Abstract
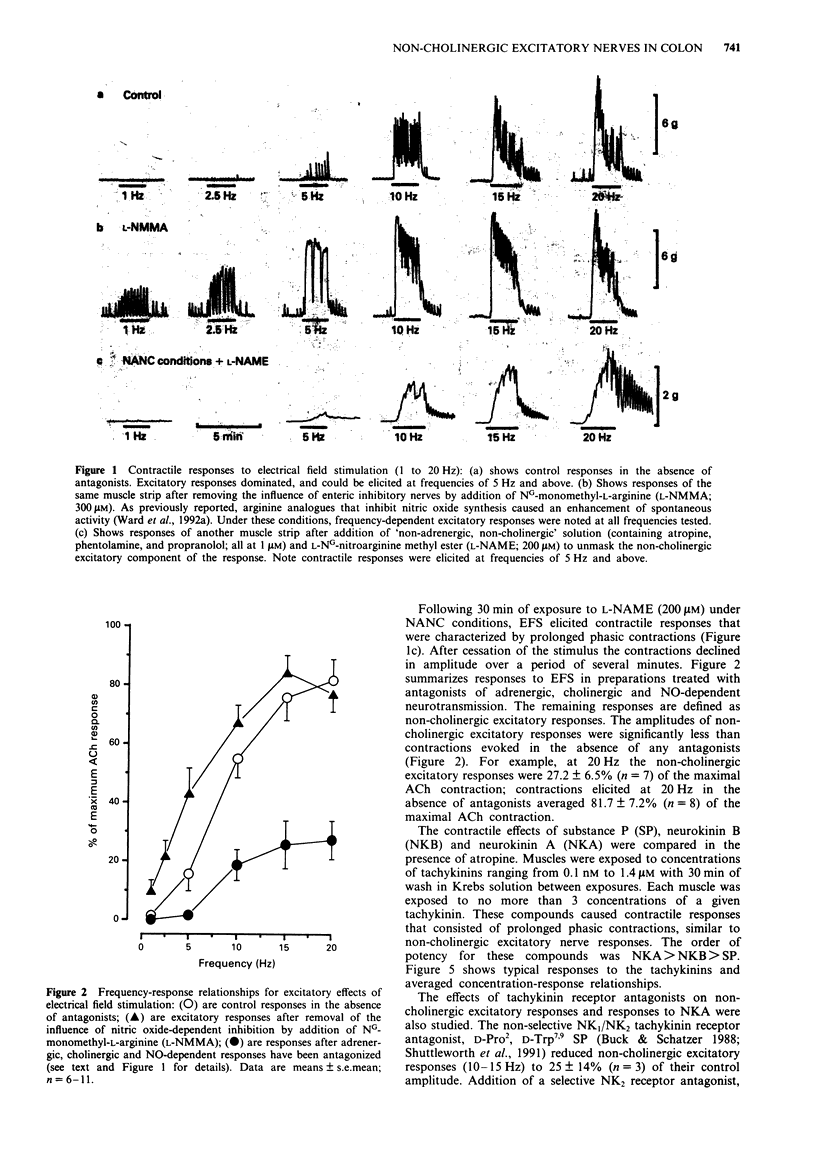
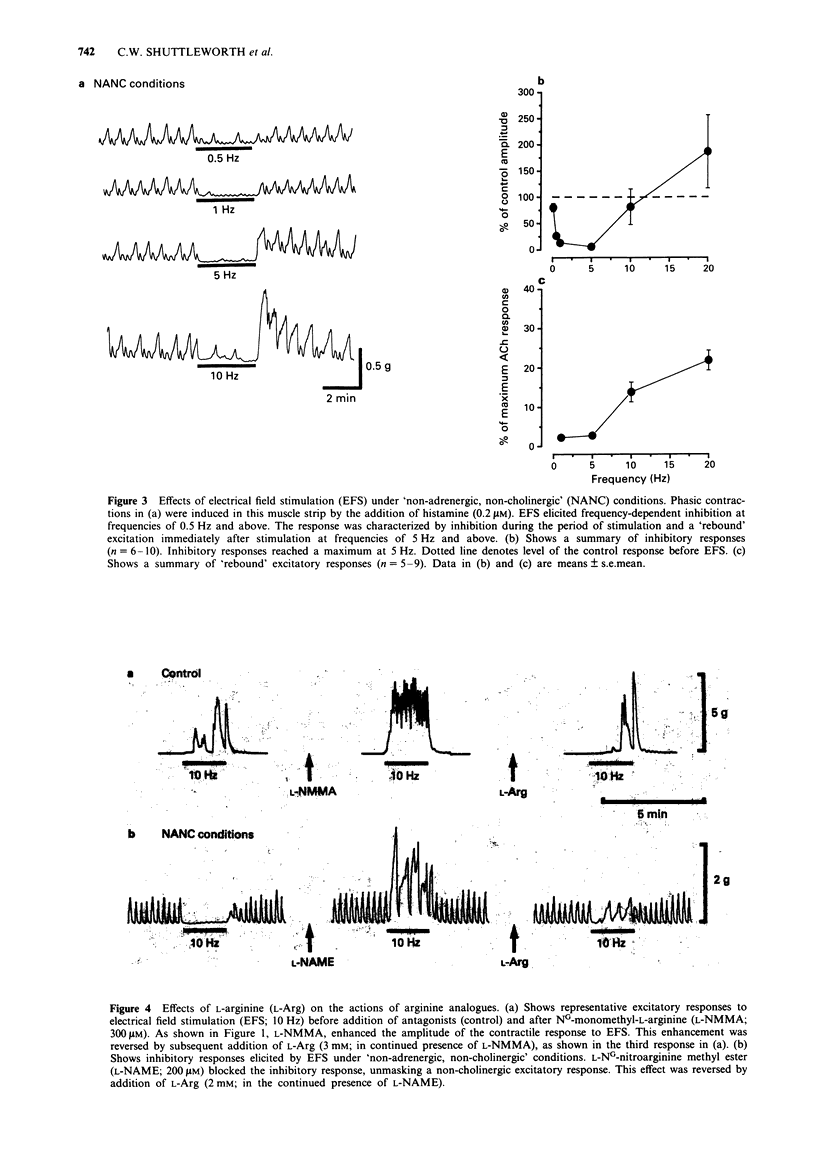
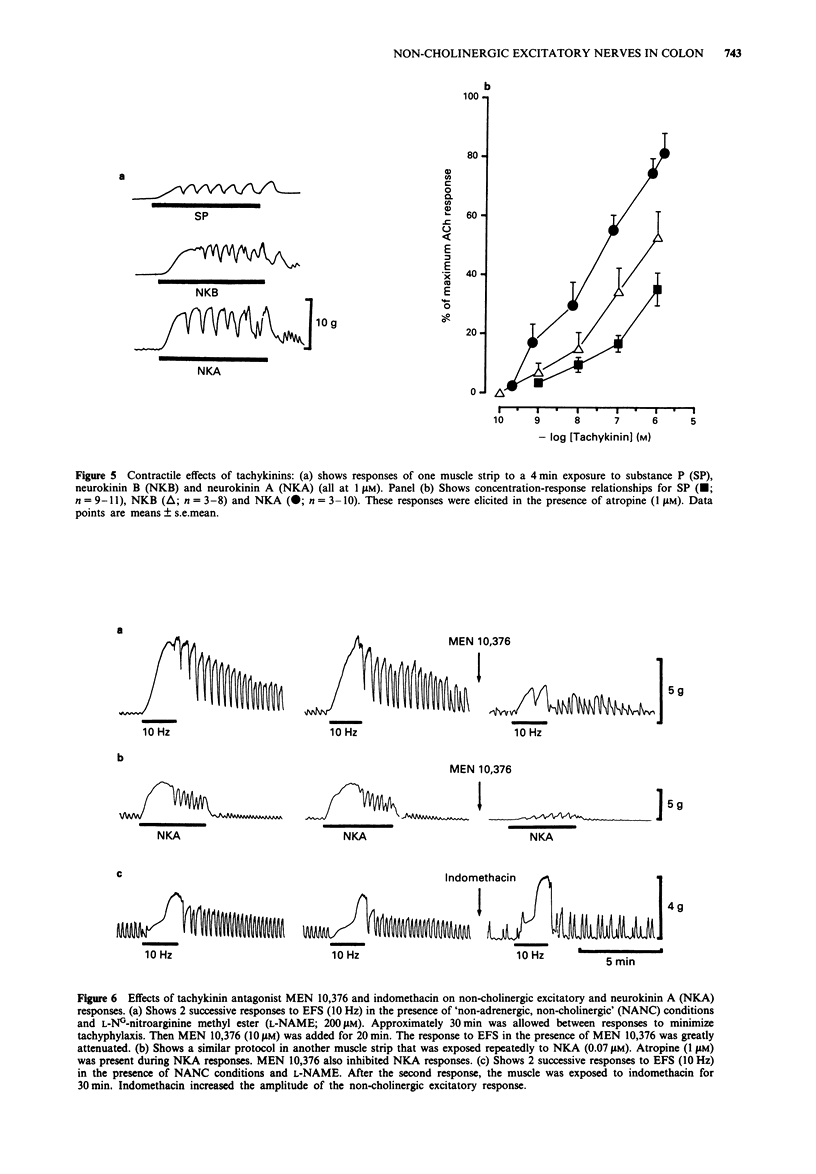
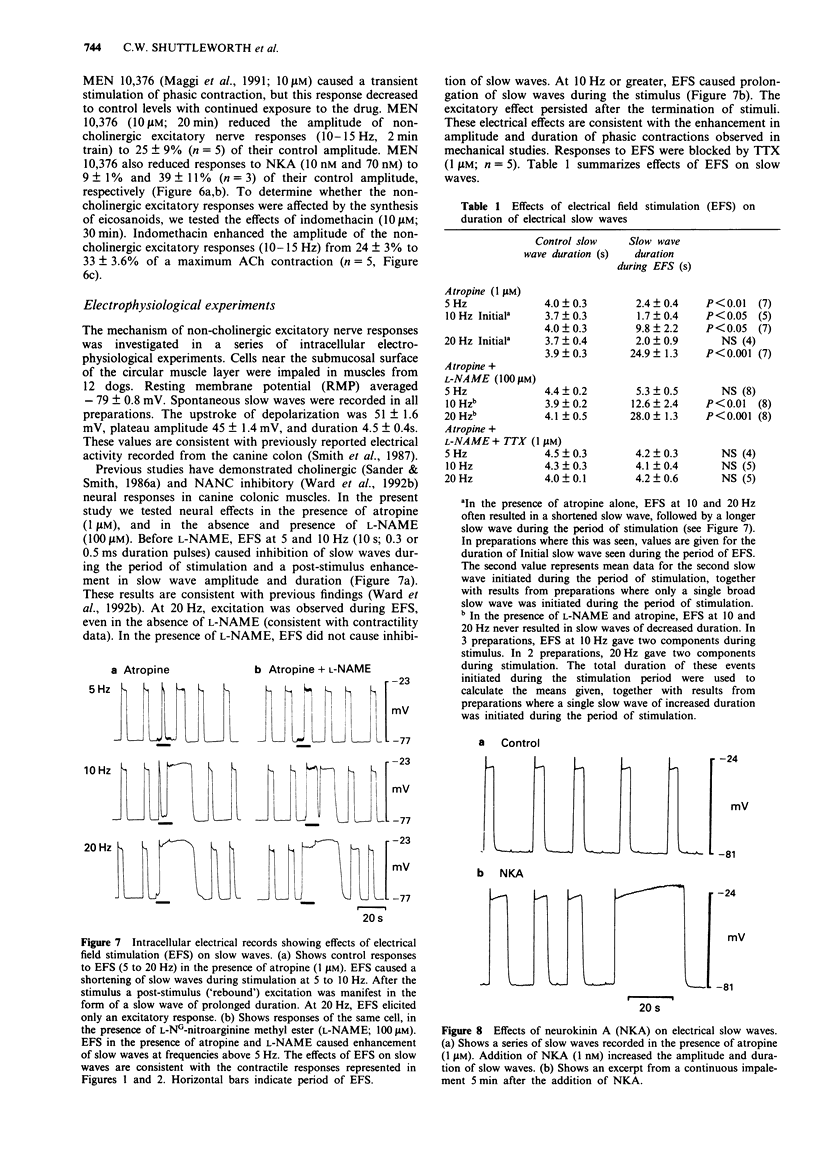
1. Neuromuscular transmission in the circular muscle of the canine proximal colon was examined, in the presence and absence of nitric oxide synthase inhibitors, by use of mechanical and intracellular microelectrode recording techniques. 2. Electrical field stimulation (EFS; 0.1-20 HZ) produced frequency-dependent contractions of circular muscle strips which reached a maximum at 15 Hz. These responses were enhanced by NG-monomethyl-L-arginine (L-NMMA; 300 microM) and reduced by atropine (1 microM). The effects of L-NMMA were reversed by L-arginine (3 mM). All responses to EFS were abolished by tetrodotoxin (1 microM). 3. In the presence of atropine, phentolamine and propranolol (all at 1 microM; 'non-adrenergic, non-cholingergic (NANC) conditions'), EFS evoked frequency-dependent inhibition of phasic contractions which reached a maximum at 5 Hz. At higher frequencies of EFS, inhibition diminished, and these responses were followed by post-stimulus excitation. 4. Under NANC conditions and in the presence of L-NG-nitroarginine methyl ester (L-NAME; 200 microM), EFS evoked contractions at frequencies of 5 Hz or greater. These contractions were reduced by co-incubation with L-arginine (2 mM) and abolished by tetrodotoxin (1 microM). 5. In the presence of atropine (1 microM), EFS (5-20 Hz) caused frequency-dependent inhibition of electrical slow waves. In the presence of L-NAME (100 microM) and atropine, the inhibitory response to EFS was abolished and an increase in slow wave duration was seen at stimulation frequencies greater than 5 Hz. The effects of EFS on slow wave duration were abolished by tetrodotoxin (1 microM).