Abstract
In comparison to the well characterized role of the principal subunit of voltage-gated Ca2+ channels, the pore-forming, antagonist-binding α1 subunit, considerably less is understood about how β subunits contribute to neuronal Ca2+ channel function. We studied the role of the Ca2+ channel β3 subunit, the major Ca2+ channel β subunit in neurons, by using a gene-targeting strategy. The β3 deficient (β3−/−) animals were indistinguishable from the wild type (wt) with no gross morphological or histological differences. However, in sympathetic β3−/− neurons, the L- and N-type current was significantly reduced relative to wt. Voltage-dependent activation of P/Q-type Ca2+ channels was described by two Boltzmann components with different voltage dependence, analogous to the “reluctant” and “willing” states reported for N-type channels. The absence of the β3 subunit was associated with a hyperpolarizing shift of the “reluctant” component of activation. Norepinephrine inhibited wt and β3−/− neurons similarly but the voltage sensitive component was greater for N-type than P/Q-type Ca2+ channels. The reduction in the expression of N-type Ca2+ channels in the β3−/− mice may be expected to impair Ca2+ entry and therefore synaptic transmission in these animals. This effect may be reversed, at least in part, by the increase in the proportion of P/Q channels activated at less depolarized voltage levels.
Voltage-gated calcium (Ca2+) channels are multimers composed of α1, β, and α2δ subunits (1, 2). Unlike the pore-forming α1 subunit, the contribution of the other subunits to Ca2+ channel function is less well understood. The cloning of four β subunit types has permitted the investigation of their function in heterologous systems (3–6). Yet the role of the β subunit appears complex as it regulates the Ca2+ entry into the cell by increasing the peak Ca2+ current (7–9), by shifting the voltage dependence of activation and inactivation (5, 9), and by modulating G protein inhibition of the α1 subunit (10–12). Deletion of the β1 subunit in skeletal muscle causes a loss of excitation–contraction coupling due to profound decreases in the surface expression of α1S (13). This establishes that β subunits can affect the level of Ca2+ current by controlling α1 membrane targeting in a cell type containing only one type of high voltage-activated Ca2+ channel (α1S,α2δ, β1,γ) but begs the question of what happens in cells such as neurons where multiple types of α1 and β subunits coexist. Accordingly, we have looked for changes in the properties of neuronal Ca2+ channels resulting from removal of the β3 subunit, the most abundant β subunit in the brain (14). This β subunit is an intriguing candidate for investigation because β3 is associated with 56% of the N-type channel complexes in the brain (15), and N-type Ca2+ channels play an important role in regulating Ca2+ entry in neurons(16, 17). Further interest arises from a report that the β3 subunit significantly magnifies differences in G protein modulation of α1A and α1B in oocytes(18). With these results in mind, we have used a gene-targeting strategy to approach questions about the role of the β3 subunit in neurons. Ca2+ channel currents recorded from wild-type (wt) and β3−/− superior cervical ganglion (SCG) neurons were compared in terms of the prevalence of multiple Ca2+ channel types, their voltage-dependence, and their responsiveness to modulation by G proteins.
MATERIALS AND METHODS
Generation of β3 Knockout Mice.
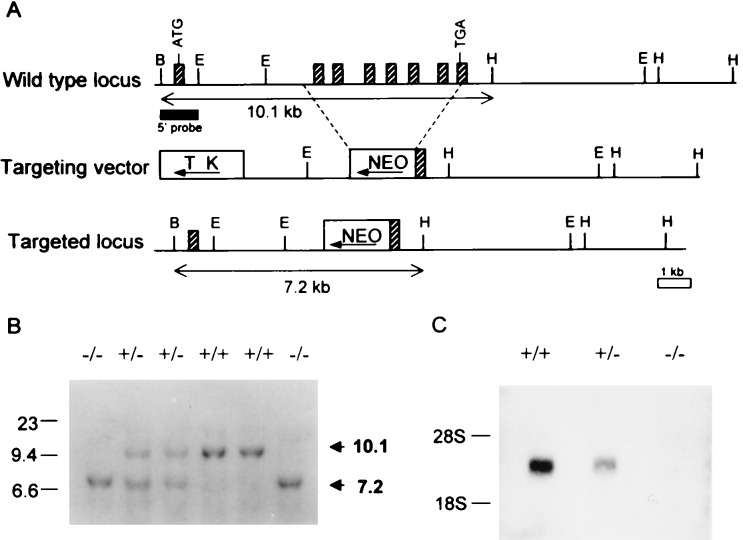
β3-Deficient mice were generated by a conventional gene-targeting method (19). A β3 genomic DNA clone having all the coding exons was isolated from the λ DASH library made from genomic DNA of 129/sv mice by using the 0.47-kb EcoRI–BglII fragment, corresponding to the 5′ end of mouse β3 cDNA, as a probe. For the 5′ homology region, the 3.3-kb EcoRI–SmaI fragment of the β3 genomic DNA was inserted between the neo cassette and the tk cassette of pPNT. For the 3′ homology region, the 8.9-kb ApaI–HindIII was inserted into the XhoI site 5′ of the neo gene. Thus, the final targeting construct was identical to the corresponding regions in the mouse genome, except that in the 4.8-kb SmaI–ApaI fragment all coding exons but the first had been replaced by the positive selection marker, the neo cassette (Fig. 1A).
Figure 1.
Targeted disruption of the Ca2+ channel β3 (ccβ3) gene by homologous recombination. (A) Restriction maps of the murine ccβ3 locus, the targeting vector, and the targeted ccβ3 locus. Hatched boxes represent partially identified exons by DNA sequencing and Southern blot analysis. Solid box underneath the wt locus indicates the probe used for Southern blot analysis; B, BamHI; E, EcoRI; H, HindIII. (B) Southern blotting for genotyping. Ten micrograms of DNA from each tail tip was digested with BamHI and HindIII and hybridized with the probe. The 10.1-kb fragment is the wt allele, and the 7.2-kb fragment is the targeted allele. (C) Northern blot analysis. Total brain RNAs (30 μg each) from ccβ3 (+/+), (+/−), and (−/−) mice were hybridized with BglII–SacI (0.8 kb) fragment of murine ccβ3 cDNA.
