Abstract
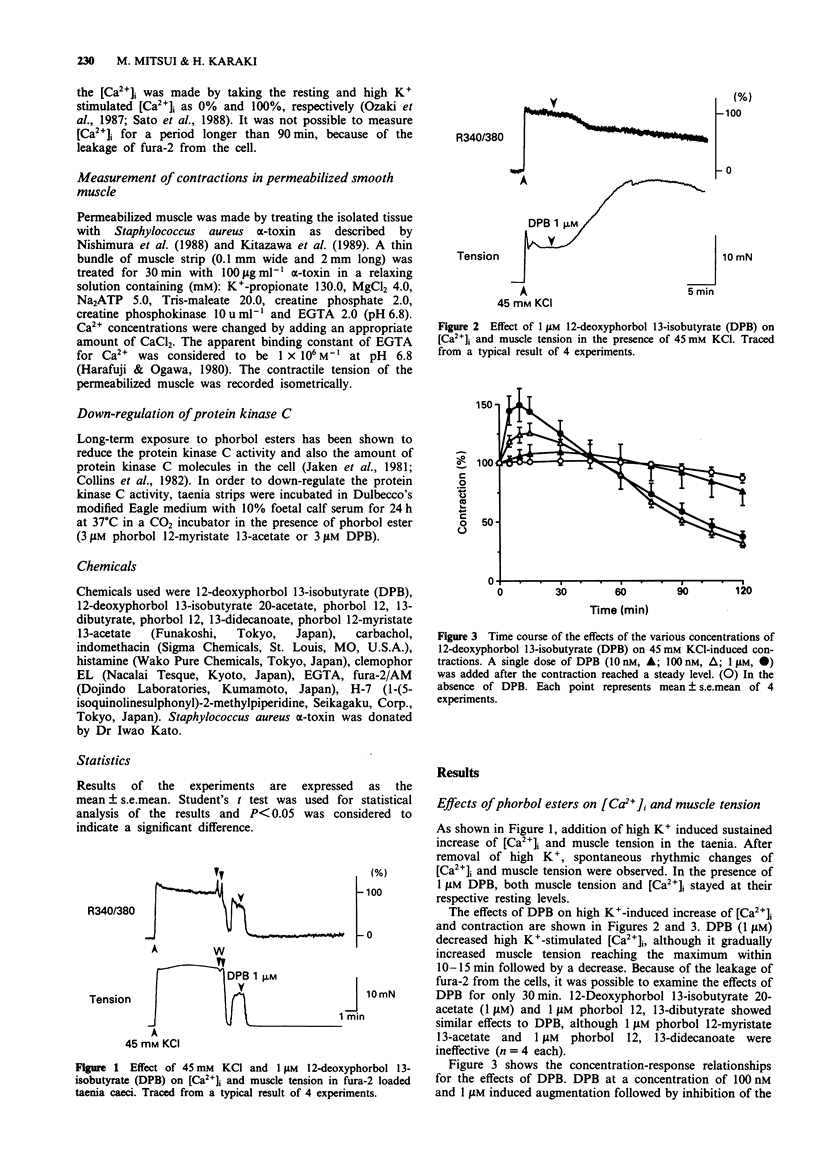
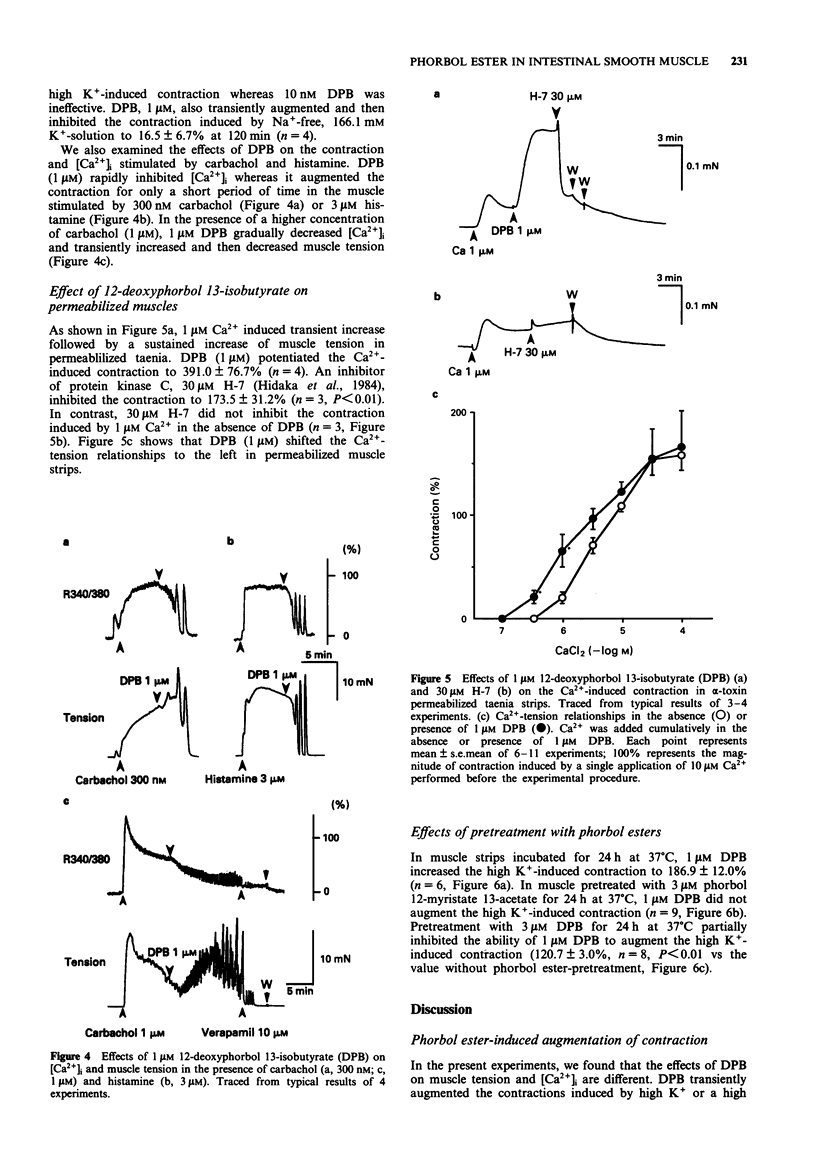
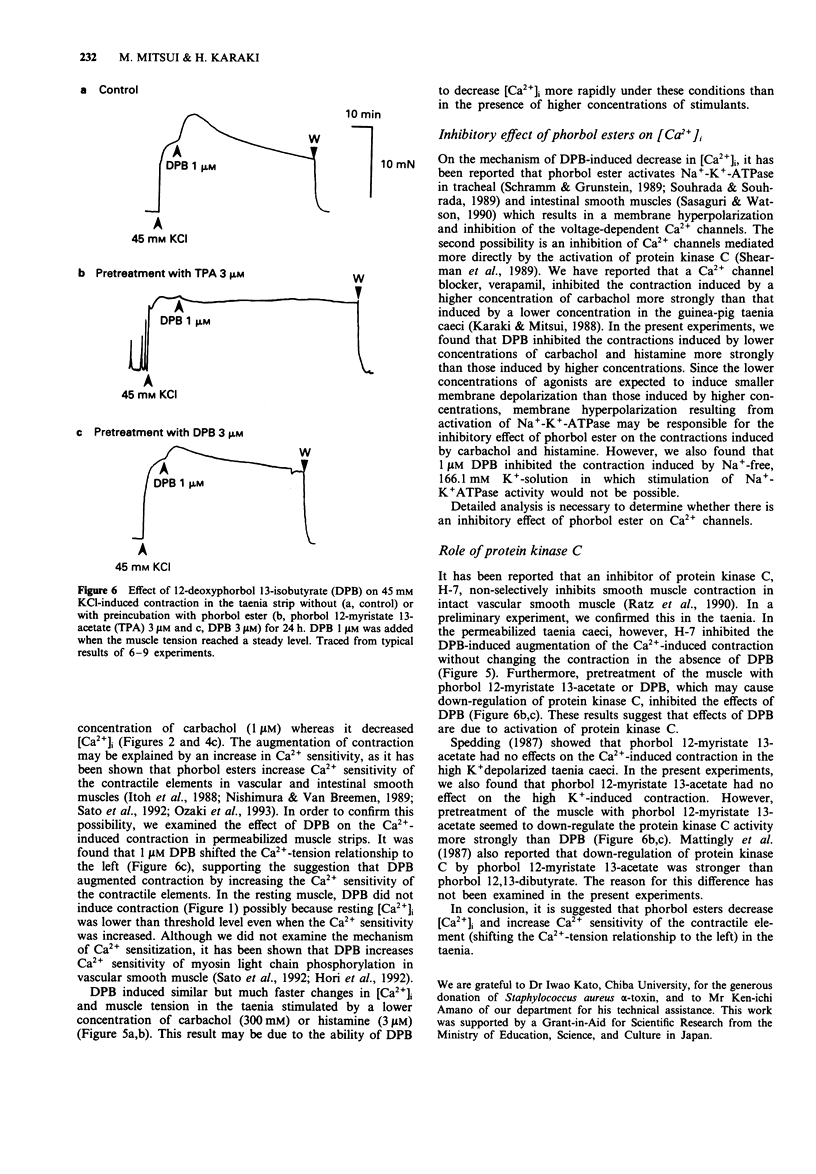
1. Effects of phorbol esters on the cytosolic Ca2+ level ([Ca2+]i) and muscle tension in the intestinal smooth muscle of guinea-pig taenia caeci were examined. 2. 12-Deoxyphorbol 13-isobutyrate (DPB, 1 microM) did not change the [Ca2+]i and tension in resting muscle. 3. In high K(+)-stimulated muscle, 1 microM DPB transiently augmented the contraction and decreased [Ca2+]i. 12-Deoxyphorbol 13-isobutyrate 20-acetate (1 microM) and phorbol 12, 13-dibutyrate (1 microM) showed similar effects to DPB whereas phorbol 12-myristate 13-acetate (1 microM) and phorbol 12, 13-didecanoate (1 microM) were ineffective. 4. DPB (1 microM) inhibited both [Ca2+]i and tension stimulated by 300 nM carbachol or 3 microM histamine. In the presence of a higher concentration of carbachol (1 microM), DPB decreased [Ca2+]i and transiently increased muscle tension. 5. In the muscle strips permeabilized with bacterial alpha-toxin, 1 microM DPB shifted the Ca(2+)-tension curve to the left. An inhibitor of protein kinase C, H-7 (30 microM), inhibited the effect of DPB. 6. DPB did not change the high K(+)-induced contraction in the muscle strips pretreated with 3 microM phorbol 12-myristate 13-acetate for 24 h. 7. These results suggest that activation of protein kinase C has dual effects; it augments contraction by increasing the Ca2+ sensitivity of the contractile elements and it inhibits contraction by decreasing [Ca2+]i.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baraban J. M., Gould R. J., Peroutka S. J., Snyder S. H. Phorbol ester effects on neurotransmission: interaction with neurotransmitters and calcium in smooth muscle. Proc Natl Acad Sci U S A. 1985 Jan;82(2):604–607. doi: 10.1073/pnas.82.2.