Abstract
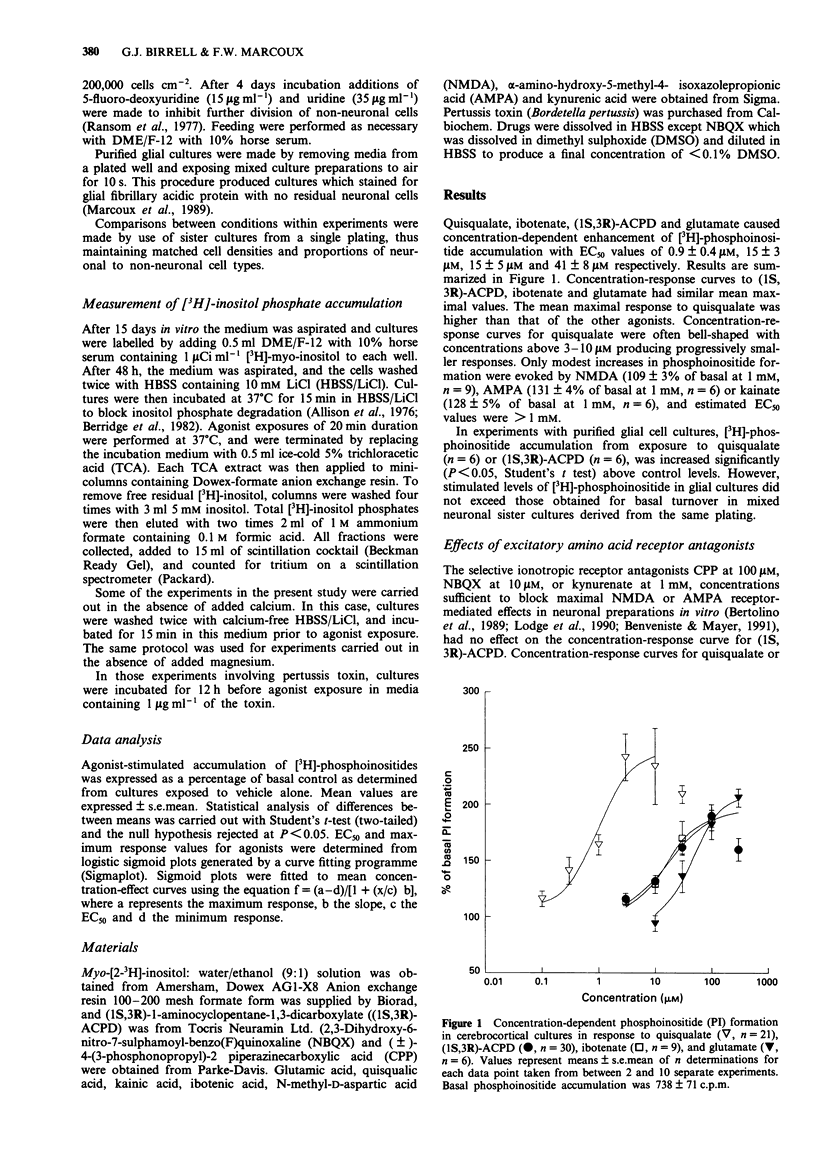
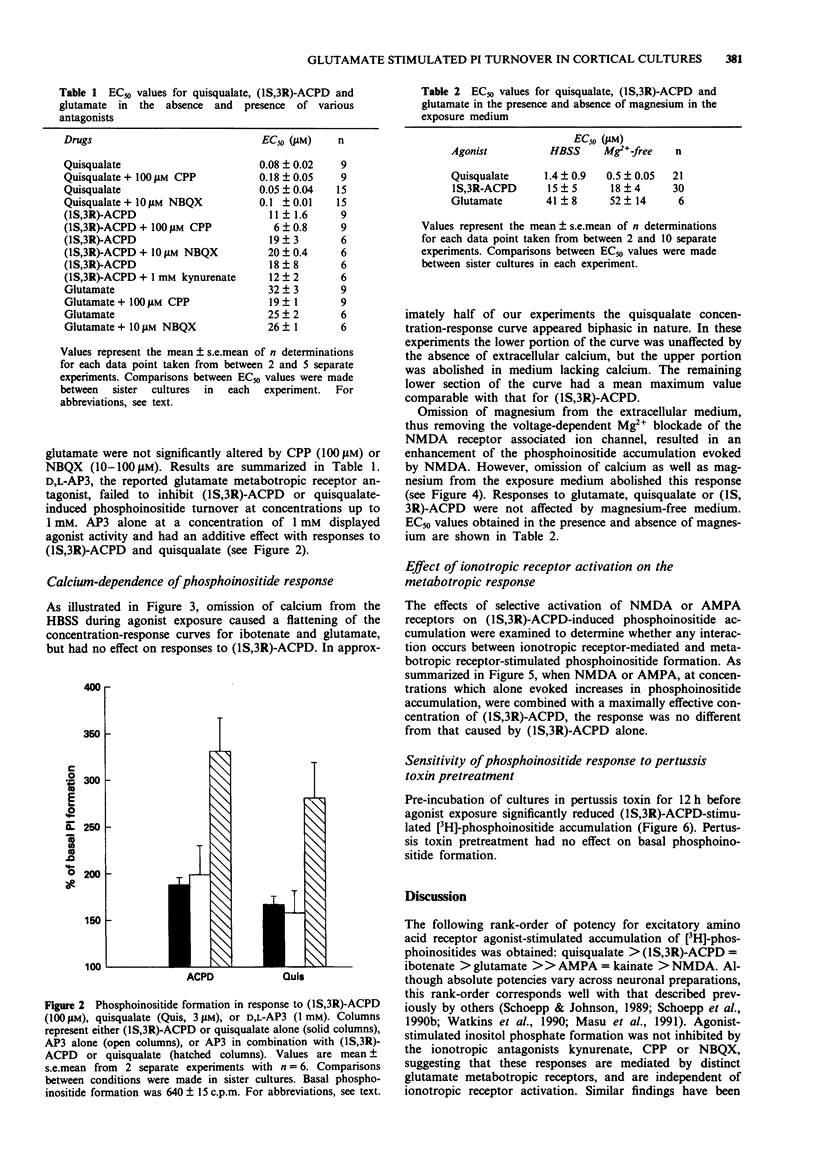
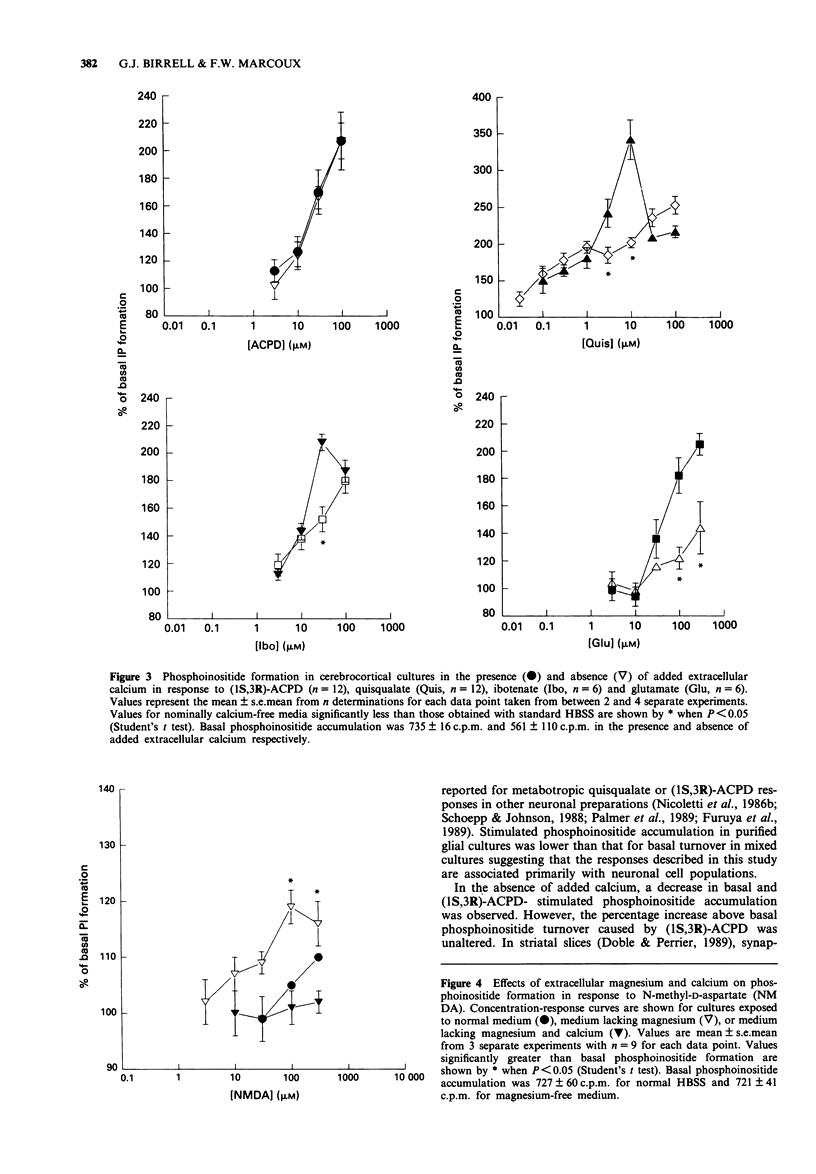
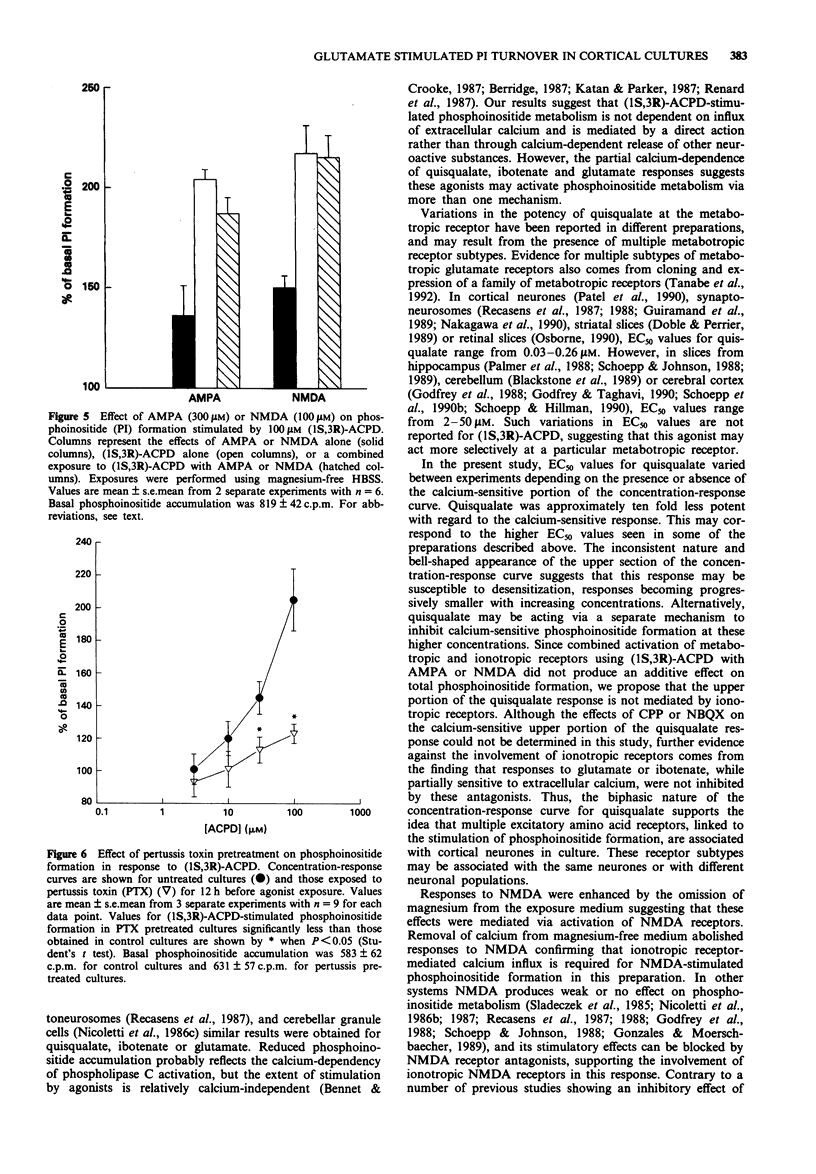
1. Characterization of excitatory amino acid-induced accumulation of [3H]-phosphoinositides was carried out in primary cerebrocortical cultures isolated from foetal rats. 2. All of the excitatory amino acid receptor agonists examined caused concentration-dependent enhancement of phosphoinositide (PI) formation. The most potent excitatory amino acid receptor agonists were quisqualate, (1S,3R)-1-aminocyclopentane-1,3-dicarboxylic acid ((1S,3R)-ACPD), ibotenate and glutamate with mean EC50 values of 0.9 +/- 0.4 microM, 15 +/- 5 microM, 15 +/- 3 microM and 41 +/- 8 microM respectively. 3. The selective ionotropic receptor antagonists kynurenic acid (1 mM), 2,3-dihydroxy-6-nitro-7-sulphamoyl-benzo(F)quinoxaline (NBQX, 10 microM) and (+/-)-4-(3-phosphonopropyl)-2 piperazinecarboxylic acid (CPP, 100 microM), failed to block responses to quisqualate, (1S,3R)-ACPD or glutamate. D,L-2-Amino-3-phosphonopropionate (D,L-AP3) did not block 1S,3R-ACPD or quisqualate-induced PI turnover, but had an additive effect with quisqualate or (1S,3R)-ACPD. 4. Exposure of cultures to agonists in the absence of added extracellular calcium reduced the maximal quisqualate response by approximately 45%, revealing a two-component concentration-response curve. Concentration-response curves to ibotenate and glutamate became flattened by omission of extracellular calcium, whereas (1S,3R)-ACPD-stimulated PI turnover was unaffected. 5. Pretreatment of cultures with pertussis toxin markedly inhibited PI responses evoked by (1S,3R)-ACPD. 6. These results suggest that excitatory amino acid-stimulated PI turnover in cerebrocortical cultures is independent of ionotropic receptor activation and is mediated via specific G-protein-linked metabotropic receptors.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison J. H., Blisner M. E., Holland W. H., Hipps P. P., Sherman W. R. Increased brain myo-inositol 1-phosphate in lithium-treated rats. Biochem Biophys Res Commun. 1976 Jul 26;71(2):664–670. doi: 10.1016/0006-291x(76)90839-1. [DOI] [PubMed] [Google Scholar]
