Abstract
1. The effects of propafenone on the inward calcium current (ICa) were investigated in isolated single ventricular myocytes of the guinea-pig by the whole-cell clamp method. Propafenone inhibited ICa in a dose-dependent manner at concentrations of propafenone ranging from 1 x 10(-8) to 1 x 10(-3) M and half maximal block of ICa occurred at a propafenone concentration of 1.5 x 10(-6) M. Propafenone did not change the current-voltage relation of ICa other than a reduction in amplitude and showed no clear use- or frequency-dependent effects upon ICa (stimulation frequencies from 0.03 to 2 Hz). Propafenone did not alter the steady-state inactivation process: the half maximal activation potentials were 18.5 +/- 2.2 mV in the control state and 20.9 +/- 5.0 mV in the presence of 1 x 10(-6) M propafenone (n = 12, NS). Propafenone (1 x 10(-6) M) increased the half-time of reactivation by 73.9% (n = 6, 212.3 +/- 1.2 ms vs 369.2 +/- 1.5 ms, P < 0.05). 2. We conclude that propafenone blocks ICa in a concentration-dependent and a channel state-, use- or frequency-independent manner. The ICa blockade elicited by propafenone at clinically therapeutic plasma concentration is significant and may be involved in its anti-arrhythmic effects.
Full text
PDF
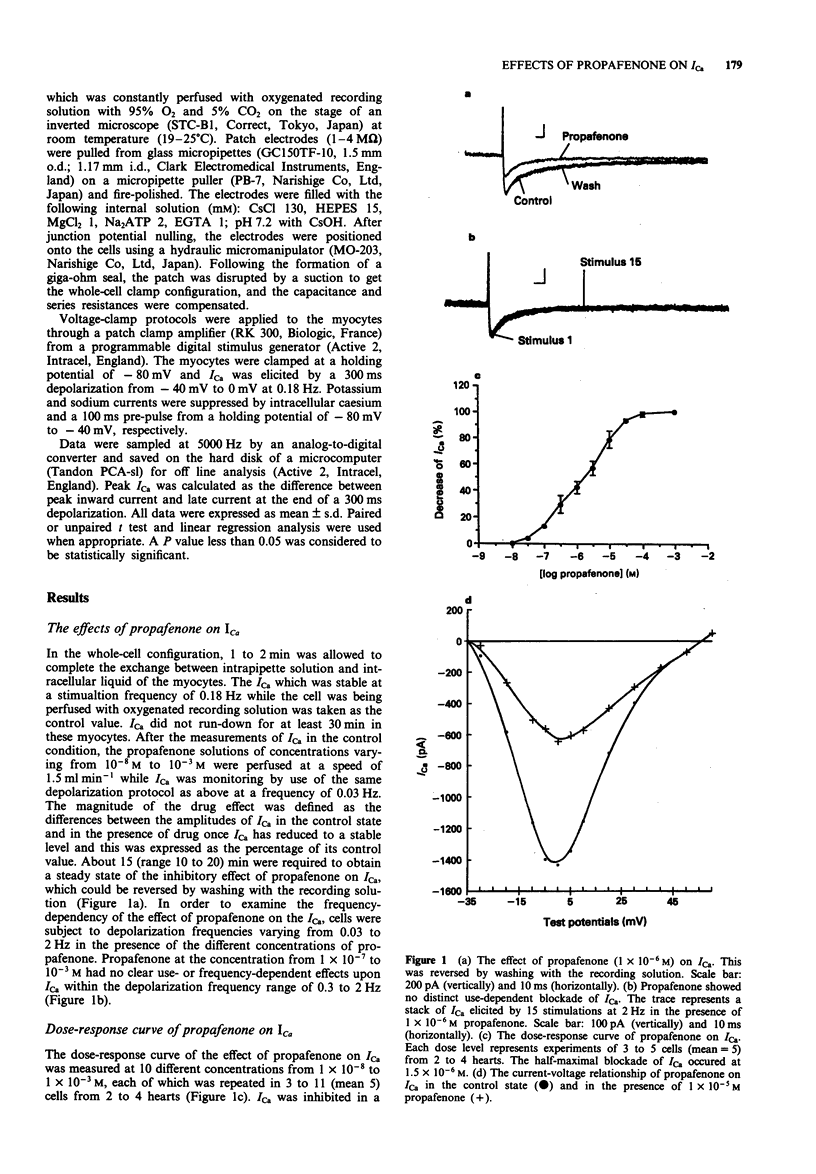
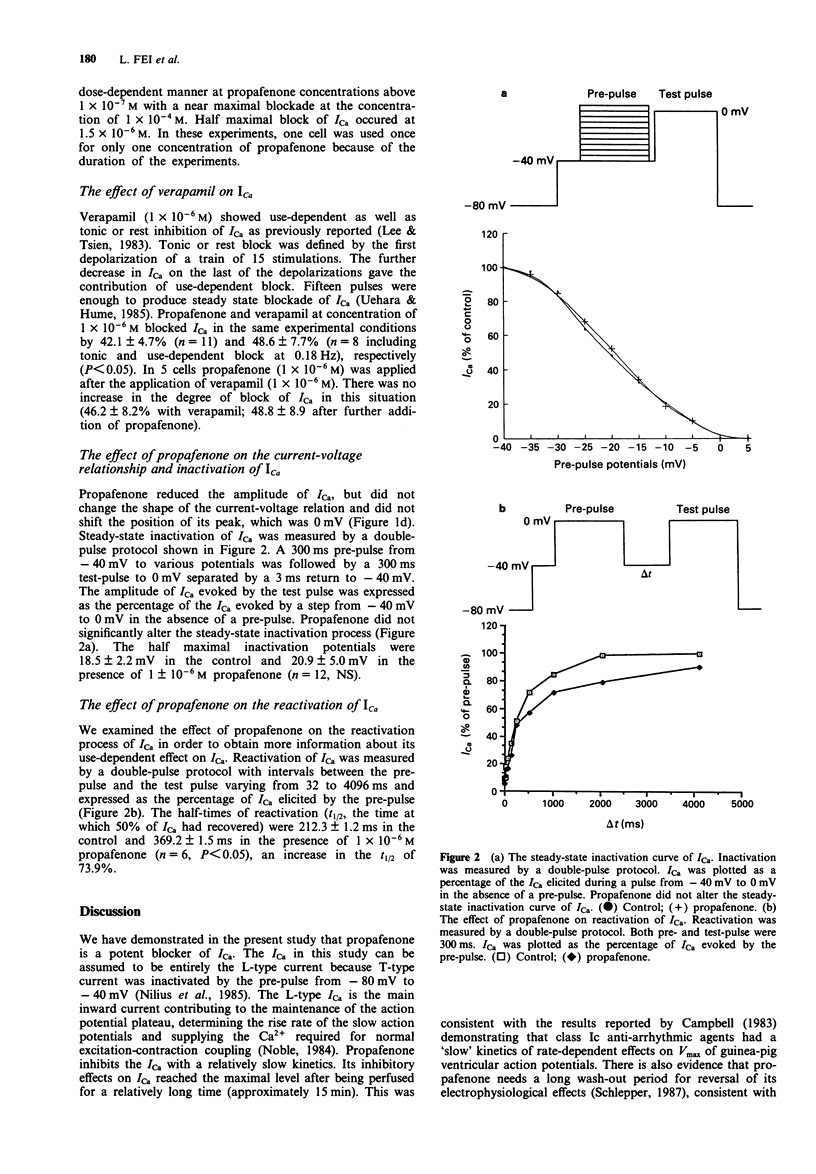


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell T. J. Kinetics of onset of rate-dependent effects of Class I antiarrhythmic drugs are important in determining their effects on refractoriness in guinea-pig ventricle, and provide a theoretical basis for their subclassification. Cardiovasc Res. 1983 Jun;17(6):344–352. doi: 10.1093/cvr/17.6.344. [DOI] [PubMed] [Google Scholar]
- Clarkson C. W., Hondeghem L. M. Evidence for a specific receptor site for lidocaine, quinidine, and bupivacaine associated with cardiac sodium channels in guinea pig ventricular myocardium. Circ Res. 1985 Apr;56(4):496–506. doi: 10.1161/01.res.56.4.496. [DOI] [PubMed] [Google Scholar]