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barajas-López C., Huizinga J. D. Different mechanisms of contraction generation in circular muscle of canine colon. Am J Physiol. 1989 Mar;256(3 Pt 1):G570–G580. doi: 10.1152/ajpgi.1989.256.3.G570. [DOI] [PubMed] [Google Scholar]
- Baron S. A., Jaffe B. M., Gintzler A. R. Release of substance P from the enteric nervous system: direct quantitation and characterization. J Pharmacol Exp Ther. 1983 Nov;227(2):365–368. [PubMed] [Google Scholar]
- Brodin E., Nilsson G. Concentration of substance P-like immunoreactivity (SPLI) in tissues of dog, rat and mouse. Acta Physiol Scand. 1981 Jul;112(3):305–312. doi: 10.1111/j.1748-1716.1981.tb06821.x. [DOI] [PubMed] [Google Scholar]
- Brookes S. J., Song Z. M., Steele P. A., Costa M. Identification of motor neurons to the longitudinal muscle of the guinea pig ileum. Gastroenterology. 1992 Sep;103(3):961–973. doi: 10.1016/0016-5085(92)90030-3. [DOI] [PubMed] [Google Scholar]
- Buck S. H., Shatzer S. A. Agonist and antagonist binding to tachykinin peptide NK-2 receptors. Life Sci. 1988;42(26):2701–2708. doi: 10.1016/0024-3205(88)90246-9. [DOI] [PubMed] [Google Scholar]
- Buxton I. L., Cheek D. J., Eckman D., Westfall D. P., Sanders K. M., Keef K. D. NG-nitro L-arginine methyl ester and other alkyl esters of arginine are muscarinic receptor antagonists. Circ Res. 1993 Feb;72(2):387–395. doi: 10.1161/01.res.72.2.387. [DOI] [PubMed] [Google Scholar]
- Costa M., Furness J. B., Pullin C. O., Bornstein J. Substance P enteric neurons mediate non-cholinergic transmission to the circular muscle of the guinea-pig intestine. Naunyn Schmiedebergs Arch Pharmacol. 1985 Feb;328(4):446–453. doi: 10.1007/BF00692914. [DOI] [PubMed] [Google Scholar]
