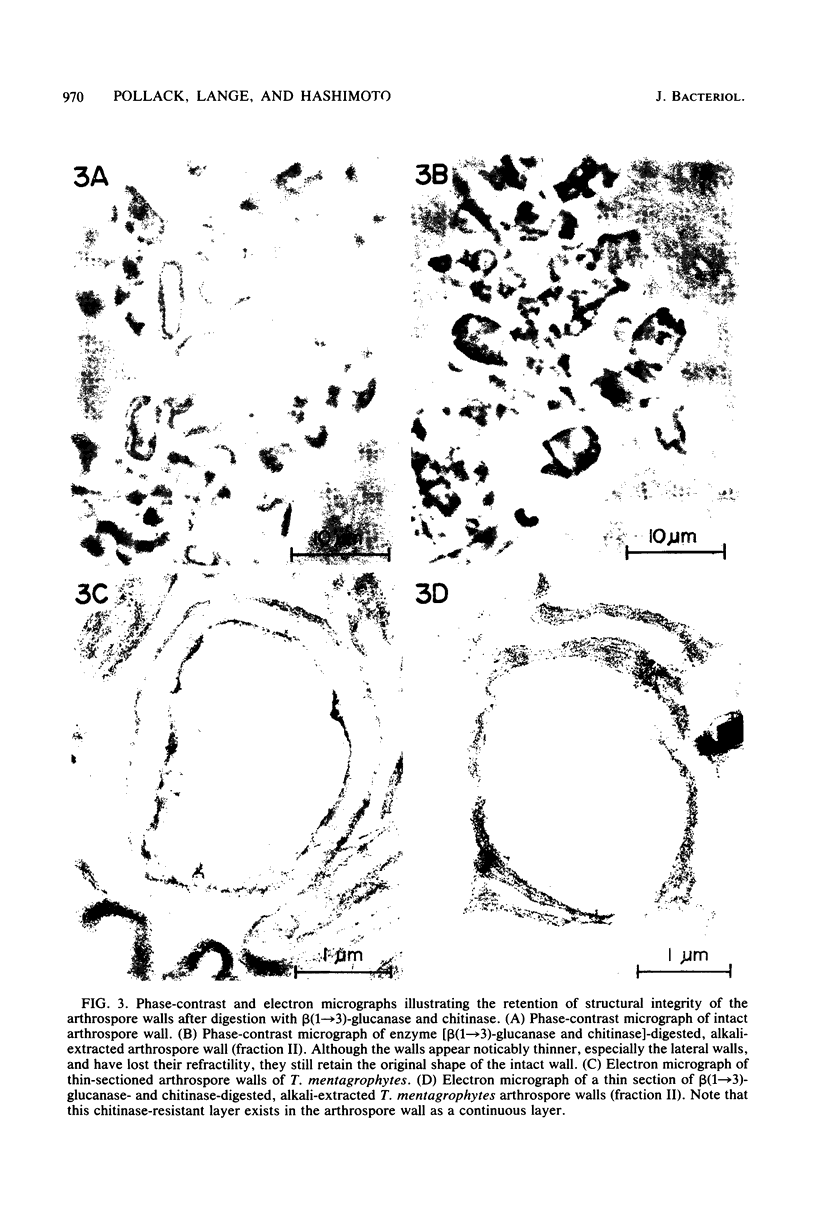
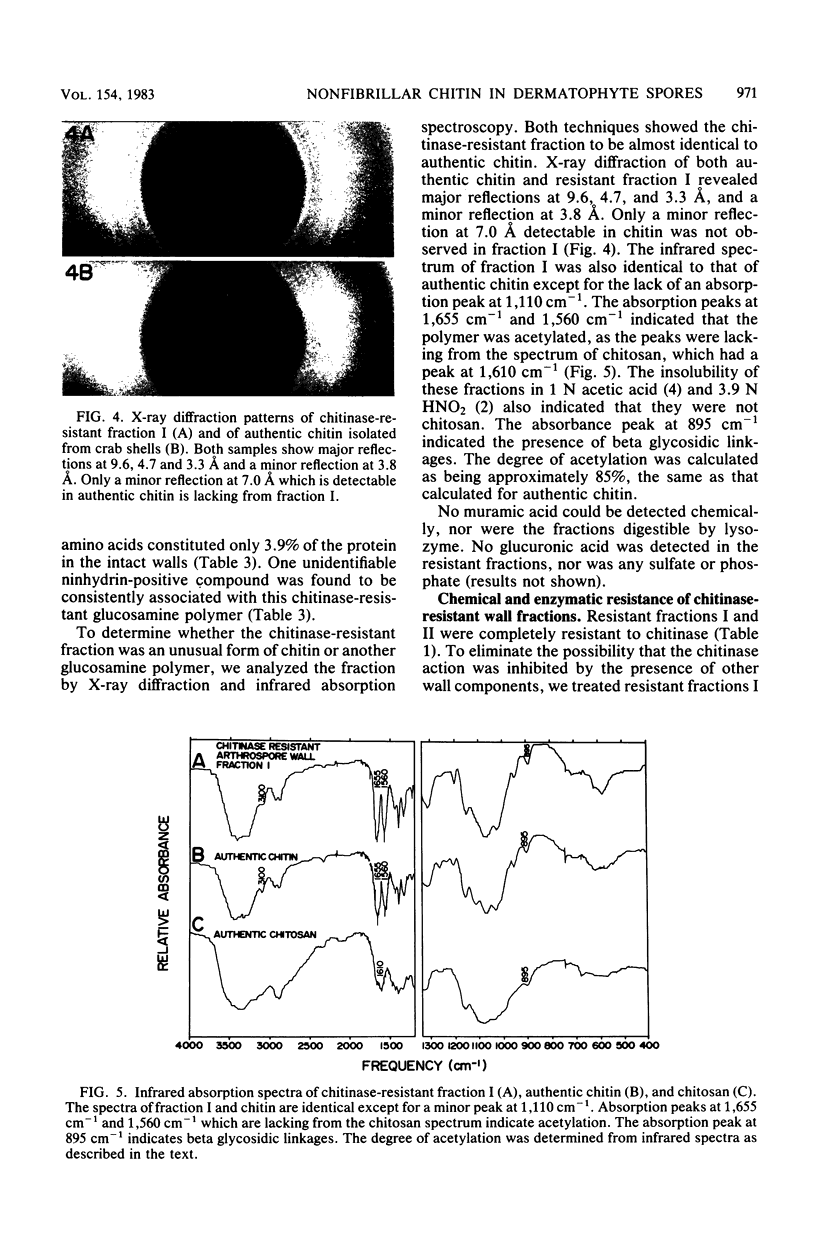
Two morphologically distinct forms of chitin were found in the arthrospore walls and septa of Trichophyton mentagrophytes. Two-thirds of the total wall chitin was the microfibrillar and chitinase-sensitive form. The remaining chitin existed in a previously uncharacterized "nonfibrillar" form and was insensitive to the action of Streptomyces chitinase. Exhaustive digestion of the arthrospore walls and septa with beta (1 leads to 3)-glucanase and chitinase followed by extraction with NaOH (1 N, 100 degrees C, 3 h) resulted in a fraction which retained the original wall shape. This fraction consisted of 85% N-acetylglucosamine, 2.0% galactosamine, 2.5% glucose, and 0.4% amino acids, 74% of which were lysine. Both its infrared spectrum and its X-ray diffraction pattern were almost identical to those of authentic chitin. There was no evidence of the presence of muramic acid, hexuronic acid, phosphate, or sulfate in this fraction. Its resistance to chitinase was due neither to the presence of protective wall layers or melanin nor to its close or covalent association with beta-glucan. Aside from its nonfibrillarity, this hexosamine polymer differed from authentic chitin in that it was soluble in 6 N HCl and 7.5 N NaOH. The development of this nonfibrillar chitin layer in the cell wall during arthrosporogenesis of T. mentagrophytes may be related to the arthrospores being resistant to a variety of antifungal agents.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTNICKI-GARCIA S., NICKERSON W. J. Isolation, composition, and structure of cell walls of filamentous and yeast-like forms of Mucor rouxii. Biochim Biophys Acta. 1962 Mar 26;58:102–119. doi: 10.1016/0006-3002(62)90822-3. [DOI] [PubMed] [Google Scholar]
- Ballesta J. P., Alexander M. Resistance of Zygorhynchus species to lysis. J Bacteriol. 1971 Jun;106(3):938–945. doi: 10.1128/jb.106.3.938-945.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardalaye P. C., Nordin J. H. Galactosaminogalactan from cell walls of Aspergillus niger. J Bacteriol. 1976 Feb;125(2):655–669. doi: 10.1128/jb.125.2.655-669.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnicki-Garcia S. Cell wall chemistry, morphogenesis, and taxonomy of fungi. Annu Rev Microbiol. 1968;22:87–108. doi: 10.1146/annurev.mi.22.100168.000511. [DOI] [PubMed] [Google Scholar]
