Abstract
Most proteins in the secretory pathway are translated, folded, and subjected to quality control at the endoplasmic reticulum (ER). These processes must be flexible enough to process diverse protein conformations, yet specific enough to recognize when a protein should be degraded. Molecular chaperones are responsible for this decision making process. ER associated chaperones assist in polypeptide translocation, protein folding, and ER associated degradation (ERAD). Nevertheless, we are only beginning to understand how chaperones function, how they are recruited to specific substrates and assist in folding/degradation, and how unique chaperone classes make quality control “decisions.”
Keywords: heat shock proteins, ERAD, proteasome, lectin, degradation
1. Introduction: Protein Biogenesis in the ER
The endoplasmic reticulum (ER) is the entry point for proteins in the secretory pathway. Approximately 30% of all newly synthesized proteins are delivered to their final cellular destinations via this pathway [1]. After translation is initiated in the cytosol, the ribosome-nascent chain complex docks at the Sec61 translocon in the ER membrane and protein synthesis resumes. Soluble proteins are translocated into the ER lumen, whereas transmembrane proteins are cotranslationally integrated into the lipid bilayer [2]. Proteins that contain an Asn-X-Ser/Thr sequence are glycosylated by oligosacchhryl transferase as the polypeptide emerges from the translocon. Next, the protein will fold properly and traffic to its final destination, or if folding is inefficient the protein can be targeted for ER associated degradation (ERAD) by the cytosolic proteasome [3-7]. These three events: protein translocation, protein folding, and protein degradation, are each facilitated by a group of factors termed molecular chaperones.
Before the ribosome-nascent chain complex even docks at the ER, molecular chaperones play an important role in ER physiology: The ER lumenal Hsp70 chaperone, BiP, maintains the permeability barrier between the cytosol and the ER lumen by gating the Sec61 translocon [8,9]. Next, as translocating proteins leave the translocon, folding and post-translational modifications are mediated by a collection of chaperones and enzymes. Three major groups of ER chaperones act on the nascent chain, heat shock protein homologues (e.g., Hsp70, Hsp40, Hsp90), the ER lectins (e.g., calnexin, calreticulin), and the thiol oxidoreductases (e.g., PDI). Several ER lumenal heat shock protein homologues prevent protein aggregation and promote folding. Similarly, the ER lectins bind to immature glycoproteins to promote substrate folding and will retain proteins in the ER in the event folding is compromised. The thiooxidoreductase proteins mediate proper disulfide bond formation and may also act as chaperones. Because transmembrane proteins have domains exposed in the ER lumen, in the cytosol, and in the membrane, this group of proteins may interact with factors in each of these compartments. For example, a different set of chaperones is responsible for recognizing the defect and targeting the protein for degradation depending upon where the folding lesion is located within the protein [6,10,11].
The same chaperones involved in “folding” the substrate may also trigger its proteolysis. In fact, all three chaperone classes are involved in targeting proteins for degradation in addition to catalyzing folding. Not surprisingly, many diseases are linked to defects in the ER associated quality control machinery (ERQC). For example, cystic fibrosis, antitrypsin deficiency, Alzheimer’s disease, Huntington’s Disease, and nephrogenic diabetes insipidus result when specific proteins either fail to fold properly and are targeted for degradation, or are hidden from the degradation machinery and accumulate as toxic aggregates [12,13].
In most cases, the accumulation of misfolded proteins in the ER leads to the activation of the unfolded protein response (UPR). In yeast, misfolded proteins in the ER are sensed by the transmembrane kinase, Ire1, which when activated promotes the splicing of the message for the Hac1 transcription factor. Hac1 regulates the transcription of UPR targets that include chaperones and ERAD effectors. In mammals, the ER sensor, ATF6, upregulates the synthesis of ER chaperones, and the ER transmembrane kinase PERK activates the synthesis of the ATF4 transcription factor but triggers a general inhibition of translation. Along with activation of Ire1, these cascades reduce the overall protein load in the mammalian secretory pathway. If, however, this load cannot be decreased, ATF4 and ATF6 dependent apoptosis may ensue [14-16].
2. Molecular chaperones, chaperone-like proteins, and their roles during protein biogenesis in the ER
In this section we describe the major classes of chaperones, cochaperones, and chaperone-like proteins that act on substrates in the ER. Because of their role in ER homeostasis, we will focus our discussion on eukaryotic systems. Due to space constrictions select examples in the literature will be highlighted.
2.1 Hsp70s
Members of the Hsp70/Hsc70 class of molecular chaperones are essential for protein folding, membrane translocation, disrupting protein aggregates, and rearranging multiprotein complexes. Indeed, Hsp70s are perhaps the best-studied class of chaperones [17]. Hsp70s are highly conserved and are found in all organisms. Hsp70 function and specificity are highly regulated by cochaperones such as Hsp40s and nucleotide exchange factors (NEFs) (described below).
All Hsp70s are composed of three domains, an ~44 kDa N-terminal ATPase domain, an ~15 kDa substrate binding domain, and an ~10 kDa C-terminal lid [18-20]. The extreme C-terminus contains an EEVD motif that interacts with cochaperones that possess degenerate 34 amino acid tetratricopeptide repeats (TPRs). Crystallographic studies of bovine Hsc70 show that the ATPase domain contains two globular lobes, which are separated by a deep ATP binding cleft [18]. A change in the tertiary structure of this domain is seen when ATP or ADP is bound in the active site [21]. The structure of the substrate-binding domain was first determined for the E.coli Hsp70, DnaK, and is primarily composed of β sheets that bind the hydrophobic peptide substrate [19]. The substrate is sequestered by, but does not interact with the α helical lid [19], but directly interacts with the substrate-binding domain in an extended conformation.
Hsp70s function by binding substrates and using the energy from ATP hydrolysis to influence substrate binding. The unstimulated rate of ATP hydrolysis is slow and ranges from 0.02 to 0.2 min-1 [22-24]. When Hsp70 is found in the ATP bound state, the lid is open and substrates have a low affinity for the peptide-binding domain, resulting in a high on/off rate. However, substrate binding significantly stimulates Hsp70 ATP hydrolysis and causes the lid to clamp down onto the substrate [25,26]. When ADP is bound the substrate interacts with the peptide-binding domain with high affinity, and thus has a low on/off rate [24,27]. After ADP is released, the Hsp70 rebinds ATP, the lid domain opens, and the peptide dissociates [28,29].
Nucleotide-and substrate-induced conformational changes in Hsp70 are becoming better defined and in one recent example were shown to require a proline at residue 143 and an arginine at residue 151 in the DnaK ATPase domain [30]. It has been proposed that ATP binding is relayed to the substrate-binding domain via the proline, most likely by a cis-trans isomerization, which then shifts the arginine toward the peptide-binding domain. When substrate binds to the peptide-binding domain and Hsp40 (see below) interacts with the ATPase domain, the arginine is moved inward and the conformation of the proline is altered. This conformation positions residues in the ATPase domain to facilitate ATP hydrolysis [30]. The coupling between the ATPase and substrate binding domains is also dependent on the movement of an interdomain linker. Overall, it is likely that a continued refinement of the ATP-dependent conformational changes will better elucidate how this intricate machine functions [31].
For most Hsp70s ATP hydrolysis represents the rate-limiting step [23,24]. In addition to peptide stimulation, the Hsp70 ATPase cycle is stimulated by cochaperones such as the Hsp40s and NEFs. Hsp40s stimulate ATP hydrolysis [32-34], whereas NEFs aid in the release of ADP [35,36]. Thus, these cofactors and the bound substrate function together to regulate Hsp70 function. The NEFs and Hsp40s will be discussed in subsequent sections.
Both the cytosolic and ER lumenal Hsp70s affect protein biogenesis at the ER. As noted above, the ER lumenal Hsp70, BiP/Kar2 may gate the translocon and is therefore required for the co-and post-translational translocation of proteins into the ER [8,9]. As the nascent chain emerges from the ribosome, or during post-translational translocation, BiP binds the chain and promotes translocation through a ratcheting mechanism [37]. As the chain continues to lengthen and increasing polypeptide lengths enter the ER, BiP “holds” the nascent chain in the ER and prevents it from moving back into the cytoplasm. Mathematical modeling suggests that Brownian motion and the action of BiP is a plausible mechanism for translocation [38]. Further, in vesicles lacking BiP, antibodies that bind the peptide chain during translocation can replace BiP, suggesting that protein binding alone is sufficient to facilitate translocation [37].
Upon the completion of translation, BiP assists in protein folding in the ER. For example, unfolded carboxypeptidase Y (CPY), a soluble protein targeted to the vacuole in the secretory pathway, fails to mature in yeast strains with mutations in BiP; instead, CPY is recovered in high molecular weight aggregates associated with BiP [39]. Mutations that decrease BiP ATPase activity increase the BiP-CPY association, and because the mutant BiP does not release CPY, folding is inefficient [39]. More recently, it has become apparent that BiP’s role in protein folding may be coordinated with Lhs1, a distant Hsp70 homologue in the ER lumen [40]. BiP and Lhs1 reciprocally stimulate each other’s ATPase activity, and Lhs1 acts as an NEF for BiP [40]. Both proteins are necessary for protein folding in the ER, and BiP and Lhs1 may alternatively and/or sequentially bind unfolded proteins since the substrate specificities of different classes of Hsp70s are variable [40,41]. In support of this hypothesis, BiP and the mammalian Lhs1 homolog, GRP170, were both found to associate with unfolded immunoglobulin chains [41,42].
In the event that unfolded proteins accumulate in the ER lumen, the UPR is activated. In one model, under nonstressed conditions BiP binds to the lumenal domain of Ire1, but under stressed conditions BiP is titrated away from this UPR transducer by misfolded proteins. This results in the activation of both Ire1 and consequently the UPR [43,44]. Alternatively, more recent studies suggest that BiP may play only an indirect or regulatory role in UPR induction because deleting or mutating the BiP binding region in Ire1 had little effect on Ire1’s response to unfolded proteins [45]. In addition, a recent crystal structure of the lumenal domain of yeast Ire1 suggests that this UPR sensor binds directly to unfolded proteins [46].
