Abstract
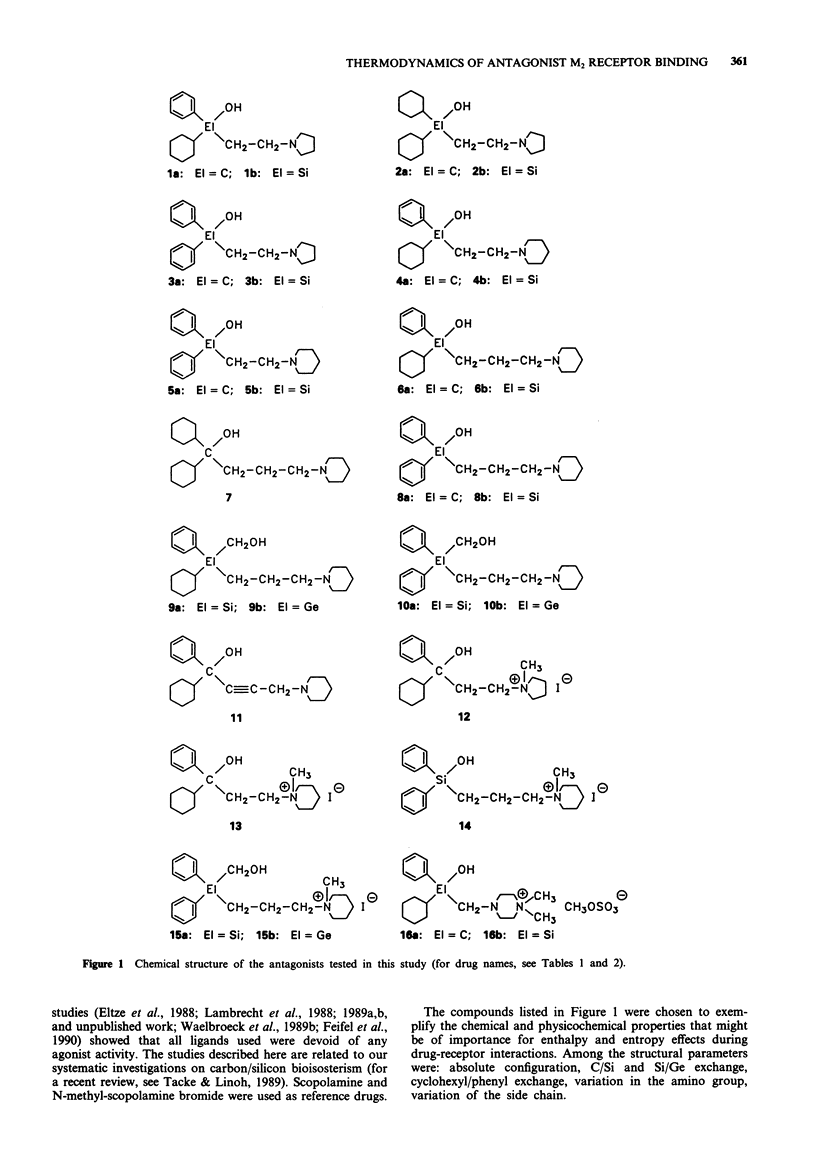
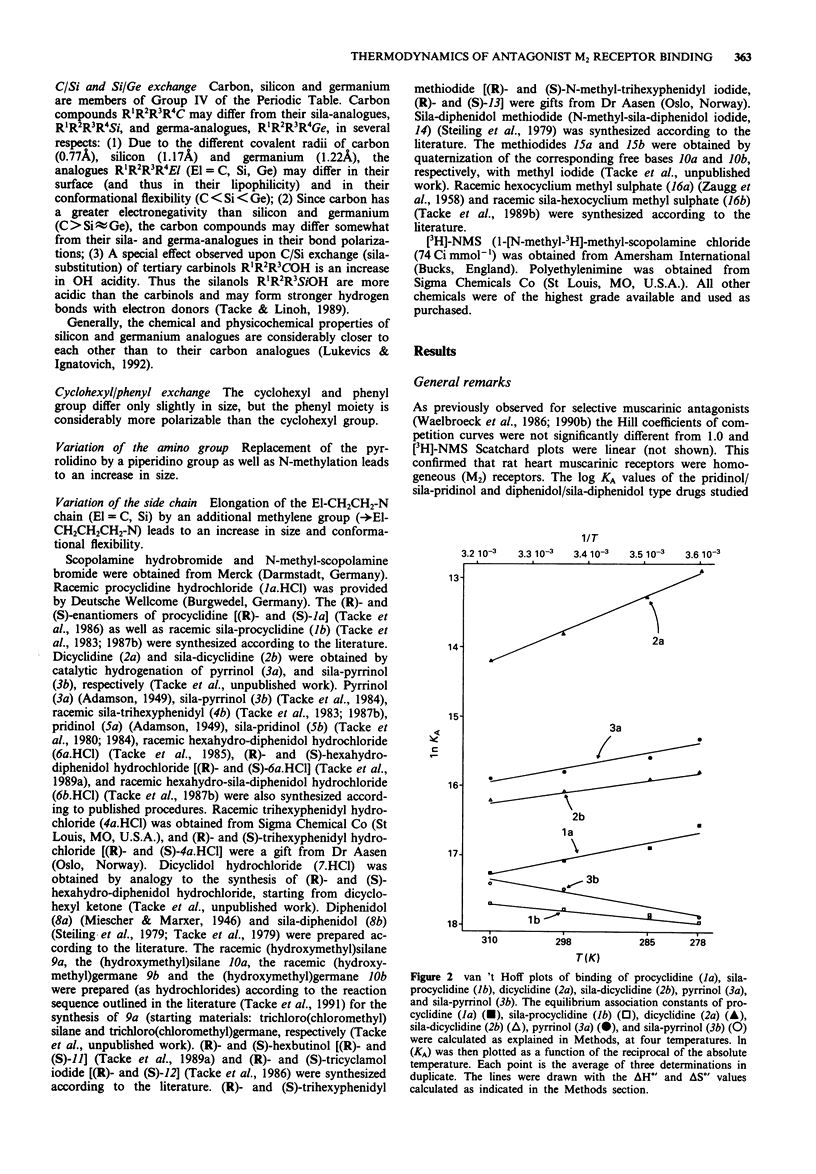
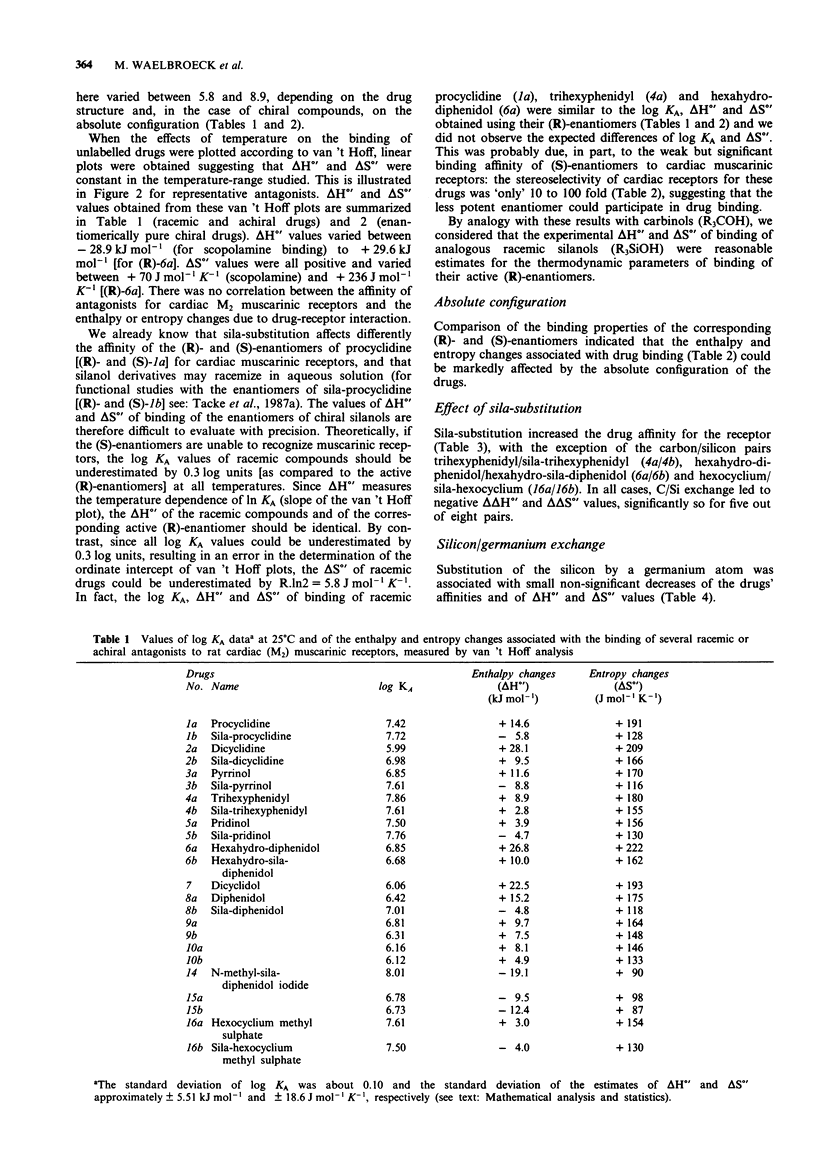
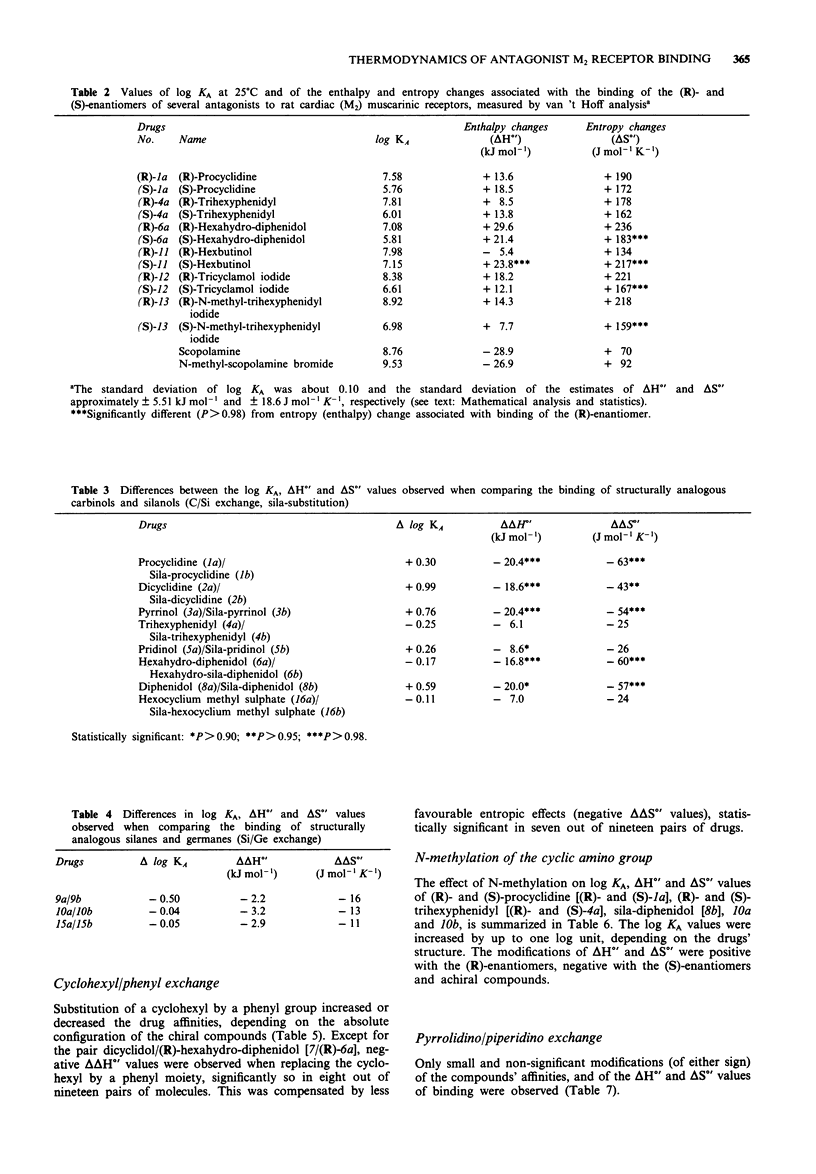
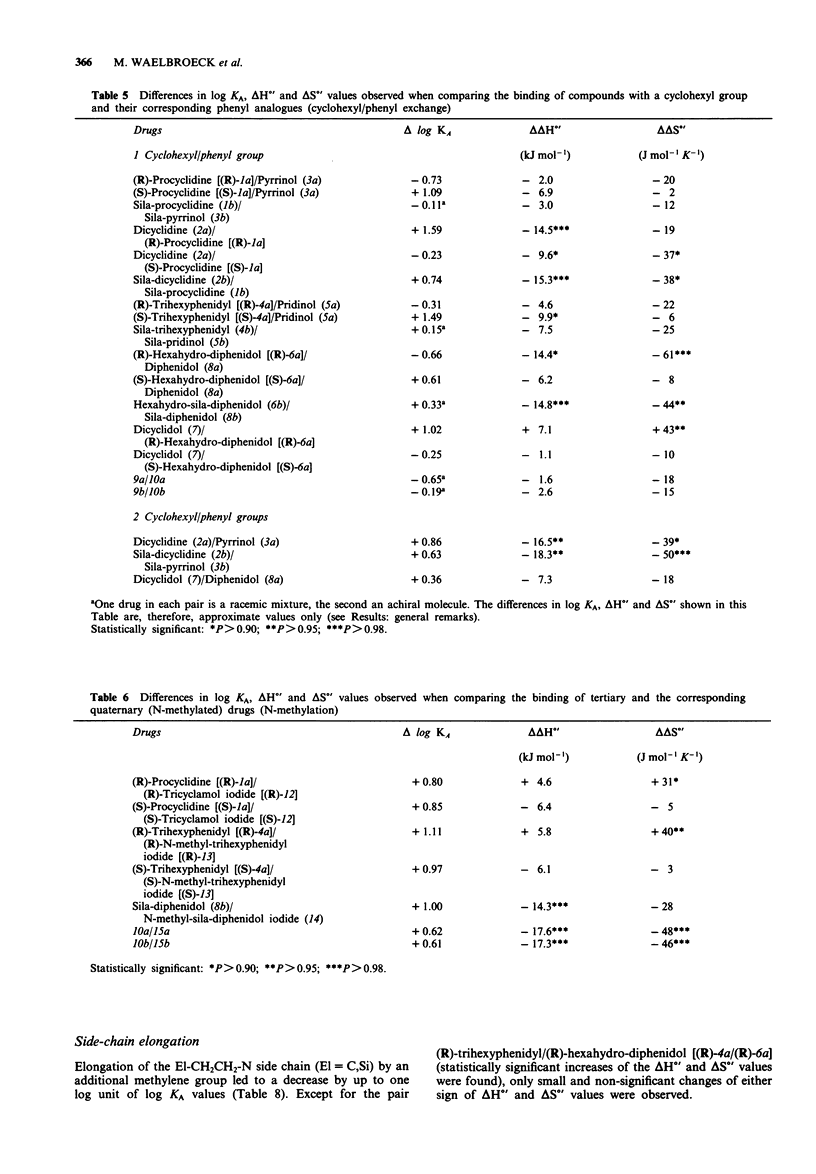
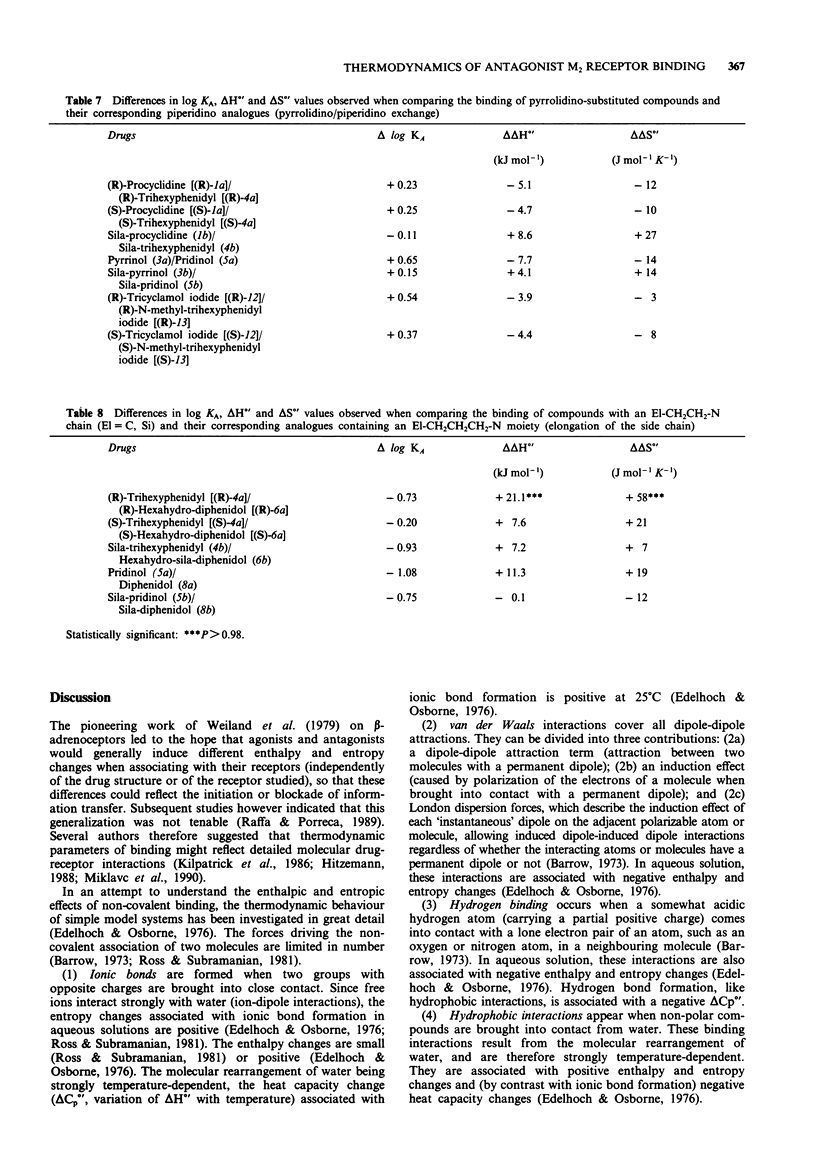
1. We studied the effect of temperature on the binding to rat heart M2 muscarinic receptors of antagonists related to the carbon/silicon pairs pridinol/sila-pridinol and diphenidol/sila-diphenidol (including three germanium compounds) and six structurally related pairs of enantiomers [(R)- and (S)-procyclidine, (R)- and (S)-trihexyphenidyl, (R)- and (S)-tricyclamol, (R)- and (S)-trihexyphenidyl methiodide, (R)- and (S)-hexahydro-diphenidol and (R)- and (S)-hexbutinol]. Binding affinities were determined in competition experiments using [3H]-N-methyl-scopolamine chloride as radioligand. The reference drugs were scopolamine and N-methyl-scopolamine bromide. 2. The affinity of the antagonists either increased or decreased with temperature. van't Hoff plots were linear in the 278-310 degrees K temperature range. Binding of all antagonists was entropy driven. Enthalpy changes varied from large negative values (down to -29 kJ mol-1) to large positive values (up to +30 kJ mol-1). 3. (R)-configurated drugs had a 10 to 100 fold greater affinity for M2 receptors than the corresponding (S)-enantiomers. Enthalpy and entropy changes of the respective enantiomers were different but no consistent pattern was observed. 