Abstract
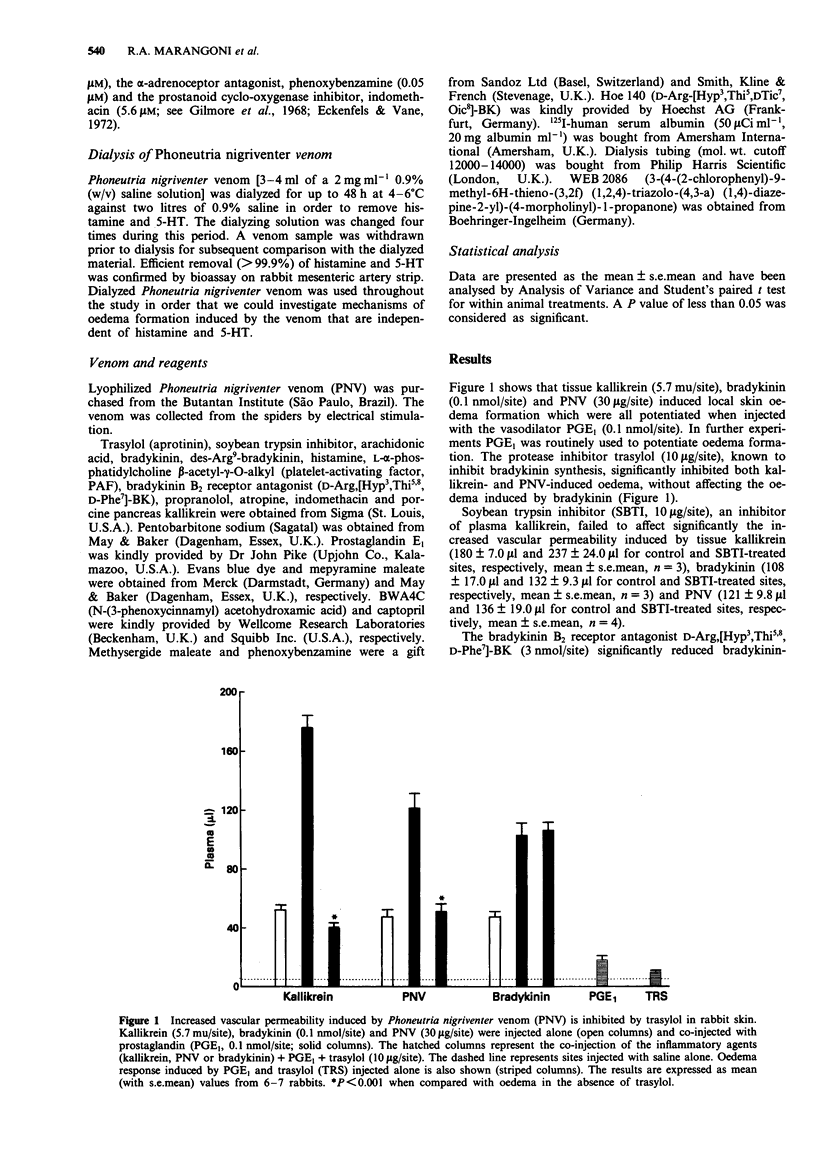
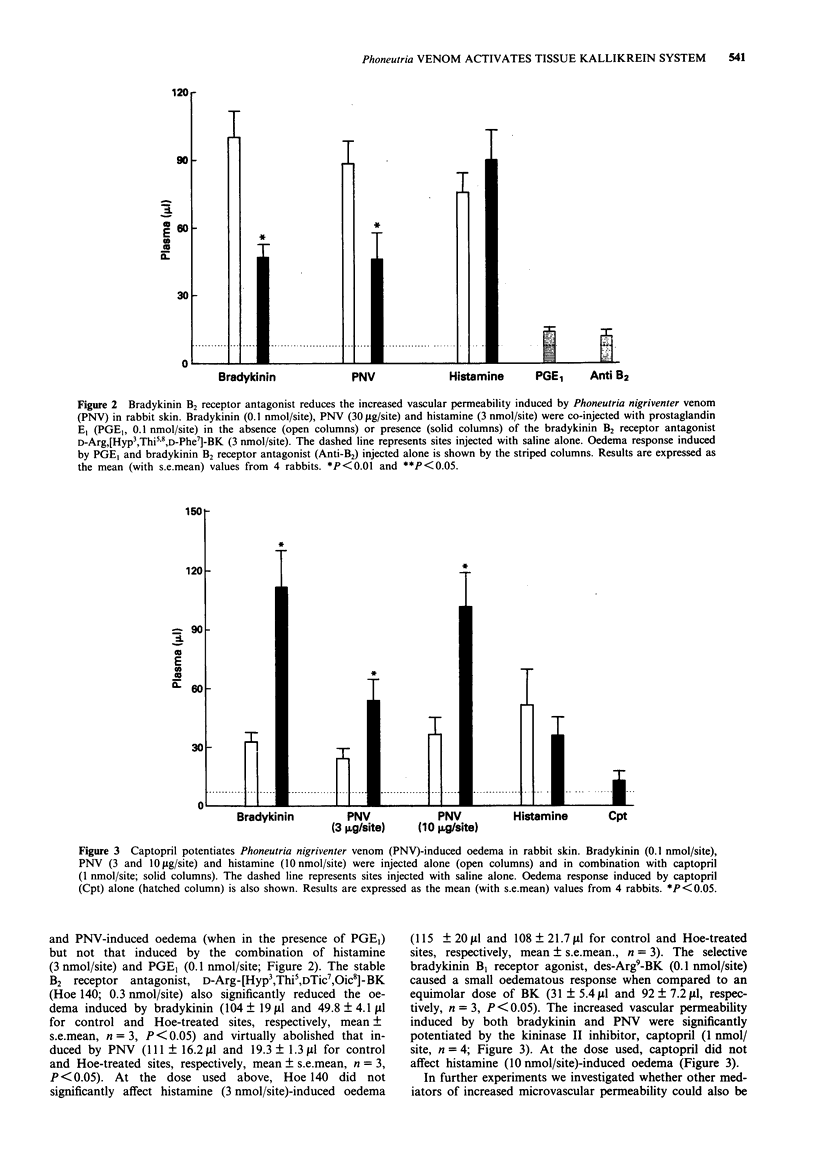
1. The purpose of the present study was to investigate the mechanisms by which venom from Phoneutria nigriventer spider induces increases in vascular permeability in rabbit skin. 2. Local oedema formation, in response to intradermally-injected agents, was measured in male New Zealand white rabbits as the local accumulation of i.v. injected 125I-labelled human serum albumin into skin sites. 3. Phoneutria nigriventer venom (10-30 micrograms/site) increased vascular permeability, which was inhibited by trasylol (10 micrograms/site) and the bradykinin B2 receptor antagonists D-Arg,[Hyp3,Thi5,8,D-Phe7]-BK (3 nmol/site) and Hoe 140 (0.3 nmol/site). In addition, the oedema induced by the venom was potentiated by the kinase II inhibitor, captopril (1 nmol/site). The lipoxygenase inhibitor, BWA4C (10 nmol/site) and the PAF antagonist, WEB 2086 (100 nmol/site) had no effect on the venom-induced increase in vascular permeability. 4. Incubation of rabbit plasma with Phoneutria nigriventer venom in vitro did not cause bradykinin formation. Further, the plasma kallikrein inhibitor, soybean trypsin inhibitor (10 micrograms/site), had no effect on the venom-induced increase in vascular permeability in rabbit skin. 5. These results indicate that the oedema produced by Phoneutria nigriventer venom is dependent on the activation of the tissue kallikrein-kinin system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antunes E., Marangoni R. A., Borges N. C., Hyslop S., Fontana M. D., de-Nucci G. Effects of Phoneutria nigriventer venom on rabbit vascular smooth muscle. Braz J Med Biol Res. 1993;26(1):81–91. [PubMed] [Google Scholar]
- Antunes E., Marangoni R. A., Brain S. D., de Nucci G. Phoneutria nigriventer (armed spider) venom induces increased vascular permeability in rat and rabbit skin in vivo. Toxicon. 1992 Sep;30(9):1011–1016. doi: 10.1016/0041-0101(92)90045-7. [DOI] [PubMed] [Google Scholar]