ER lumenal Hsp70s are not only important for protein folding and sensing the UPR, but also for targeting soluble proteins for ERAD. For example, yeast BiP (also known as Kar2) is required for the degradation of the soluble ERAD substrate, CPY*, because it retains CPY* in a soluble conformation [47]. In accordance with this result, a BiP mutant that is proficient for translocation but deficient for ERAD leads to the accumulation of unfolded proteins in the ER and UPR induction [48]. Therefore, the roles BiP plays in translocation and targeting proteins for degradation are distinct.
The cytosolic Hsp70s are also conserved and fall into two classes: Hsp70s, which are stress-inducible and Hsc70s, which are constitutively expressed Hsp70 homologues. Historically, the major difference between Hsps and Hscs was thought to be their mode of expression. However, recent work has shown that Hsp70 and Hsc70 have different effects on the biogenesis of membrane spanning proteins. Whereas Hsc70 decreased the functional expression of the epithelial sodium channel, ENaC, Hsp70 had the opposite effect and promoted the surface expression of the channel [49]. Along with its cofactors, Hsc70 was also proposed to facilitate the early biogenesis of CFTR whereas Hsp70 may be important for ERAD [50]. It was suggested that the Hsc70 chaperone complex functions by stabilizing CFTR’s large cytoplasmic domains during translation, and consistent with this model Hsc70 and its cochaperones precipitate with newly translated CFTR [51].
The ERAD of most membrane-spanning proteins does not require the ER lumenal Hsp70s, but does require the cytosolic Hsp70s. For example, the degradation of CFTR was inhibited in yeast strains with mutations in the cytosolic Hsp70, Ssa1. In contrast, there was no effect on CFTR degradation in strains with mutations in BiP [52]. Integral membrane ERAD substrates that require neither the cytosolic nor lumenal Hsp70s have also been identified [11].
2.2 Hsp40s
Members of the Hsp40 class of chaperones can act as Hsp70 cochaperones. Although their best-defined role is to stimulate Hsp70 ATP hydrolysis, some Hsp40 homologues also have a substrate-binding domain and deliver substrates to Hsp70s [53-57]. The prototypical Hsp40 is the E. coli DnaJ [32]. All Hsp40s contain a J domain, named for a conserved ~70 amino acid motif in DnaJ, and are often referred to as J domain containing proteins or J-proteins. The structures of several J domains have been elucidated and indicate the presence of four alpha helices [58]. Helices II and III interact to form a finger-like projection and the loop between Helix II and III contains an invariant HPD motif, which is essential for stimulating Hsp70 ATP hydrolysis. DnaJ is a type I Hsp40, and contains an N-terminal J domain, a glycine/phenylalanine-rich domain (G/F), and a cysteine-rich domain. In contrast, type II Hsp40s lack a cysteine-rich domain, but generally have a longer glycine/phenylalanine rich domain. These differences may dictate substrate specificity [59]. A third class of J-domain containing proteins, the type III proteins, contains only the J domain, and may employ this domain to recruit Hsp70.
The Bukau lab determined that DnaJ preferentially binds short polypeptides that are enriched for aromatic and/or hydrophobic residues [55]. But, between similar Hsp40s some degree of divergence in peptide substrate has been observed [59]. The preferred peptides for Hsp40 are slightly different than Hsp70 [60], but most peptides that bind to Hsp40 also bind to Hsp70 and two hypotheses for substrate transfer from Hsp40 to Hsp70 have been proposed [55]. The “handover” mechanism suggests that Hsp70 displaces Hsp40 from the substrate and binds the same residues. A second hypothesis proposes that Hsp40 and Hsp70 bind residues in close proximity and each protein occupies discrete positions on the substrate simultaneously, which has been experimentally for select substrates [53].
Because each cell contains multiple Hsp70s and Hsp40s, the nature of substrate-specific interactions involving individual Hsp70 and Hsp40 is mysterious. Some degree of specificity is certainly created simply by compartmentalization. Membrane enclosed compartments such as the ER and mitochondria have dedicated Hsp70s and Hsp40s, which are physically separated from the cytosolic chaperones. However, specificity is also seen within a single compartment. For example, the yeast cytosolic Hsp70, Ssa1, is stimulated ~6 fold by a cytosolic partner, Ydj1, but only 1.5 fold by the J domain of Sec63, which resides in the ER lumen. Similarly, BiP is stimulated ~13 fold by the Sec63 J domain, but only 1.4 fold by Ydj1 [61]. J domain swapping experiments have also been performed to address Hsp70/Hsp40 specificity [58]. These studies have shown that not all J domains can functionally substitute for each other and that J domains must encode information for interaction specificity. J domain swaps between the yeast cytosolic J-protein, Sis1, mitochondrial J-protein, Mdj1, and the ER lumenal J-protein, Scj1, with Sec63 were performed and indicated that the J domains of mitochondrial Mdj1p and cytosolic Sis1 do not substitute for the ER lumenal Sec63 J domain. However, ER lumenal Scj1 functionally substituted for Sec63, which is perhaps not surprising since both proteins interact with BiP [62]. In some J-proteins the J domain appears sufficient to dictate specificity. For example, the exposed regions of some type III J-proteins (e.g., polyomavirus tiny tumor antigen (tAg) and Sec63) are extremely short. Despite its size, the Sec63 J domain allows productive interactions with BiP, but not cytoplasmic Hsp70, Ssa1 [61]. However, none of these studies rule-out the possibility that residues beyond the J domain play an additional role in determining specificity.
The Hsp40 Sec63 acts as a cochaperone for BiP to facilitate translocation into the ER lumen [63]. But, BiP interacts with two other ER lumenal Hsp40s, Scj1 and Jem1, to retain proteins in a soluble conformation [47,64]. In the event that a soluble, secreted protein fails to mature, Scj1 and Jem1 are also required for ERAD. In contrast, these lumenal Hsp40s have no effect on the ERAD of membrane-spanning proteins that have been examined, but in mammals the cytosolic Ydj1 homologue, Hdj2, acts a cochaperone for Hsc70 to facilitate early folding events during the biogenesis of CFTR. The Hsc70-Hdj2 complex associates at ~2 fold higher levels with the disease-causing, mutant form of CFTR (ÄF508 CFTR) than WT CFTR, which is consistent with the fact that ÄF508 folds less efficiently than WT. Further evidence that this complex plays a role in folding is its ability to maintain the solubility of a large, cytoplasmic nucleotide binding domain in CFTR during the translation of the C-terminal portion of the protein [51] (also see Figure 1). Overexpression of another cytosolic Hsp40, Csp, in CFTR expressing cells blocks CFTR maturation by “holding” it in the ER where it will either complete folding or be subjected to ERAD [65]. Similarly, the cytosolic Hsp40s, Ydj1 and Hlj1, in yeast function redundantly to promote CFTR degradation in yeast [66]. Overall, it is clear that Hsp40s are important mediators of ERQC but the rules governing whether distinct Hsp40s help fold and/or degrade a given substrate have yet to be defined.
Figure 1. Models for chaperone involvement in the ER associated biogenesis and degradation of the soluble CPY/CPY* and the transmembrane spanning CFTR proteins.
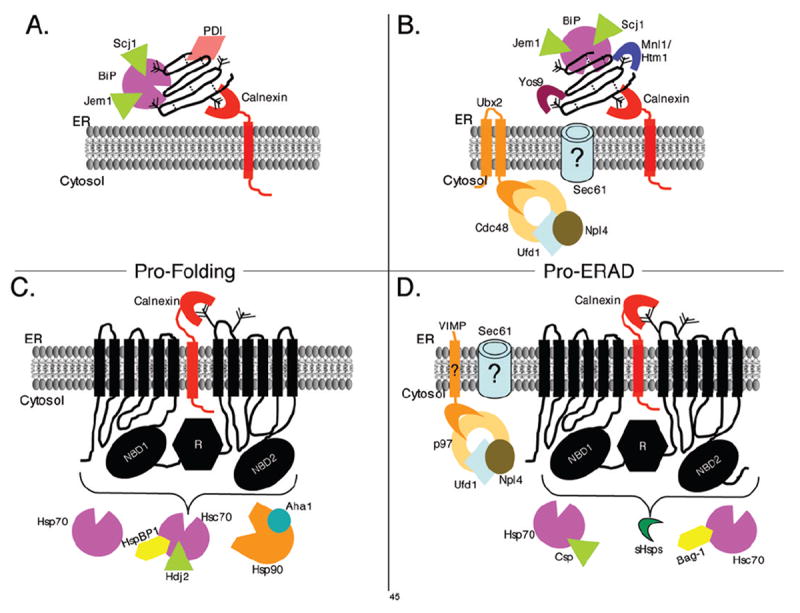
Model for molecular chaperones and cofactors required for CPY folding (A) and degradation (B) based on data from yeast. Similarly, a model is presented demonstrating the molecular chaperone and cofactors identified to date in the folding (C) and ERAD (D) of CFTR in mammalian cells. The models highlight the differential requirements for folding and ERAD of a soluble versus membrane spanning protein. The examples in these models focus on select molecular chaperones, and other proteins involved are not included in the model (i.e. proteins involved in the ubiquitination cascade). In (A) and (B) the dotted lines depict disulfide bonds and in each panel the “tree” depicts the core oligosaccharide with a varying number of mannoses.
2.3 Nucleotide Exchange Factors (NEFs)
In the presence of a J domain containing protein, the rate-limiting step in the Hsp70 cycle becomes the release of ADP [67]. Not surprisingly, then, NEFs have evolved to enhance the liberation of ADP from Hsp70s. ATP then preferentially rebinds because of its higher intracellular concentration. Interestingly, three structurally distinct Hsp70 NEFs have been identified, GrpE in bacteria, and BAG-1 and HspBP1 in eukaryotes. Also, recent studies have shown that the yeast Hsp110 homologue, Sse1p, acts as a NEF for Ssa1p [68,69], and is likely to enhance ADP release through yet another mechanism. Each of these factors is considered below.