- Delgado C., Tamargo J., Tejerina T. Electrophysiological effects of propafenone in untreated and propafenone-pretreated guinea-pig atrial and ventricular muscle fibres. Br J Pharmacol. 1985 Dec;86(4):765–775. doi: 10.1111/j.1476-5381.1985.tb11098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducouret P. The effect of quinidine on membrane electrical activity in frog auricular fibres studied by current and voltage clamp. Br J Pharmacol. 1976 Jun;57(2):163–184. doi: 10.1111/j.1476-5381.1976.tb07465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukes I. D., Vaughan Williams E. M. The multiple modes of action of propafenone. Eur Heart J. 1984 Feb;5(2):115–125. doi: 10.1093/oxfordjournals.eurheartj.a061621. [DOI] [PubMed] [Google Scholar]
- Grant A. O., Katzung B. G. The effects of quinidine and verapamil on electrically induced automaticity in the ventricular myocardium of guinea pig. J Pharmacol Exp Ther. 1976 Feb;196(2):407–419. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hondeghem L. M., Katzung B. G. Antiarrhythmic agents: the modulated receptor mechanism of action of sodium and calcium channel-blocking drugs. Annu Rev Pharmacol Toxicol. 1984;24:387–423. doi: 10.1146/annurev.pa.24.040184.002131. [DOI] [PubMed] [Google Scholar]
- Honjo H., Watanabe T., Kamiya K., Kodama I., Toyama J. Effects of propafenone on electrical and mechanical activities of single ventricular myocytes isolated from guinea-pig hearts. Br J Pharmacol. 1989 Jul;97(3):731–738. doi: 10.1111/j.1476-5381.1989.tb12010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonason T., Ringqvist I., Bandh S., Nilsson G., Nilsson H., Lidell C., Bjerle P., Olofsson B. O. Propafenone versus disopyramide for treatment of chronic symptomatic ventricular arrhythmias. A multicenter study. Acta Med Scand. 1988;223(6):515–523. doi: 10.1111/j.0954-6820.1988.tb17689.x. [DOI] [PubMed] [Google Scholar]
- Ledda F., Mantelli L., Manzini S., Amerini S., Mugelli A. Electrophysiological and antiarrhythmic properties of propafenon in isolated cardiac preparations. J Cardiovasc Pharmacol. 1981 Nov-Dec;3(6):1162–1173. doi: 10.1097/00005344-198111000-00002. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature. 1983 Apr 28;302(5911):790–794. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- Malfatto G., Zaza A., Forster M., Sodowick B., Danilo P., Jr, Rosen M. R. Electrophysiologic, inotropic and antiarrhythmic effects of propafenone, 5-hydroxypropafenone and N-depropylpropafenone. J Pharmacol Exp Ther. 1988 Aug;246(2):419–426. [PubMed] [Google Scholar]
