Abstract
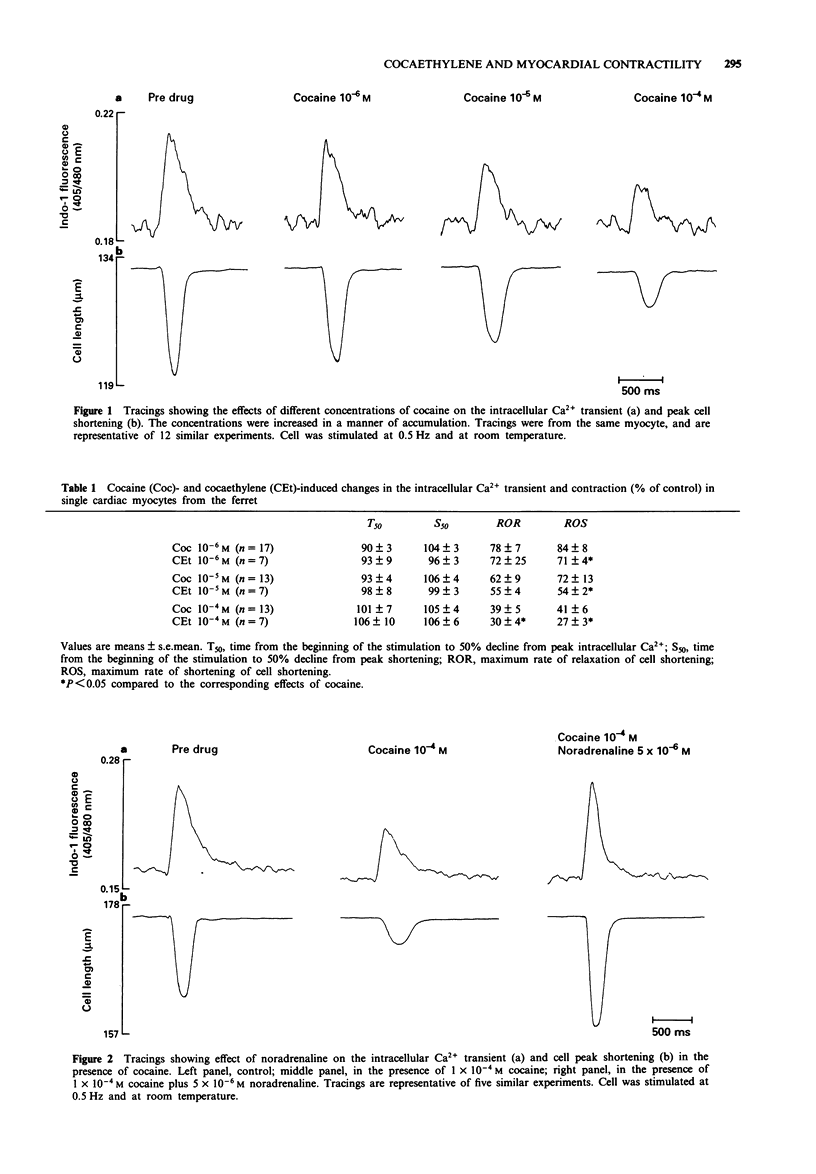
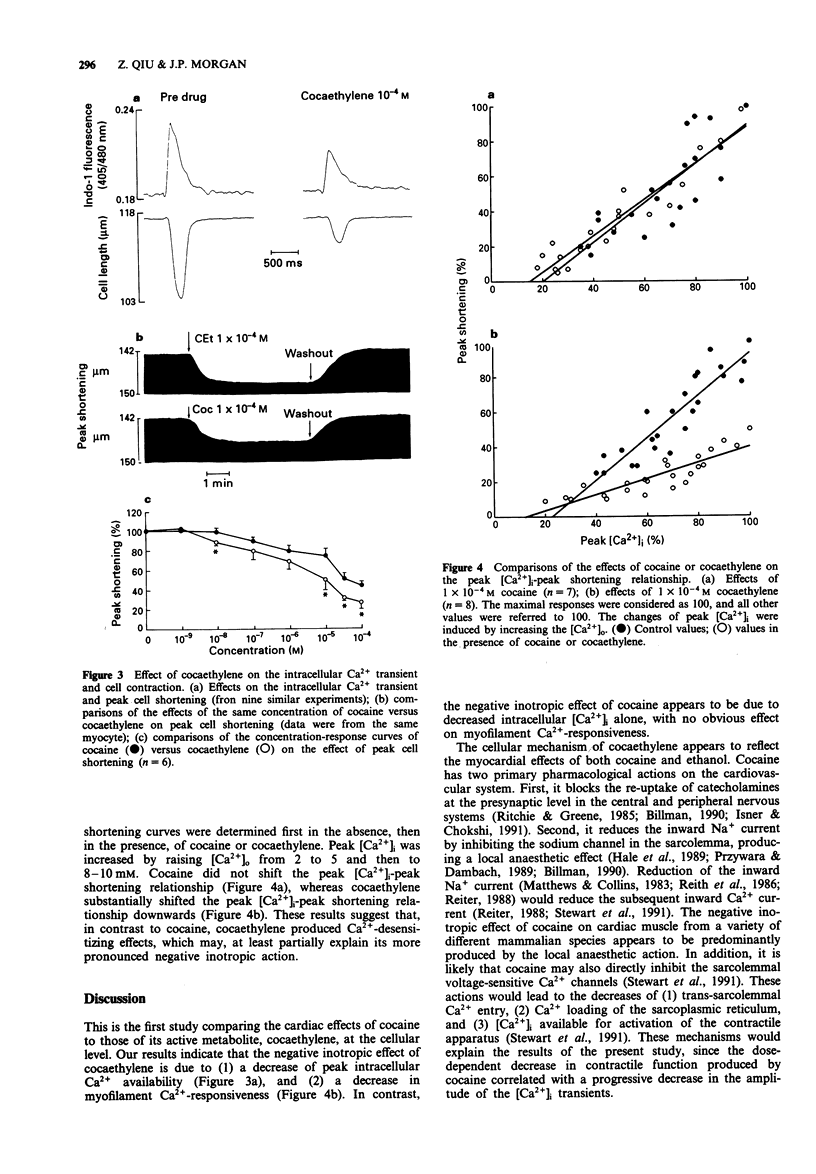
1. Isolated cardiac myocytes of the ferret were used to investigate the influence of cocaine and cocaethylene on the intracellular Ca2+ transient indicated by the indo-1 405/480 nm ratio signal, and peak cell shortening. 2. Both cocaine and cocaethylene produced significant decreases in peak intracellular Ca2+ and peak cell shortening in a dose-dependent manner. Of interest, (1) the minimally effective dose of cocaethylene was ten fold lower (10(-8)M versus 10(-7)M) than that of cocaine; (2) the log EC50 of cocaethylene was -5.99 +/- 0.13 (1.0 x 10(-6) M), which was about ten fold lower than that of cocaine (-5.02 +/- 0.11, 9.6 x 10(-6) M); and (3) 1 x 10(-4)M cocaethylene decreased the contraction amplitude by 71 +/- 7%, while the same concentration of cocaine decreased the amplitude only by 55 +/- 5%, indicating that cocaethylene is more potent than cocaine. 3. The negative inotropic effects of either cocaine or cocaethylene could be overcome by noradrenaline (approximately 5 microM) or calcium. 4. In contrast to cocaine, cocaethylene shifted the peak [Ca2+]i-peak shortening relationship downward, indicating that cocaethylene decreased myofilament Ca(2+)-responsiveness. 5. These data indicate that both cocaine and cocaethylene act directly on cardiac myocytes to produce a negative inotropic effect that is due to decreased Ca2+ availability. In contrast to cocaine, cocaethylene produces more potent inhibition by an additional action to decrease myofilament Ca(2+)-responsiveness.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett G., Hawks R., Resnick R. Cocaine pharmacokinetics in humans. J Ethnopharmacol. 1981 Mar-May;3(2-3):353–366. doi: 10.1016/0378-8741(81)90063-5. [DOI] [PubMed] [Google Scholar]
- Billman G. E. Mechanisms responsible for the cardiotoxic effects of cocaine. FASEB J. 1990 May;4(8):2469–2475. doi: 10.1096/fasebj.4.8.2185973. [DOI] [PubMed] [Google Scholar]
- Carpenter J. R. A method for presenting and comparing dose-response curves. J Pharmacol Methods. 1986 Jul;15(4):283–303. doi: 10.1016/0160-5402(86)90009-4. [DOI] [PubMed] [Google Scholar]
- Chang C. C., Lee M. C. Studies of the effect of phenoxybenzamine on the uptake of noradrenaline and on the response to sympathetic stimulation of rat hearts. Arch Int Pharmacodyn Ther. 1973 Feb;201(2):400–414. [PubMed] [Google Scholar]