GrpE was the first Hsp70 NEF identified and acts on DnaK. GrpE homologues have only been identified in bacteria and eukaryotic organelles of prokaryotic origin, such as the mitochondria [35]. GrpE was discovered as a protein required for λ phage replication [70] that removes both ADP and ATP from DnaK [71], though the affinity of GrpE for DnaK is decreased in the presence of ATP [72]. GrpE crystallizes as a dimer, which is consistent with the observed 1:2 ratio of DnaK to bound to GrpE [73,74]. GrpE interferes with nucleotide binding by loosening the conformation of DnaK’s ATPase domain.
The mammalian BAG-1 NEF was first identified as a Bcl-2 associated athanogene, which binds anti-apoptotic Bcl-2 and was then discovered to associate with other proteins, including Hsp70 [75]. All of the functions of BAG-1 in the cell are currently unclear, but BAG-1 has convincingly been shown to be a NEF for Hsp70 [76]. A BAG-1 homologue, Snl1p, has been identified in S. cerevisiae, which interacts with cytosolic Hsp70s and stimulates nucleotide exchange [77]. The BAG domain is required for NEF activity, and is structurally distinct from GrpE [78]. Unlike GrpE, BAG-1 is selective and only releases ADP from Hsp70 [71]. Interestingly, BAG-1 has differing effects on Hsp70 substrate folding, depending on the Hsp70:BAG-1 stoichiometry [79].
A third NEF class is exemplified by the Hsp Binding Protein 1, HspBP1, which was first identified as a cytosolic inhibitor of Hsp70 refolding at high concentrations [80]. As with BAG-1 it now appears that HspBP1 also facilitates protein folding when the HspBP1:Hsp70 ratio is adjusted [81]. Two other HspBP1 homologues have been identified, Fes1 in the yeast cytosol and BAP in the mammalian ER [82,83]. Interestingly, Fes1 is an NEF for two yeast cytosolic chaperones. In addition, fes1 cells are sensitive to cycloheximide, an inhibitor of protein translation, and contain somewhat increased levels of 80S ribosomes relative to wild-type cells [82,84]. Taken together, these results suggest that Fes1 may be important for folding newly synthesized polypeptides as they exit the ribosome. These NEFs are structurally distinct from GrpE and BAG-1 and utilize a unique mechanism of action [85].
Recently, the yeast Hsp110 molecular chaperone, Sse1, was shown to exhibit nucleotide exchange activity [68,69]. Hsp110s are distantly related to Hsp70s and have similar N-terminal ATPase domains, but the C-terminal, putative substrate-binding domain is not homologous to Hsp70. In addition, Hsp110s have extended regions between the ATPase and C-terminal domains and at the extreme C-terminus [86,87]. Hsp110 acts as a holdase, and prevents protein aggregation but cannot catalyze protein folding [88]. Sse1 is found in chaperone complexes with yeast Hsp90 and Hsp70 [89,90]. A role in Hsp70 function was hinted at by the discovery of SSE1 as a multi-copy suppressor of the ydj1-151 thermosensitive allele of the yeast Hsp40, YDJ1 [91]. This suggested that Sse1, like Ydj1, stimulates the Hsp70 ATP hydrolysis cycle. In fact, Sse1 stimulates the steady state ATPase activity of Hsp70, but does not stimulate Hsp70 in single turnover ATPase assays, suggesting it is involved in nucleotide release. Fluorescent ADP-Hsp70 release assays in the presence Sse1 confirmed this hypothesis [68]. Sse1 also stimulates substrate release from Hsp70 [84]. While Sse1 cannot fold a model substrate independently, it stimulates folding in the presence of Hsp70 in vivo and in vitro [68,84]. To date, a role for Sse1 in ERAD has only been established for one substrate, apolipoprotein B (apoB). In a yeast model system, loss of Sse1 led to decreased ERAD of the lipoprotein, and over-expression of Hsp110 increased apoB secretion from hepatic cells. Therefore, Sse1 seems to be important for stabilizing apoB [92].
Although cellular roles for NEFs are still being defined, the data discussed above suggest that NEFs play varied roles in protein biogenesis. Consistent with this view, HspBP1 stimulates CFTR folding, possibly by inhibiting the binding of a ubiquitin ligase, CHIP [93]. CHIP, in association with Hsc70, targets CFTR for proteasome-mediated degradation. In contrast, BAG-1 facilitates CFTR degradation by stimulating the Hsc70-CHIP complex [94,95]. Thus, distinct NEFs can play opposing roles during the biogenesis of a given substrate.
2.4 Hsp90s
Hsp90s, like Hsp70s, bind and release substrates concomitant with ATP binding/hydrolysis. However, unlike Hsp70s and Hsp40s, which are involved in folding newly synthesized polypeptides, Hsp90s fold a specific set of client proteins that have neared their native conformations (http://www.picard.ch/downloads/Hsp90interactors.pdf). Specifically, Hsp90 is responsible for activating proteins or holding them in a conformation conducive for interaction with required hormones or other proteins. Prominent Hsp90 clients include hormone receptors, protein kinases, and transcription factors [96]. Hsp90 homologues reside in bacteria, yeast, and human cells, and in humans include the cytoplasmic Hsp90α and Hsp90β proteins and mitochondrial and ER forms [97].
Hsp90s contain a ~25 kDa N-terminal ATP binding domain, a middle region, and a C-terminal ~12 kDa domain. The ATP binding domain contains an ATP binding pocket, which also binds the competitive inhibitor, geldanamycin, and other ansamycin antibiotics. Hsp90 contains a split ATPase domain, and ATPase activity requires dimerization. The middle region contains an acceptor site for the γ phosphate of ATP and may aid in the removal of the γ phosphate during ATP hydrolysis [98], and a large hydrophobic patch that is responsible for client protein binding [98]. The C-terminal domain is dedicated to Hsp90 dimerization [99,100].
In the nucleotide free state, the Hsp90 dimer exists in the “open” conformation in which the N-terminal ATP binding domains are separated and client proteins interact in the region between the two dimers. However, upon ATP binding, the N-terminal domains interact and form a “closed” state around the client protein. Hsp90 has a weak ATPase activity [101-103] and many cochaperones, including Hsp70 and Hsp40, are required for Hsp90 function. Other co-chaperones either enhance or inhibit Hsp90 ATPase activity. Components of the Hsp90 complex can differ depending on the nature of the bound substrate, but after ATP hydrolysis, client proteins are released and Hsp90 is recycled.
The role of Hsp90 during protein biogenesis in the ER has been difficult to reconcile with a single mechanism of action. Blocking the association between Hsp90 and CFTR using geldanamycin accelerated CFTR degradation [104]. Consistent with this result, studies in yeast showed that Hsp90 is required for the folding/stabilization of CFTR and a related, misfolded yeast, Ste6p* [66,105]. In contrast, Hsp90 promotes the degradation of apoB [106]. A recent study by Wang et al. [107] demonstrated that the Hsp90 cochaperone, Aha1, which enhances the ATPase activity of Hsp90, is crucial for retaining the ΔF508 mutant form of CFTR in the mammalian ER. When Aha1 expression was reduced by siRNA the defects in trafficking and plasma membrane expression of ΔF508 CFTR were rescued, which suggests a role for Hsp90 not only in folding but in acting as a gate-keeper for ER exit.
2.5 Thiol oxidoreductases
The thiol oxidoreductases are an ER localized class of chaperone-like enzymes that are involved in disulfide bond formation, protein folding, and recognizing and targeting aberrant proteins for ERAD [108-110]. The best studied thiol oxidoreductase is PDI (protein disulfide isomerase). PDI is a ~110 kDa homodimeric protein that is widely distributed and makes up nearly 2% of the total protein content of the ER [111]. The mammalian ER has at least 17 disulfide isomerases, 9 of which have been shown to participate in the formation of disulphide bonds through redox reactions (Table 1) [112].
The family of PDI-like proteins is characterized by the presence of catalytic CxxC active site motif. The sequence and number of these domains varies among family members. The crystal structure of yeast PDI revealed that the protein has a twisted U shape with two inactive catalytic sites between the two active thioredoxin domains that face one another [113]. The interior of the “U” is hydrophobic and is most likely responsible for binding hydrophobic stretches on unfolded proteins [113], which probably explains why PDI has an essential chaperone-like function [114,115]. The structure also predicts that the C-terminal tail stabilizes one of the catalytically active domains. This is in contrast to mammalian PDI, which is predicted to possess a C-terminal tail that does not seem to play a critical role in enzyme activity [116,117].
Unlike the cytosol, the ER provides an oxidative environment for disulfide bond formation. Therefore, to catalyze disulfide bond formation PDI must be maintained in an oxidized state, which is accomplished by Ero1 [118,119]. Ero1 then passes electrons to molecular oxygen via FAD, potentially creating reactive oxygen species (ROS). These ROS can give rise to a significant degree of oxidative stress and even induce the UPR if the level of PDI substrates is high. The yeast ER has one Ero1 and mammals have two isozymes, Ero1α and Ero1β. Ero1α is induced by hypoxia and may play a role in ERAD [120,121] whereas Ero1β is induced by the unfolded protein response [122].
It is currently unknown why there are so many PDIs in the ER. One proposal is that these serve as sources of oxidative equivalents, or participate in an oxidative relay [123]. However, it has been shown that the oxidative activities of 5 different PDIs are very similar, suggesting that they are not dependent upon one another [124]. Other explanations are that specialized PDI members act on unique substrates, or the PDIs act sequentially. For example, the transmembrane TMX proteins may preferentially act on membrane spanning substrates, and ERp57 may interact preferentially with glycoproteins bound to CNX/CRT [125,126]. In contrast, PDI works alone or with BiP [127,128]. Recent work by Forster et al. demonstrated that two PDI family members have opposite effects on cholera toxin protein: PDI promoted the unfolding and subsequent retrotranslocation of cholera toxin A and A1 chains whereas ERp72 promotes folding [129,130].