The targeting vector was transfected into J1 embryonic stem (ES) cells (Gift from En Li, Whitehead Institute, M.I.T.). Targeted ES clones were identified by Southern blot analysis. Chimeric mice, generated by injecting targeted ES cells into C57BL/6J blastocysts, were mated to 129/sv female mice to obtain the transmission of the β3 mutation to their offspring. Southern blotting was performed for genotyping (Fig. 1B) by using the genomic DNA fragment shown in Fig. 1A as a probe. PCR analysis also was used for rapid genotyping with the following primer set: S primer (5′-TGGACCGGATCTTCACAGCG-3′), A2 primer (5′-GGATGCAGAACACGGCTAGT-3′), and PGK primer (5′-CTGACTAGGGGAGGAGTAGAAG-3′). The wt locus yields a 452-bp fragment amplified by the S and A2 primers whereas the mutant locus yields a 290-bp fragment amplified by the A2 and PGK primers. Northern blot analysis was carried out by using the BglII–SacI (0.8 kb) fragment of murine ccβ3 cDNA as a probe (Fig. 1C).
Animals.
Animals used were 129/sv inbred mice. Chimeric males were mated to 129/sv females to obtain germ line transmission in the 129/sv background.
Histological Analysis.
Seven-month-old mice were perfused transcardially with Bouin’s solution under anesthesia. Organs were removed, fixed overnight, dehydrated in ethanol, embedded in paraffin wax, sectioned, and stained with hematoxylin/eosin.
Isolation of SCG.
SCG neurons were prepared by using a modification of methods described previously (20). Fourteen- to twenty-day-old mice were killed with isoflurane. The SCGs were excised, stripped of their capsule, and cut up. The pieces were incubated at 36°C in a 1.5 mg⋅ml−1 collagenase and 6 mg⋅ml−1 albumin for 15 min and then rinsed before incubation in 1 mg⋅ml−1 trypsin for 30 min. Cells were dissociated by vigorous shaking. After centrifugation, the dispersed neurons were resuspended in L15 medium supplemented with 10% FBS, 24 mM NaHCO3, 38 mM glucose, 2 mM l-glutamine, and 50 μg⋅ml−1 penicillin-streptomycin. Neurons were plated onto glass or poly-l-lysine-coated coverslips and maintained in a humidified atmosphere containing 5% CO2 in air at 37°C. Neurons were used 6–30 hr after plating.
Electrophysiology.
Whole-cell recordings were made from the process-free soma of SCG neurons with 1–3 MΩ electrodes. Recordings were obtained with an Axopatch 200A amplifier (Axon Instruments, Foster City, CA). Series resistance ranged from 2–10 MΩ and was compensated by 80–85%. pulse control (21) and igor pro 3.01 software were used to control data acquisition by computer (Instrutech ITC-16, Great Neck, NY and Macintosh Quadra 950, Apple, Cupertino, CA). Currents were filtered at 5–10 kHz, then digitized at 10–20 μs per point, and leak-subtracted by using a −P/4 procedure.
Solutions.
The pipettes were filled with (mM) cesium methanesulfonate 108, MgCl2 4, Hepes 10, EGTA 9, phosphocreatine 14, Na2ATP 4, and Na2GTP 0.3 (pH 7.3). The external solution (pH 7.4) consisted of TEACl 160, BaCl2 5, Hepes 10, glucose 15, tetrodotoxin 0.001, and cytochrome c (0.1 mg⋅ml−1). Drugs were applied by gravity from a linear array of glass capillaries. Membrane voltages have been corrected for liquid junction potentials. All recordings were made at 23–24°C. Data are presented as mean ± SE.
RESULTS
No Apparent Structural or Behavioral Abnormality in β3-Deficient Mice.
Crossing of heterozygous (β3+/−) mice produced a Mendelian distribution among wt, β3+/−, and β3−/− animals (Fig. 1B). Northern analysis (Fig. 1C) and a reverse transcriptase (RT)-PCR assay confirmed the absence of β3 transcripts in the brain RNA of β3−/− mice, whereas β1, β2, and β4 subunits were each detected in both wt and β3−/− brains (data not shown). SCG neurons were different inasmuch as RT-PCR analysis did not detect β2 subunit in either wt or β3−/− cells.
The β3−/− mice exhibited no obvious behavioral or gross morphological phenotype. Homozygous mutant pairs gave rise to healthy litters of normal size. Histological analysis of brain showed no apparent difference between β3−/− and wt animals. Specifically, the olfactory bulb, hippocampus, habenula, and Purkinje cells, in which the β3 subunit was known to be predominantly expressed, were all apparently normal in β3−/− brain. No abnormality was detected on histological analysis of other organs of the mutant, including the heart, lung, kidney, spleen, adrenal gland, pancreas, liver, ovary, and testis. The apparently normal morphology of β3−/− mice suggests that the β3 subunit is not essential for mouse development.
Ca2+ Channel Currents in Mouse SCG Neurons Arising from N-, P/Q-, and L-Type Channels.