4. When silanols (R3SiOH) were compared to carbinols (R3COH), the affinity increase caused by C/Si exchange varied between 3 and 10 fold for achiral drugs but was negligible in the case of chiral drugs. Silanols induced more favourable enthalpy and less favourable entropy changes than the corresponding carbinols when binding. Organogermanium compounds (R4Ge) when compared to their silicon counterparts (R4Si) showed no significant difference in affinity as well as in enthalpy and entropy changes. 5. Exchange of a cyclohexyl by a phenyl moiety was associated with an increase or a decrease in drug affinity (depending on the absolute configuration in the case of chiral drugs) and generally also with a more favourable enthalpy change and a less favourable entropy change of drug binding. 6. Replacement of a pyrrolidino by a piperidino group and increasing the length of the alkylene chain bridging the amino group and the central carbon or silicon atom were associated with either an increase or a decrease of entropy and enthalpy changes of drug binding. However, there was no clear correlation between these structural variations and the thermodynamic effects. 7. Taken together, these results suggest that hydrogen bond-forming OH groups and, to a lesser extent, polarizable phenyl groups contribute significantly to the thermodynamics of interactions between these classes of muscarinic antagonists and M2 muscarinic receptors.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow R. B., Birdsall N. J., Hulme E. C. Temperature coefficients of affinity constants for the binding of antagonists to muscarinic receptors in the rat cerebral cortex. Br J Pharmacol. 1979 Aug;66(4):587–590. doi: 10.1111/j.1476-5381.1979.tb13698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow R. B., Burston K. N. Temperature coefficients of affinity and entropies of adsorption from enantiomeric pairs of compounds acting at muscarinic receptors in the guinea-pig ileum. Br J Pharmacol. 1979 Aug;66(4):581–585. doi: 10.1111/j.1476-5381.1979.tb13697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Dörje F., Wess J., Lambrecht G., Tacke R., Mutschler E., Brann M. R. Antagonist binding profiles of five cloned human muscarinic receptor subtypes. J Pharmacol Exp Ther. 1991 Feb;256(2):727–733. [PubMed] [Google Scholar]
- Edelhoch H., Osborne J. C., Jr The thermodynamic basis of the stability of proteins, nucleic acids, and membranes. Adv Protein Chem. 1976;30:183–250. doi: 10.1016/s0065-3233(08)60480-5. [DOI] [PubMed] [Google Scholar]