- McLeod A. A., Stiles G. L., Shand D. G. Demonstration of beta adrenoceptor blockade by propafenone hydrochloride: clinical pharmacologic, radioligand binding and adenylate cyclase activation studies. J Pharmacol Exp Ther. 1984 Feb;228(2):461–466. [PubMed] [Google Scholar]
- Nilius B., Hess P., Lansman J. B., Tsien R. W. A novel type of cardiac calcium channel in ventricular cells. Nature. 1985 Aug 1;316(6027):443–446. doi: 10.1038/316443a0. [DOI] [PubMed] [Google Scholar]
- Noble D. The surprising heart: a review of recent progress in cardiac electrophysiology. J Physiol. 1984 Aug;353:1–50. doi: 10.1113/jphysiol.1984.sp015320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D. G., Sarmiento J. G., Herbette L. G. Kinetics of binding of membrane-active drugs to receptor sites. Diffusion-limited rates for a membrane bilayer approach of 1,4-dihydropyridine calcium channel antagonists to their active site. Mol Pharmacol. 1985 Jun;27(6):612–623. [PubMed] [Google Scholar]
- Scamps F., Undrovinas A., Vassort G. Inhibition of ICa in single frog cardiac cells by quinidine, flecainide, ethmozin, and ethacizin. Am J Physiol. 1989 Mar;256(3 Pt 1):C549–C559. doi: 10.1152/ajpcell.1989.256.3.C549. [DOI] [PubMed] [Google Scholar]
- Schlepper M. Propafenone, a review of its profile. Eur Heart J. 1987 Mar;8 (Suppl A):27–32. doi: 10.1093/eurheartj/8.suppl_a.27. [DOI] [PubMed] [Google Scholar]
- Starmer C. F., Grant A. O. Phasic ion channel blockade. A kinetic model and parameter estimation procedure. Mol Pharmacol. 1985 Oct;28(4):348–356. [PubMed] [Google Scholar]
- Uehara A., Hume J. R. Interactions of organic calcium channel antagonists with calcium channels in single frog atrial cells. J Gen Physiol. 1985 May;85(5):621–647. doi: 10.1085/jgp.85.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]