A role for a yeast PDI homologue in a specific aspect of ERQC has also been uncovered. Eps1 is an ER membrane, PDI-like, protein. In strains deleted for EPS1 a mutant plasma membrane [H+]ATPase was not ER retained, but trafficked to the cell surface. When Eps1 is expressed, the ATPase is degraded. These data suggest that Eps1 acts as a novel chaperone-like protein to block the trafficking of mutant Pma1 and can target the protein for ERAD [131]. More generally, these data suggest that specialized roles for other PDI homologues are likely to exist in both yeast and mammals.
2.6 AAA ATPase
p97 (Cdc48 in yeast) is a homohexameric ring that is involved in diverse cellular processes, including membrane fusion [132,133], activation of membrane associated transcription factors [134], spindle disassembly [135], apoptosis [136], and ERAD [137-139]. Interestingly, p97 binds distinct co-factors that specifies its function. The AAA proteins contain two ATP-binding domains, D1 and D2 domains, and a ~200 residue N-terminal domain (N) that varies among the AAA proteins. Multiple mechanisms for p97 function have been proposed based on the structures of p97 and other AAA proteins. The “molecular ratchet” model suggests that p97 uses ATP-driven conformation changes to propel substrates that are bound to the N-terminal domain. This model predicts that p97, with one of its cofactors, p47, forms a propeller-like structure [140]. In contrast, the “denaturation-collar” model predicts that secondary structures are unfolded by an inherent denaturation-like activity within a pore. Substrates are then threaded through grooves within this pore [141].
p97 binds both to ubiquitinated proteins and to the proteasome [142,143]. Several studies have implicated p97/Cdc48 and two of its potential co-factors, Npl4 and Ufd1, in ERAD [144]. These studies are consistent with p97 providing the energy necessary to extract most proteins for ERAD [137,138,143]. Mutations in the yeast Cdc48 ATPase domain block ER export, although at least two ERAD substrates are instead extracted by AAA proteins in the proteasome “cap” [145-147]. The mechanism for this process is poorly defined. Yeast Cdc48 is tethered to the ER by the transmembrane Ubx2 protein, which links the Cdc48 complex to ubiquitin ligases [148]. Deletion of Ubx2 leads to the stabilization of the soluble ERAD substrate CPY* as well as a membrane spanning substrate [148]. Surprisingly, in cells lacking Ubx2 a significant amount of Cdc48 is still localized to the ER membrane, suggesting that other proteins are involved in tethering [148]. p97 also interacts with a ubiquitin ligase gp78 at the ER membrane to couple ubiquitination and retrotranslocation [149]. In mammals, Ataxin-3, a deubiquitinating enzyme, was recently identified as a modulator of p97 activity. Ataxin-3 appears to displace Ufd1 and inhibits retrotranslocation by decreasing the interaction between p97 and both Ufd1 and ubiquitinated substrates [150]. These data suggest that p97 plays several roles in the selection and modification of proteins during ERAD.
2.7 lectin like
The lectin-like class of chaperones recognizes the oligosaccharyl-appended N-glycan to moderate binding and facilitate protein folding and/or ERAD. This class includes calnexin/calreticulin, Yos9 and the EDEM1-3 proteins. Through cycles of binding and release these chaperones help fold and/or retain misfolded proteins in the ER so that they can be targeted for degradation [151,152]. Although the lectin-like chaperones will be discussed in detail in another chapter in this volume, it is important to note here that these chaperone-like proteins have been found in multi-protein complexes with other effectors of protein biogenesis and degradation.
2.8 Other
Another class of chaperones that has recently been connected to the biogenesis/degradation of ER associated proteins are the cytosolic small heat shock proteins (sHsps). This class of chaperones is widespread, but except for a ~90 amino acid “α-crystallin” domain are poorly conserved. Recently, Ahner et al. found that mammalian α-crytallin preferentially selected the ΔF508 form of mutant CFTR for degradation over WT CFTR [153]. These studies derived from observations that CFTR expression induced the synthesis of two sHsps in yeast. Because α-crystallin maintained the solubility of the first nucleotide binding domain of CFTR, the sHsps may help deliver ΔF508 CFTR to the proteasome. A recent study by Kashlan et al. also showed that sHsps target the membrane spanning epithelial sodium channel for degradation [154].
Membrane-spanning, chaperone-like proteins that exhibit substrate specificity have also been identified in bacteria and yeast [155-157]. For example, the Ljungdahl group identified four ER membrane-spanning proteins that assist in the folding of unique substrates in yeast. Gsf2, Pho86, Chs7 and Shr3 assist in folding hexose transporters, phosphate transporters, chitin synthase-III and amino acid permeases, respectively. In the absence of their unique chaperones each of these substrates fails to properly fold, forms high molecular weight aggregates, and is ER retained [155,156]. It remains to be seen if “specialized” chaperones are common in higher eukaryotes as well.
3. Final Comments
The functions of the chaperone classes discussed above are in no way mutually exclusive. In fact, many of these proteins act at the same time or sequentially on the same substrates and have been found in complexes. For example, Meunier et al. identified a chaperone complex associated with unfolded IgG heavy chain that includes BiP (Hsp70), Grp94 (ER Hsp90 homologue), CaBP1 (calcium binding protein), PDI, ERdj3 (Hsp40), cyclophilin B (peptidyl-prolyl isomerase), ERp72 (thiol oxidoreductase), Grp170 (Hsp110), UDP-glucosyltransferase, and SDF2-L1; BiP, GRP94, and GRP170 exist as a pre-formed complex even in the absence of substrate [42]. The mutant form of α1-antitrypsin, α1-AT Z is found in a complex with BiP, Grp94, Grp170, UGGT, and calnexin [158]. Complexes similar to these have been found for cytosolic Hsp70 and Hsp90, which together bind unfolded proteins and appear to recruit other chaperones [159]. Together, these studies suggest that chaperones do not work in isolation, but function together as part of a dynamic and complex network of folding and degradation effectors.
What determines the make-up of these complexes is only beginning to be understood. The location and type of protein substrate seems to play some role. For example, the soluble protein, CPY, and the transmembrane protein, CFTR, require different chaperone complements for both their folding and degradation (Figure 1). Whereas CPY requires the ER lumenal chaperones BiP, Scj1, Jem1, PDI, and calnexin for folding, CFTR has large domains exposed to the cytosol that require Hsp70, Hsc70, HspBP1, Hdj2, Hsp90 and Aha1 to attain a mature conformation.
It is also unclear precisely how chaperones act as “decision makers” for the ER quality control system. As discussed above distinct chaperone classes have unique effects depending upon the ERAD substrate, and even at times different effects on the same substrate. For example, as shown in Figure 1, BiP/Jem1/Scj1 are required not only for the folding of CPY, but for the degradation of the ERAD target, CPY* [47,64]. Similarly, both Hsp70 and Hsc70 are required for CFTR folding and degradation, although the cochaperones may vary [51,66,95]. What, specifically, these chaperones recognize and how they decide whether a protein is properly folded or will be degraded is still unknown, even for these two well-studied proteins.
Abbreviations
- ER
endoplasmic reticulum
- ERAD
endoplasmic reticulum associated degradation
- NEF
nucleotide exchange factor
- PDI
protein disulfide isomerase
- CPY
carboxypeptidase Y
- CFTR
cystic fibrosis transmembrane conductance regulator
- UPR
unfolded protein response
- Hsp
heat shock protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ghaemmaghami S, Huh WK, Bower K, Howson RW, Belle A, Dephoure N, et al. Global analysis of protein expression in yeast. Nature. 2003;425:737–41. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- 2.Higy M, Junne T, Spiess M. Topogenesis of membrane proteins at the endoplasmic reticulum. Biochemistry. 2004;43:12716–22. doi: 10.1021/bi048368m. [DOI] [PubMed] [Google Scholar]
- 3.Tsai B, Ye Y, Rapoport TA. Retro-translocation of proteins from the endoplasmic reticulum into the cytosol. Nat Rev Mol Cell Biol. 2002;3:246–55. doi: 10.1038/nrm780. [DOI] [PubMed] [Google Scholar]
- 4.Sayeed A, Ng DT. Search and destroy: ER quality control and ER-associated protein degradation. Crit Rev Biochem Mol Biol. 2005;40:75–91. doi: 10.1080/10409230590918685. [DOI] [PubMed] [Google Scholar]