- Ambrosini A., Meldolesi J. Muscarinic and quisqualate receptor-induced phosphoinositide hydrolysis in primary cultures of striatal and hippocampal neurons. Evidence for differential mechanisms of activation. J Neurochem. 1989 Sep;53(3):825–833. doi: 10.1111/j.1471-4159.1989.tb11779.x. [DOI] [PubMed] [Google Scholar]
- Baird J. G., Nahorski S. R. Stimulatory and inhibitory effects of N-methyl-D-aspartate on 3H-inositol polyphosphate accumulation in rat cortical slices. J Neurochem. 1991 Aug;57(2):629–635. doi: 10.1111/j.1471-4159.1991.tb03794.x. [DOI] [PubMed] [Google Scholar]
- Baskys A. Metabotropic receptors and 'slow' excitatory actions of glutamate agonists in the hippocampus. Trends Neurosci. 1992 Mar;15(3):92–96. doi: 10.1016/0166-2236(92)90018-4. [DOI] [PubMed] [Google Scholar]
- Baudry M., Evans J., Lynch G. Excitatory amino acids inhibit stimulation of phosphatidylinositol metabolism by aminergic agonists in hippocampus. Nature. 1986 Jan 23;319(6051):329–331. doi: 10.1038/319329a0. [DOI] [PubMed] [Google Scholar]
- Bennett C. F., Crooke S. T. Purification and characterization of a phosphoinositide-specific phospholipase C from guinea pig uterus. Phosphorylation by protein kinase C in vivo. J Biol Chem. 1987 Oct 5;262(28):13789–13797. [PubMed] [Google Scholar]
- Benveniste M., Mayer M. L. Structure-activity analysis of binding kinetics for NMDA receptor competitive antagonists: the influence of conformational restriction. Br J Pharmacol. 1991 Sep;104(1):207–221. doi: 10.1111/j.1476-5381.1991.tb12409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Bertolino M., Vicini S., Costa E. Kynurenic acid inhibits the activation of kainic and N-methyl-D-aspartic acid-sensitive ionotropic receptors by a different mechanism. Neuropharmacology. 1989 May;28(5):453–457. doi: 10.1016/0028-3908(89)90078-6. [DOI] [PubMed] [Google Scholar]