- Copelas L., Briggs M., Grossman W., Morgan J. P. A method for recording isometric tension development by isolated cardiac myocytes: transducer attachment with fibrin glue. Pflugers Arch. 1987 Mar;408(3):315–317. doi: 10.1007/BF02181475. [DOI] [PubMed] [Google Scholar]
- Cregler L. L., Mark H. Medical complications of cocaine abuse. N Engl J Med. 1986 Dec 4;315(23):1495–1500. doi: 10.1056/NEJM198612043152327. [DOI] [PubMed] [Google Scholar]
- Danziger R. S., Sakai M., Capogrossi M. C., Spurgeon H. A., Hansford R. G., Lakatta E. G. Ethanol acutely and reversibly suppresses excitation-contraction coupling in cardiac myocytes. Circ Res. 1991 Jun;68(6):1660–1668. doi: 10.1161/01.res.68.6.1660. [DOI] [PubMed] [Google Scholar]
- Foltin R. W., Fischman M. W. Ethanol and cocaine interactions in humans: cardiovascular consequences. Pharmacol Biochem Behav. 1988 Dec;31(4):877–883. doi: 10.1016/0091-3057(88)90399-1. [DOI] [PubMed] [Google Scholar]
- Hale S. L., Lehmann M. H., Kloner R. A. Electrocardiographic abnormalities after acute administration of cocaine in the rat. Am J Cardiol. 1989 Jun 15;63(20):1529–1530. doi: 10.1016/0002-9149(89)90024-6. [DOI] [PubMed] [Google Scholar]
- Hardman J. G., Mayer S. E., Clark B. Cocaine potentiation of the cardiac inotropic and phosphorylase responses to catecholamines as related to the uptake of H3-catecholamines. J Pharmacol Exp Ther. 1965 Dec;150(3):341–348. [PubMed] [Google Scholar]
- Hearn W. L., Flynn D. D., Hime G. W., Rose S., Cofino J. C., Mantero-Atienza E., Wetli C. V., Mash D. C. Cocaethylene: a unique cocaine metabolite displays high affinity for the dopamine transporter. J Neurochem. 1991 Feb;56(2):698–701. doi: 10.1111/j.1471-4159.1991.tb08205.x. [DOI] [PubMed] [Google Scholar]
- Hearn W. L., Rose S., Wagner J., Ciarleglio A., Mash D. C. Cocaethylene is more potent than cocaine in mediating lethality. Pharmacol Biochem Behav. 1991 Jun;39(2):531–533. doi: 10.1016/0091-3057(91)90222-n. [DOI] [PubMed] [Google Scholar]
- Isner J. M., Chokshi S. K. Cardiovascular complications of cocaine. Curr Probl Cardiol. 1991 Feb;16(2):89–123. doi: 10.1016/0146-2806(91)90013-z. [DOI] [PubMed] [Google Scholar]
- Isner J. M., Estes N. A., 3rd, Thompson P. D., Costanzo-Nordin M. R., Subramanian R., Miller G., Katsas G., Sweeney K., Sturner W. Q. Acute cardiac events temporally related to cocaine abuse. N Engl J Med. 1986 Dec 4;315(23):1438–1443. doi: 10.1056/NEJM198612043152302. [DOI] [PubMed] [Google Scholar]