- 5.Hilt W, Wolf DH. The ubiquitin-proteasome system: past, present and future. Cell Mol Life Sci. 2004;61:1545. doi: 10.1007/s00018-004-4128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahner A, Brodsky JL. Checkpoints in ER-associated degradation: excuse me, which way to the proteasome? Trends Cell Biol. 2004;14:474–8. doi: 10.1016/j.tcb.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–51. doi: 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Brodsky JL, Goeckeler J, Schekman R. BiP and Sec63p are required for both co- and posttranslational protein translocation into the yeast endoplasmic reticulum. Proc Natl Acad Sci U S A. 1995;92:9643–6. doi: 10.1073/pnas.92.21.9643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamman BD, Hendershot LM, Johnson AE. BiP maintains the permeability barrier of the ER membrane by sealing the lumenal end of the translocon pore before and early in translocation. Cell. 1998;92:747–58. doi: 10.1016/s0092-8674(00)81403-8. [DOI] [PubMed] [Google Scholar]
- 10.Vashist S, Ng DT. Misfolded proteins are sorted by a sequential checkpoint mechanism of ER quality control. Journal of Cell Biology. 2004;165:41–52. doi: 10.1083/jcb.200309132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taxis C, Hitt R, Park SH, Deak PM, Kostova Z, Wolf DH. Use of modular substrates demonstrates mechanistic diversity and reveals differences in chaperone requirement of ERAD. Journal of Biological Chemistry. 2003;278:35903–13. doi: 10.1074/jbc.M301080200. [DOI] [PubMed] [Google Scholar]
- 12.Kreplak L, Aebi U. From the polymorphism of amyloid fibrils to their assembly mechanism and cytotoxicity. Adv Protein Chem. 2006;73:217–33. doi: 10.1016/S0065-3233(06)73007-8. [DOI] [PubMed] [Google Scholar]
- 13.McCracken AA, Brodsky JL. Evolving questions and paradigm shifts in endoplasmic-reticulum-associated degradation (ERAD) Bioessays. 2003;25:868–77. doi: 10.1002/bies.10320. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida H. ER stress and diseases. Febs J. 2007;274:630–58. doi: 10.1111/j.1742-4658.2007.05639.x. [DOI] [PubMed] [Google Scholar]
- 15.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–89. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 16.Bernales S, Papa FR, Walter P. Intracellular signaling by the unfolded protein response. Annu Rev Cell Dev Biol. 2006;22:487–508. doi: 10.1146/annurev.cellbio.21.122303.120200. [DOI] [PubMed] [Google Scholar]
- 17.Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670–84. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flaherty KM, DeLuca-Flaherty C, McKay DB. Three-dimensional structure of the ATPase fragment of a 70K heat-shock cognate protein. Nature. 1990;346:623–8. doi: 10.1038/346623a0. [DOI] [PubMed] [Google Scholar]
- 19.Zhu X, Zhao X, Burkholder WF, Gragerov A, Ogata CM, Gottesman ME, et al. Structural analysis of substrate binding by the molecular chaperone DnaK. Science. 1996;272:1606–14. doi: 10.1126/science.272.5268.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sousa R, Lafer EM. Keep the traffic moving: mechanism of the Hsp70 motor. Traffic. 2006;7:1596–603. doi: 10.1111/j.1600-0854.2006.00497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Revington M, Zhang Y, Yip GN, Kurochkin AV, Zuiderweg ER. NMR investigations of allosteric processes in a two-domain Thermus thermophilus Hsp70 molecular chaperone. J Mol Biol. 2005;349:163–83. doi: 10.1016/j.jmb.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 22.Ha JH, McKay DB. ATPase kinetics of recombinant bovine 70 kDa heat shock cognate protein and its amino-terminal ATPase domain. Biochemistry. 1994;33:14625–35. doi: 10.1021/bi00252a031. [DOI] [PubMed] [Google Scholar]
- 23.Theyssen H, Schuster HP, Packschies L, Bukau B, Reinstein J. The second step of ATP binding to DnaK induces peptide release. J Mol Biol. 1996;263:657–70. doi: 10.1006/jmbi.1996.0606. [DOI] [PubMed] [Google Scholar]
- 24.McCarty JS, Buchberger A, Reinstein J, Bukau B. The role of ATP in the functional cycle of the DnaK chaperone system. J Mol Biol. 1995;249:126–37. doi: 10.1006/jmbi.1995.0284. [DOI] [PubMed] [Google Scholar]
- 25.Flynn GC, Chappell TG, Rothman JE. Peptide binding and release by proteins implicated as catalysts of protein assembly. Science. 1989;245:385–90. doi: 10.1126/science.2756425. [DOI] [PubMed] [Google Scholar]
- 26.Jordan R, McMacken R. Modulation of the ATPase activity of the molecular chaperone DnaK by peptides and the DnaJ and GrpE heat shock proteins. J Biol Chem. 1995;270:4563–9. doi: 10.1074/jbc.270.9.4563. [DOI] [PubMed] [Google Scholar]
- 27.Russell R, Wali Karzai A, Mehl AF, McMacken R. DnaJ dramatically stimulates ATP hydrolysis by DnaK: insight into targeting of Hsp70 proteins to polypeptide substrates. Biochemistry. 1999;38:4165–76. doi: 10.1021/bi9824036. [DOI] [PubMed] [Google Scholar]
- 28.Gisler SM, Pierpaoli EV, Christen P. Catapult mechanism renders the chaperone action of Hsp70 unidirectional. J Mol Biol. 1998;279:833–40. doi: 10.1006/jmbi.1998.1815. [DOI] [PubMed] [Google Scholar]
- 29.Mayer MP, Schroder H, Rudiger S, Paal K, Laufen T, Bukau B. Multistep mechanism of substrate binding determines chaperone activity of Hsp70. Nat Struct Biol. 2000;7:586–93. doi: 10.1038/76819. [DOI] [PubMed] [Google Scholar]
- 30.Vogel M, Bukau B, Mayer MP. Allosteric regulation of Hsp70 chaperones by a proline switch. Mol Cell. 2006;21:359–67. doi: 10.1016/j.molcel.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 31.Swain JF, Dinler G, Sivendran R, Montgomery DL, Stotz M, Gierasch LM. Hsp70 Chaperone Ligands Control Domain Association via an Allosteric Mechanism Mediated by the Interdomain Linker. Mol Cell. 2007;26:27–39. doi: 10.1016/j.molcel.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheetham ME, Caplan AJ. Structure, function and evolution of DnaJ: conservation and adaptation of chaperone function. Cell Stress Chaperones. 1998;3:28–36. doi: 10.1379/1466-1268(1998)003<0028:sfaeod>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gassler CS, Buchberger A, Laufen T, Mayer MP, Schroder H, Valencia A, et al. Mutations in the DnaK chaperone affecting interaction with the DnaJ cochaperone. Proc Natl Acad Sci U S A. 1998;95:15229–34. doi: 10.1073/pnas.95.26.15229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suh WC, Burkholder WF, Lu CZ, Zhao X, Gottesman ME, Gross CA. Interaction of the Hsp70 molecular chaperone, DnaK, with its cochaperone DnaJ. Proc Natl Acad Sci U S A. 1998;95:15223–8. doi: 10.1073/pnas.95.26.15223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harrison C. GrpE, a nucleotide exchange factor for DnaK. Cell Stress Chaperones. 2003;8:218–24. doi: 10.1379/1466-1268(2003)008<0218:ganeff>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alberti S, Esser C, Hohfeld J. BAG-1--a nucleotide exchange factor of Hsc70 with multiple cellular functions. Cell Stress Chaperones. 2003;8:225–31. doi: 10.1379/1466-1268(2003)008<0225:bnefoh>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matlack KE, Misselwitz B, Plath K, Rapoport TA. BiP acts as a molecular ratchet during posttranslational transport of prepro-alpha factor across the ER membrane. Cell. 1999;97:553–64. doi: 10.1016/s0092-8674(00)80767-9. [DOI] [PubMed] [Google Scholar]
- 38.Liebermeister W, Rapoport TA, Heinrich R. Ratcheting in post-translational protein translocation: a mathematical model. J Mol Biol. 2001;305:643–56. doi: 10.1006/jmbi.2000.4302. [DOI] [PubMed] [Google Scholar]
- 39.Simons JF, Ferro-Novick S, Rose MD, Helenius A. BiP/Kar2p serves as a molecular chaperone during carboxypeptidase Y folding in yeast. J Cell Biol. 1995;130:41–9. doi: 10.1083/jcb.130.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steel GJ, Fullerton DM, Tyson JR, Stirling CJ. Coordinated activation of Hsp70 chaperones. Science. 2004;303:98–101. doi: 10.1126/science.1092287. [DOI] [PubMed] [Google Scholar]
- 41.Lin HY, Masso-Welch P, Di YP, Cai JW, Shen JW, Subjeck JR. The 170-kDa glucose-regulated stress protein is an endoplasmic reticulum protein that binds immunoglobulin. Mol Biol Cell. 1993;4:1109–19. doi: 10.1091/mbc.4.11.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meunier L, Usherwood YK, Chung KT, Hendershot LM. A subset of chaperones and folding enzymes form multiprotein complexes in endoplasmic reticulum to bind nascent proteins. Molecular Biology of the Cell. 2002;13:4456–69. doi: 10.1091/mbc.E02-05-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okamura K, Kimata Y, Higashio H, Tsuru A, Kohno K. Dissociation of Kar2p/BiP from an ER sensory molecule, Ire1p, triggers the unfolded protein response in yeast. Biochem Biophys Res Commun. 2000;279:445–50. doi: 10.1006/bbrc.2000.3987. [DOI] [PubMed] [Google Scholar]