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M., Rozengurt E. Stimulation of DNA synthesis in murine fibroblasts by the tumour promoter teleocidin: relationship to phorbol esters and vasopressin. Biochem Biophys Res Commun. 1982 Feb 26;104(4):1159–1166. doi: 10.1016/0006-291x(82)91372-9. [DOI] [PubMed] [Google Scholar]
- Harafuji H., Ogawa Y. Re-examination of the apparent binding constant of ethylene glycol bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid with calcium around neutral pH. J Biochem. 1980 May;87(5):1305–1312. doi: 10.1093/oxfordjournals.jbchem.a132868. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Himpens B., Somlyo A. P. Free-calcium and force transients during depolarization and pharmacomechanical coupling in guinea-pig smooth muscle. J Physiol. 1988 Jan;395:507–530. doi: 10.1113/jphysiol.1988.sp016932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P., Lippe I. T. Protein kinase C may regulate the tonic component of intestinal smooth muscle contraction in response to substance P. Naunyn Schmiedebergs Arch Pharmacol. 1989 Jan-Feb;339(1-2):214–220. doi: 10.1007/BF00165146. [DOI] [PubMed] [Google Scholar]
- Hori M., Sato K., Sakata K., Ozaki H., Takano-Ohmuro H., Tsuchiya T., Sugi H., Kato I., Karaki H. Receptor agonists induce myosin phosphorylation-dependent and phosphorylation-independent contractions in vascular smooth muscle. J Pharmacol Exp Ther. 1992 May;261(2):506–512. [PubMed] [Google Scholar]
- Itoh T., Kubota Y., Kuriyama H. Effects of a phorbol ester on acetylcholine-induced Ca2+ mobilization and contraction in the porcine coronary artery. J Physiol. 1988 Mar;397:401–419. doi: 10.1113/jphysiol.1988.sp017008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaken S., Tashjian A. H., Jr, Blumberg P. M. Characterization of phorbol ester receptors and their down-modulation in GH4C1 rat pituitary cells. Cancer Res. 1981 Jun;41(6):2175–2181. [PubMed] [Google Scholar]