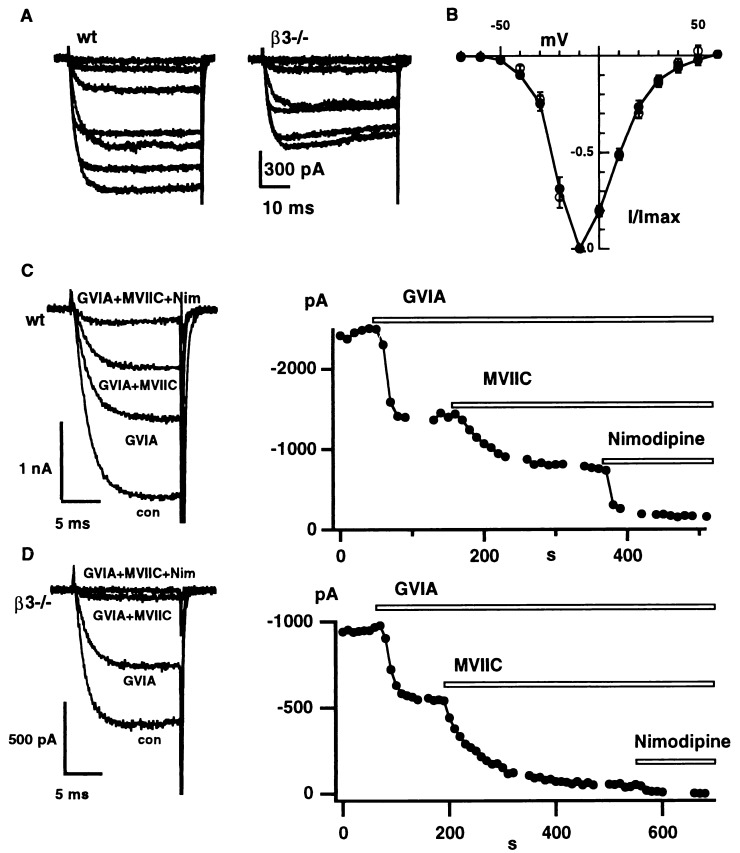
Ca2+ channel currents in SCG neurons from wt or β3 knockout mice were activated by step depolarizations from a holding potential of −90 mV. Families of current records (5 mM Ba2+) are shown (Fig. 2A). The voltage dependence of currents in wt and β3 knockout neurons was very similar, as seen in a plot of normalized peak current (Fig. 2B). In both wt and β3−/− cells, current activation was first detected with a test depolarization beyond −55 mV, and the current reached maximum amplitude near −10 mV.
Figure 2.
The voltage dependence and pharmacological dissection of Ca2+ channel currents in wt and β3−/− SCG neurons. (A) Examplar current records of IBa taken from a wt neuron and a β3−/− (mut) neuron. Depolarizations from −90 mV to levels ranging between −50 and 10 mV in 10 mV increments. (B) The current voltage plot (mean ± SE) normalized to the peak current at −10 mV for eight wt neurons (closed circles) and seven β3−/− neurons (open circles). (C and D) The response of peak IBa in wt (C) and β3−/− (D) neurons to application of 1 μM ω-conotoxin GVIA, 10 μM ω-CTx-MVIIC, and 10 μM nimodipine. IBa was activated by depolarizations from −80 mV to −10 mV every 10 s. Representative current traces are shown on the left.
In the absence of the Ca2+ channel β1 subunit, L-type current in skeletal myotubes is reduced drastically (13). By analogy, we hypothesized that deletion of the β3 subunit might alter the relative proportions of specific Ca2+ channel types in neurons. Extensive studies of sympathetic neurons from rat and bullfrog (17, 26, 39) have demonstrated the predominance of N-type channels, and a small proportion of L-type channels, but the prevalence of various Ca2+ channels in mouse SCG neurons has not been reported. Accordingly, we used an array of inhibitors to define the contribution of N-, P/Q-, and L-type channels to global Ba2+ current (IBa) (Fig. 2 C and D). IBa was activated by depolarizing pulses from −80 mV to −10 mV. In the exemplar wt recording (Fig. 2C), total current was decreased by ≈45% with application of 1 μM ω-conotoxin GVIA, which was sufficient to block all of the N-type current. The subsequent application of 10 μM ω-conotoxin MVIIC was used to define the contribution of P/Q-type current (28), ≈26% of total in this example. Further application of nimodipine (10 μM) caused an additional inhibition of 23%, representing the contribution of L-type current, leaving a residual component of 6%. Fig. 2D illustrates another experiment studying a β3−/− neuron. In this case, the N-, P/Q-, and L-type currents comprised 44%, 51%, and 5% of the total current.
Pooled data from wt and β3−/− neurons were compared with regard to current densities, expressed by using total cell capacitance as an index of membrane area. Values for L-type current density were 4.1 ± 0.9 (n = 16) vs. 1.9 ± 0.4 pA/pF (n = 10), indicating a significant reduction in the β3−/− neurons (P < 0.05) (see Fig. 2 C and D). However (in wt and β3−/− neurons), values for total current density were [44.0 ± 5.3 (n = 24) and 35.5 ± 5.1 pA/pF (n = 23)]; for N-type current density [21.2 ± 4.2 (n = 24) vs. 14.7 ± 3.6 pA/pF (n = 23)]; and for P/Q-type current density [17.7 ± 3.0 (n = 14) vs. 20.4 ± 3.2 pA/pF (n = 12)], respectively, were not different by t test.
Further Analysis Reveals that β3 −/− SCG Neurons Have Less N-Type Current than wt.
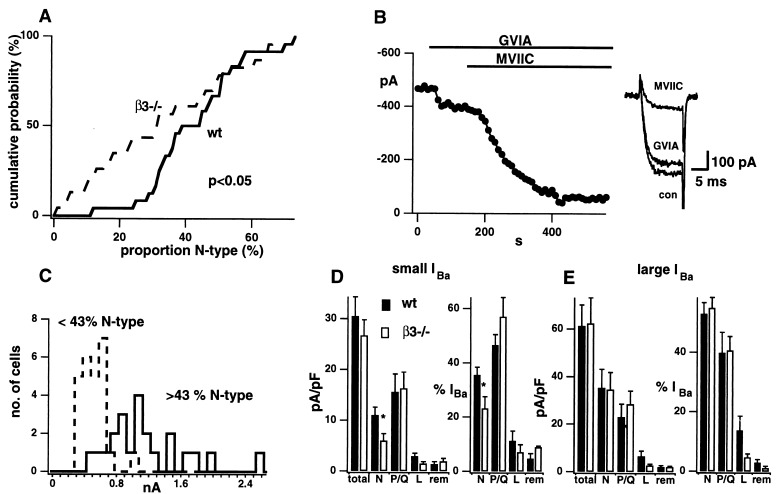
A striking feature of the pharmacological data was the wide range in the relative contributions of various channel types. This was most marked for N-type current as seen in cumulative distributions of its percentage share of total IBa (Fig. 3A). The N-type percentage ranged from 12 to 73% in wt neurons and from 1 to 67% in the β3−/− cells. The non-Gaussian form of the distribution of percentages necessitated the use of a nonparametric test. Indeed, the Kolmogorov–Smirnoff test indicated that the wt and β3−/− distributions in Fig. 3A were significantly different (P < 0.05). This arose because β3−/− neurons often displayed <30% N-type current whereas this rarely occurred in wt neurons. Fig. 3B shows an example of a β3−/− cell, containing 16% N-type and 68% P/Q-type current.