- 44.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–32. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 45.Oikawa D, Kimata Y, Takeuchi M, Kohno K. An essential dimer-forming subregion of the endoplasmic reticulum stress sensor Ire1. Biochem J. 2005;391:135–42. doi: 10.1042/BJ20050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Credle JJ, Finer-Moore JS, Papa FR, Stroud RM, Walter P. On the mechanism of sensing unfolded protein in the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2005;102:18773–84. doi: 10.1073/pnas.0509487102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishikawa SI, Fewell SW, Kato Y, Brodsky JL, Endo T. Molecular chaperones in the yeast endoplasmic reticulum maintain the solubility of proteins for retrotranslocation and degradation. J Cell Biol. 2001;153:1061–70. doi: 10.1083/jcb.153.5.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kabani M, Kelley SS, Morrow MW, Montgomery DL, Sivendran R, Rose MD, et al. Dependence of endoplasmic reticulum-associated degradation on the peptide binding domain and concentration of BiP. Mol Biol Cell. 2003;14:3437–48. doi: 10.1091/mbc.E02-12-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldfarb SB, Kashlan OB, Watkins JN, Suaud L, Yan W, Kleyman TR, et al. Differential effects of Hsc70 and Hsp70 on the intracellular trafficking and functional expression of epithelial sodium channels. Proc Natl Acad Sci U S A. 2006;103:5817–22. doi: 10.1073/pnas.0507903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rubenstein RC, Zeitlin PL. Sodium 4-phenylbutyrate downregulates Hsc70: implications for intracellular trafficking of DeltaF508-CFTR. Am J Physiol Cell Physiol. 2000;278:C259–67. doi: 10.1152/ajpcell.2000.278.2.C259. [DOI] [PubMed] [Google Scholar]
- 51.Meacham GC, Lu Z, King S, Sorscher E, Tousson A, Cyr DM. The Hdj-2/Hsc70 chaperone pair facilitates early steps in CFTR biogenesis. Embo J. 1999;18:1492–505. doi: 10.1093/emboj/18.6.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Nijbroek G, Sullivan ML, McCracken AA, Watkins SC, Michaelis S, et al. Hsp70 molecular chaperone facilitates endoplasmic reticulum-associated protein degradation of cystic fibrosis transmembrane conductance regulator in yeast. Mol Biol Cell. 2001;12:1303–14. doi: 10.1091/mbc.12.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han W, Christen P. Mechanism of the targeting action of DnaJ in the DnaK molecular chaperone system. J Biol Chem. 2003;278:19038–43. doi: 10.1074/jbc.M300756200. [DOI] [PubMed] [Google Scholar]
- 54.Langer T, Lu C, Echols H, Flanagan J, Hayer MK, Hartl FU. Successive action of DnaK, DnaJ and GroEL along the pathway of chaperone-mediated protein folding. Nature. 1992;356:683–9. doi: 10.1038/356683a0. [DOI] [PubMed] [Google Scholar]
- 55.Rudiger S, Schneider-Mergener J, Bukau B. Its substrate specificity characterizes the DnaJ co-chaperone as a scanning factor for the DnaK chaperone. Embo J. 2001;20:1042–50. doi: 10.1093/emboj/20.5.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Szabo A, Korszun R, Hartl FU, Flanagan J. A zinc finger-like domain of the molecular chaperone DnaJ is involved in binding to denatured protein substrates. Embo J. 1996;15:408–17. [PMC free article] [PubMed] [Google Scholar]
- 57.Wickner S, Hoskins J, McKenney K. Function of DnaJ and DnaK as chaperones in origin-specific DNA binding by RepA. Nature. 1991;350:165–7. doi: 10.1038/350165a0. [DOI] [PubMed] [Google Scholar]
- 58.Hennessy F, Nicoll WS, Zimmermann R, Cheetham ME, Blatch GL. Not all domains are created equal: implications for the specificity of Hsp40-Hsp70 interactions. Protein Sci. 2005;14:1697–709. doi: 10.1110/ps.051406805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fan CY, Lee S, Ren HY, Cyr DM. Exchangeable chaperone modules contribute to specification of type I and type II Hsp40 cellular function. Mol Biol Cell. 2004;15:761–73. doi: 10.1091/mbc.E03-03-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rudiger S, Germeroth L, Schneider-Mergener J, Bukau B. Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. Embo J. 1997;16:1501–7. doi: 10.1093/emboj/16.7.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McClellan AJ, Endres JB, Vogel JP, Palazzi D, Rose MD, Brodsky JL. Specific molecular chaperone interactions and an ATP-dependent conformational change are required during posttranslational protein translocation into the yeast ER. Mol Biol Cell. 1998;9:3533–45. doi: 10.1091/mbc.9.12.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schlenstedt G, Harris S, Risse B, Lill R, Silver PA. A yeast DnaJ homologue, Scj1p, can function in the endoplasmic reticulum with BiP/Kar2p via a conserved domain that specifies interactions with Hsp70s. J Cell Biol. 1995;129:979–88. doi: 10.1083/jcb.129.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Corsi AK, Schekman R. The lumenal domain of Sec63p stimulates the ATPase activity of BiP and mediates BiP recruitment to the translocon in Saccharomyces cerevisiae. J Cell Biol. 1997;137:1483–93. doi: 10.1083/jcb.137.7.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Silberstein S, Schlenstedt G, Silver PA, Gilmore R. A role for the DnaJ homologue Scj1p in protein folding in the yeast endoplasmic reticulum. J Cell Biol. 1998;143:921–33. doi: 10.1083/jcb.143.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang H, Schmidt BZ, Sun F, Condliffe SB, Butterworth MB, Youker RT, et al. Cysteine string protein monitors late steps in cystic fibrosis transmembrane conductance regulator biogenesis. J Biol Chem. 2006;281:11312–21. doi: 10.1074/jbc.M512013200. [DOI] [PubMed] [Google Scholar]
- 66.Youker RT, Walsh P, Beilharz T, Lithgow T, Brodsky JL. Distinct roles for the Hsp40 and Hsp90 molecular chaperones during cystic fibrosis transmembrane conductance regulator degradation in yeast. Mol Biol Cell. 2004;15:4787–97. doi: 10.1091/mbc.E04-07-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–66. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- 68.Raviol H, Sadlish H, Rodriguez F, Mayer MP, Bukau B. Chaperone network in the yeast cytosol: Hsp110 is revealed as an Hsp70 nucleotide exchange factor. Embo J. 2006;25:2510–8. doi: 10.1038/sj.emboj.7601139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dragovic Z, Broadley SA, Shomura Y, Bracher A, Hartl FU. Molecular chaperones of the Hsp110 family act as nucleotide exchange factors of Hsp70s. Embo J. 2006;25:2519–28. doi: 10.1038/sj.emboj.7601138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saito H, Uchida H. Initiation of the DNA replication of bacteriophage lambda in Escherichia coli K12. J Mol Biol. 1977;113:1–25. doi: 10.1016/0022-2836(77)90038-9. [DOI] [PubMed] [Google Scholar]
- 71.Brehmer D, Rudiger S, Gassler CS, Klostermeier D, Packschies L, Reinstein J, et al. Tuning of chaperone activity of Hsp70 proteins by modulation of nucleotide exchange. Nat Struct Biol. 2001;8:427–32. doi: 10.1038/87588. [DOI] [PubMed] [Google Scholar]
- 72.Zylicz M, Ang D, Georgopoulos C. The grpE protein of Escherichia coli. Purification and properties. J Biol Chem. 1987;262:17437–42. [PubMed] [Google Scholar]
- 73.Schonfeld HJ, Schmidt D, Schroder H, Bukau B. The DnaK chaperone system of Escherichia coli: quaternary structures and interactions of the DnaK and GrpE components. J Biol Chem. 1995;270:2183–9. doi: 10.1074/jbc.270.5.2183. [DOI] [PubMed] [Google Scholar]
- 74.Harrison CJ, Hayer-Hartl M, Di Liberto M, Hartl F, Kuriyan J. Crystal structure of the nucleotide exchange factor GrpE bound to the ATPase domain of the molecular chaperone DnaK. Science. 1997;276:431–5. doi: 10.1126/science.276.5311.431. [DOI] [PubMed] [Google Scholar]
- 75.Takayama S, Bimston DN, Matsuzawa S, Freeman BC, Aime-Sempe C, Xie Z, et al. BAG-1 modulates the chaperone activity of Hsp70/Hsc70. Embo J. 1997;16:4887–96. doi: 10.1093/emboj/16.16.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hohfeld J, Jentsch S. GrpE-like regulation of the hsc70 chaperone by the anti-apoptotic protein BAG-1. Embo J. 1997;16:6209–16. doi: 10.1093/emboj/16.20.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sondermann H, Ho AK, Listenberger LL, Siegers K, Moarefi I, Wente SR, et al. Prediction of novel Bag-1 homologs based on structure/function analysis identifies Snl1p as an Hsp70 co-chaperone in Saccharomyces cerevisiae. J Biol Chem. 2002;277:33220–7. doi: 10.1074/jbc.M204624200. [DOI] [PubMed] [Google Scholar]
- 78.Sondermann H, Scheufler C, Schneider C, Hohfeld J, Hartl FU, Moarefi I. Structure of a Bag/Hsc70 complex: convergent functional evolution of Hsp70 nucleotide exchange factors. Science. 2001;291:1553–7. doi: 10.1126/science.1057268. [DOI] [PubMed] [Google Scholar]
- 79.Gassler CS, Wiederkehr T, Brehmer D, Bukau B, Mayer MP. Bag-1M accelerates nucleotide release for human Hsc70 and Hsp70 and can act concentration-dependent as positive and negative cofactor. J Biol Chem. 2001;276:32538–44. doi: 10.1074/jbc.M105328200. [DOI] [PubMed] [Google Scholar]
- 80.Raynes DA, Guerriero V., Jr Inhibition of Hsp70 ATPase activity and protein renaturation by a novel Hsp70-binding protein. J Biol Chem. 1998;273:32883–8. doi: 10.1074/jbc.273.49.32883. [DOI] [PubMed] [Google Scholar]
- 81.Kabani M, McLellan C, Raynes DA, Guerriero V, Brodsky JL. HspBP1, a homologue of the yeast Fes1 and Sls1 proteins, is an Hsc70 nucleotide exchange factor. FEBS Lett. 2002;531:339–42. doi: 10.1016/s0014-5793(02)03570-6. [DOI] [PubMed] [Google Scholar]
- 82.Kabani M, Beckerich JM, Brodsky JL. Nucleotide exchange factor for the yeast Hsp70 molecular chaperone Ssa 1p. Mol Cell Biol. 2002;22:4677–89. doi: 10.1128/MCB.22.13.4677-4689.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chung KT, Shen Y, Hendershot LM. BAP, a mammalian BiP-associated protein, is a nucleotide exchange factor that regulates the ATPase activity of BiP. J Biol Chem. 2002;277:47557–63. doi: 10.1074/jbc.M208377200. [DOI] [PubMed] [Google Scholar]