- Karaki H. Ca2+ localization and sensitivity in vascular smooth muscle. Trends Pharmacol Sci. 1989 Aug;10(8):320–325. doi: 10.1016/0165-6147(89)90066-7. [DOI] [PubMed] [Google Scholar]
- Karaki H., Mitsui M. Verapamil-sensitive and less sensitive contractions in the intestinal smooth muscle of the guinea-pig taenia caeci. Jpn J Pharmacol. 1988 Apr;46(4):325–330. doi: 10.1254/jjp.46.325. [DOI] [PubMed] [Google Scholar]
- Karaki H., Suzuki T., Urakawa N., Ishida Y., Shibata S. High K+,Na+-deficient solution inhibits tension, O2 consumption, and ATP synthesis in smooth muscle. Jpn J Pharmacol. 1982 Aug;32(4):727–733. doi: 10.1254/jjp.32.727. [DOI] [PubMed] [Google Scholar]
- Kitazawa T., Kobayashi S., Horiuti K., Somlyo A. V., Somlyo A. P. Receptor-coupled, permeabilized smooth muscle. Role of the phosphatidylinositol cascade, G-proteins, and modulation of the contractile response to Ca2+. J Biol Chem. 1989 Apr 5;264(10):5339–5342. [PubMed] [Google Scholar]
- Mattingly R. R., Dreher M. L., Hanley M. R. Down-regulation of phorbol diester binding to NG115-401L neuronal cells is dependent on structure, concentration and time. FEBS Lett. 1987 Oct 19;223(1):11–14. doi: 10.1016/0014-5793(87)80500-8. [DOI] [PubMed] [Google Scholar]
- Menkes H., Baraban J. M., Snyder S. H. Protein kinase C regulates smooth muscle tension in guinea-pig trachea and ileum. Eur J Pharmacol. 1986 Mar 11;122(1):19–27. doi: 10.1016/0014-2999(86)90153-6. [DOI] [PubMed] [Google Scholar]
- Mitsui M., Karaki H. Dual effects of carbachol on cytosolic Ca2+ and contraction in intestinal smooth muscle. Am J Physiol. 1990 May;258(5 Pt 1):C787–C793. doi: 10.1152/ajpcell.1990.258.5.C787. [DOI] [PubMed] [Google Scholar]