- Choi D. W., Rothman S. M. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- Farooqui A. A., Horrocks L. A. Excitatory amino acid receptors, neural membrane phospholipid metabolism and neurological disorders. Brain Res Brain Res Rev. 1991 May-Aug;16(2):171–191. doi: 10.1016/0165-0173(91)90004-r. [DOI] [PubMed] [Google Scholar]
- Furuya S., Ohmori H., Shigemoto T., Sugiyama H. Intracellular calcium mobilization triggered by a glutamate receptor in rat cultured hippocampal cells. J Physiol. 1989 Jul;414:539–548. doi: 10.1113/jphysiol.1989.sp017702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Godfrey P. P., Wilkins C. J., Tyler W., Watson S. P. Stimulatory and inhibitory actions of excitatory amino acids on inositol phospholipid metabolism in rat cerebral cortex. Br J Pharmacol. 1988 Sep;95(1):131–138. doi: 10.1111/j.1476-5381.1988.tb16556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales R. A., Moerschbaecher J. M. A phencyclidine recognition site is associated with N-methyl-D-aspartate inhibition of carbachol-stimulated phosphoinositide hydrolysis in rat cortical slices. Mol Pharmacol. 1989 Jun;35(6):787–794. [PubMed] [Google Scholar]
- Guiramand J., Sassetti I., Recasens M. Developmental changes in the chemosensitivity of rat brain synaptoneurosomes to excitatory amino acids, estimated by inositol phosphate formation. Int J Dev Neurosci. 1989;7(3):257–266. doi: 10.1016/0736-5748(89)90030-0. [DOI] [PubMed] [Google Scholar]
- Hwang P. M., Bredt D. S., Snyder S. H. Autoradiographic imaging of phosphoinositide turnover in the brain. Science. 1990 Aug 17;249(4970):802–804. doi: 10.1126/science.1975122. [DOI] [PubMed] [Google Scholar]
- Katan M., Parker P. J. Purification of phosphoinositide-specific phospholipase C from a particulate fraction of bovine brain. Eur J Biochem. 1987 Oct 15;168(2):413–418. doi: 10.1111/j.1432-1033.1987.tb13435.x. [DOI] [PubMed] [Google Scholar]