- Eltz M., Gmelin G., Wess J., Strohmann C., Tacke R., Mutschler E., Lambrecht G. Presynaptic muscarinic receptors mediating inhibition of neurogenic contractions in rabbit vas deferens are of the ganglionic M1-type. Eur J Pharmacol. 1988 Dec 13;158(3):233–242. doi: 10.1016/0014-2999(88)90072-6. [DOI] [PubMed] [Google Scholar]
- Feifel R., Wagner-Röder M., Strohmann C., Tacke R., Waelbroeck M., Christophe J., Mutschler E., Lambrecht G. Stereoselective inhibition of muscarinic receptor subtypes by the enantiomers of hexahydro-difenidol and acetylenic analogues. Br J Pharmacol. 1990 Mar;99(3):455–460. doi: 10.1111/j.1476-5381.1990.tb12949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gies J. P., Ilien B., Landry Y. Muscarinic acetylcholine receptor: thermodynamic analysis of the interaction of agonists and antagonists. Biochim Biophys Acta. 1986 Oct 31;889(1):103–115. doi: 10.1016/0167-4889(86)90014-5. [DOI] [PubMed] [Google Scholar]
- Hitzemann R. Thermodynamic aspects of drug-receptor interactions. Trends Pharmacol Sci. 1988 Nov;9(11):408–411. doi: 10.1016/0165-6147(88)90068-5. [DOI] [PubMed] [Google Scholar]
- Hulme E. C., Birdsall N. J., Buckley N. J. Muscarinic receptor subtypes. Annu Rev Pharmacol Toxicol. 1990;30:633–673. doi: 10.1146/annurev.pa.30.040190.003221. [DOI] [PubMed] [Google Scholar]