- Dalziel H. H., Thornbury K. D., Ward S. M., Sanders K. M. Involvement of nitric oxide synthetic pathway in inhibitory junction potentials in canine proximal colon. Am J Physiol. 1991 May;260(5 Pt 1):G789–G792. doi: 10.1152/ajpgi.1991.260.5.G789. [DOI] [PubMed] [Google Scholar]
- Donnerer J., Barthó L., Holzer P., Lembeck F. Intestinal peristalsis associated with release of immunoreactive substance P. Neuroscience. 1984 Apr;11(4):913–918. doi: 10.1016/0306-4522(84)90202-1. [DOI] [PubMed] [Google Scholar]
- Franco R., Costa M., Furness J. B. Evidence for the release of endogenous substance P from intestinal nerves. Naunyn Schmiedebergs Arch Pharmacol. 1979 Apr;306(3):195–201. doi: 10.1007/BF00507103. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Bornstein J. C., Murphy R., Pompolo S. Roles of peptides in transmission in the enteric nervous system. Trends Neurosci. 1992 Feb;15(2):66–71. doi: 10.1016/0166-2236(92)90029-8. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Lloyd K. C., Sternini C., Walsh J. H. Projections of substance P, vasoactive intestinal peptide and tyrosine hydroxylase immunoreactive nerve fibres in the canine intestine, with special reference to the innervation of the circular muscle. Arch Histol Cytol. 1990 May;53(2):129–140. doi: 10.1679/aohc.53.129. [DOI] [PubMed] [Google Scholar]
- Gonda T., Daniel E. E., Kostolanska F., Oki M., Fox J. E. Neural control of canine colon motor function: studies in vitro. Can J Physiol Pharmacol. 1988 Apr;66(4):359–368. doi: 10.1139/y88-061. [DOI] [PubMed] [Google Scholar]
- Gustafsson L. E., Wiklund C. U., Wiklund N. P., Persson M. G., Moncada S. Modulation of autonomic neuroeffector transmission by nitric oxide in guinea pig ileum. Biochem Biophys Res Commun. 1990 Nov 30;173(1):106–110. doi: 10.1016/s0006-291x(05)81028-9. [DOI] [PubMed] [Google Scholar]