- Karch S. B., Billingham M. E. The pathology and etiology of cocaine-induced heart disease. Arch Pathol Lab Med. 1988 Mar;112(3):225–230. [PubMed] [Google Scholar]
- Katz A. M. Effects of ethanol on ion transport in muscle membranes. Fed Proc. 1982 Jun;41(8):2456–2459. [PubMed] [Google Scholar]
- Levy M. N., Blattberg B. The influence of cocaine and desipramine on the cardiac responses to exogenous and endogenous norepinephrine. Eur J Pharmacol. 1978 Mar 1;48(1):37–49. doi: 10.1016/0014-2999(78)90042-0. [DOI] [PubMed] [Google Scholar]
- Masuda Y., Levy M. N. Responses of the atrial myocardium to cardiac sympathetic nerve stimulation. Am J Physiol. 1985 Aug;249(2 Pt 2):H207–H211. doi: 10.1152/ajpheart.1985.249.2.H207. [DOI] [PubMed] [Google Scholar]
- Masur J., Souza-Formigoni M. L., Pires M. L. Increased stimulatory effect by the combined administration of cocaine and alcohol in mice. Alcohol. 1989 May-Jun;6(3):181–182. doi: 10.1016/0741-8329(89)90015-3. [DOI] [PubMed] [Google Scholar]
- Matthews J. C., Collins A. Interactions of cocaine and cocaine congeners with sodium channels. Biochem Pharmacol. 1983 Feb 1;32(3):455–460. doi: 10.1016/0006-2952(83)90523-3. [DOI] [PubMed] [Google Scholar]
- McCulloch M. W., Rand M. J., Story D. F. Resting and stimulation-induced efflux of tritium from guinea-pig atria incubated with 3-H-noradrenaline. Clin Exp Pharmacol Physiol. 1974 Jul-Aug;1(4):275–289. doi: 10.1111/j.1440-1681.1974.tb00550.x. [DOI] [PubMed] [Google Scholar]
- Perreault C. L., Hague N. L., Ransil B. J., Morgan J. P. The effects of cocaine on intracellular Ca2+ handling and myofilament Ca2+ responsiveness of ferret ventricular myocardium. Br J Pharmacol. 1990 Nov;101(3):679–685. doi: 10.1111/j.1476-5381.1990.tb14140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polimeni P. I., Otten M. D., Hoeschen L. E. In vivo effects of ethanol on the rat myocardium: evidence for a reversible, non-specific increase of sarcolemmal permeability. J Mol Cell Cardiol. 1983 Feb;15(2):113–122. doi: 10.1016/0022-2828(83)90287-0. [DOI] [PubMed] [Google Scholar]
- Przywara D. A., Dambach G. E. Direct actions of cocaine on cardiac cellular electrical activity. Circ Res. 1989 Jul;65(1):185–192. doi: 10.1161/01.res.65.1.185. [DOI] [PubMed] [Google Scholar]