- Kilpatrick G. J., el Tayar N., Van de Waterbeemd H., Jenner P., Testa B., Marsden C. D. The thermodynamics of agonist and antagonist binding to dopamine D-2 receptors. Mol Pharmacol. 1986 Sep;30(3):226–234. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lambrecht G., Feifel R., Moser U., Aasen A. J., Waelbroeck M., Christophe J., Mutschler E. Stereoselectivity of the enantiomers of trihexyphenidyl and its methiodide at muscarinic receptor subtypes. Eur J Pharmacol. 1988 Oct 11;155(1-2):167–170. doi: 10.1016/0014-2999(88)90417-7. [DOI] [PubMed] [Google Scholar]
- Lambrecht G., Feifel R., Wagner-Röder M., Strohmann C., Zilch H., Tacke R., Waelbroeck M., Christophe J., Boddeke H., Mutschler E. Affinity profiles of hexahydro-sila-difenidol analogues at muscarinic receptor subtypes. Eur J Pharmacol. 1989 Sep 1;168(1):71–80. doi: 10.1016/0014-2999(89)90634-1. [DOI] [PubMed] [Google Scholar]
- Miklavc A., Kocjan D., Mavri J., Koller J., Hadzi D. On the fundamental difference in the thermodynamics of agonist and antagonist interactions with beta-adrenergic receptors and the mechanism of entropy-driven binding. Biochem Pharmacol. 1990 Aug 15;40(4):663–669. doi: 10.1016/0006-2952(90)90299-z. [DOI] [PubMed] [Google Scholar]
- Muzio F., Malandrino S., Ferrari M., Tonon G. Temperature effect on subclasses of muscarinic receptors in rat colon, heart and cerebral cortex. Life Sci. 1986 Jul 28;39(4):365–371. doi: 10.1016/0024-3205(86)90655-7. [DOI] [PubMed] [Google Scholar]