- Bathon J. M., Proud D. Bradykinin antagonists. Annu Rev Pharmacol Toxicol. 1991;31:129–162. doi: 10.1146/annurev.pa.31.040191.001021. [DOI] [PubMed] [Google Scholar]
- Brain S. D., Williams T. J. Inflammatory oedema induced by synergism between calcitonin gene-related peptide (CGRP) and mediators of increased vascular permeability. Br J Pharmacol. 1985 Dec;86(4):855–860. doi: 10.1111/j.1476-5381.1985.tb11107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray M. A., Cunningham F. M., Ford-Hutchinson A. W., Smith M. J. Leukotriene B4: a mediator of vascular permeability. Br J Pharmacol. 1981 Mar;72(3):483–486. doi: 10.1111/j.1476-5381.1981.tb11000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DINIZ C. R., CARVALHO I. F. A micromethod for determination of bradykininogen under several conditions. Ann N Y Acad Sci. 1963 Feb 4;104:77–89. doi: 10.1111/j.1749-6632.1963.tb17654.x. [DOI] [PubMed] [Google Scholar]
- Eckenfels A., Vane J. R. Prostaglandins, oxygen tension and smooth muscle tone. Br J Pharmacol. 1972 Jul;45(3):451–462. doi: 10.1111/j.1476-5381.1972.tb08101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans B. A., Yun Z. X., Close J. A., Tregear G. W., Kitamura N., Nakanishi S., Callen D. F., Baker E., Hyland V. J., Sutherland G. R. Structure and chromosomal localization of the human renal kallikrein gene. Biochemistry. 1988 May 3;27(9):3124–3129. doi: 10.1021/bi00409a003. [DOI] [PubMed] [Google Scholar]
- Ferreira S. H., Nakamura M., de Abreu Castro M. S. The hyperalgesic effects of prostacyclin and prostaglandin E2. Prostaglandins. 1978 Jul;16(1):31–37. doi: 10.1016/0090-6980(78)90199-5. [DOI] [PubMed] [Google Scholar]
- Ferreira S. H. Prostaglandins, aspirin-like drugs and analgesia. Nat New Biol. 1972 Dec 13;240(102):200–203. doi: 10.1038/newbio240200a0. [DOI] [PubMed] [Google Scholar]
- Fontana M. D., Vital-Brazil O., Vital-Brasil O. Mode of action of Phoneutria nigriventer spider venom at the isolated phrenic nerve-diaphragm of the rat. Braz J Med Biol Res. 1985;18(4):557–565. [PubMed] [Google Scholar]
