Abstract
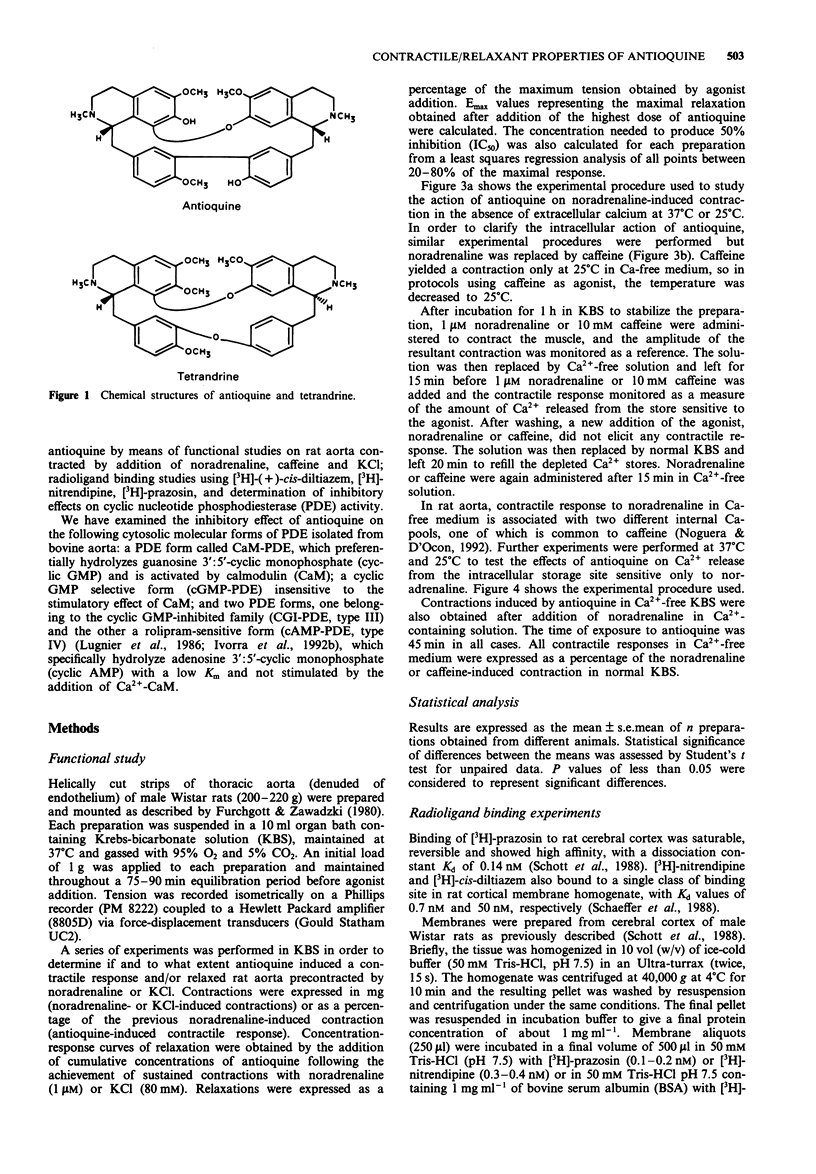
1. In the present study we assessed the activity of antioquine, a bisbenzyltetrahydroisoquinoline alkaloid isolated from Pseudoxandra sclerocarpa, by examining its effects on the contractile activity of rat isolated aorta, specific binding of [3H]-(+)-cis-diltiazem, [3H]-nitrendipine and [3H]-prazosin to cerebral cortical membranes and the different molecular forms of cyclic nucleotide phosphodiesterases (PDE) isolated from bovine aorta. 2. Contractions in rat aorta induced by high concentrations of KCl (80 mM) and noradrenaline (1 microM) were inhibited by antioquine in a concentration-dependent manner (0.1 microM- 300 microM). The alkaloid appeared more potent against KCl-induced contractions. This inhibitory effect was observed at both 37 degrees C and 25 degrees C. 3. Paradoxically, at the highest concentration tested (300 microM) antioquine induced a contractile response of similar magnitude in the presence and absence of extracellular calcium, at 37 degrees C. This activity was greatly attenuated at 25 degrees C. Antioquine-induced contractions were not inhibited by prazosin (0.1 microM), nifedipine (1 microM) or diltiazem (100 microM). On the contrary, prazosin and nifedipine slightly increased the contractions in the presence of extracellular calcium. Papaverine (100 microM) partially inhibited the contractile response to antioquine both in the presence and absence of extracellular calcium. 4. At 25 degrees C, in Ca(2+)-free solution, antioquine (300 microM) did not modify the contractile response (phasic and tonic) evoked by noradrenaline, but increased the phasic contraction induced by caffeine. At 37 degrees C, the contraction elicited by antioquine made it impossible to observe the noradrenaline-induced one.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- D'Ocon M. P., Candenas M. L., Anselmi E., Zafra-Polo M. C., Cortes D. Antioquine: a new bisbenzylisoquinoleine alkaloid with calcium antagonist activity. Arch Int Pharmacodyn Ther. 1989 Jan-Feb;297:205–216. [PubMed] [Google Scholar]
- Dong H., Lee C. M., Huang W. L., Peng S. X. Cardiovascular effects of substituted tetrahydroisoquinolines in rats. Br J Pharmacol. 1992 Sep;107(1):262–268. doi: 10.1111/j.1476-5381.1992.tb14496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang D. C., Jiang M. X. Studies on tetrandrine calcium antagonistic action. Chin Med J (Engl) 1986 Aug;99(8):638–644. [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Zech C., Striessnig J., Staudinger R., Hall L., Greenberg R., Armah B. I. Very high affinity interaction of DPI 201-106 and BDF 8784 enantiomers with the phenylalkylamine-sensitive Ca2(+)-channel in Drosophila head membranes. Br J Pharmacol. 