- Raffa R. B., Porreca F. Thermodynamic analysis of the drug-receptor interaction. Life Sci. 1989;44(4):245–258. doi: 10.1016/0024-3205(89)90182-3. [DOI] [PubMed] [Google Scholar]
- Ross P. D., Subramanian S. Thermodynamics of protein association reactions: forces contributing to stability. Biochemistry. 1981 May 26;20(11):3096–3102. doi: 10.1021/bi00514a017. [DOI] [PubMed] [Google Scholar]
- Strader C. D., Candelore M. R., Hill W. S., Sigal I. S., Dixon R. A. Identification of two serine residues involved in agonist activation of the beta-adrenergic receptor. J Biol Chem. 1989 Aug 15;264(23):13572–13578. [PubMed] [Google Scholar]
- Tacke R., Strecker M. Sila-Pharmaka, 29. Mitt. Bioisosterer C/Si-Austausch bei Parasympatholytika vom Typ des Pridinols. Arch Pharm (Weinheim) 1984 Mar;317(3):207–214. doi: 10.1002/ardp.19843170305. [DOI] [PubMed] [Google Scholar]
- Waelbroeck M., Camus J., Tastenoy M., Lambrecht G., Mutschler E., Tacke R., Christophe J. Stereoselectivity of procyclidine binding to muscarinic receptor subtypes M1, M2 and M4. Eur J Pharmacol. 1990 Sep 18;189(2-3):135–142. doi: 10.1016/0922-4106(90)90017-r. [DOI] [PubMed] [Google Scholar]
- Waelbroeck M., Camus J., Tastenoy M., Mutschler E., Strohmann C., Tacke R., Lambrecht G., Christophe J. Binding affinities of hexahydro-difenidol and hexahydro-sila-difenidol analogues at four muscarinic receptor subtypes: constitutional and stereochemical aspects. Eur J Pharmacol. 1991 Feb 25;206(2):95–103. doi: 10.1016/0922-4106(91)90017-c. [DOI] [PubMed] [Google Scholar]