- Helke C. J., Krause J. E., Mantyh P. W., Couture R., Bannon M. J. Diversity in mammalian tachykinin peptidergic neurons: multiple peptides, receptors, and regulatory mechanisms. FASEB J. 1990 Apr 1;4(6):1606–1615. [PubMed] [Google Scholar]
- Holzer P. Characterization of the stimulus-induced release of immunoreactive substance P from the myenteric plexus of the guinea-pig small intestine. Brain Res. 1984 Apr 9;297(1):127–136. doi: 10.1016/0006-8993(84)90549-3. [DOI] [PubMed] [Google Scholar]
- Huizinga J. D., Chang G., Diamant N. E., El-Sharkawy T. Y. Electrophysiological basis of excitation of canine colonic circular muscle by cholinergic agents and substance P. J Pharmacol Exp Ther. 1984 Dec;231(3):692–699. [PubMed] [Google Scholar]
- Huizinga J. D., Tomlinson J., Pintin-Quezada J. Involvement of nitric oxide in nerve-mediated inhibition and action of vasoactive intestinal peptide in colonic smooth muscle. J Pharmacol Exp Ther. 1992 Feb;260(2):803–808. [PubMed] [Google Scholar]
- Keef K. D., Ward S. M., Stevens R. J., Frey B. W., Sanders K. M. Electrical and mechanical effects of acetylcholine and substance P in subregions of canine colon. Am J Physiol. 1992 Feb;262(2 Pt 1):G298–G307. doi: 10.1152/ajpgi.1992.262.2.G298. [DOI] [PubMed] [Google Scholar]
- Knudsen M. A., Tøttrup A. A possible role of the L-arginine-nitric oxide pathway in the modulation of cholinergic transmission in the guinea-pig taenia coli. Br J Pharmacol. 1992 Nov;107(3):837–841. doi: 10.1111/j.1476-5381.1992.tb14533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi C. A., Giuliani S., Ballati L., Lecci A., Manzini S., Patacchini R., Renzetti A. R., Rovero P., Quartara L., Giachetti A. In vivo evidence for tachykininergic transmission using a new NK-2 receptor-selective antagonist, MEN 10,376. J Pharmacol Exp Ther. 1991 Jun;257(3):1172–1178. [PubMed] [Google Scholar]