1991 Feb;102(2):446–452. doi: 10.1111/j.1476-5381.1991.tb12193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann-Frank A., Darling E., Meissner G. Functional characterization of the Ca(2+)-gated Ca2+ release channel of vascular smooth muscle sarcoplasmic reticulum. Pflugers Arch. 1991 May;418(4):353–359. doi: 10.1007/BF00550873. [DOI] [PubMed] [Google Scholar]
- Hisayama T., Takayanagi I., Okamoto Y. Ryanodine reveals multiple contractile and relaxant mechanisms in vascular smooth muscle: simultaneous measurements of mechanical activity and of cytoplasmic free Ca2+ level with fura-2. Br J Pharmacol. 1990 Aug;100(4):677–684. doi: 10.1111/j.1476-5381.1990.tb14075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara H., Ozaki H., Sato K., Hori M., Karaki H., Watabe S., Kato Y., Fusetani N., Hashimoto K., Uemura D. Calcium-independent activation of contractile apparatus in smooth muscle by calyculin-A. J Pharmacol Exp Ther. 1989 Jul;250(1):388–396. [PubMed] [Google Scholar]
- Itoh H., Higuchi H., Hiraoka N., Ito M., Konishi T., Nakano T., Lederis K. Contraction of rat thoracic aorta strips by endothelin-1 in the absence of extracellular Ca2+. Br J Pharmacol. 1991 Dec;104(4):847–852. doi: 10.1111/j.1476-5381.1991.tb12516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivorra M. D., Cercós A., Zafra-Polo M. C., Perez-Prieto J., Sáez J., Cortes D., D'Ocon P. Selective chiral inhibition of Ca2+ entry promoted by bisbenzyltetrahydroisoquinolines in rat uterus. Eur J Pharmacol. 1992 Aug 25;219(2):303–309. doi: 10.1016/0014-2999(92)90310-z. [DOI] [PubMed] [Google Scholar]
- Ivorra M. D., Le Bec A., Lugnier C. Characterization of membrane-bound cyclic nucleotide phosphodiesterases from bovine aortic smooth muscle. J Cardiovasc Pharmacol. 1992 Apr;19(4):532–540. doi: 10.1097/00005344-199204000-00009. [DOI] [PubMed] [Google Scholar]
- Ivorra M. D., Lugnier C., Schott C., Catret M., Noguera M. A., Anselmi E., D'Ocon P. Multiple actions of glaucine on cyclic nucleotide phosphodiesterases, alpha 1-adrenoceptor and benzothiazepine binding site at the calcium channel. Br J Pharmacol. 1992 Jun;106(2):387–394. doi: 10.1111/j.1476-5381.1992.tb14345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A. Physiologic functions of normal endothelial cells. Ann N Y Acad Sci. 1985;454:279–291. doi: 10.1111/j.1749-6632.1985.tb11868.x. [DOI] [PubMed] [Google Scholar]