- Fukushima D., Kitamura N., Nakanishi S. Nucleotide sequence of cloned cDNA for human pancreatic kallikrein. Biochemistry. 1985 Dec 31;24(27):8037–8043. doi: 10.1021/bi00348a030. [DOI] [PubMed] [Google Scholar]
- Gilmore N., Vane J. R., Wyllie J. H. Prostaglandins released by the spleen. Nature. 1968 Jun 22;218(5147):1135–1140. doi: 10.1038/2181135a0. [DOI] [PubMed] [Google Scholar]
- Higgs G. A., Follenfant R. L., Garland L. G. Selective inhibition of arachidonate 5-lipoxygenase by novel acetohydroxamic acids: effects on acute inflammatory responses. Br J Pharmacol. 1988 Jun;94(2):547–551. doi: 10.1111/j.1476-5381.1988.tb11559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S. B., Li C. L., Lam M. H., Shen T. Y. Characterization of cutaneous vascular permeability induced by platelet-activating factor in guinea pigs and rats and its inhibition by a platelet-activating factor receptor antagonist. Lab Invest. 1985 Jun;52(6):617–630. [PubMed] [Google Scholar]
- Lucas S. Spiders in Brazil. Toxicon. 1988;26(9):759–772. doi: 10.1016/0041-0101(88)90317-0. [DOI] [PubMed] [Google Scholar]
- Margolius H. S. Tissue kallikreins and kinins: regulation and roles in hypertensive and diabetic diseases. Annu Rev Pharmacol Toxicol. 1989;29:343–364. doi: 10.1146/annurev.pa.29.040189.002015. [DOI] [PubMed] [Google Scholar]
- McGivern D. V., Basran G. S. Synergism between platelet-activating factor (PAF-acether) and prostaglandin E2 in man. Eur J Pharmacol. 1984 Jun 15;102(1):183–185. doi: 10.1016/0014-2999(84)90356-x. [DOI] [PubMed] [Google Scholar]
- Morley J., Page C. P., Paul W. Inflammatory actions of platelet activating factor (Pafacether) in guinea-pig skin. Br J Pharmacol. 1983 Nov;80(3):503–509. doi: 10.1111/j.1476-5381.1983.tb10722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piek T. Neurotoxic kinins from wasp and ant venoms. Toxicon. 1991;29(2):139–149. doi: 10.1016/0041-0101(91)90098-c. [DOI] [PubMed] [Google Scholar]