- 84.Dragovic Z, Shomura Y, Tzvetkov N, Hartl FU, Bracher A. Fes1p acts as a nucleotide exchange factor for the ribosome-associated molecular chaperone Ssb1p. Biol Chem. 2006;387:1593–600. doi: 10.1515/BC.2006.198. [DOI] [PubMed] [Google Scholar]
- 85.Shomura Y, Dragovic Z, Chang HC, Tzvetkov N, Young JC, Brodsky JL, et al. Regulation of Hsp70 function by HspBP1: structural analysis reveals an alternate mechanism for Hsp70 nucleotide exchange. Mol Cell. 2005;17:367–79. doi: 10.1016/j.molcel.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 86.Shaner L, Morano KA. All in the family: atypical Hsp70 chaperones are conserved modulators of Hsp70 activity. Cell Stress Chaperones. 2007;12:1–8. doi: 10.1379/CSC-245R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Easton DP, Kaneko Y, Subjeck JR. The hsp110 and Grp1 70 stress proteins: newly recognized relatives of the Hsp70s. Cell Stress Chaperones. 2000;5:276–90. doi: 10.1379/1466-1268(2000)005<0276:thagsp>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oh HJ, Chen X, Subjeck JR. Hsp110 protects heat-denatured proteins and confers cellular thermoresistance. J Biol Chem. 1997;272:31636–40. doi: 10.1074/jbc.272.50.31636. [DOI] [PubMed] [Google Scholar]
- 89.Shaner L, Wegele H, Buchner J, Morano KA. The yeast Hsp110 Sse1 functionally interacts with the Hsp70 chaperones Ssa and Ssb. J Biol Chem. 2005;280:41262–9. doi: 10.1074/jbc.M503614200. [DOI] [PubMed] [Google Scholar]
- 90.Liu XD, Morano KA, Thiele DJ. The yeast Hsp110 family member, Sse1, is an Hsp90 cochaperone. J Biol Chem. 1999;274:26654–60. doi: 10.1074/jbc.274.38.26654. [DOI] [PubMed] [Google Scholar]
- 91.Goeckeler JL, Stephens A, Lee P, Caplan AJ, Brodsky JL. Overexpression of yeast Hsp110 homolog Sse1p suppresses ydj1-151 thermosensitivity and restores Hsp90-dependent activity. Mol Biol Cell. 2002;13:2760–70. doi: 10.1091/mbc.02-04-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hrizo SL, Gusarova V, Habiel DM, Goeckeler JL, Fisher EA, Brodsky JL. The Hsp110 Molecular Chaperone Stabilizes Apolipoprotein B from Endoplasmic Reticulum Associated Degradation (ERAD) J Cell Biol. 2007 doi: 10.1074/jbc.M705216200. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alberti S, Bohse K, Arndt V, Schmitz A, Hohfeld J. The cochaperone HspBP1 inhibits the CHIP ubiquitin ligase and stimulates the maturation of the cystic fibrosis transmembrane conductance regulator. Mol Biol Cell. 2004;15:4003–10. doi: 10.1091/mbc.E04-04-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Connell P, Ballinger CA, Jiang J, Wu Y, Thompson LJ, Hohfeld J, et al. The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nature Cell Biology. 2001;3:93–6. doi: 10.1038/35050618. [DOI] [PubMed] [Google Scholar]
- 95.Meacham GC, Patterson C, Zhang W, Younger JM, Cyr DM. The Hsc70 co-chaperone CHIP targets immature CFTR for proteasomal degradation. Nature Cell Biology. 2001;3:100–5. doi: 10.1038/35050509. [DOI] [PubMed] [Google Scholar]
- 96.Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood) 2003;228:111–33. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- 97.Zhao R, Houry WA. Hsp90: a chaperone for protein folding and gene regulation. Biochem Cell Biol. 2005;83:703–10. doi: 10.1139/o05-158. [DOI] [PubMed] [Google Scholar]
- 98.Meyer P, Prodromou C, Hu B, Vaughan C, Roe SM, Panaretou B, et al. Structural and functional analysis of the middle segment of hsp90: implications for ATP hydrolysis and client protein and cochaperone interactions. Mol Cell. 2003;11:647–58. doi: 10.1016/s1097-2765(03)00065-0. [DOI] [PubMed] [Google Scholar]
- 99.Harris SF, Shiau AK, Agard DA. The crystal structure of the carboxy-terminal dimerization domain of htpG, the Escherichia coli Hsp90, reveals a potential substrate binding site. Structure. 2004;12:1087–97. doi: 10.1016/j.str.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 100.Prodromou C, Panaretou B, Chohan S, Siligardi G, O’Brien R, Ladbury JE, et al. The ATPase cycle of Hsp90 drives a molecular ‘clamp’ via transient dimerization of the N-terminal domains. Embo J. 2000;19:4383–92. doi: 10.1093/emboj/19.16.4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Obermann WM, Sondermann H, Russo AA, Pavletich NP, Hartl FU. In vivo function of Hsp90 is dependent on ATP binding and ATP hydrolysis. J Cell Biol. 1998;143:901–10. doi: 10.1083/jcb.143.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Panaretou B, Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, et al. ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo. Embo J. 1998;17:4829–36. doi: 10.1093/emboj/17.16.4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grenert JP, Johnson BD, Toft DO. The importance of ATP binding and hydrolysis by hsp90 in formation and function of protein heterocomplexes. J Biol Chem. 1999;274:17525–33. doi: 10.1074/jbc.274.25.17525. [DOI] [PubMed] [Google Scholar]
- 104.Loo MA, Jensen TJ, Cui L, Hou Y, Chang XB, Riordan JR. Perturbation of Hsp90 interaction with nascent CFTR prevents its maturation and accelerates its degradation by the proteasome. Embo J. 1998;17:6879–87. doi: 10.1093/emboj/17.23.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Huyer G, Piluek WF, Fansler Z, Kreft SG, Hochstrasser M, Brodsky JL, et al. Distinct machinery is required in Saccharomyces cerevisiae for the endoplasmic reticulum-associated degradation of a multispanning membrane protein and a soluble luminal protein. J Biol Chem. 2004;279:38369–78. doi: 10.1074/jbc.M402468200. [DOI] [PubMed] [Google Scholar]
- 106.Gusarova V, Caplan AJ, Brodsky JL, Fisher EA. Apoprotein B degradation is promoted by the molecular chaperones hsp90 and hsp70. J Biol Chem. 2001;276:24891–900. doi: 10.1074/jbc.M100633200. [DOI] [PubMed] [Google Scholar]
- 107.Wang X, Venable J, LaPointe P, Hutt DM, Koulov AV, Coppinger J, et al. Hsp90 cochaperone Aha1 downregulation rescues misfolding of CFTR in cystic fibrosis. Cell. 2006;127:803–15. doi: 10.1016/j.cell.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 108.Jordan PA, Gibbins JM. Extracellular disulfide exchange and the regulation of cellular function. Antioxid Redox Signal. 2006;8:312–24. doi: 10.1089/ars.2006.8.312. [DOI] [PubMed] [Google Scholar]
- 109.Nishikawa S, Brodsky JL, Nakatsukasa K. Roles of molecular chaperones in endoplasmic reticulum (ER) quality control and ER-associated degradation (ERAD) J Biochem (Tokyo) 2005;137:551–5. doi: 10.1093/jb/mvi068. [DOI] [PubMed] [Google Scholar]
- 110.Tu BP, Weissman JS. Oxidative protein folding in eukaryotes: mechanisms and consequences. J Cell Biol. 2004;164:341–6. doi: 10.1083/jcb.200311055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Goldberger RF, Epstein CJ, Anfinsen CB. Acceleration of reactivation of reduced bovine pancreatic ribonuclease by a microsomal system from rat liver. J Biol Chem. 1963;238:628–35. [PubMed] [Google Scholar]
- 112.Ellgaard L, Ruddock LW. The human protein disulphide isomerase family: substrate interactions and functional properties. EMBO Rep. 2005;6:28–32. doi: 10.1038/sj.embor.7400311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tian G, Xiang S, Noiva R, Lennarz WJ, Schindelin H. The crystal structure of yeast protein disulfide isomerase suggests cooperativity between its active sites. Cell. 2006;124:61–73. doi: 10.1016/j.cell.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 114.Cai H, Wang CC, Tsou CL. Chaperone-like activity of protein disulfide isomerase in the refolding of a protein with no disulfide bonds. J Biol Chem. 1994;269:24550–2. [PubMed] [Google Scholar]
- 115.Song JL, Wang CC. Chaperone-like activity of protein disulfide-isomerase in the refolding of rhodanese. European Journal of Biochemistry. 1995;231:312–6. doi: 10.1111/j.1432-1033.1995.tb20702.x. [DOI] [PubMed] [Google Scholar]
- 116.Darby NJ, Penka E, Vincentelli R. The multi-domain structure of protein disulfide isomerase is essential for high catalytic efficiency. J Mol Biol. 1998;276:239–47. doi: 10.1006/jmbi.1997.1504. [DOI] [PubMed] [Google Scholar]
- 117.Koivunen P, Pirneskoski A, Karvonen P, Ljung J, Helaakoski T, Notbohm H, et al. The acidic C-terminal domain of protein disulfide isomerase is not critical for the enzyme subunit function or for the chaperone or disulfide isomerase activities of the polypeptide. Embo J. 1999;18:65–74. doi: 10.1093/emboj/18.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tu BP, Ho-Schleyer SC, Travers KJ, Weissman JS. Biochemical basis of oxidative protein folding in the endoplasmic reticulum. Science. 2000;290:1571–4. doi: 10.1126/science.290.5496.1571. [DOI] [PubMed] [Google Scholar]
- 119.Frand AR, Kaiser CA. Ero1p oxidizes protein disulfide isomerase in a pathway for disulfide bond formation in the endoplasmic reticulum. Mol Cell. 1999;4:469–77. doi: 10.1016/s1097-2765(00)80198-7. [DOI] [PubMed] [Google Scholar]
- 120.Gess B, Hofbauer KH, Wenger RH, Lohaus C, Meyer HE, Kurtz A. The cellular oxygen tension regulates expression of the endoplasmic oxidoreductase ERO1-Lalpha. Eur J Biochem. 2003;270:2228–35. doi: 10.1046/j.1432-1033.2003.03590.x. [DOI] [PubMed] [Google Scholar]