- Bracker C. E., Ruiz-Herrera J., Bartnicki-Garcia S. Structure and transformation of chitin synthetase particles (chitosomes) during microfibril synthesis in vitro. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4570–4574. doi: 10.1073/pnas.73.12.4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell L. M., Kanetsuna F., Gil F. Chemical morphology of glucan and chitin in the cell wall of the yeast phase of Paracoccidioides brasiliensis. J Bacteriol. 1970 Feb;101(2):636–642. doi: 10.1128/jb.101.2.636-642.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman R. H., Goldberg M. Studies on chitin. VI. The nature of alpha- and beta-chitins. Aust J Biol Sci. 1965 Aug;18(4):935–946. doi: 10.1071/bi9650935. [DOI] [PubMed] [Google Scholar]
- Hadzija O. A simple method for the quantitative determination of muramic acid. Anal Biochem. 1974 Aug;60(2):512–517. doi: 10.1016/0003-2697(74)90261-9. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Blumenthal H. J. Survival and resistance of Trichophyton mentagrophytes arthrospores. Appl Environ Microbiol. 1978 Feb;35(2):274–277. doi: 10.1128/aem.35.2.274-277.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Wu C. D., Blumenthal H. J. Characterization of L-leucine-induced germination of Trichophyton mentagrophytes microconidia. J Bacteriol. 1972 Nov;112(2):967–976. doi: 10.1128/jb.112.2.967-976.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herth W. Calcofluor white and Congo red inhibit chitin microfibril assembly of Poterioochromonas: evidence for a gap between polymerization and microfibril formation. J Cell Biol. 1980 Nov;87(2 Pt 1):442–450. doi: 10.1083/jcb.87.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S., Yagi Y. The effects of N-substitution of chitosan and the physical form of the products on the rate of hydrolysis by chitinase from Streptomyces griseus. Carbohydr Res. 1980 Aug 1;83(1):103–108. doi: 10.1016/s0008-6215(00)85369-0. [DOI] [PubMed] [Google Scholar]
- Hunsley D., Gooday G. W. The structure and development of septa in Neurospora crassa. Protoplasma. 1974;82(1):125–146. doi: 10.1007/BF01276876. [DOI] [PubMed] [Google Scholar]
- Johnson A. R. Improved method of hexosamine determination. Anal Biochem. 1971 Dec;44(2):628–635. doi: 10.1016/0003-2697(71)90252-1. [DOI] [PubMed] [Google Scholar]
- Kanetsuna F., Carbonell L. M. Cell wall glucans of the yeast and mycelial forms of Paracoccidioides brasiliensis. J Bacteriol. 1970 Mar;101(3):675–680. doi: 10.1128/jb.101.3.675-680.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazima Y., Banno Y., Noguchi T., Nozawa Y., Ito Y. Effects of chemical modification of structural polymer upon the cell wall integrity of Trichophyton. Arch Biochem Biophys. 1972 Oct;152(2):811–820. doi: 10.1016/0003-9861(72)90277-9. [DOI] [PubMed] [Google Scholar]