- Lodge D., Jones M. G., Palmer A. J. Excitatory amino acids: new tools for old stories or pharmacological subtypes of glutamate receptors: electrophysiological studies. Can J Physiol Pharmacol. 1991 Jul;69(7):1123–1128. doi: 10.1139/y91-164. [DOI] [PubMed] [Google Scholar]
- Manzoni O. J., Poulat F., Do E., Sahuquet A., Sassetti I., Bockaert J., Sladeczek F. A. Pharmacological characterization of the quisqualate receptor coupled to phospholipase C (Qp) in striatal neurons. Eur J Pharmacol. 1991 Jul 12;207(3):231–241. doi: 10.1016/0922-4106(91)90035-g. [DOI] [PubMed] [Google Scholar]
- Masu M., Tanabe Y., Tsuchida K., Shigemoto R., Nakanishi S. Sequence and expression of a metabotropic glutamate receptor. Nature. 1991 Feb 28;349(6312):760–765. doi: 10.1038/349760a0. [DOI] [PubMed] [Google Scholar]
- Meldrum B., Garthwaite J. Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends Pharmacol Sci. 1990 Sep;11(9):379–387. doi: 10.1016/0165-6147(90)90184-a. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Bridges R. J., Cotman C. W. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- Morari M., Calo G., Antonelli T., Gaist G., Acciarri N., Fabrizi A., Bianchi C., Beani L. Inhibitory effect of NMDA receptor activation on quisqualate-stimulated phosphatidylinositol turnover in the human cerebral cortex. Brain Res. 1991 Jul 5;553(1):14–17. doi: 10.1016/0006-8993(91)90223-i. [DOI] [PubMed] [Google Scholar]
- Morrisett R. A., Chow C. C., Sakaguchi T., Shin C., McNamara J. O. Inhibition of muscarinic-coupled phosphoinositide hydrolysis by N-methyl-D-aspartate is dependent on depolarization via channel activation. J Neurochem. 1990 May;54(5):1517–1525. doi: 10.1111/j.1471-4159.1990.tb01199.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y., Saitoh K., Ishihara T., Ishida M., Shinozaki H. (2S,3S,4S) alpha-(carboxycyclopropyl)glycine is a novel agonist of metabotropic glutamate receptors. Eur J Pharmacol. 1990 Aug 2;184(1):205–206. doi: 10.1016/0014-2999(90)90686-z. [DOI] [PubMed] [Google Scholar]