Figure 3.
The N-type current is reduced in β3−/− SCG neurons. (A) The cumulative probability plot for the proportion of IBa that is N-type for 24 wt (solid) and 23 β3−/− (broken) SCG neurons. Plots were significantly different by Kolmogorov–Smirnov test (P < 0.05). (B) Histogram of IBa magnitude in which neurons have been divided according to whether the percentage of IBa carried by N-type channels was <43 (dashed histogram) or >43 (solid histogram). The difference in average IBa between these groups was clearly significant, regardless of whether wt and β3−/− cells were lumped together as shown, or treated separately. Average values of IBa were 440 ± 55 vs. 1061 ± 153 pA for wt neurons (P < 0.01) and 425 ± 38 vs. 1013 ± 146 pA for β3−/− cells (P < 0.001). (C) Effect of 1 μM GVIA and 10 μM MVIIC on IBa in β3−/− neuron with IBa < 700 pA. Exemplar traces on the right. Histograms of the current density and proportion of small (D; n = 6–16) or large (E; n = 3–11) IBa carried by total, N, P/Q, L ,or remainder channels types for wt (solid) and β3−/− (open). N-type current density and proportion are significantly reduced in small IBa β3−/− neurons (∗ indicates P < 0.05 by Student’s t test).
We looked for an explanation of the wide variability, including possible cellular heterogeneity, which might be expected because neurons in intact SCG are functionally heterogeneous (22). A possible correlation between effect of β3 deletion and cell size was suggested by the findings of different patterns of Ca2+ current expression in sensory neurons of various diameters (23). However, when neurons were compared by using a cut-off (43%) to divide the cells into roughly equal groups, there was no clear difference in cell size between cells with low or high percentages of N-type current (15.4 ± 1.8 pF (n = 12) vs. 21.0 ± 2.7 pF (n = 13) for wt, 18.3 ± 2.2 pF (n = 14) vs. 19.0 ± 1.4 pF (n = 9) for β3−/−). However, clear evidence for heterogeneity emerged when we considered the distribution of total IBa magnitude (Fig. 3C). The overall distribution showed two distinct peaks: the left peak consisting almost entirely of neurons with a low proportion of N-type current (dashed histogram) and the right peak almost completely made up of cells with a high proportion (solid histogram). The difference in total IBa was also significant for cells with low and high proportions of N-type current, when wt and β3−/− cells were treated separately (Fig. 3C, legend).
We divided the SCG neurons into small IBa and large IBa groups by using 0.7 nA as a watershed and reanalyzed their properties (Fig. 3 D and E). The large IBa group (Fig. 3E) failed to show significant changes in either the absolute density or proportion of any of the individual currents upon deletion of β3. In contrast, the neurons with small IBa displayed clear differences between wt and β3−/− cells (Fig. 3D). Here, elimination of the β3 subunit caused a significant reduction in the prevalence of N-type current, whether expressed as current density (11.0 ± 1.6 in control vs. 6.0 ± 1.4 pA/pF in β3−/−, P < 0.05) or as a proportion (35 ± 3 vs. 24 ± 4%, P < 0.05). Among the cells with small IBa, the current densities and proportions for other types of Ca2+ channel were not significantly affected (Fig. 3D). The trend toward less L-type current in β3−/− neurons is still apparent in the subdivided data but fails to reach statistical significance due to the more limited sample sizes.
Shift in Preponderance of Modes of Gating of P/Q-type Channels in β3−/− Neurons.
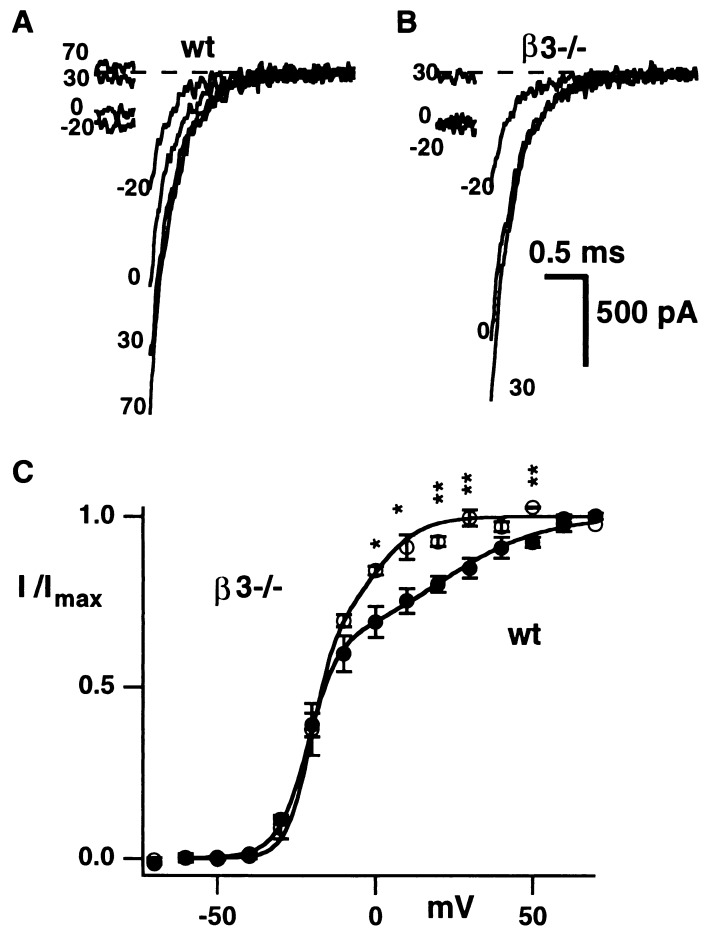
Single channel analysis of P/Q-type channels generated by expression of α1A in HEK293 cells has demonstrated three modes of gating and a relationship between the prevalence of each mode and the specific β subunit type (24). The mode characterized by the lowest probability of opening and the most depolarized activation curve was associated with the β3 subunit. To find out whether β subunits have an influence on gating of P/Q-type channels in neurons, we looked for differences in the voltage-dependence of activation of P/Q currents in whole-cell recordings from wt and β3−/− SCG cells. Tail currents were evoked by repolarizations to −80 mV following 50-ms depolarizing pulses to various potentials (Fig. 4A and B). Within 300 μs of repolarization, tail currents could be fitted by a single exponential, with similar time constants for