- Reiter M. Calcium mobilization and cardiac inotropic mechanisms. Pharmacol Rev. 1988 Sep;40(3):189–217. [PubMed] [Google Scholar]
- Reith M. E., Kim S. S., Lajtha A. Structural requirements for cocaine congeners to interact with [3H]batrachotoxinin A 20-alpha-benzoate binding sites on sodium channels in mouse brain synaptosomes. J Biol Chem. 1986 Jun 5;261(16):7300–7305. [PubMed] [Google Scholar]
- Smith R. M. Ethyl esters of arylhydroxy- and arylhydroxymethoxycocaines in the urines of simultaneous cocaine and ethanol users. J Anal Toxicol. 1984 Jan-Feb;8(1):38–42. doi: 10.1093/jat/8.1.38. [DOI] [PubMed] [Google Scholar]
- Spurgeon H. A., Stern M. D., Baartz G., Raffaeli S., Hansford R. G., Talo A., Lakatta E. G., Capogrossi M. C. Simultaneous measurement of Ca2+, contraction, and potential in cardiac myocytes. Am J Physiol. 1990 Feb;258(2 Pt 2):H574–H586. doi: 10.1152/ajpheart.1990.258.2.H574. [DOI] [PubMed] [Google Scholar]
- Stewart D. J., Inaba T., Lucassen M., Kalow W. Cocaine metabolism: cocaine and norcocaine hydrolysis by liver and serum esterases. Clin Pharmacol Ther. 1979 Apr;25(4):464–468. doi: 10.1002/cpt1979254464. [DOI] [PubMed] [Google Scholar]
- Stewart G., Rubin E., Thomas A. P. Inhibition by cocaine of excitation-contraction coupling in isolated cardiomyocytes. Am J Physiol. 1991 Jan;260(1 Pt 2):H50–H57. doi: 10.1152/ajpheart.1991.260.1.H50. [DOI] [PubMed] [Google Scholar]
- Taraschi T. F., Rubin E. Effects of ethanol on the chemical and structural properties of biologic membranes. Lab Invest. 1985 Feb;52(2):120–131. [PubMed] [Google Scholar]
- Tazelaar H. D., Karch S. B., Stephens B. G., Billingham M. E. Cocaine and the heart. Hum Pathol. 1987 Feb;18(2):195–199. doi: 10.1016/s0046-8177(87)80338-6. [DOI] [PubMed] [Google Scholar]
- WEIDMANN S. Effects of calcium ions and local anesthetics on electrical properties of Purkinje fibres. J Physiol. 1955 Sep 28;129(3):568–582. doi: 10.1113/jphysiol.1955.sp005379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. D., Mirin S. M., Griffin M. L., Michael J. L. Psychopathology in cocaine abusers. Changing trends. J Nerv Ment Dis. 1988 Dec;176(12):719–725. doi: 10.1097/00005053-198812000-00004. [DOI] [PubMed] [Google Scholar]
- Wilkinson P., Van Dyke C., Jatlow P., Barash P., Byck R. Intranasal and oral cocaine kinetics. Clin Pharmacol Ther. 1980 Mar;27(3):386–394. doi: 10.1038/clpt.1980.52. [DOI] [PubMed] [Google Scholar]