- Nicoletti F., Iadarola M. J., Wroblewski J. T., Costa E. Excitatory amino acid recognition sites coupled with inositol phospholipid metabolism: developmental changes and interaction with alpha 1-adrenoceptors. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1931–1935. doi: 10.1073/pnas.83.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti F., Meek J. L., Iadarola M. J., Chuang D. M., Roth B. L., Costa E. Coupling of inositol phospholipid metabolism with excitatory amino acid recognition sites in rat hippocampus. J Neurochem. 1986 Jan;46(1):40–46. doi: 10.1111/j.1471-4159.1986.tb12922.x. [DOI] [PubMed] [Google Scholar]
- Nicoletti F., Wroblewski J. T., Costa E. Magnesium ions inhibit the stimulation of inositol phospholipid hydrolysis by endogenous excitatory amino acids in primary cultures of cerebellar granule cells. J Neurochem. 1987 Mar;48(3):967–973. doi: 10.1111/j.1471-4159.1987.tb05611.x. [DOI] [PubMed] [Google Scholar]
- Nicoletti F., Wroblewski J. T., Fadda E., Costa E. Pertussis toxin inhibits signal transduction at a specific metabolotropic glutamate receptor in primary cultures of cerebellar granule cells. Neuropharmacology. 1988 Jun;27(6):551–556. doi: 10.1016/0028-3908(88)90174-8. [DOI] [PubMed] [Google Scholar]
- Nicoletti F., Wroblewski J. T., Novelli A., Alho H., Guidotti A., Costa E. The activation of inositol phospholipid metabolism as a signal-transducing system for excitatory amino acids in primary cultures of cerebellar granule cells. J Neurosci. 1986 Jul;6(7):1905–1911. doi: 10.1523/JNEUROSCI.06-07-01905.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble E. P., Sincini E., Bergmann D., ten Bruggencate G. Excitatory amino acids inhibit stimulated phosphoinositide hydrolysis in the rat prefrontal cortex. Life Sci. 1989;44(1):19–26. doi: 10.1016/0024-3205(89)90213-0. [DOI] [PubMed] [Google Scholar]
- Osborne N. N. Stimulatory and inhibitory actions of excitatory amino acids on inositol phospholipid metabolism in rabbit retina. Evidence for a specific quisqualate receptor subtype associated with neurones. Exp Eye Res. 1990 Apr;50(4):397–405. doi: 10.1016/0014-4835(90)90141-g. [DOI] [PubMed] [Google Scholar]