- 121.Tsai B, Rapoport TA. Unfolded cholera toxin is transferred to the ER membrane and released from protein disulfide isomerase upon oxidation by Ero1. J Cell Biol. 2002;159:207–16. doi: 10.1083/jcb.200207120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pagani M, Fabbri M, Benedetti C, Fassio A, Pilati S, Bulleid NJ, et al. Endoplasmic reticulum oxidoreductin 1-lbeta (ERO1-Lbeta), a human gene induced in the course of the unfolded protein response. J Biol Chem. 2000;275:23685–92. doi: 10.1074/jbc.M003061200. [DOI] [PubMed] [Google Scholar]
- 123.Maattanen P, Kozlov G, Gehring K, Thomas DY. ERp57 and PDI: multifunctional protein disulfide isomerases with similar domain architectures but differing substrate-partner associations. Biochem Cell Biol. 2006;84:881–9. doi: 10.1139/o06-186. [DOI] [PubMed] [Google Scholar]
- 124.Alanen HI, Salo KE, Pirneskoski A, Ruddock LW. pH dependence of the peptide thiol-disulfide oxidase activity of six members of the human protein disulfide isomerase family. Antioxid Redox Signal. 2006;8:283–91. doi: 10.1089/ars.2006.8.283. [DOI] [PubMed] [Google Scholar]
- 125.Zapun A, Darby NJ, Tessier DC, Michalak M, Bergeron JJ, Thomas DY. Enhanced catalysis of ribonuclease B folding by the interaction of calnexin or calreticulin with ERp57. J Biol Chem. 1998;273:6009–12. doi: 10.1074/jbc.273.11.6009. [DOI] [PubMed] [Google Scholar]
- 126.Williams DB. Beyond lectins: the calnexin/calreticulin chaperone system of the endoplasmic reticulum. J Cell Sci. 2006;119:615–23. doi: 10.1242/jcs.02856. [DOI] [PubMed] [Google Scholar]
- 127.Kulp MS, Frickel EM, Ellgaard L, Weissman JS. Domain architecture of protein-disulfide isomerase facilitates its dual role as an oxidase and an isomerase in Ero1p-mediated disulfide formation. J Biol Chem. 2006;281:876–84. doi: 10.1074/jbc.M511764200. [DOI] [PubMed] [Google Scholar]
- 128.Mayer M, Kies U, Kammermeier R, Buchner J. BiP and PDI cooperate in the oxidative folding of antibodies in vitro. J Biol Chem. 2000;275:29421–5. doi: 10.1074/jbc.M002655200. [DOI] [PubMed] [Google Scholar]
- 129.Forster ML, Sivick K, Park YN, Arvan P, Lencer WI, Tsai B. Protein disulfide isomerase-like proteins play opposing roles during retrotranslocation. J Cell Biol. 2006;173:853–9. doi: 10.1083/jcb.200602046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tsai B, Rodighiero C, Lencer WI, Rapoport TA. Protein disulfide isomerase acts as a redox-dependent chaperone to unfold cholera toxin. Cell. 2001;104:937–48. doi: 10.1016/s0092-8674(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 131.Wang Q, Chang A. Substrate recognition in ER-associated degradation mediated by Eps1, a member of the protein disulfide isomerase family. Embo J. 2003;22:3792–802. doi: 10.1093/emboj/cdg378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Patel SK, Indig FE, Olivieri N, Levine ND, Latterich M. Organelle membrane fusion: a novel function for the syntaxin homolog Ufe1p in ER membrane fusion. Cell. 1998;92:611–20. doi: 10.1016/s0092-8674(00)81129-0. [DOI] [PubMed] [Google Scholar]
- 133.Kondo H, Rabouille C, Newman R, Levine TP, Pappin D, Freemont P, et al. p47 is a cofactor for p97-mediated membrane fusion. Nature. 1997;388:75–8. doi: 10.1038/40411. [DOI] [PubMed] [Google Scholar]
- 134.Rape M, Hoppe T, Gorr I, Kalocay M, Richly H, Jentsch S. Mobilization of processed, membrane-tethered SPT23 transcription factor by CDC48(UFD1/NPL4), a ubiquitin-selective chaperone. Cell. 2001;107:667–77. doi: 10.1016/s0092-8674(01)00595-5. [DOI] [PubMed] [Google Scholar]
- 135.Cao K, Nakajima R, Meyer HH, Zheng Y. The AAA-ATPase Cdc48/p97 regulates spindle disassembly at the end of mitosis. Cell. 2003;115:355–67. doi: 10.1016/s0092-8674(03)00815-8. [DOI] [PubMed] [Google Scholar]
- 136.Madeo F, Frohlich E, Frohlich KU. A yeast mutant showing diagnostic markers of early and late apoptosis. J Cell Biol. 1997;139:729–34. doi: 10.1083/jcb.139.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bays NW, Wilhovsky SK, Goradia A, Hodgkiss-Harlow K, Hampton RY. HRD4/NPL4 is required for the proteasomal processing of ubiquitinated ER proteins. Mol Biol Cell. 2001;12:4114–28. doi: 10.1091/mbc.12.12.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jarosch E, Taxis C, Volkwein C, Bordallo J, Finley D, Wolf DH, et al. Protein dislocation from the ER requires polyubiquitination and the AAA-ATPase Cdc48. Nature Cell Biology. 2002;4:134–9. doi: 10.1038/ncb746. [DOI] [PubMed] [Google Scholar]
- 139.Braun S, Matuschewski K, Rape M, Thoms S, Jentsch S. Role of the ubiquitin-selective CDC48(UFD1/NPL4)chaperone (segregase) in ERAD of OLE1 and other substrates. EMBO Journal. 2002;21:615–21. doi: 10.1093/emboj/21.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Beuron F, Dreveny I, Yuan X, Pye VE, McKeown C, Briggs LC, et al. Conformational changes in the AAA ATPase p97-p47 adaptor complex. Embo J. 2006;25:1967–76. doi: 10.1038/sj.emboj.7601055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.DeLaBarre B, Christianson JC, Kopito RR, Brunger AT. Central pore residues mediate the p97/VCP activity required for ERAD. Mol Cell. 2006;22:451–62. doi: 10.1016/j.molcel.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 142.Dai RM, Li CC. Valosin-containing protein is a multi-ubiquitin chain-targeting factor required in ubiquitin-proteasome degradation. Nat Cell Biol. 2001;3:740–4. doi: 10.1038/35087056. [DOI] [PubMed] [Google Scholar]
- 143.Ye Y, Meyer HH, Rapoport TA. The AAA ATPase Cdc48/p97 and its partners transport proteins from the ER into the cytosol. Nature. 2001;414:652–6. doi: 10.1038/414652a. [DOI] [PubMed] [Google Scholar]
- 144.Jentsch S, Rumpf S. Cdc48 (p97): a “molecular gearbox” in the ubiquitin pathway? Trends Biochem Sci. 2007;32:6–11. doi: 10.1016/j.tibs.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 145.Kothe M, Ye Y, Wagner JS, De Luca HE, Kern E, Rapoport TA, et al. Role of p97 AAA-ATPase in the retrotranslocation of the cholera toxin A1 chain, a non-ubiquitinated substrate. J Biol Chem. 2005;280:28127–32. doi: 10.1074/jbc.M503138200. [DOI] [PubMed] [Google Scholar]
- 146.Wahlman J, DeMartino GN, Skach W, Bulleid NJ, Brodsky JL, Johnson AE. Real-time fluorescence detection of ERAD substrate retro-translocation in a mammalian in vitro system. Cell. 2007 doi: 10.1016/j.cell.2007.03.046. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lee RJ, Liu CW, Harty C, McCracken AA, Latterich M, Romisch K, et al. Uncoupling retro-translocation and degradation in the ER-associated degradation of a soluble protein. EMBO Journal. 2004;23:2206–15. doi: 10.1038/sj.emboj.7600232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Neuber O, Jarosch E, Volkwein C, Walter J, Sommer T. Ubx2 links the Cdc48 complex to ER-associated protein degradation. Nat Cell Biol. 2005;7:993–8. doi: 10.1038/ncb1298. [DOI] [PubMed] [Google Scholar]
- 149.Zhong X, Shen Y, Ballar P, Apostolou A, Agami R, Fang S. AAA ATPase p97/valosin-containing protein interacts with gp78, a ubiquitin ligase for endoplasmic reticulum-associated degradation. J Biol Chem. 2004;279:45676–84. doi: 10.1074/jbc.M409034200. [DOI] [PubMed] [Google Scholar]
- 150.Zhong X, Pittman RN. Ataxin-3 binds VCP/p97 and regulates retrotranslocation of ERAD substrates. Hum Mol Genet. 2006;15:2409–20. doi: 10.1093/hmg/ddl164. [DOI] [PubMed] [Google Scholar]
- 151.Helenius A, Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annual Review of Biochemistry. 2004;73:1019–49. doi: 10.1146/annurev.biochem.73.011303.073752. [DOI] [PubMed] [Google Scholar]
- 152.Carvalho P, Goder V, Rapoport TA. Distinct ubiquitin-ligase complexes define convergent pathways for the degradation of ER proteins. Cell. 2006;126:361–73. doi: 10.1016/j.cell.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 153.Ahner A, Nakatsukasa K, Zhang H, Frizzell RA, Brodsky JL. Small Heat-Shock Proteins Select {Delta}F508-CFTR for Endoplasmic Reticulum-associated Degradation. Mol Biol Cell. 2007;18:806–14. doi: 10.1091/mbc.E06-05-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kashlan OB, Mueller GM, Qamar MZ, Poland PA, Ahner A, Rubenstein RC, et al. Small heat shock protein alphaA-crystallin regulates epithelial sodium channel expression. J Biol Chem. 2007 doi: 10.1074/jbc.M703409200. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kota J, Gilstring CF, Ljungdahl PO. Membrane chaperone Shr3 assists in folding amino acid permeases preventing precocious ERAD. J Cell Biol. 2007;176:617–28. doi: 10.1083/jcb.200612100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kota J, Ljungdahl PO. Specialized membrane-localized chaperones prevent aggregation of polytopic proteins in the ER. J Cell Biol. 2005;168:79–88. doi: 10.1083/jcb.200408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nagamori S, Smirnova IN, Kaback HR. Role of YidC in folding of polytopic membrane proteins. J Cell Biol. 2004;165:53–62. doi: 10.1083/jcb.200402067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Schmidt BZ, Perlmutter DH. Grp78, Grp94, and Grp170 interact with alpha1-antitrypsin mutants that are retained in the endoplasmic reticulum. Am J Physiol Gastrointest Liver Physiol. 2005;289:G444–55. doi: 10.1152/ajpgi.00237.2004. [DOI] [PubMed] [Google Scholar]
- 159.Buchner J. Hsp90 & Co.- a holding for folding. Trends Biochem Sci. 1999;24:136–41. doi: 10.1016/s0968-0004(99)01373-0. [DOI] [PubMed] [Google Scholar]