- Proud D., Kaplan A. P. Kinin formation: mechanisms and role in inflammatory disorders. Annu Rev Immunol. 1988;6:49–83. doi: 10.1146/annurev.iy.06.040188.000405. [DOI] [PubMed] [Google Scholar]
- Regoli D., Rhaleb N. E., Dion S., Drapeau G. New selective bradykinin receptor antagonists and bradykinin B2 receptor characterization. Trends Pharmacol Sci. 1990 Apr;11(4):156–161. doi: 10.1016/0165-6147(90)90067-I. [DOI] [PubMed] [Google Scholar]
- Ueno A., Tanaka K., Katori M., Hayashi M., Arai Y. Species difference in increased vascular permeability by synthetic leukotriene C4 and D4. Prostaglandins. 1981 Apr;21(4):637–648. doi: 10.1016/0090-6980(81)90012-5. [DOI] [PubMed] [Google Scholar]
- Williams T. J., Morley J. Prostaglandins as potentiators of increased vascular permeability in inflammation. Nature. 1973 Nov 23;246(5430):215–217. doi: 10.1038/246215a0. [DOI] [PubMed] [Google Scholar]
- Williams T. J., Peck M. J. Role of prostaglandin-mediated vasodilatation in inflammation. Nature. 1977 Dec 8;270(5637):530–532. doi: 10.1038/270530a0. [DOI] [PubMed] [Google Scholar]
- Williams T. J. Prostaglandin E2, prostaglandin I2 and the vascular changes of inflammation. Br J Pharmacol. 1979 Mar;65(3):517–524. doi: 10.1111/j.1476-5381.1979.tb07860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth K., Hock F. J., Albus U., Linz W., Alpermann H. G., Anagnostopoulos H., Henk S., Breipohl G., König W., Knolle J. Hoe 140 a new potent and long acting bradykinin-antagonist: in vivo studies. Br J Pharmacol. 1991 Mar;102(3):774–777. doi: 10.1111/j.1476-5381.1991.tb12249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nucci G., Gryglewski R. J., Warner T. D., Vane J. R. Receptor-mediated release of endothelium-derived relaxing factor and prostacyclin from bovine aortic endothelial cells is coupled. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2334–2338. doi: 10.1073/pnas.85.7.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]