- Palmer E., Monaghan D. T., Cotman C. W. Glutamate receptors and phosphoinositide metabolism: stimulation via quisqualate receptors is inhibited by N-methyl-D-aspartate receptor activation. Brain Res. 1988 Sep;464(2):161–165. doi: 10.1016/0169-328x(88)90008-3. [DOI] [PubMed] [Google Scholar]
- Palmer E., Monaghan D. T., Cotman C. W. Trans-ACPD, a selective agonist of the phosphoinositide-coupled excitatory amino acid receptor. Eur J Pharmacol. 1989 Aug 3;166(3):585–587. doi: 10.1016/0014-2999(89)90383-x. [DOI] [PubMed] [Google Scholar]
- Patel J., Moore W. C., Thompson C., Keith R. A., Salama A. I. Characterization of the quisqualate receptor linked to phosphoinositide hydrolysis in neurocortical cultures. J Neurochem. 1990 May;54(5):1461–1466. doi: 10.1111/j.1471-4159.1990.tb01192.x. [DOI] [PubMed] [Google Scholar]
- Ransom B. R., Neale E., Henkart M., Bullock P. N., Nelson P. G. Mouse spinal cord in cell culture. I. Morphology and intrinsic neuronal electrophysiologic properties. J Neurophysiol. 1977 Sep;40(5):1132–1150. doi: 10.1152/jn.1977.40.5.1132. [DOI] [PubMed] [Google Scholar]
- Renard D., Poggioli J., Berthon B., Claret M. How far does phospholipase C activity depend on the cell calcium concentration? A study in intact cells. Biochem J. 1987 Apr 15;243(2):391–398. doi: 10.1042/bj2430391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Récasens M., Sassetti I., Nourigat A., Sladeczek F., Bockaert J. Characterization of subtypes of excitatory amino acid receptors involved in the stimulation of inositol phosphate synthesis in rat brain synaptoneurosomes. Eur J Pharmacol. 1987 Sep 2;141(1):87–93. doi: 10.1016/0014-2999(87)90413-4. [DOI] [PubMed] [Google Scholar]
- Schmidt B. H., Weiss S., Sebben M., Kemp D. E., Bockaert J., Sladeczek F. Dual action of excitatory amino acids on the metabolism of inositol phosphates in striatal neurons. Mol Pharmacol. 1987 Sep;32(3):364–368. [PubMed] [Google Scholar]