- Mahadevan P. R., Tatum E. L. Localization of structural polymers in the cell wall of Neurospora crassa. J Cell Biol. 1967 Nov;35(2):295–302. doi: 10.1083/jcb.35.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monreal J., Reese E. T. The chitinase of Serratia marcescens. Can J Microbiol. 1969 Jul;15(7):689–696. doi: 10.1139/m69-122. [DOI] [PubMed] [Google Scholar]
- Morris D. L. Quantitative Determination of Carbohydrates With Dreywood's Anthrone Reagent. Science. 1948 Mar 5;107(2775):254–255. doi: 10.1126/science.107.2775.254. [DOI] [PubMed] [Google Scholar]
- Polacheck I., Rosenberger R. F. Aspergillus nidulans mutant lacking alpha-(1,3)-glucan, melanin, and cleistothecia. J Bacteriol. 1977 Nov;132(2):650–656. doi: 10.1128/jb.132.2.650-656.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potgieter H. J., Alexander M. Susceptibility and resistance of several fungi to microbial lysis. J Bacteriol. 1966 Apr;91(4):1526–1532. doi: 10.1128/jb.91.4.1526-1532.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- Reiss E., Miller S. E., Kaplan W., Kaufman L. Antigenic, chemical, and structural properties of cell walls of Histoplasma capsulatum yeast-form chemotypes 1 and 2 after serial enzymatic hydrolysis. Infect Immun. 1977 May;16(2):690–700. doi: 10.1128/iai.16.2.690-700.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Herrera J., Sing V. O., Van der Woude W. J., Bartnicki-Garcia S. Microfibril assembly by granules of chitin synthetase. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2706–2710. doi: 10.1073/pnas.72.7.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sietsma J. H., Wessels J. G. Solubility of (1 leads to 3)-beta-D/(1 leads to 6)-beta-D-glucan in fungal walls: importance of presumed linkage between glucan and chitin. J Gen Microbiol. 1981 Jul;125(1):209–212. doi: 10.1099/00221287-125-1-209. [DOI] [PubMed] [Google Scholar]
- Skujins J. J., Potgieter H. J., Alexander M. Dissolution of fungal cell walls by a streptomycete chitinase and beta-(1-3) glucanase. Arch Biochem Biophys. 1965 Aug;111(2):358–364. doi: 10.1016/0003-9861(65)90197-9. [DOI] [PubMed] [Google Scholar]
- Torres-Bauzá L. J., Riggsby W. S. Protoplasts from yeast and mycelial forms of Candida albicans. J Gen Microbiol. 1980 Aug;119(2):341–349. doi: 10.1099/00221287-119-2-341. [DOI] [PubMed] [Google Scholar]
- Van Etten J. L., Freer S. N. Simple procedure for disruption of fungal spores. Appl Environ Microbiol. 1978 Mar;35(3):622–623. doi: 10.1128/aem.35.3.622-623.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaar K., Beyer P., Kleinig H. The spherule wall of Physarum polycephalum: chemical analysis and electron microscopy. Biochim Biophys Acta. 1979 Jan 4;582(1):21–32. doi: 10.1016/0304-4165(79)90285-x. [DOI] [PubMed] [Google Scholar]