- Waelbroeck M., Camus J., Tastenoy M., Mutschler E., Strohmann C., Tacke R., Lambrecht G., Christophe J. Stereoselectivity of (R)- and (S)-hexahydro-difenidol binding to neuroblastoma M1, cardiac M2, pancreatic M3, and striatum M4 muscarinic receptors. Chirality. 1991;3(2):118–123. doi: 10.1002/chir.530030207. [DOI] [PubMed] [Google Scholar]
- Waelbroeck M., Camus J., Tastenoy M., Mutschler E., Strohmann C., Tacke R., Schjelderup L., Aasen A., Lambrecht G., Christophe J. Stereoselective interaction of procyclidine, hexahydro-difenidol, hexbutinol and oxyphencyclimine, and of related antagonists, with four muscarinic receptors. Eur J Pharmacol. 1992 Sep 1;227(1):33–42. doi: 10.1016/0922-4106(92)90139-m. [DOI] [PubMed] [Google Scholar]
- Waelbroeck M., Gillard M., Robberecht P., Christophe J. Kinetic studies of [3H]-N-methylscopolamine binding to muscarinic receptors in the rat central nervous system: evidence for the existence of three classes of binding sites. Mol Pharmacol. 1986 Oct;30(4):305–314. [PubMed] [Google Scholar]