wt (328 ± 51 μs, n = 6) and β3−/− neurons (427 ± 170 μs, n = 3). Tail current amplitudes were measured 400 μs after the repolarization to reduce the series resistance error (25, 26). The dependence of tail current amplitude on voltage could be described as the sum of two Boltzmann components (see Fig. 4, legend). The parameters describing each of the components are half-activation voltage (V0.5), fractional current amplitude (I) and slope factor (k). Fits to tail-derived activation curves gave comparable values for wt and β3−/− neurons for I′ (0.61 ± 0.05 vs. 0.67 ± 0.03), I′′ (0.39 ± 0.05 vs. 0.33 ± 0.03), k′ (5.1 ± 0.7 vs. 3.9 ± 0.1 mV), k′′ (16.8 ± 1.8 vs. 9.3 ± 2.7 mV) and V′0.5 (−21 ± 1 vs. −20 ± 2 mV). However, the V′′0.5 for β3−/− neurons (−1 ± 1 mV) was 21 mV more negative than V′0.5 for wt neurons (20 ± 5 mV; P < 0.05). This difference resulted in a prominent leftward shift of the upper limb of the activation curve for the β3−/− neurons (Fig. 4B).
Figure 4.
The activation curve for P/Q currents from β3−/− neurons is shifted in a hyperpolarizing direction. Recordings performed in solutions containing 10 μM nimodipine and 1 μM ω-conotoxin GVIA to block L- and N-type channels. Examples of tail currents recorded at −80 mV from wt (A) and β3−/− (B) neurons. (C) The plot of mean (± SE) normalized tail current amplitude for wt (n = 6) and β3−/− (n = 3) neurons is different between 0 and 50 mV (∗ indicates P < 0.05 and ∗∗ P < 0.01 by Student’s t test). The curves are drawn by using the mean values given in the text and the equation I/Imax = I′ (1 + exp(V′0.5 − V)/k′)−1 + I′′(1 + exp(V′′0.5 − V)/k′′)−1 where V is the test voltage.
We also compared the rising and decaying phases of IBa supported by various Ca2+ channel types in wt and β3−/− neurons because the kinetics of activation and inactivation have been found to vary with the type of β subunit in oocytes or HEK293 cells. For both N- or P/Q-type currents, there were no significant differences between wt and β3−/− neurons in the time constants of single exponential functions fitted to the rising phase of current activated at −20, −10, or 0 mV. Likewise, there were no detectable differences between wt or β3−/− neurons in the inactivation kinetics of N- or P/Q-type Ca2+ channels at the same test potentials, analyzed by fitting a single exponential to the current decay during 500-ms depolarizing pulses.
The Relative Importance of α1 and β Subunit Type on G Protein-Modulated Inhibition.
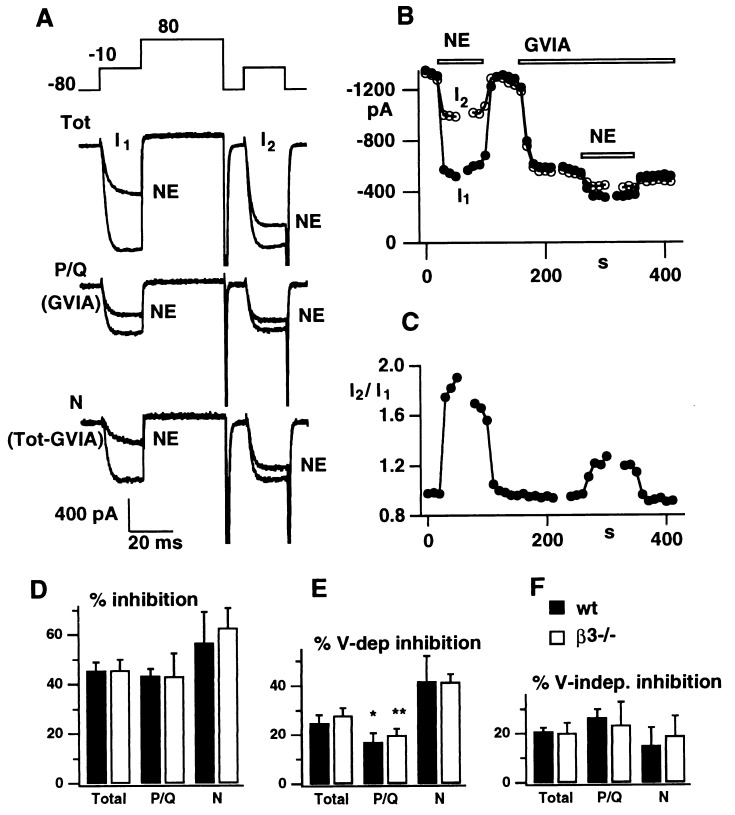
Ca2+ channel β subunits play an important role in the regulation of Ca2+ channels by G protein-coupled receptors (27–30). The β subunits interact with at least two distinct domains of α1 subunits, which also bind G protein βγ subunits (11, 31–33). Thus, the β subunit may help regulate channel function by competing with Gβγ (34). We investigated the possibility of a specific interaction between individual β subunits and G proteins by comparing the responses of wt and β3−/− neurons to G protein-mediated signaling. Norepinephrine (NE) was chosen as a modulatory agent because it represents the class of agents that inhibit Ca2+ currents in a voltage-dependent manner (26, 35, 36) and can be released by sympathetic neurons themselves. Fig. 5 illustrates the action of NE on N- and P/Q-type Ca2+ channel currents, recorded with nimodipine present to eliminate L-type current. The current traces and analysis in Fig. 5A–C were obtained from a representative β3−/− neuron by using a voltage protocol that distinguishes voltage-dependent and voltage-independent components of inhibition (26, 37). In each trial, IBa was activated by two voltage pulses from −80 mV to −10 mV, the second pulse being preceded by a strong conditioning depolarization (+80 mV), sufficient to remove any voltage-dependent component of G protein inhibition. Current amplitudes, measured 10 ms after the onset of the first and second test pulses (I1 and I2), are plotted separately (Fig. 5B) and as a ratio (Fig. 5C). In the absence of NE, I1 and I2 were almost identical (I2/I1 = 0.96 ± 0.01 and 1.00 ± 0.02 for total current from wt and β3−/− neurons). The application of NE caused a prompt decrease in peak IBa and a slowing of its activation during the first test pulse. The NE-induced inhibition was partially relieved by the strong conditioning depolarization, resulting in a sharp increase in the I2/I1 ratio (Fig. 5C).