- Nishimura J., Kolber M., van Breemen C. Norepinephrine and GTP-gamma-S increase myofilament Ca2+ sensitivity in alpha-toxin permeabilized arterial smooth muscle. Biochem Biophys Res Commun. 1988 Dec 15;157(2):677–683. doi: 10.1016/s0006-291x(88)80303-6. [DOI] [PubMed] [Google Scholar]
- Nishimura J., van Breemen C. Direct regulation of smooth muscle contractile elements by second messengers. Biochem Biophys Res Commun. 1989 Sep 15;163(2):929–935. doi: 10.1016/0006-291x(89)92311-5. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Ozaki H., Gerthoffer W. T., Hori M., Karaki H., Sanders K. M., Publicover N. G. Ca2+ regulation of the contractile apparatus in canine gastric smooth muscle. J Physiol. 1993 Jan;460:33–50. doi: 10.1113/jphysiol.1993.sp019457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H., Sato K., Satoh T., Karaki H. Simultaneous recordings of calcium signals and mechanical activity using fluorescent dye fura 2 in isolated strips of vascular smooth muscle. Jpn J Pharmacol. 1987 Nov;45(3):429–433. doi: 10.1254/jjp.45.429. [DOI] [PubMed] [Google Scholar]
- Ratz P. H. Effect of the kinase inhibitor, H-7, on stress, crossbridge phosphorylation, muscle shortening and inositol phosphate production in rabbit arteries. J Pharmacol Exp Ther. 1990 Jan;252(1):253–259. [PubMed] [Google Scholar]
- Sasaguri T., Watson S. P. Phorbol esters inhibit smooth muscle contractions through activation of Na(+)-K(+)-ATPase. Br J Pharmacol. 1990 Feb;99(2):237–242. doi: 10.1111/j.1476-5381.1990.tb14687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaguri T., Watson S. P. Protein kinase C regulates the tonic but not the phasic component of contraction in guinea-pig ileum. Br J Pharmacol. 1989 Nov;98(3):791–798. doi: 10.1111/j.1476-5381.1989.tb14607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Hori M., Ozaki H., Takano-Ohmuro H., Tsuchiya T., Sugi H., Karaki H. Myosin phosphorylation-independent contraction induced by phorbol ester in vascular smooth muscle. J Pharmacol Exp Ther. 1992 May;261(2):497–505. [PubMed] [Google Scholar]