- Schoepp D. D., Johnson B. G. Excitatory amino acid agonist-antagonist interactions at 2-amino-4-phosphonobutyric acid-sensitive quisqualate receptors coupled to phosphoinositide hydrolysis in slices of rat hippocampus. J Neurochem. 1988 May;50(5):1605–1613. doi: 10.1111/j.1471-4159.1988.tb03050.x. [DOI] [PubMed] [Google Scholar]
- Schoepp D. D., Johnson B. G. Inhibition of excitatory amino acid-stimulated phosphoinositide hydrolysis in the neonatal rat hippocampus by 2-amino-3-phosphonopropionate. J Neurochem. 1989 Dec;53(6):1865–1870. doi: 10.1111/j.1471-4159.1989.tb09254.x. [DOI] [PubMed] [Google Scholar]
- Schoepp D. D., Johnson B. G., Smith E. C., McQuaid L. A. Stereoselectivity and mode of inhibition of phosphoinositide-coupled excitatory amino acid receptors by 2-amino-3-phosphonopropionic acid. Mol Pharmacol. 1990 Aug;38(2):222–228. [PubMed] [Google Scholar]
- Schoepp D., Bockaert J., Sladeczek F. Pharmacological and functional characteristics of metabotropic excitatory amino acid receptors. Trends Pharmacol Sci. 1990 Dec;11(12):508–515. doi: 10.1016/0165-6147(90)90052-a. [DOI] [PubMed] [Google Scholar]
- Sladeczek F., Pin J. P., Récasens M., Bockaert J., Weiss S. Glutamate stimulates inositol phosphate formation in striatal neurones. Nature. 1985 Oct 24;317(6039):717–719. doi: 10.1038/317717a0. [DOI] [PubMed] [Google Scholar]
- Sugiyama H., Ito I., Hirono C. A new type of glutamate receptor linked to inositol phospholipid metabolism. Nature. 1987 Feb 5;325(6104):531–533. doi: 10.1038/325531a0. [DOI] [PubMed] [Google Scholar]
- Tanabe Y., Masu M., Ishii T., Shigemoto R., Nakanishi S. A family of metabotropic glutamate receptors. Neuron. 1992 Jan;8(1):169–179. doi: 10.1016/0896-6273(92)90118-w. [DOI] [PubMed] [Google Scholar]
- Watkins J. C., Krogsgaard-Larsen P., Honoré T. Structure-activity relationships in the development of excitatory amino acid receptor agonists and competitive antagonists. Trends Pharmacol Sci. 1990 Jan;11(1):25–33. doi: 10.1016/0165-6147(90)90038-a. [DOI] [PubMed] [Google Scholar]