Figure 5.
The voltage dependent component of NE inhibition is greater for N-type currents than for P/Q-type. (A) Examples of “total” (Top trace), P/Q-type (Middle trace) and N-type currents (Bottom trace), all in the presence of 10 μM nimodipine, in a β3−/− neuron following the “double pulse” protocol (Inset), before and after the application of 10 μM NE. Time course of inhibition of currents (I1 and I2; B) and ratio (I2/I1), measured 10 ms after the step to −10 mV after application of NE (10 μM) and GVIA (1 μM). (D) Histograms of the total, voltage-dependent, and voltage-independent components of NE inhibition of total, N-type, and P/Q-type currents in wt (solid) and β3−/− (open) SCG neurons (n = 5–9). ∗, P < 0.05; ∗∗, P < 0.01.
The effect of NE on the combination of N- and P/Q-type current (Fig. 5A, upper traces) was compared with the effect on P/Q-type current alone (in the presence of ω-CTx-GVIA- middle traces). The P/Q-type current also was inhibited, but the voltage dependent relief was considerably smaller than for total current (note smaller rise of I2/I1 in Fig. 5C). N-type current, obtained by subtracting records taken in the absence and presence of ω-CTx-GVIA (bottom traces), showed a larger degree of voltage-dependent relief. This observation was confirmed in pooled results from experiments in wt and β3−/− neurons in which NE inhibition was broken down according to channel type and whether dependent or independent of conditioning voltage (Fig. 5D–F). The most notable finding is that voltage-dependent inhibition of N type current was significantly greater than for P/Q current in both wt neurons (P < 0.05) and β3−/− neurons (P < 0.01). In contrast, no significant difference was detected between wt and β3−/− neurons in any of the other categories. Thus, these experiments confirm the key nature of the α1 subunit in conferring characteristics of G protein-modulated inhibition but leave open the question of the specific importance of particular β subunits.
DISCUSSION
An overall conclusion of this study is that deletion of a specific β subunit can cause deficits or alterations in multiple components of neuronal Ca2+ channel current. The loss of N-type current density was prominent in sympathetic neurons that displayed a small total current, but the N-type component was reduced, not abolished. L-type current also was markedly diminished, although not completely eliminated. P/Q-type currents were not detectably reduced in amplitude but displayed a clear alteration in their voltage-dependence of activation. Thus, the impact of deleting β3 was found to extend across different current components, generated by distinct α1 subunits. In this sense, our experiments provided direct evidence in neurons that a single kind of β subunit can play a significant role in supporting expression of multiple α1 subunits. On the other hand, the persistence of each of the channel types lends weight to the notion that individual α1 subunits can rely on a variety of β subunits. The β1 and β4 subunits that we found present in β3−/− SCG neurons may partially substitute for the absent β3 subunit and thereby attenuate the resulting disturbance of channel function.
In hindsight, the choice of SCG neurons was fortunate because these cells expressed a variety of high voltage-activated currents, including N-, P/Q, and L-type currents in relative abundance. Our findings for currents in the cell body are in line with previous data on sympathetic transmission in mice, showing a prominent contribution of P/Q-type channels (38). Mouse SCG neurons clearly differ from rat or bullfrog sympathetic neurons, which display >90% N-type current but no P/Q-type current (17, 39).
Our tail current analysis demonstrated that the voltage-dependent activation of P/Q-type channels in wt neurons could not be fitted with a single Boltzmann function but required the sum of two Boltzmann functions with midpoint voltages separated by ≈40 mV. This result is in contrast to activation of P-type currents in Purkinje neurons, which can be well described by a single Boltzmann function (40). The more complex activation characteristics of P/Q channels in our cells are reminiscent of the voltage-dependent activation of N-type channels (25, 26), commonly ascribed to “willing” and “reluctant” modes of gating (26). β3 deletion produced a hyperpolarizing shift in the more depolarized limb of the activation curve, corresponding to the reluctant mode. Single channel recordings from expressed α1A subunits (24) showed that coexpression of β3 subunits favors occupancy of low probability-gating modes, possibly corresponding to the more depolarized component of the activation curve reported here for P/Q Ca2+ channels and the reluctant state found in N-type currents (26). The lack of a reluctant component of activation of P-type current in Purkinje neurons may reflect a predominance of β2 or another β subunit other than β3 (41, 42). Given the absence of β2 in SCG neurons, we were interested to find that the P/Q-type channels in SCG neurons were insensitive to 20 nM ω-Aga-IVA (S.M.S., Y.N., and R.W.T., unpublished observation) and inactivated with prolonged depolarizations (τ = 245 ± 27 ms at −10 mV), both consistent with “Q-type” behavior.