- Sato K., Ozaki H., Karaki H. Changes in cytosolic calcium level in vascular smooth muscle strip measured simultaneously with contraction using fluorescent calcium indicator fura 2. J Pharmacol Exp Ther. 1988 Jul;246(1):294–300. [PubMed] [Google Scholar]
- Satoh T., Karaki H. Release of prostaglandins during the contraction of rat aorta induced by ouabain and K+-free solution. Jpn J Pharmacol. 1988 Oct;48(2):249–255. doi: 10.1254/jjp.48.249. [DOI] [PubMed] [Google Scholar]
- Schramm C. M., Grunstein M. M. Mechanisms of protein kinase C regulation of airway contractility. J Appl Physiol (1985) 1989 Apr;66(4):1935–1941. doi: 10.1152/jappl.1989.66.4.1935. [DOI] [PubMed] [Google Scholar]
- Shearman M. S., Sekiguchi K., Nishizuka Y. Modulation of ion channel activity: a key function of the protein kinase C enzyme family. Pharmacol Rev. 1989 Jun;41(2):211–237. [PubMed] [Google Scholar]
- Souhrada M., Souhrada J. F. Sodium and calcium influx induced by phorbol esters in airway smooth muscle cells. Am Rev Respir Dis. 1989 Apr;139(4):927–932. doi: 10.1164/ajrccm/139.4.927. [DOI] [PubMed] [Google Scholar]
- Spedding M. Interaction of phorbol esters with Ca2+ channels in smooth muscle. Br J Pharmacol. 1987 Jun;91(2):377–384. doi: 10.1111/j.1476-5381.1987.tb10292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. A., Fay F. S. Intracellular calibration of the fluorescent calcium indicator Fura-2. Cell Calcium. 1990 Feb-Mar;11(2-3):75–83. doi: 10.1016/0143-4160(90)90061-x. [DOI] [PubMed] [Google Scholar]
- Xu S. F., Collins M. A., Chang K. J. Phorbol esters induce oscillatory contractions of intestinal smooth muscles. Eur J Pharmacol. 1991 Aug 29;201(2-3):215–222. doi: 10.1016/0014-2999(91)90348-t. [DOI] [PubMed] [Google Scholar]