- Mantyh P. W., Mantyh C. R., Gates T., Vigna S. R., Maggio J. E. Receptor binding sites for substance P and substance K in the canine gastrointestinal tract and their possible role in inflammatory bowel disease. Neuroscience. 1988 Jun;25(3):817–837. doi: 10.1016/0306-4522(88)90038-3. [DOI] [PubMed] [Google Scholar]
- McDonald T. J., Ahmad S., Allescher H. D., Kostka P., Daniel E. E., Barnett W., Brodin E. Canine myenteric, deep muscular, and submucosal plexus preparations of purified nerve varicosities: content and chromatographic forms of certain neuropeptides. Peptides. 1990 Jan-Feb;11(1):95–102. doi: 10.1016/0196-9781(90)90116-m. [DOI] [PubMed] [Google Scholar]
- Niel J. P., Bywater R. A., Taylor G. S. Effect of substance P on non-cholinergic fast and slow post-stimulus depolarization in the guinea-pig ileum. J Auton Nerv Syst. 1983 Dec;9(4):573–584. doi: 10.1016/0165-1838(83)90114-5. [DOI] [PubMed] [Google Scholar]
- Rand M. J. Nitrergic transmission: nitric oxide as a mediator of non-adrenergic, non-cholinergic neuro-effector transmission. Clin Exp Pharmacol Physiol. 1992 Mar;19(3):147–169. doi: 10.1111/j.1440-1681.1992.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Sanders K. M., Smith T. K. Enteric neural regulation of slow waves in circular muscle of the canine proximal colon. J Physiol. 1986 Aug;377:297–313. doi: 10.1113/jphysiol.1986.sp016188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders K. M., Smith T. K. Motoneurones of the submucous plexus regulate electrical activity of the circular muscle of canine proximal colon. J Physiol. 1986 Nov;380:293–310. doi: 10.1113/jphysiol.1986.sp016286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth C. W., Murphy R., Furness J. B., Pompolo S. Comparison of the presence and actions of substance P and neurokinin A in guinea-pig taenia coli. Neuropeptides. 1991 May;19(1):23–34. doi: 10.1016/0143-4179(91)90070-y. [DOI] [PubMed] [Google Scholar]