- Waelbroeck M., Robberecht P., Chatelain P., De Neef P., Christophe J. Effects of temperature and ethanol on agonist and antagonist binding to rat heart muscarinic receptors in the absence and presence of GTP. Biochem J. 1985 Oct 15;231(2):469–476. doi: 10.1042/bj2310469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waelbroeck M., Tastenoy M., Camus J., Christophe J. Binding kinetics of quinuclidinyl benzilate and methyl-quinuclidinyl benzilate enantiomers at neuronal (M1), cardiac (M2), and pancreatic (M3) muscarinic receptors. Mol Pharmacol. 1991 Sep;40(3):413–420. [PubMed] [Google Scholar]
- Waelbroeck M., Tastenoy M., Camus J., Christophe J. Binding of selective antagonists to four muscarinic receptors (M1 to M4) in rat forebrain. Mol Pharmacol. 1990 Aug;38(2):267–273. [PubMed] [Google Scholar]
- Waelbroeck M., Tastenoy M., Camus J., Christophe J., Strohmann C., Linoh H., Zilch H., Tacke R., Mutschler E., Lambrecht G. Binding and functional properties of antimuscarinics of the hexocyclium/sila-hexocyclium and hexahydro-diphenidol/hexahydro-sila-diphenidol type to muscarinic receptor subtypes. Br J Pharmacol. 1989 Sep;98(1):197–205. doi: 10.1111/j.1476-5381.1989.tb16882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland G. A., Minneman K. P., Molinoff P. B. Fundamental difference between the molecular interactions of agonists and antagonists with the beta-adrenergic receptor. Nature. 1979 Sep 13;281(5727):114–117. doi: 10.1038/281114a0. [DOI] [PubMed] [Google Scholar]