- Karaki H., Mitsui M., Nagase H., Ozaki H., Shibata S., Uemura D. Inhibitory effect of a toxin okadaic acid, isolated from the black sponge on smooth muscle and platelets. Br J Pharmacol. 1989 Oct;98(2):590–596. doi: 10.1111/j.1476-5381.1989.tb12633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King V. F., Garcia M. L., Himmel D., Reuben J. P., Lam Y. K., Pan J. X., Han G. Q., Kaczorowski G. J. Interaction of tetrandrine with slowly inactivating calcium channels. Characterization of calcium channel modulation by an alkaloid of Chinese medicinal herb origin. J Biol Chem. 1988 Feb 15;263(5):2238–2244. [PubMed] [Google Scholar]
- Lu Y. M., Liu G. Q. The effects of (-)-daurisoline on Ca2+ influx in presynaptic nerve terminals. Br J Pharmacol. 1990 Sep;101(1):45–48. doi: 10.1111/j.1476-5381.1990.tb12086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugnier C., Schoeffter P., Le Bec A., Strouthou E., Stoclet J. C. Selective inhibition of cyclic nucleotide phosphodiesterases of human, bovine and rat aorta. Biochem Pharmacol. 1986 May 15;35(10):1743–1751. doi: 10.1016/0006-2952(86)90333-3. [DOI] [PubMed] [Google Scholar]
- Lüllmann H., Timmermans P. B., Ziegler A. Accumulation of drugs by resting or beating cardiac tissue. Eur J Pharmacol. 1979 Dec 20;60(4):277–285. doi: 10.1016/0014-2999(79)90231-0. [DOI] [PubMed] [Google Scholar]
- Meissner G. Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J Biol Chem. 1986 May 15;261(14):6300–6306. [PubMed] [Google Scholar]
- Nakajima H., Hoshiyama M., Yamashita K., Kiyomoto A. Effect of diltiazem on electrical and mechanical activity of isolated cardiac ventricular muscle of guinea pig. Jpn J Pharmacol. 1975 Aug;25(4):383–392. doi: 10.1254/jjp.25.383. [DOI] [PubMed] [Google Scholar]
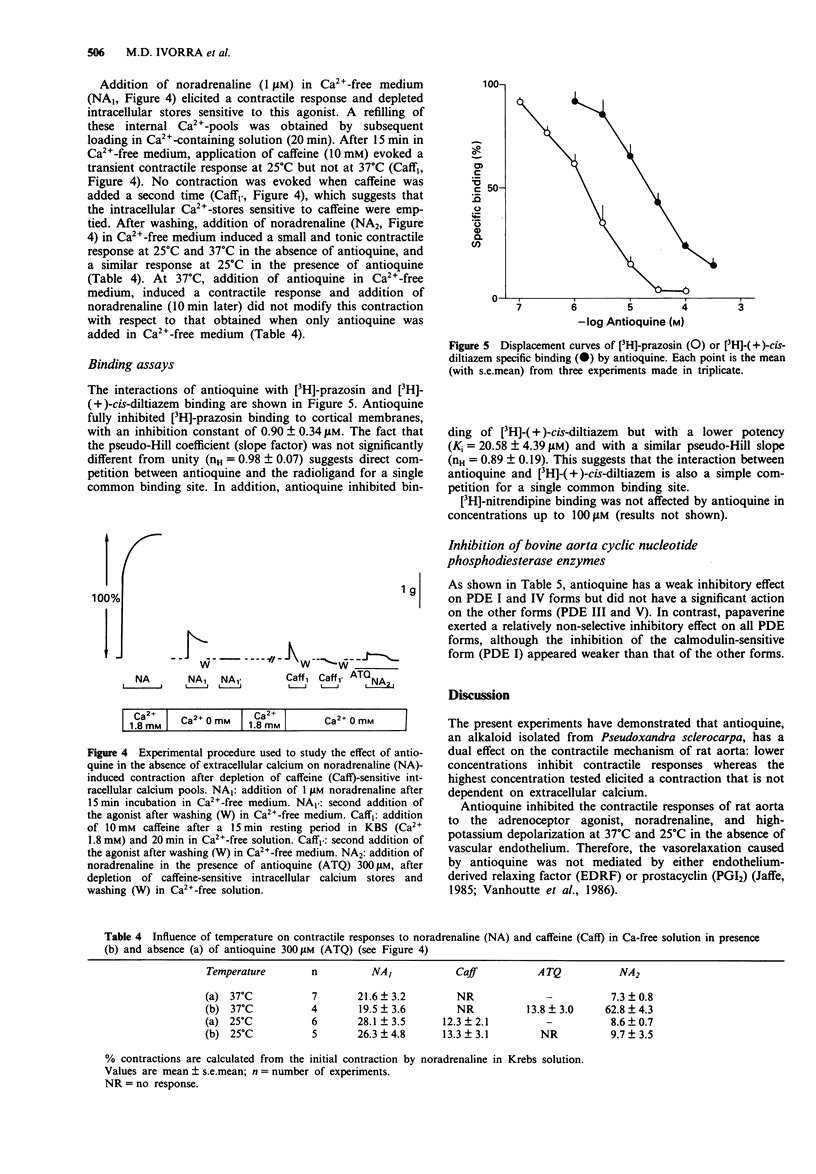
- Noguera M. A., D'Ocon M. P. Different and common intracellular calcium-stores mobilized by noradrenaline and caffeine in vascular smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 1992 Mar;345(3):333–341. doi: 10.1007/BF00168695. [DOI] [PubMed] [Google Scholar]
- Obara K., Takai A., Ruegg J. C., de Lanerolle P. Okadaic acid, a phosphatase inhibitor, produces a Ca2+ and calmodulin-independent contraction of smooth muscle. Pflugers Arch. 1989 Jun;414(2):134–138. doi: 10.1007/BF00580954. [DOI] [PubMed] [Google Scholar]
- Pang D. C., Sperelakis N. Uptake of [3H]nitrendipine into cardiac and smooth muscles. Biochem Pharmacol. 1983 May 15;32(10):1660–1663. doi: 10.1016/0006-2952(83)90347-7. [DOI] [PubMed] [Google Scholar]