Another topic of interest was the possible impact of β3 on G protein-mediated inhibition of Ca2+ currents. The voltage-dependent component of NE inhibition of N-type Ca2+ channels was nearly twofold greater than for P/Q-type channels (Fig. 5E). This difference was observed in both wt and β3−/− neurons and suggested that the type of α1 subunit is more important than the type of β subunit in determining the Ca2+ channel response to G protein modulation. The greater degree of voltage-dependent inhibition of N-type channels relative to P/Q-type has been already described for native currents in chromaffin cells (43) and for α1A and α1B expressed in oocytes (18, 44). Because N- and P/Q-type Ca2+ channels both play prominent roles in regulating transmitter release, differences in their susceptibility to relief of G protein-mediated inhibition may be useful for fine tuning of synaptic strength. The viability of the β3 knockout stands in contrast with the embryonic lethality of deleting the β1 subunit (13), which arises from the reliance of skeletal muscle excitation–contraction coupling on this particular β subunit. An impact of β3 deletion might be detected in the intact mouse with further testing. Cardiovascular changes might be anticipated in view of the importance of sympathetic neurons in controlling vascular tone and the prominent expression of β3 subunits in smooth muscle (45). Nociceptive signaling would be another interesting possibility because N-type currents play a key role in pain transmission (46). Meanwhile, our electrophysiological data may help explain the absence of overt changes in β3−/− mice. Their neurological status will depend on changes in the functional properties of N- and P/Q-type Ca2+ channels, which often work together to regulate Ca2+ influx and exocytosis at presynaptic terminals in the CNS (16, 47). A reduction in N-type current like that found in SCG neurons would impair transmitter release while the hyperpolarizing shift in the activation curve of P/Q-type channels would favor increased activation and enhanced synaptic transmission. The opposing nature of these actions, along with likely compensation for loss of β3 by other β subunits, may help explain the lack of an obvious impact on CNS function in the whole animal.
Acknowledgments
We thank F. Horrigan for providing software, M. Dayrell and J. Yang for advice on culturing SCG neurons, K. S. Jun for primary library screening, D. Kim and N. G. Kang for their generous teaching, M. P. Kong for animal care, and all members of the Tsien laboratory for helpful discussion. In addition we thank E. Kavalali, D. Lipscombe, P. Mermelstein, J. Nargeot, E. Piedras-Renteria, and D. Pietrobon for comments on the manuscript. This work was supported in part by a Creative Research Initiative program grant from the Ministry of Science and Technology, a medical science grant from the Ministry of Health and Welfare, a genetic engineering grant from the Ministry of Education of Korea and grants from the National Institutes of Health, Howard Hughes Medical Institute, and the McKnight Foundation. S.M.S. is a Howard Hughes Medical Institute Physician Postdoctoral Fellow.
ABBREVIATIONS
- SCG
superior cervical ganglion
- wt
wild type
- IBa
Ba2+ current
- I1 and I2
first and second test pulses
- V0.5
half-activation voltage
References
- 1. Flockerzi V, Oeken H J, Hofmann F, Pelzer D, Cavalie A, Trautwein W. Nature (London) 1986;323:66–68. doi: 10.1038/323066a0. [DOI] [PubMed] [Google Scholar]
- 2.Witcher D R, De Waard M, Liu H, Pragnell M, Campbell K P. J Biol Chem. 1995;270:18088–18093. doi: 10.1074/jbc.270.30.18088. [DOI] [PubMed] [Google Scholar]
- 3.Pragnell M, Sakamoto J, Jay S D, Campbell K P. FEBS Lett. 1991;291:253–258. doi: 10.1016/0014-5793(91)81296-k. [DOI] [PubMed] [Google Scholar]
- 4.Perez-Reyes E, Castellano A, Kim H S, Bertrand P, Baggstrom E, Lacerda A E, Wei X Y, Birnbaumer L. J Biol Chem. 1992;267:1792–1797. [PubMed] [Google Scholar]
- 5.Castellano A, Wei X, Birnbaumer L, Perez-Reyes E. J Biol Chem. 1993;268:3450–3455. [PubMed] [Google Scholar]
- 6.Castellano A, Wei X, Birnbaumer L, Perez-Reyes E. J Biol Chem. 1993;268:12359–12366. [PubMed] [Google Scholar]
- 7.Stea A, Dubel S J, Pragnell M, Leonard J P, Campbell K P, Snutch T P. Neuropharmacology. 1993;32:1103–1116. doi: 10.1016/0028-3908(93)90005-n. [DOI] [PubMed] [Google Scholar]
- 8.Mori Y, Friedrich T, Kim M S, Mikami A, Nakai J, Ruth P, Bosse E, Hofmann F, Flockerzi V, Furuichi T, et al. Nature (London) 1991;350:398–402. doi: 10.1038/350398a0. [DOI] [PubMed] [Google Scholar]
- 9.De Waard M, Witcher D R, Pragnell M, Liu H, Campbell K P. J Biol Chem. 1995;270:12056–12064. doi: 10.1074/jbc.270.20.12056. [DOI] [PubMed] [Google Scholar]
- 10.Campbell V, Berrow N S, Fitzgerald E M, Brickley K, Dolphin A C. J Physiol (London) 1995;485:365–372. doi: 10.1113/jphysiol.1995.sp020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin N, Platano D, Olcese R, Stefani E, Birnbaumer L. Proc Natl Acad Sci USA. 1997;94:8866–8871. doi: 10.1073/pnas.94.16.8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roche J P, Anantharam V, Treistman S N. FEBS Lett. 1995;371:43–46. doi: 10.1016/0014-5793(95)00860-c. [DOI] [PubMed] [Google Scholar]