- Smith T. K., Reed J. B., Sanders K. M. Origin and propagation of electrical slow waves in circular muscle of canine proximal colon. Am J Physiol. 1987 Feb;252(2 Pt 1):C215–C224. doi: 10.1152/ajpcell.1987.252.2.C215. [DOI] [PubMed] [Google Scholar]
- Thornbury K. D., Ward S. M., Dalziel H. H., Carl A., Westfall D. P., Sanders K. M. Nitric oxide and nitrosocysteine mimic nonadrenergic, noncholinergic hyperpolarization in canine proximal colon. Am J Physiol. 1991 Sep;261(3 Pt 1):G553–G557. doi: 10.1152/ajpgi.1991.261.3.G553. [DOI] [PubMed] [Google Scholar]
- Ward S. M., Dalziel H. H., Bradley M. E., Buxton I. L., Keef K., Westfall D. P., Sanders K. M. Involvement of cyclic GMP in non-adrenergic, non-cholinergic inhibitory neurotransmission in dog proximal colon. Br J Pharmacol. 1992 Dec;107(4):1075–1082. doi: 10.1111/j.1476-5381.1992.tb13409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S. M., Dalziel H. H., Thornbury K. D., Westfall D. P., Sanders K. M. Nonadrenergic, noncholinergic inhibition and rebound excitation in canine colon depend on nitric oxide. Am J Physiol. 1992 Feb;262(2 Pt 1):G237–G243. doi: 10.1152/ajpgi.1992.262.2.G237. [DOI] [PubMed] [Google Scholar]
- Ward S. M., Sanders K. M. Upstroke component of electrical slow waves in canine colonic smooth muscle due to nifedipine-resistant calcium current. J Physiol. 1992 Sep;455:321–337. doi: 10.1113/jphysiol.1992.sp019304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. D., Marsh D. R. Effects of atropine, tetrodotoxin and lidocaine on rebound excitation of guinea-pig small intestine. J Pharmacol Exp Ther. 1973 Mar;184(3):590–598. [PubMed] [Google Scholar]