- Salaices M., Balfagon G., Arribas S., de Sagarra M. R., Marín J. Effects of phorbol 12,13-dibutyrate on the vascular tone and on norepinephrine- and potassium-induced contractions of cat cerebral arteries. J Pharmacol Exp Ther. 1990 Oct;255(1):66–73. [PubMed] [Google Scholar]
- Sato K., Ozaki H., Karaki H. Multiple effects of caffeine on contraction and cytosolic free Ca2+ levels in vascular smooth muscle of rat aorta. Naunyn Schmiedebergs Arch Pharmacol. 1988 Oct;338(4):443–448. doi: 10.1007/BF00172125. [DOI] [PubMed] [Google Scholar]
- Sato M., Nagao T., Yamaguchi I., Nakajima H., Kiyomoto A. Pharmacological studies on a new l,5-benzothiazepine derivative (CRD-401). Arzneimittelforschung. 1971 Sep;21(9):1338–1343. [PubMed] [Google Scholar]
- Schaeffer P., Lugnier C., Stoclet J. C. Interaction of calmodulin and calcium antagonists with [3H]diltiazem and [3H]nitrendipine binding sites. J Cardiovasc Pharmacol. 1988;12 (Suppl 4):S102–S103. doi: 10.1097/00005344-198806124-00021. [DOI] [PubMed] [Google Scholar]
- Schott C., Tetsi L., Heitz C., Stambach J. F., Jung L., Stoclet J. C. Stereoselective blockade of alpha-adrenoceptors by berbine derivatives. Arzneimittelforschung. 1988 Nov;38(11):1567–1571. [PubMed] [Google Scholar]
- Tagami M., Nara Y., Kubota A., Sunaga T., Maezawa H., Horie R., Yamori Y. Electronmicroscopic autoradiographic study of the distribution of 3H-diltiazem in myocardial cells. Jpn Heart J. 1985 Sep;26(5):823–832. doi: 10.1536/ihj.26.823. [DOI] [PubMed] [Google Scholar]
- Triggle D. J., Langs D. A., Janis R. A. Ca2+ channel ligands: structure-function relationships of the 1,4-dihydropyridines. Med Res Rev. 1989 Apr-Jun;9(2):123–180. doi: 10.1002/med.2610090203. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M., Rubanyi G. M., Miller V. M., Houston D. S. Modulation of vascular smooth muscle contraction by the endothelium. Annu Rev Physiol. 1986;48:307–320. doi: 10.1146/annurev.ph.48.030186.001515. [DOI] [PubMed] [Google Scholar]
- Wells J. N., Baird C. E., Hardman Y. J., Wu J. G. Cyclic nucleotide phosphodiesterase activities of pig coronary arteries. Biochim Biophys Acta. 1975 Apr 19;384(2):430–442. doi: 10.1016/0005-2744(75)90044-3. [DOI] [PubMed] [Google Scholar]
- Yao W. X., Xia G. J., Fang D. C., Jiang M. X. Studies on the calcium antagonistic action of tetrandrine: XIV. Influence of tetrandrine and verapamil on hemodynamic action of ouabain in guinea pigs. J Tongji Med Univ. 1987;7(2):80–83. doi: 10.1007/BF02888166. [DOI] [PubMed] [Google Scholar]