- 13.Gregg R G, Messing A, Strube C, Beurg M, Moss R, Behan M, Sukhareva M, Haynes S, Powell J A, Coronado R, Powers P A. Proc Natl Acad Sci USA. 1996;93:13961–13966. doi: 10.1073/pnas.93.24.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witcher D R, De Waard M, Kahl S D, Campbell K P. Methods Enzymol. 1994;238:335–348. doi: 10.1016/0076-6879(94)38030-9. [DOI] [PubMed] [Google Scholar]
- 15.Scott V E, De Waard M, Liu H, Gurnett C A, Venzke D P, Lennon V A, Campbell K P. J Biol Chem. 1996;271:3207–3212. doi: 10.1074/jbc.271.6.3207. [DOI] [PubMed] [Google Scholar]
- 16.Wheeler D B, Randall A, Tsien R W. Science. 1994;264:107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- 17.Plummer M R, Logothetis D E, Hess P. Neuron. 1989;2:1453–1463. doi: 10.1016/0896-6273(89)90191-8. [DOI] [PubMed] [Google Scholar]
- 18.Roche J P, Treistman S N. J Neurosci. 1998;18:878–886. doi: 10.1523/JNEUROSCI.18-03-00878.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim D, Jun K S, Lee S B, Kang N G, Min D S, Kim Y H, Ryu S H, Suh P G, Shin H S. Nature (London) 1997;389:290–293. doi: 10.1038/38508. [DOI] [PubMed] [Google Scholar]
- 20.Zhu Y, Ikeda S R. Neuron. 1994;13:657–669. doi: 10.1016/0896-6273(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 21.Herrington J, Newton K R, Bookman R J. Pulse Control: Igor XOPs for Patch Clamp Data Acquisition and Capacitance Measurements. Miami: University of Miami; 1995. , Version 4.5. [Google Scholar]
- 22.Eccles J C. J Physiol (London) 1935;85:179–206. doi: 10.1113/jphysiol.1935.sp003313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scroggs R S, Fox A P. J Physiol (London) 1992;445:639–658. doi: 10.1113/jphysiol.1992.sp018944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luvisetto S, Hivert B, Spagnolo M, Brust P, Williams M, Stauderman K, Harpold M, Pietrobon D. Biophys J. 1998;74:120. (abstr.). [Google Scholar]
- 25.Ikeda S R. J Physiol (London) 1991;439:181–214. doi: 10.1113/jphysiol.1991.sp018663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bean B P. Nature (London) 1989;340:153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- 27.Dolphin A C. Exp Physiol. 1995;80:1–36. doi: 10.1113/expphysiol.1995.sp003825. [DOI] [PubMed] [Google Scholar]
- 28.Zamponi G W, Bourinet E, Nelson D, Nargeot J, Snutch T P. Nature (London) 1997;385:442–446. doi: 10.1038/385442a0. [DOI] [PubMed] [Google Scholar]
- 29.De Waard M, Pragnell M, Campbell K P. Neuron. 1994;13:495–503. doi: 10.1016/0896-6273(94)90363-8. [DOI] [PubMed] [Google Scholar]
- 30.Herlitze S, Hockerman G H, Scheuer T, Catterall W A. Proc Natl Acad Sci USA. 1997;94:1512–1516. doi: 10.1073/pnas.94.4.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tareilus E, Roux M, Qin N, Olcese R, Zhou J, Stefani E, Birnbaumer L. Proc Natl Acad Sci USA. 1997;94:1703–1708. doi: 10.1073/pnas.94.5.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Waard M, Liu H, Walker D, Scott V E, Gurnett C A, Campbell K P. Nature (London) 1997;385:446–450. doi: 10.1038/385446a0. [DOI] [PubMed] [Google Scholar]
- 33.Page K M, Stephens G J, Berrow N S, Dolphin A C. J Neurosci. 1997;17:1330–1338. doi: 10.1523/JNEUROSCI.17-04-01330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campbell V, Berrow N, Brickley K, Page K, Wade R, Dolphin A C. FEBS Lett. 1995;370:135–140. doi: 10.1016/0014-5793(95)00813-o. [DOI] [PubMed] [Google Scholar]
- 35.Tsien R W, Lipscombe D, Madison D V, Bley K R, Fox A P. Trends Neurosci. 1988;11:431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- 36.Ikeda S R. Nature (London) 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- 37.Elmslie K S, Kammermeier P J, Jones S W. Neuron. 1994;13:217–228. doi: 10.1016/0896-6273(94)90471-5. [DOI] [PubMed] [Google Scholar]
- 38.Waterman S A. Br J Pharmacol. 1997;120:393–398. doi: 10.1038/sj.bjp.0700948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones S W, Marks T N. J Gen Physiol. 1989;94:151–167. doi: 10.1085/jgp.94.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McDonough S I, Lampe R A, Keith R A, Bean B P. Mol Pharmacol. 1997;52:1095–1104. doi: 10.1124/mol.52.6.1095. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka O, Sakagami H, Kondo H. Brain Res Mol Brain Res. 1995;30:1–16. doi: 10.1016/0169-328x(94)00265-g. [DOI] [PubMed] [Google Scholar]
- 42.Moreno H, Rudy B, Llinas R. Proc Natl Acad Sci USA. 1997;94:14042–14047. doi: 10.1073/pnas.94.25.14042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Currie K P, Fox A P. J Neurosci. 1997;17:4570–4579. doi: 10.1523/JNEUROSCI.17-12-04570.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J F, Ellinor P T, Aldrich R W, Tsien R W. Neuron. 1996;17:991–1003. doi: 10.1016/s0896-6273(00)80229-9. [DOI] [PubMed] [Google Scholar]
- 45.Collin T, Lory P, Taviaux S, Courtieu C, Guilbault P, Berta P, Nargeot J. Eur J Biochem. 1994;220:257–262. doi: 10.1111/j.1432-1033.1994.tb18621.x. [DOI] [PubMed] [Google Scholar]
- 46.Miljanich G P, Ramachandran J. Annu Rev Pharmacol Toxicol. 1995;35:707–734. doi: 10.1146/annurev.pa.35.040195.003423. [DOI] [PubMed] [Google Scholar]
- 47.Dunlap K, Luebke J I, Turner T J. Trends Neurosci. 1995;18:89–98. [PubMed] [Google Scholar]